User login
Guidance covers glycemia in dexamethasone-treated COVID-19 patients
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
New guidance from the U.K. National Diabetes COVID-19 Response Group addresses glucose management in patients with COVID-19 who are receiving dexamethasone therapy.
Although there are already guidelines that address inpatient management of steroid-induced hyperglycemia, the authors of the new document wrote that this new expert opinion paper was needed “given the ‘triple insult’ of dexamethasone-induced–impaired glucose metabolism, COVID-19–induced insulin resistance, and COVID-19–impaired insulin production.”
RECOVERY trial spurs response
The document, which is the latest in a series from the Association of British Clinical Diabetologists, was published online Aug. 2 in Diabetic Medicine. The group is chaired by Gerry Rayman, MD, consultant physician at the diabetes centre and diabetes research unit, East Suffolk (England) and North East NHS Foundation Trust.
The guidance was developed in response to the recent “breakthrough” Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which showed that dexamethasone reduced deaths in patients with COVID-19 on ventilators or receiving oxygen therapy. The advice is not intended for critical care units but can be adapted for that use.
The dose used in RECOVERY – 6 mg daily for 10 days – is 400%-500% greater than the therapeutic glucocorticoid replacement dose. High glucocorticoid doses can exacerbate hyperglycemia in people with established diabetes, unmask undiagnosed diabetes, precipitate hyperglycemia or new-onset diabetes, and can also cause hyperglycemic hyperosmolar state (HHS), the authors explained.
They recommended a target glucose of 6.0-10.0 mmol/L (108-180 mg/dL), although they say up to 12 mmol/L (216 mg/dL) is “acceptable.” They then gave advice on frequency of monitoring for people with and without known diabetes, exclusion of diabetic ketoacidosis and HHS, correction of initial hyperglycemia and maintenance of glycemic control using subcutaneous insulin, and prevention of hypoglycemia at the end of dexamethasone therapy (day 10) with insulin down-titration, discharge, and follow-up.
The detailed insulin guidance covers dose escalation for both insulin-treated and insulin-naive patients. A table suggests increasing correction doses of rapid-acting insulin based on prior total daily dose or weight.
Use of once- or twice-daily NPH insulin is recommended for patients whose glucose has risen above 12 mmol/L, in some cases with the addition of a long-acting analog. A second chart gives dose adjustments for those insulins. Additional guidance addresses patients on insulin pumps.
Guidance useful for U.S. physicians
Francisco Pasquel, MD, assistant professor of medicine in the division of endocrinology at Emory University, Atlanta, said in an interview that he believes the guidance is “acceptable” for worldwide use, and that “it’s coherent and consistent with what we typically do.”
However, Dr. Pasquel, who founded COVID-in-Diabetes, an online repository of published guidance and shared experience – to which this new document has now been added – did take issue with one piece of advice. The guidance says that patients already taking premixed insulin formulations can continue using them while increasing the dose by 20%-40%. Given the risk of hypoglycemia associated with those formulations, Dr. Pasquel said he would switch those patients to NPH during the time that they’re on dexamethasone.
He also noted that the rapid-acting insulin dose range of 2-10 units provided in the first table, for correction of initial hyperglycemia, are more conservative than those used at his hospital, where correction doses of up to 14-16 units are sometimes necessary.
But Dr. Pasquel praised the group’s overall efforts since the pandemic began, noting that “they’re very organized and constantly updating their recommendations. They have a unified system in the [National Health Service], so it’s easier to standardize. They have a unique [electronic health record] which is far superior to what we do from a public health perspective.”
Dr. Rayman reported no relevant financial relationships. Dr. Pasquel reported receiving research funding from Dexcom, Merck, and the National Institutes of Health, and consulting for AstraZeneca, Eli Lilly, Merck, and Boehringer Ingelheim.
A version of this article originally appeared on Medscape.com.
Antiaffirmative action paper blasted on Twitter now retracted
An article published in March in the Journal of the American Heart Association that raised a ruckus on #medtwitter this week has now been retracted.
It’s unclear what prompted the public explosion of anger, sadness, and recrimination that ultimately led to the retraction of this article – which flew almost completely under the radar when it first appeared online and in print – but it’s crystal clear why it might offend.
To many readers, the paper, written by Norman C. Wang, MD, MSc, an electrophysiologist at the University of Pittsburgh Medical Center, is a “racist” rant that relies on half-truths (J Am Heart Assoc. 2020 Mar 24. doi: 10.1161/JAHA.120.015959).
Officially, the article, “Diversity, Inclusion, and Equity: Evolution of Race and Ethnicity Considerations for the Cardiology Workforce in the United States of America From 1969 to 2019,” was retracted after the American Heart Association “became aware of serious concerns after publication. The author’s institution, the University of Pittsburgh Medical Center, has notified the Editor‐in‐Chief that the article contains many misconceptions and misquotes and that together those inaccuracies, misstatements, and selective misreading of source materials strip the paper of its scientific validity,” the retraction reads (J Am Heart Assoc. 2020 Aug 6. doi: 10.1161/JAHA.119.014602).
The journal will be publishing a detailed rebuttal, the notice adds: “This retraction notice will be updated with a link to the rebuttal when it publishes.”
“The Editor‐in‐Chief deeply regrets publishing the article and offers his apologies,” it further reads. “The American Heart Association and the Editor‐in‐Chief have determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The author does not agree to the retraction.”
In the paper, Dr. Wang argues that affirmative action policies designed to increase minority representation in medical schools and cardiovascular training programs result in unqualified applicants being admitted, where they will struggle to succeed.
The article itself is a dense review of the topic of diversity, inclusion, and equity, aiming to “critically assess current paradigms, and to consider potential solutions to anticipated challenges,” according to its author. Supported by 108 references, Dr. Wang concludes with a lengthy quote from tennis great Arthur Ashe, an opponent of affirmative action who died in 1993.
Affirmative action, said Mr. Ashe, is “an insult to the people it intended to help.” Dr. Wang suggests that “racial and ethnic preferences for undergraduate and medical school admissions should be gradually rolled back with a target end year of 2028.”
He cites the $16 billion in federal funding that cardiovascular disease training programs receive every year to support graduate medical education in support of this contention.
#Medtwitter explodes
“My entire lived experience contradicts everything in that racist @JAHA_AHA article, as does the experience of so many others. So, I know it’s just a bad opinion piece passed off as ‘research’ that shouldn’t have been published. Still the damage has been done. We MUST do better,” tweeted Bryan A. Smith, MD, University of Chicago Medicine.
According to its Altmetric score, the article received very little attention back in March and April. There were three tweets referencing it, including one from JAHA announcing its publication. Since Aug. 2, an additional 390-odd Tweets from 347 Twitter users have been registered. None appear to be complimentary. Several days into the Twitter storm, the article was officially retracted.
“This article is shocking and makes me sad,” Martha Gulati, MD, University of Arizona, Phoenix, said in an interview. “We are all working so hard to make cardiology more inclusive and diverse, and this takes us like 1,000 steps backwards.”
For her part, Dr. Gulati would have liked a retraction earlier in the week. “The analysis was selective and incorrect, and the statements made intimate that minority trainees were selected based on affirmative action rather than their merits,” she said. It also suggested that their presence was representative of a decline in standards in cardiology programs that take underrepresented minorities (URMs).
Standard arguments against affirmative action
According to Dr. Wang, who did not respond to a request to comment for this article, allowing minority students into medical school with academic records that are weaker than their classmates sets them up for failure.
“Many do not complete their intended programs or do not attain academic success to be attractive candidates for subsequent educational programs or employment,” he wrote.
This is a standard argument of opponents to affirmative action, said Quinn Capers IV, MD. Dr. Capers, a longtime advocate for diversity in medicine, acknowledges that, “on average,” test scores for Blacks, Hispanics, and Native Americans tend to be lower than for White applicants for a wide range of reasons, many of which are related to systemic racism.
“This is the strongest weapon opponents to affirmative action have, and they keep coming back to it, but it’s out of step with how many in academic medicine feel,” said Dr. Capers, who is an interventional cardiologist and the vice dean for faculty affairs at Ohio State University, Columbus.
This is why, he added, most medical schools have embraced the Association of American Medical Colleges’ concept of “holistic review,” which judges potential physicians on their academic records, their personal experiences, and their individual attributes.
“Standardized tests and academic records are important, but so are the experiences one has gone through and the individual attributes they may have. How resilient are you? How compassionate? Our embrace of this more holistic approach, I believe, is helping many medical schools move toward having a more diverse class that is closer to reflecting the needs of our multicultural and multiracial society,” Dr. Capers said.
To be clear, Dr. Capers is not afraid of having a discussion on this topic and denies that the uproar against this article represents “cancel culture.”
“Hey, I love to debate and I’m not against hearing divisive voices, but then let’s have a debate and hear both sides. But there are several problems with the way they did this. No. 1, they called it a ‘white paper,’ which to most people means it reflects the views of the organization, not a specific individual, and, secondly, it’s more than an opinion piece in that he manipulates facts to make his points, with no chance for rebuttal.”
Several have also questioned how this paper, which is written by a nonexpert in the field, passed peer review.
The article contains some accurate historical references, said Dr. Capers, but intertwined with this history the author editorializes in a fashion that is “charged with racism.” In other places, Dr. Wang is just outright wrong, he added.
“I can also tell you that, in one place where he quotes me specifically, what he says is quite damaging and completely wrong. He quotes something we wrote but cuts off the final sentence, making it seem as though we acknowledged that we had to artificially rank minority applicants high, just so we could say we have a diverse fellowship program.
“It’s frankly very hard to believe that was an accident,” Dr. Capers added.
AHA backs away, promises investigation
The article has been disowned by all levels of the AHA leadership – past, present, and future.
In an Editor’s Note, Barry London, MD, PhD, the Editor in chief of the Journal of the American Heart Association, apologized for his role and the role of his staff in publishing the article.
“JAHA will support all efforts to correct this error, including but not limited to the publication of alternate viewpoints, which we solicited at the time of publication but have not yet been submitted to the journal. In addition, we will work to improve our peer review system to prevent future missteps of this type,” Dr. London wrote. “I can only hope that igniting a discussion around diversity in cardiology will ultimately fuel new ideas and lead to real advances.”
“I want to emphasize in the strongest possible terms that this paper does not represent the views of the AHA as an organization or its leadership. This paper should never have been published. A thorough investigation is rightly being conducted,” tweeted Mitchell S.V. Elkind, MD, MPhil, who took over the AHA presidency last month.
“Author’s views are racist and not consistent with my values nor AHA,” tweeted Robert Harrington, MD, immediate past president of the AHA. ‘Investigation is underway into how it made it through the editorial process. Like you, I want to know what happened. I am angry, frustrated and disappointed that this piece was published; expect review soon.’
“Agree with @HeartBobH. It is impossible not to hear and feel the hurt and pain out there on a very personal level, especially among our young colleagues. You are valued, and worthy. Please stay tuned and then help all of us work to be better,” tweeted Donald Lloyd-Jones, MD, president-elect of AHA.
A version of this article originally appeared on Medscape.com.
An article published in March in the Journal of the American Heart Association that raised a ruckus on #medtwitter this week has now been retracted.
It’s unclear what prompted the public explosion of anger, sadness, and recrimination that ultimately led to the retraction of this article – which flew almost completely under the radar when it first appeared online and in print – but it’s crystal clear why it might offend.
To many readers, the paper, written by Norman C. Wang, MD, MSc, an electrophysiologist at the University of Pittsburgh Medical Center, is a “racist” rant that relies on half-truths (J Am Heart Assoc. 2020 Mar 24. doi: 10.1161/JAHA.120.015959).
Officially, the article, “Diversity, Inclusion, and Equity: Evolution of Race and Ethnicity Considerations for the Cardiology Workforce in the United States of America From 1969 to 2019,” was retracted after the American Heart Association “became aware of serious concerns after publication. The author’s institution, the University of Pittsburgh Medical Center, has notified the Editor‐in‐Chief that the article contains many misconceptions and misquotes and that together those inaccuracies, misstatements, and selective misreading of source materials strip the paper of its scientific validity,” the retraction reads (J Am Heart Assoc. 2020 Aug 6. doi: 10.1161/JAHA.119.014602).
The journal will be publishing a detailed rebuttal, the notice adds: “This retraction notice will be updated with a link to the rebuttal when it publishes.”
“The Editor‐in‐Chief deeply regrets publishing the article and offers his apologies,” it further reads. “The American Heart Association and the Editor‐in‐Chief have determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The author does not agree to the retraction.”
In the paper, Dr. Wang argues that affirmative action policies designed to increase minority representation in medical schools and cardiovascular training programs result in unqualified applicants being admitted, where they will struggle to succeed.
The article itself is a dense review of the topic of diversity, inclusion, and equity, aiming to “critically assess current paradigms, and to consider potential solutions to anticipated challenges,” according to its author. Supported by 108 references, Dr. Wang concludes with a lengthy quote from tennis great Arthur Ashe, an opponent of affirmative action who died in 1993.
Affirmative action, said Mr. Ashe, is “an insult to the people it intended to help.” Dr. Wang suggests that “racial and ethnic preferences for undergraduate and medical school admissions should be gradually rolled back with a target end year of 2028.”
He cites the $16 billion in federal funding that cardiovascular disease training programs receive every year to support graduate medical education in support of this contention.
#Medtwitter explodes
“My entire lived experience contradicts everything in that racist @JAHA_AHA article, as does the experience of so many others. So, I know it’s just a bad opinion piece passed off as ‘research’ that shouldn’t have been published. Still the damage has been done. We MUST do better,” tweeted Bryan A. Smith, MD, University of Chicago Medicine.
According to its Altmetric score, the article received very little attention back in March and April. There were three tweets referencing it, including one from JAHA announcing its publication. Since Aug. 2, an additional 390-odd Tweets from 347 Twitter users have been registered. None appear to be complimentary. Several days into the Twitter storm, the article was officially retracted.
“This article is shocking and makes me sad,” Martha Gulati, MD, University of Arizona, Phoenix, said in an interview. “We are all working so hard to make cardiology more inclusive and diverse, and this takes us like 1,000 steps backwards.”
For her part, Dr. Gulati would have liked a retraction earlier in the week. “The analysis was selective and incorrect, and the statements made intimate that minority trainees were selected based on affirmative action rather than their merits,” she said. It also suggested that their presence was representative of a decline in standards in cardiology programs that take underrepresented minorities (URMs).
Standard arguments against affirmative action
According to Dr. Wang, who did not respond to a request to comment for this article, allowing minority students into medical school with academic records that are weaker than their classmates sets them up for failure.
“Many do not complete their intended programs or do not attain academic success to be attractive candidates for subsequent educational programs or employment,” he wrote.
This is a standard argument of opponents to affirmative action, said Quinn Capers IV, MD. Dr. Capers, a longtime advocate for diversity in medicine, acknowledges that, “on average,” test scores for Blacks, Hispanics, and Native Americans tend to be lower than for White applicants for a wide range of reasons, many of which are related to systemic racism.
“This is the strongest weapon opponents to affirmative action have, and they keep coming back to it, but it’s out of step with how many in academic medicine feel,” said Dr. Capers, who is an interventional cardiologist and the vice dean for faculty affairs at Ohio State University, Columbus.
This is why, he added, most medical schools have embraced the Association of American Medical Colleges’ concept of “holistic review,” which judges potential physicians on their academic records, their personal experiences, and their individual attributes.
“Standardized tests and academic records are important, but so are the experiences one has gone through and the individual attributes they may have. How resilient are you? How compassionate? Our embrace of this more holistic approach, I believe, is helping many medical schools move toward having a more diverse class that is closer to reflecting the needs of our multicultural and multiracial society,” Dr. Capers said.
To be clear, Dr. Capers is not afraid of having a discussion on this topic and denies that the uproar against this article represents “cancel culture.”
“Hey, I love to debate and I’m not against hearing divisive voices, but then let’s have a debate and hear both sides. But there are several problems with the way they did this. No. 1, they called it a ‘white paper,’ which to most people means it reflects the views of the organization, not a specific individual, and, secondly, it’s more than an opinion piece in that he manipulates facts to make his points, with no chance for rebuttal.”
Several have also questioned how this paper, which is written by a nonexpert in the field, passed peer review.
The article contains some accurate historical references, said Dr. Capers, but intertwined with this history the author editorializes in a fashion that is “charged with racism.” In other places, Dr. Wang is just outright wrong, he added.
“I can also tell you that, in one place where he quotes me specifically, what he says is quite damaging and completely wrong. He quotes something we wrote but cuts off the final sentence, making it seem as though we acknowledged that we had to artificially rank minority applicants high, just so we could say we have a diverse fellowship program.
“It’s frankly very hard to believe that was an accident,” Dr. Capers added.
AHA backs away, promises investigation
The article has been disowned by all levels of the AHA leadership – past, present, and future.
In an Editor’s Note, Barry London, MD, PhD, the Editor in chief of the Journal of the American Heart Association, apologized for his role and the role of his staff in publishing the article.
“JAHA will support all efforts to correct this error, including but not limited to the publication of alternate viewpoints, which we solicited at the time of publication but have not yet been submitted to the journal. In addition, we will work to improve our peer review system to prevent future missteps of this type,” Dr. London wrote. “I can only hope that igniting a discussion around diversity in cardiology will ultimately fuel new ideas and lead to real advances.”
“I want to emphasize in the strongest possible terms that this paper does not represent the views of the AHA as an organization or its leadership. This paper should never have been published. A thorough investigation is rightly being conducted,” tweeted Mitchell S.V. Elkind, MD, MPhil, who took over the AHA presidency last month.
“Author’s views are racist and not consistent with my values nor AHA,” tweeted Robert Harrington, MD, immediate past president of the AHA. ‘Investigation is underway into how it made it through the editorial process. Like you, I want to know what happened. I am angry, frustrated and disappointed that this piece was published; expect review soon.’
“Agree with @HeartBobH. It is impossible not to hear and feel the hurt and pain out there on a very personal level, especially among our young colleagues. You are valued, and worthy. Please stay tuned and then help all of us work to be better,” tweeted Donald Lloyd-Jones, MD, president-elect of AHA.
A version of this article originally appeared on Medscape.com.
An article published in March in the Journal of the American Heart Association that raised a ruckus on #medtwitter this week has now been retracted.
It’s unclear what prompted the public explosion of anger, sadness, and recrimination that ultimately led to the retraction of this article – which flew almost completely under the radar when it first appeared online and in print – but it’s crystal clear why it might offend.
To many readers, the paper, written by Norman C. Wang, MD, MSc, an electrophysiologist at the University of Pittsburgh Medical Center, is a “racist” rant that relies on half-truths (J Am Heart Assoc. 2020 Mar 24. doi: 10.1161/JAHA.120.015959).
Officially, the article, “Diversity, Inclusion, and Equity: Evolution of Race and Ethnicity Considerations for the Cardiology Workforce in the United States of America From 1969 to 2019,” was retracted after the American Heart Association “became aware of serious concerns after publication. The author’s institution, the University of Pittsburgh Medical Center, has notified the Editor‐in‐Chief that the article contains many misconceptions and misquotes and that together those inaccuracies, misstatements, and selective misreading of source materials strip the paper of its scientific validity,” the retraction reads (J Am Heart Assoc. 2020 Aug 6. doi: 10.1161/JAHA.119.014602).
The journal will be publishing a detailed rebuttal, the notice adds: “This retraction notice will be updated with a link to the rebuttal when it publishes.”
“The Editor‐in‐Chief deeply regrets publishing the article and offers his apologies,” it further reads. “The American Heart Association and the Editor‐in‐Chief have determined that the best interest of the public and the research community will be served by issuing this notice of retraction. The author does not agree to the retraction.”
In the paper, Dr. Wang argues that affirmative action policies designed to increase minority representation in medical schools and cardiovascular training programs result in unqualified applicants being admitted, where they will struggle to succeed.
The article itself is a dense review of the topic of diversity, inclusion, and equity, aiming to “critically assess current paradigms, and to consider potential solutions to anticipated challenges,” according to its author. Supported by 108 references, Dr. Wang concludes with a lengthy quote from tennis great Arthur Ashe, an opponent of affirmative action who died in 1993.
Affirmative action, said Mr. Ashe, is “an insult to the people it intended to help.” Dr. Wang suggests that “racial and ethnic preferences for undergraduate and medical school admissions should be gradually rolled back with a target end year of 2028.”
He cites the $16 billion in federal funding that cardiovascular disease training programs receive every year to support graduate medical education in support of this contention.
#Medtwitter explodes
“My entire lived experience contradicts everything in that racist @JAHA_AHA article, as does the experience of so many others. So, I know it’s just a bad opinion piece passed off as ‘research’ that shouldn’t have been published. Still the damage has been done. We MUST do better,” tweeted Bryan A. Smith, MD, University of Chicago Medicine.
According to its Altmetric score, the article received very little attention back in March and April. There were three tweets referencing it, including one from JAHA announcing its publication. Since Aug. 2, an additional 390-odd Tweets from 347 Twitter users have been registered. None appear to be complimentary. Several days into the Twitter storm, the article was officially retracted.
“This article is shocking and makes me sad,” Martha Gulati, MD, University of Arizona, Phoenix, said in an interview. “We are all working so hard to make cardiology more inclusive and diverse, and this takes us like 1,000 steps backwards.”
For her part, Dr. Gulati would have liked a retraction earlier in the week. “The analysis was selective and incorrect, and the statements made intimate that minority trainees were selected based on affirmative action rather than their merits,” she said. It also suggested that their presence was representative of a decline in standards in cardiology programs that take underrepresented minorities (URMs).
Standard arguments against affirmative action
According to Dr. Wang, who did not respond to a request to comment for this article, allowing minority students into medical school with academic records that are weaker than their classmates sets them up for failure.
“Many do not complete their intended programs or do not attain academic success to be attractive candidates for subsequent educational programs or employment,” he wrote.
This is a standard argument of opponents to affirmative action, said Quinn Capers IV, MD. Dr. Capers, a longtime advocate for diversity in medicine, acknowledges that, “on average,” test scores for Blacks, Hispanics, and Native Americans tend to be lower than for White applicants for a wide range of reasons, many of which are related to systemic racism.
“This is the strongest weapon opponents to affirmative action have, and they keep coming back to it, but it’s out of step with how many in academic medicine feel,” said Dr. Capers, who is an interventional cardiologist and the vice dean for faculty affairs at Ohio State University, Columbus.
This is why, he added, most medical schools have embraced the Association of American Medical Colleges’ concept of “holistic review,” which judges potential physicians on their academic records, their personal experiences, and their individual attributes.
“Standardized tests and academic records are important, but so are the experiences one has gone through and the individual attributes they may have. How resilient are you? How compassionate? Our embrace of this more holistic approach, I believe, is helping many medical schools move toward having a more diverse class that is closer to reflecting the needs of our multicultural and multiracial society,” Dr. Capers said.
To be clear, Dr. Capers is not afraid of having a discussion on this topic and denies that the uproar against this article represents “cancel culture.”
“Hey, I love to debate and I’m not against hearing divisive voices, but then let’s have a debate and hear both sides. But there are several problems with the way they did this. No. 1, they called it a ‘white paper,’ which to most people means it reflects the views of the organization, not a specific individual, and, secondly, it’s more than an opinion piece in that he manipulates facts to make his points, with no chance for rebuttal.”
Several have also questioned how this paper, which is written by a nonexpert in the field, passed peer review.
The article contains some accurate historical references, said Dr. Capers, but intertwined with this history the author editorializes in a fashion that is “charged with racism.” In other places, Dr. Wang is just outright wrong, he added.
“I can also tell you that, in one place where he quotes me specifically, what he says is quite damaging and completely wrong. He quotes something we wrote but cuts off the final sentence, making it seem as though we acknowledged that we had to artificially rank minority applicants high, just so we could say we have a diverse fellowship program.
“It’s frankly very hard to believe that was an accident,” Dr. Capers added.
AHA backs away, promises investigation
The article has been disowned by all levels of the AHA leadership – past, present, and future.
In an Editor’s Note, Barry London, MD, PhD, the Editor in chief of the Journal of the American Heart Association, apologized for his role and the role of his staff in publishing the article.
“JAHA will support all efforts to correct this error, including but not limited to the publication of alternate viewpoints, which we solicited at the time of publication but have not yet been submitted to the journal. In addition, we will work to improve our peer review system to prevent future missteps of this type,” Dr. London wrote. “I can only hope that igniting a discussion around diversity in cardiology will ultimately fuel new ideas and lead to real advances.”
“I want to emphasize in the strongest possible terms that this paper does not represent the views of the AHA as an organization or its leadership. This paper should never have been published. A thorough investigation is rightly being conducted,” tweeted Mitchell S.V. Elkind, MD, MPhil, who took over the AHA presidency last month.
“Author’s views are racist and not consistent with my values nor AHA,” tweeted Robert Harrington, MD, immediate past president of the AHA. ‘Investigation is underway into how it made it through the editorial process. Like you, I want to know what happened. I am angry, frustrated and disappointed that this piece was published; expect review soon.’
“Agree with @HeartBobH. It is impossible not to hear and feel the hurt and pain out there on a very personal level, especially among our young colleagues. You are valued, and worthy. Please stay tuned and then help all of us work to be better,” tweeted Donald Lloyd-Jones, MD, president-elect of AHA.
A version of this article originally appeared on Medscape.com.
ED visits for mental health, substance use doubled in 1 decade
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).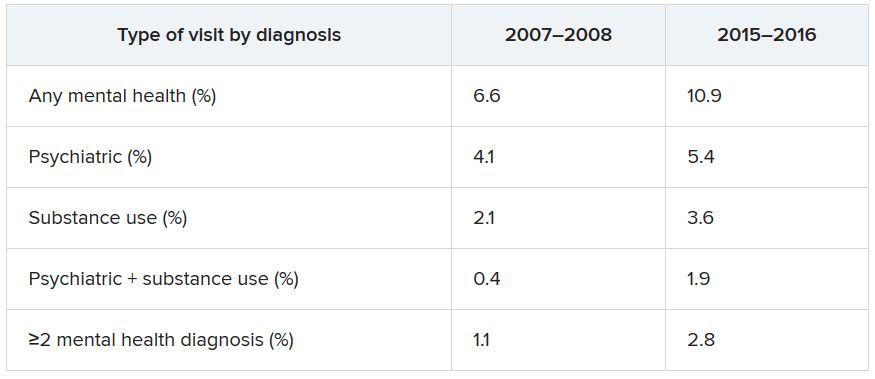
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Chronicles of cancer: A history of mammography, part 1
Technological imperatives
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Technological imperatives
Technological imperatives
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
The history of mammography provides a powerful example of the connection between social factors and the rise of a medical technology. It is also an object lesson in the profound difficulties that the medical community faces when trying to evaluate and embrace new discoveries in such a complex area as cancer diagnosis and treatment, especially when tied to issues of sex-based bias and gender identity. Given its profound ties to women’s lives and women’s bodies, mammography holds a unique place in the history of cancer. Part 1 will examine the technological imperatives driving mammography forward, and part 2 will address the social factors that promoted and inhibited the developing technology.
All that glitters
Innovations in technology have contributed so greatly to the progress of medical science in saving and improving patients’ lives that the lure of new technology and the desire to see it succeed and to embrace it has become profound.
In a debate on the adoption of new technologies, Michael Rosen, MD, a surgeon at the Cleveland Clinic, Ohio, pointed out the inherent risks in the life cycle of medical technology: “The stages of surgical innovation have been well described as moving from the generation of a hypothesis with an early promising report to being accepted conclusively as a new standard without formal testing. As the life cycle continues and comparative effectiveness data begin to emerge slowly through appropriately designed trials, the procedure or device is often ultimately abandoned.”1
The history of mammography bears out this grim warning in example after example as an object lesson, revealing not only the difficulties involved in the development of new medical technologies, but also the profound problems involved in validating the effectiveness and appropriateness of a new technology from its inception to the present.
A modern failure?
In fact, one of the more modern developments in mammography technology – digital imaging – has recently been called into question with regard to its effectiveness in saving lives, even as the technology continues to spread throughout the medical community.
A recent meta-analysis has shown that there is little or no improvement in outcomes of breast cancer screening when using digital analysis and screening mammograms vs. traditional film recording.
The meta-analysis assessed 24 studies with a combined total of 16,583,743 screening examinations (10,968,843 film and 5,614,900 digital). The study found that the difference in cancer detection rate using digital rather than film screening showed an increase of only 0.51 detections per 1,000 screens.
The researchers concluded “that while digital mammography is beneficial for medical facilities due to easier storage and handling of images, these results suggest the transition from film to digital mammography has not resulted in health benefits for screened women.”2
In fact, the researchers added that “This analysis reinforces the need to carefully evaluate effects of future changes in technology, such as tomosynthesis, to ensure new technology leads to improved health outcomes and beyond technical gains.”2
None of the nine main randomized clinical trials that were used to determine the effectiveness of mammography screening from the 1960s to the 1990s used digital or 3-D digital mammography (digital breast tomosynthesis or DBT). The earliest trial used direct-exposure film mammography and the others relied upon screen-film mammography.3 And yet the assumptions of the validity of the new digital technologies were predicated on the generalized acceptance of the validity of screening derived from these studies, and a corollary assumption that any technological improvement in the quality of the image must inherently be an improvement of the overall results of screening.
The failure of new technologies to meet expectations is a sobering corrective to the high hopes of researchers, practitioners, and patient groups alike, and is perhaps destined to contribute more to the parallel history of controversy and distrust concerning the risk/benefits of mammography that has been a media and scientific mainstay.
Too often the history of medical technology has found disappointment at the end of the road for new discoveries. But although the disappointing results of digital screening might be considered a failure in the progress of mammography, it is likely just another pause on the road of this technology, the history of which has been rocky from the start.
The need for a new way of looking
The rationale behind the original and continuing development of mammography is a simple one, common to all cancer screening methods – the belief that the earlier the detection of a cancer, the more likely it is to be treated effectively with the therapeutic regimens at hand. While there is some controversy regarding the cost-benefit ratio of screening, especially when therapies for breast cancer are not perfect and vary widely in expense and availability globally, the driving belief has been that mammography provides an outcomes benefit in allowing early surgical and chemoradiation therapy with a curative intent.
There were two main driving forces behind the early development of mammography. The first was the highly lethal nature of breast cancer, especially when it was caught too late and had spread too far to benefit from the only available option at the time – surgery. The second was the severity of the surgical treatment, the only therapeutic option at the time, and the distressing number of women who faced the radical mastectomy procedure pioneered by physicians William Stewart Halsted (1852-1922) at Johns Hopkins University, Baltimore, and Willy Meyer (1858-1932) in New York.
In 1894, in an era when the development of anesthetics and antisepsis made ever more difficult surgical procedures possible without inevitably killing the patient, both men separately published their results of a highly extensive operation that consisted of removal of the breast, chest muscles, and axillary lymph nodes.
As long as there was no presurgical method of determining the extent of a breast cancer’s spread, much less an ability to visually distinguish malignant from benign growths, this “better safe than sorry” approach became the default approach of an increasing number of surgeons, and the drastic solution of radical mastectomy was increasingly applied universally.
But in 1895, with the discovery of x-rays, medical science recognized a nearly miraculous technology for visualizing the inside of the body, and radioactive materials were also routinely used in medical therapies, by both legitimate practitioners and hucksters.
However, in the very early days, the users of x-rays were unaware that large radiation doses could have serious biological effects and had no way of determining radiation field strength and accumulating dosage.
In fact, early calibration of x-ray tubes was based on the amount of skin reddening (erythema) produced when the operator placed a hand directly in the x-ray beam.
It was in this environment that, within only a few decades, the new x-rays, especially with the development of improvements in mammography imaging, were able in many cases to identify smaller, more curable breast cancers. This eventually allowed surgeons to develop and use less extensive operations than the highly disfiguring radical mastectomy that was simultaneously dreaded for its invasiveness and embraced for its life-saving potential.4
Pioneering era
The technological history of mammography was thus driven by the quest for better imaging and reproducibility in order to further the hopes of curative surgical approaches.
In 1913, the German surgeon Albert Salomon (1883-1976) was the first to detect breast cancer using x-rays, but its clinical use was not established, as the images published in his “Beiträge zur pathologie und klinik der mammakarzinome (Contributions to the pathology and clinic of breast cancers)” were photographs of postsurgical breast specimens that illustrated the anatomy and spread of breast cancer tumors but were not adapted to presurgical screening.
After Salomon’s work was published in 1913, there was no new mammography literature published until 1927, when German surgeon Otto Kleinschmidt (1880-1948) published a report describing the world’s first authentic mammography, which he attributed to his mentor, the plastic surgeon Erwin Payr (1871-1946).5
This was followed soon after in 1930 by the work of radiologist Stafford L. Warren (1896-1981), of the University of Rochester (N.Y.), who published a paper on the use of standard roentgenograms for the in vivo preoperative assessment of breast malignancies. His technique involved the use of a stereoscopic system with a grid mechanism and intensifying screens to amplify the image. Breast compression was not involved in his mammogram technique. “Dr. Warren claimed to be correct 92% of the time when using this technique to predict malignancy.”5
His study of 119 women with a histopathologic diagnosis (61 benign and 58 malignant) demonstrated the feasibility of the technique for routine use and “created a surge of interest.”6
But the technology of the time proved difficult to use, and the results difficult to reproduce from laboratory to laboratory, and ultimately did not gain wide acceptance. Among Warren’s other claims to fame, he was a participant in the Manhattan Project and was a member of the teams sent to assess radiation damage in Hiroshima and Nagasaki after the dropping of the atomic bombs.
And in fact, future developments in mammography and all other x-ray screening techniques included attempts to minimize radiation exposure; such attempts were driven, in part, by the tragic impact of atomic bomb radiation and the medical studies carried out on the survivors.
An image more deadly than the disease
Further improvements in mammography technique occurred through the 1930s and 1940s, including better visualization of the mammary ducts based upon the pioneering studies of Emil Ries, MD, in Chicago, who, along with Nymphus Frederick Hicken, MD (1900-1998), reported on the use of contrast mammography (also known as ductography or galactography). On a side note, Dr. Hicken was responsible for introducing the terms mammogram and mammography in 1937.
Problems with ductography, which involved the injection of a radiographically opaque contrast agent into the nipple, occurred when the early contrast agents, such as oil-based lipiodol, proved to be toxic and capable of causing abscesses.7This advance led to the development of other agents, and among the most popular at the time was one that would prove deadly to many.
Thorotrast, first used in 1928, was widely embraced because of its lack of immediately noticeable side effects and the high-quality contrast it provided. Thorotrast was a suspension of radioactive thorium dioxide particles, which gained popularity for use as a radiological imaging agent from the 1930s to 1950s throughout the world, being used in an estimated 2-10 million radiographic exams, primarily for neurosurgery.
In the 1920s and 1930s, world governments had begun to recognize the dangers of radiation exposure, especially among workers, but thorotrast was a unique case because, unbeknownst to most practitioners at the time, thorium dioxide was retained in the body for the lifetime of the patient, with 70% deposited in the liver, 20% in the spleen, and the remaining in the bony medulla and in the peripheral lymph nodes.
Nineteen years after the first use of thorotrast, the first case of a human malignant tumor attributed to its exposure was reported. “Besides the liver neoplasm cases, aplastic anemia, leukemia and an impressive incidence of chromosome aberrations were registered in exposed individuals.”8
Despite its widespread adoption elsewhere, especially in Japan, the use of thorotrast never became popular in the United States, in part because in 1932 and 1937, warnings were issued by the American Medical Association to restrict its use.9
There was a shift to the use of iodinated hydrophilic molecules as contrast agents for conventional x-ray, computed tomography, and fluoroscopy procedures.9 However, it was discovered that these agents, too, have their own risks and dangerous side effects. They can cause severe adverse effects, including allergies, cardiovascular diseases, and nephrotoxicity in some patients.
Slow adoption and limited results
Between 1930 and 1950, Dr. Warren, Jacob Gershon-Cohen, MD (1899-1971) of Philadelphia, and radiologist Raul Leborgne of Uruguay “spread the gospel of mammography as an adjunct to physical examination for the diagnosis of breast cancer.”4 The latter also developed the breast compression technique to produce better quality images and lower the radiation exposure needed, and described the differences that could be visualized between benign and malign microcalcifications.
But despite the introduction of improvements such as double-emulsion film and breast compression to produce higher-quality images, “mammographic films often remained dark and hazy. Moreover, the new techniques, while improving the images, were not easily reproduced by other investigators and clinicians,” and therefore were still not widely adopted.4
Little noticeable effect of mammography
Although the technology existed and had its popularizers, mammography had little impact on an epidemiological level.
There was no major change in the mean maximum breast cancer tumor diameter and node positivity rate detected over the 20 years from 1929 to 1948.10 However, starting in the late 1940s, the American Cancer Society began public education campaigns and early detection education, and thereafter, there was a 3% decline in mean maximum diameter of tumor size seen every 10 years until 1968.
“We have interpreted this as the effect of public education and professional education about early detection through television, print media, and professional publications that began in 1947 because no other event was known to occur that would affect cancer detection beginning in the late 1940s.”10
However, the early detection methods at the time were self-examination and clinical examination for lumps, with mammography remaining a relatively limited tool until its general acceptance broadened a few decades later.
Robert Egan, “Father of Mammography,” et al.
The broad acceptance of mammography as a screening tool and its impacts on a broad population level resulted in large part from the work of Robert L. Egan, MD (1921-2001) in the late 1950s and 1960s.
Dr. Egan’s work was inspired in 1956 by a presentation by a visiting fellow, Jean Pierre Batiani, who brought a mammogram clearly showing a breast cancer from his institution, the Curie Foundation in Paris. The image had been made using very low kilowattage, high tube currents, and fine-grain film.
Dr. Egan, then a resident in radiology, was given the task by the head of his department of reproducing the results.
In 1959, Dr. Egan, then at the University of Texas MD Anderson Cancer Center, Houston, published a combined technique that used a high-milliamperage–low-voltage technique, a fine-grain intensifying screen, and single-emulsion films for mammography, thereby decreasing the radiation exposure significantly from previous x-ray techniques and improving the visualization and reproducibility of screening.
By 1960, Dr. Egan reported on 1,000 mammography cases at MD Anderson, demonstrating the ability of proper screening to detect unsuspected cancers and to limit mastectomies on benign masses. Of 245 breast cancers ultimately confirmed by biopsy, 238 were discovered by mammography, 19 of which were in women whose physical examinations had revealed no breast pathology. One of the cancers was only 8 mm in diameter when sectioned at biopsy.
Dr. Egan’s findings prompted an investigation by the Cancer Control Program (CCP) of the U.S. Public Health Service and led to a study jointly conducted by the National Cancer Institute and MD Anderson Hospital and the CCP, which involved 24 institutions and 1,500 patients.
“The results showed a 21% false-negative rate and a 79% true-positive rate for screening studies using Egan’s technique. This was a milestone for women’s imaging in the United States. Screening mammography was off to a tentative start.”5
“Egan was the man who developed a smooth-riding automobile compared to a Model T. He put mammography on the map and made it an intelligible, reproducible study. In short, he was the father of modern mammography,” according to his professor, mentor, and fellow mammography pioneer Gerald Dodd, MD (Emory School of Medicine website biography).
In 1964 Dr. Egan published his definitive book, “Mammography,” and in 1965 he hosted a 30-minute audiovisual presentation describing in detail his technique.11
The use of mammography was further powered by improved methods of preoperative needle localization, pioneered by Richard H. Gold, MD, in 1963 at Jefferson Medical College, Philadelphia, which eased obtaining a tissue diagnosis for any suspicious lesions detected in the mammogram. Dr. Gold performed needle localization of nonpalpable, mammographically visible lesions before biopsy, which allowed surgical resection of a smaller volume of breast tissue than was possible before.
Throughout the era, there were also incremental improvements in mammography machines and an increase in the number of commercial manufacturers.
Xeroradiography, an imaging technique adapted from xerographic photocopying, was seen as a major improvement over direct film imaging, and the technology became popular throughout the 1970s based on the research of John N. Wolfe, MD (1923-1993), who worked closely with the Xerox Corporation to improve the breast imaging process.6 However, this technology had all the same problems associated with running an office copying machine, including paper jams and toner issues, and the worst aspect was the high dose of radiation required. For this reason, it would quickly be superseded by the use of screen-film mammography, which eventually completely replaced the use of both xeromammography and direct-exposure film mammography.
The march of mammography
A series of nine randomized clinical trials (RCTs) between the 1960s and 1990s formed the foundation of the clinical use of mammography. These studies enrolled more than 600,000 women in the United States, Canada, the United Kingdom, and Sweden. The nine main RCTs of breast cancer screening were the Health Insurance Plan of Greater New York (HIP) trial, the Edinburgh trial, the Canadian National Breast Screening Study, the Canadian National Breast Screening Study 2, the United Kingdom Age trial, the Stockholm trial, the Malmö Mammographic Screening Trial, the Gothenburg trial, and the Swedish Two-County Study.3
These trials incorporated improvements in the technology as it developed, as seen in the fact that the earliest, the HIP trial, used direct-exposure film mammography and the other trials used screen-film mammography.3
Meta-analyses of the major nine screening trials indicated that reduced breast cancer mortality with screening was dependent on age. In particular, the results for women aged 40-49 years and 50-59 years showed only borderline statistical significance, and they varied depending on how cases were accrued in individual trials. “Assuming that differences actually exist, the absolute breast cancer mortality reduction per 10,000 women screened for 10 years ranged from 3 for age 39-49 years; 5-8 for age 50-59 years; and 12-21 for age 60-69 years.”3 In addition the estimates for women aged 70-74 years were limited by low numbers of events in trials that had smaller numbers of women in this age group.
However, at the time, the studies had a profound influence on increasing the popularity and spread of mammography.
As mammographies became more common, standardization became an important issue and a Mammography Accreditation Program began in 1987. Originally a voluntary program, it became mandatory with the Mammography Quality Standards Act of 1992, which required all U.S. mammography facilities to become accredited and certified.
In 1986, the American College of Radiology proposed its Breast Imaging Reporting and Data System (BI-RADS) initiative to enable standardized reporting of mammography; the first report was released in 1993.
BI-RADS is now on its fifth edition and has addressed the use of mammography, breast ultrasonography, and breast magnetic resonance imaging, developing standardized auditing approaches for all three techniques of breast cancer imaging.6
The digital era and beyond
With the dawn of the 21st century, the era of digital breast cancer screening began.
The screen-film mammography (SFM) technique employed throughout the 1980s and 1990s had significant advantages over earlier x-ray films for producing more vivid images of dense breast tissues. The next technology, digital mammography, was introduced in the late 20th century, and the first system was approved by the U.S. FDA in 2000.
One of the key benefits touted for digital mammograms is the fact that the radiologist can manipulate the contrast of the images, which allows for masses to be identified that might otherwise not be visible on standard film.
However, the recent meta-analysis discussed in the introduction calls such benefits into question, and a new controversy is likely to ensue on the question of the effectiveness of digital mammography on overall clinical outcomes.
But the technology continues to evolve.
“There has been a continuous and substantial technical development from SFM to full-field digital mammography and very recently also the introduction of digital breast tomosynthesis (DBT). This technical evolution calls for new evidence regarding the performance of screening using new mammography technologies, and the evidence needed to translate new technologies into screening practice,” according to an updated assessment by the U.S. Preventive Services Task Force.12
DBT was approved by the Food and Drug Administration in 2011. The technology involves the creation of a series of images, which are assembled into a 3-D–like image of breast slices. Traditional digital mammography creates a 2-D image of a flattened breast, and the radiologist must peer through the layers to find abnormalities. DBT uses a computer algorithm to reconstruct multiple low-dose digital images of the breast that can be displayed individually or in cinematic mode.13
Early trials showed a significant benefit of DBT in detecting new and smaller breast cancers, compared with standard digital mammography.
In women in their 40s, DBT found 1.7 more cancers than digital mammography for every 1,000 exams of women with normal breast tissue. In addition, 16.3% of women in this age group who were screened using digital mammography received callbacks, versus 11.7% of those screened using DBT. For younger women with dense breasts, the advantage of DBT was even greater, with 2.27 more cancers found for every 1,000 women screened. Whether such results will lead to clinically improved outcomes remains a question. “It can still miss cancers. Also, like traditional mammography, DBT might not reduce deaths from tumors that are very aggressive and fast-growing. And some women will still be called back unnecessarily for false-positive results.”14
But such technological advances further the hopes of researchers and patients alike.
Conclusion
Medical technology is driven both by advances in science and by the demands of patients and physicians for improved outcomes. The history of mammography, for example, is tied to the scientific advancements in x-ray technology, which allowed physicians for the first time to peer inside a living body without a scalpel at hand. But mammography was also an outgrowth of the profound need of the surgeon to identify cancerous masses in the breast at an early-enough stage to attempt a cure, while simultaneously minimizing the radical nature of the surgery required.
And while seeing is believing, the need to see and verify what was seen in order to make life-and-death decisions drove the demand for improvements in the technology of mammography throughout most of the 20th century and beyond.
The tortuous path from the early and continuing snafus with contrast agents to the apparent failure of the promise of digital technology serves as a continuing reminder of the hopes and perils that developing medical technologies present. It will be interesting to see if further refinements to mammography, such as DBT, will enhance the technology enough to have a major impact on countless women’s lives, or if new developments in magnetic resonance imaging and ultrasound make traditional mammography a relic of the past.
Part 2 of this history will present the social dynamics intimately involved with the rise and promulgation of mammography and how social need and public fears and controversies affected its development and spread as much, if not more, than technological innovation.
This article could only touch upon the myriad of details and technologies involved in the history of mammography, and I urge interested readers to check out the relevant references for far more in-depth and fascinating stories from its complex and controversial past.
References
1. Felix EL, Rosen M, Earle D. “Curbing Our Enthusiasm for Surgical Innovation: Is It a Good Thing or Bad Thing?” The Great Debates, General Surgery News, 2018 Oct 17
2. J Natl Cancer Inst. 2020 Jun 23. doi: 10.1093/jnci/djaa080.
3. Nelson H et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan, pp. 29-49)4. Lerner, BH. “To See Today With the Eyes of Tomorrow: A History of Screening Mammography,” background paper for Patlak M et al., Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (Washington: National Academies Press, 2001).
5. Grady I, Hansen P. Chapter 28: Mammography in “Kuerer’s Breast Surgical Oncology”(New York: McGaw-Hill Medical, 2010)
6. Radiology. 2014 Nov;273(2 Suppl):S23-44.
7. Bassett LW, Kim CH. (2003) Chapter 1: Ductography in Dershaw DD (eds) “Imaging-Guided Interventional Breast Techniques” (New York: Springer, 2003, pp. 1-30).
8. Cuperschmid EM, Ribeiro de Campos TP. 2009 International Nuclear Atlantic Conference, Rio de Janeiro, Sept 27–Oct 2, 2009
9. Bioscience Microflora. 2000;19(2):107-16.
10. Cady B. New era in breast cancer. Impact of screening on disease presentation. Surg Oncol Clin N Am. 1997 Apr;6(2):195-202.
11. Egan R. “Mammography Technique.” Audiovisual presentation. (Washington: U.S. Public Health Service, 1965).
12. Zackrisson S, Houssami N. Chapter 13: Evolution of Mammography Screening: From Film Screen to Digital Breast Tomosynthesis in “Breast Cancer Screening: An Examination of Scientific Evidence” (Cambridge, Mass.: Academic Press, 2016, pp. 323-46).13. Melnikow J et al. Screening for breast cancer with digital breast tomosynthesis. Evidence Synthesis No. 125 (Rockville, Md.: U.S. Agency for Healthcare Research and Quality, 2016 Jan).
14. Newer breast screening technology may spot more cancers. Harvard Women’s Health Watch online, June 2019.
Mark Lesney is the editor of Hematology News and the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has worked as a writer/editor for the American Chemical Society, and has served as an adjunct assistant professor in the department of biochemistry and molecular & cellular biology at Georgetown University, Washington.
Educational intervention curbs use of antibiotics for respiratory infections
A clinician education program significantly reduced overall antibiotic prescribing during pediatric visits for acute respiratory tract infections, according to data from 57 clinicians who participated in an intervention.
In a study published in Pediatrics, Matthew P. Kronman, MD, of the University of Washington, Seattle, and associates randomized 57 clinicians at 19 pediatric practices to a stepped-wedge clinical trial. The study included visits for acute otitis media, bronchitis, pharyngitis, sinusitis, and upper respiratory infections (defined as ARTI visits) for children aged 6 months to less than 11 years, for a total of 72,723 ARTI visits by 29,762 patients. The primary outcome was overall antibiotic prescribing for ARTI visits.
For the intervention, known as the Dialogue Around Respiratory Illness Treatment (DART) quality improvement (QI) program, clinicians received three program modules containing online tutorials and webinars. These professionally-produced modules included a combination of evidence-based communication strategies and antibiotic prescribing, booster video vignettes, and individualized antibiotic prescribing feedback reports over 11 months.
Overall, the probability of antibiotic prescribing for ARTI visits decreased by 7% (adjusted relative risk 0.93) from baseline to a 2- to 8-month postintervention in an adjusted intent-to-treat analysis.
Analysis of secondary outcomes revealed that prescribing any antibiotics for viral ARTI decreased by 40% during the postintervention period compared to baseline (aRR 0.60).
In addition, second-line antibiotic prescribing decreased from baseline by 34% for streptococcal pharyngitis (aRR 0.66), and by 41% for sinusitis (aRR 0.59); however there was no significant change in prescribing for acute otitis media, the researchers said.
The study findings were limited by several factors including the potential for biased results because of the randomization of clinicians from multiple practices and the potential for clinicians to change their prescribing habits after the start of the study, Dr. Kronman and colleagues noted.
In addition, the study did not include complete data on rapid streptococcal antigen testing, which might eliminate some children from the study population, and the relatively short postintervention period “may not represent the true long-term intervention durability may not represent the true long-term intervention durability,” they said.
However, the results support the potential of the DART program. “The 7% reduction in antibiotic prescribing for all ARTIs, if extrapolated to all ambulatory ARTI visits to pediatricians nationally, would represent 1.5 million fewer antibiotic prescriptions for children with ARTI annually,” they wrote.
“Providing online communication training and evidence-based antibiotic prescribing education in combination with individualized antibiotic prescribing feedback reports may help achieve national goals of reducing unnecessary outpatient antibiotic prescribing for children,” Dr. Kronman and associates concluded.
Combining interventions are key to reducing unnecessary antibiotics use in pediatric ambulatory care, Rana F. Hamdy, MD, MPH, of Children’s National Hospital, Washington, , and Sophie E. Katz, MD, of Vanderbilt University, Nashville, Tenn., wrote in an accompanying editorial (Pediatrics. 2020 Aug 3. doi: 10.1542/peds.2020-012922).
The researchers in the current study “seem to recognize that clinicians are adult learners, and they combine interventions to implement these adult learning theory tenets to improve appropriate antibiotic prescribing,” they wrote. The DART intervention combined best practices training, communications training, and individualized antibiotic prescribing feedback reports to improve communication between providers and families “especially when faced with a situation in which a parent or guardian might expect an antibiotic prescription but the provider does not think one is necessary,” Dr. Hamdy and Dr. Katz said.
Overall, the findings suggest that the interventions work best in combination vs. being used alone, although the study did not evaluate the separate contributions of each intervention, the editorialists wrote.
“In the current study, nonengaged physicians had an increase in second-line antibiotic prescribing, whereas the engaged physicians had a decrease in second-line antibiotic prescribing,” they noted. “This suggests that the addition of communications training could mitigate the undesirable effects that may result from solely using feedback reports.”
“Each year, U.S. children are prescribed as many as 10 million unnecessary antibiotic courses for acute respiratory tract infections,” Kristina A. Bryant, MD, of the University of Louisville, Ky., said in an interview. “Some of these prescriptions result in side effects or allergic reactions, and they contribute to growing antibiotic resistance. We need effective interventions to reduce antibiotic prescribing.”
Although the DART modules are free and available online, busy clinicians might struggle to find time to view them consistently, said Dr. Bryant.
“One advantage of the study design was that information was pushed to clinicians along with communication booster videos,” she said. “We know that education and reinforcement over time works better than a one and done approach.
“Study participants also received feedback over time about their prescribing habits, which can be a powerful motivator for change, although not all clinicians may have easy access to these reports,” she noted.
To overcome some of the barriers to using the modules, clinicians who are “interested in improving their prescribing could work with their office managers to develop antibiotic prescribing reports and schedule reminders to review them,” said Dr. Bryant.
“An individual could commit to education and review of his or her own prescribing patterns, but support from one’s partners and shared accountability is likely to be even more effective,” she said. “Sharing data within a practice and exploring differences in prescribing patterns can drive improvement.
“Spaced education and regular feedback about prescribing patterns can improve antibiotic prescribing for pharyngitis and sinusitis, and reduce antibiotic prescriptions for ARTIs,” Dr. Bryant said. The take-home from the study is that it should prompt anyone who prescribes antibiotics for children to ask themselves how they can improve their own prescribing habits.
“In this study, prescribing for viral ARTIs was reduced but not eliminated. We need additional studies to further reduce unnecessary antibiotic use,” Dr. Bryant said.
In addition, areas for future research could include longer-term follow-up. “Study participants were followed for 2 to 8 months after the intervention ended in June 2018. It would be interesting to know about their prescribing practices now, and if the changes observed in the study were durable,” she concluded.
The study was supported by the National Institutes of Health, along with additional infrastructure funding from the American Academy of Pediatrics and the Health Resources and Services Administration of the Department of Health and Human Services. The researchers had no financial conflicts to disclose.
Dr. Hamdy and Dr. Katz had no financial conflicts to disclose, but Dr. Katz disclosed grant support through the Centers for Disease Control and Prevention as a recipient of the Leadership in Epidemiology, Antimicrobial Stewardship, and Public Health fellowship, sponsored by the Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, and Pediatric Infectious Diseases Society.
Dr. Bryant disclosed serving as an investigator on multicenter clinical vaccine trials funded by Pfizer (but not in the last year). She also serves as the current president of the Pediatric Infectious Diseases Society, but the opinions expressed here are her own and do not necessarily reflect the views of PIDS.
SOURCE: Kronman MP et al. Pediatrics. 2020 Aug 3. doi: 10.1542/peds.2020-0038.
A clinician education program significantly reduced overall antibiotic prescribing during pediatric visits for acute respiratory tract infections, according to data from 57 clinicians who participated in an intervention.
In a study published in Pediatrics, Matthew P. Kronman, MD, of the University of Washington, Seattle, and associates randomized 57 clinicians at 19 pediatric practices to a stepped-wedge clinical trial. The study included visits for acute otitis media, bronchitis, pharyngitis, sinusitis, and upper respiratory infections (defined as ARTI visits) for children aged 6 months to less than 11 years, for a total of 72,723 ARTI visits by 29,762 patients. The primary outcome was overall antibiotic prescribing for ARTI visits.
For the intervention, known as the Dialogue Around Respiratory Illness Treatment (DART) quality improvement (QI) program, clinicians received three program modules containing online tutorials and webinars. These professionally-produced modules included a combination of evidence-based communication strategies and antibiotic prescribing, booster video vignettes, and individualized antibiotic prescribing feedback reports over 11 months.
Overall, the probability of antibiotic prescribing for ARTI visits decreased by 7% (adjusted relative risk 0.93) from baseline to a 2- to 8-month postintervention in an adjusted intent-to-treat analysis.
Analysis of secondary outcomes revealed that prescribing any antibiotics for viral ARTI decreased by 40% during the postintervention period compared to baseline (aRR 0.60).
In addition, second-line antibiotic prescribing decreased from baseline by 34% for streptococcal pharyngitis (aRR 0.66), and by 41% for sinusitis (aRR 0.59); however there was no significant change in prescribing for acute otitis media, the researchers said.
The study findings were limited by several factors including the potential for biased results because of the randomization of clinicians from multiple practices and the potential for clinicians to change their prescribing habits after the start of the study, Dr. Kronman and colleagues noted.
In addition, the study did not include complete data on rapid streptococcal antigen testing, which might eliminate some children from the study population, and the relatively short postintervention period “may not represent the true long-term intervention durability may not represent the true long-term intervention durability,” they said.
However, the results support the potential of the DART program. “The 7% reduction in antibiotic prescribing for all ARTIs, if extrapolated to all ambulatory ARTI visits to pediatricians nationally, would represent 1.5 million fewer antibiotic prescriptions for children with ARTI annually,” they wrote.
“Providing online communication training and evidence-based antibiotic prescribing education in combination with individualized antibiotic prescribing feedback reports may help achieve national goals of reducing unnecessary outpatient antibiotic prescribing for children,” Dr. Kronman and associates concluded.
Combining interventions are key to reducing unnecessary antibiotics use in pediatric ambulatory care, Rana F. Hamdy, MD, MPH, of Children’s National Hospital, Washington, , and Sophie E. Katz, MD, of Vanderbilt University, Nashville, Tenn., wrote in an accompanying editorial (Pediatrics. 2020 Aug 3. doi: 10.1542/peds.2020-012922).
The researchers in the current study “seem to recognize that clinicians are adult learners, and they combine interventions to implement these adult learning theory tenets to improve appropriate antibiotic prescribing,” they wrote. The DART intervention combined best practices training, communications training, and individualized antibiotic prescribing feedback reports to improve communication between providers and families “especially when faced with a situation in which a parent or guardian might expect an antibiotic prescription but the provider does not think one is necessary,” Dr. Hamdy and Dr. Katz said.
Overall, the findings suggest that the interventions work best in combination vs. being used alone, although the study did not evaluate the separate contributions of each intervention, the editorialists wrote.
“In the current study, nonengaged physicians had an increase in second-line antibiotic prescribing, whereas the engaged physicians had a decrease in second-line antibiotic prescribing,” they noted. “This suggests that the addition of communications training could mitigate the undesirable effects that may result from solely using feedback reports.”
“Each year, U.S. children are prescribed as many as 10 million unnecessary antibiotic courses for acute respiratory tract infections,” Kristina A. Bryant, MD, of the University of Louisville, Ky., said in an interview. “Some of these prescriptions result in side effects or allergic reactions, and they contribute to growing antibiotic resistance. We need effective interventions to reduce antibiotic prescribing.”
Although the DART modules are free and available online, busy clinicians might struggle to find time to view them consistently, said Dr. Bryant.
“One advantage of the study design was that information was pushed to clinicians along with communication booster videos,” she said. “We know that education and reinforcement over time works better than a one and done approach.
“Study participants also received feedback over time about their prescribing habits, which can be a powerful motivator for change, although not all clinicians may have easy access to these reports,” she noted.
To overcome some of the barriers to using the modules, clinicians who are “interested in improving their prescribing could work with their office managers to develop antibiotic prescribing reports and schedule reminders to review them,” said Dr. Bryant.
“An individual could commit to education and review of his or her own prescribing patterns, but support from one’s partners and shared accountability is likely to be even more effective,” she said. “Sharing data within a practice and exploring differences in prescribing patterns can drive improvement.
“Spaced education and regular feedback about prescribing patterns can improve antibiotic prescribing for pharyngitis and sinusitis, and reduce antibiotic prescriptions for ARTIs,” Dr. Bryant said. The take-home from the study is that it should prompt anyone who prescribes antibiotics for children to ask themselves how they can improve their own prescribing habits.
“In this study, prescribing for viral ARTIs was reduced but not eliminated. We need additional studies to further reduce unnecessary antibiotic use,” Dr. Bryant said.
In addition, areas for future research could include longer-term follow-up. “Study participants were followed for 2 to 8 months after the intervention ended in June 2018. It would be interesting to know about their prescribing practices now, and if the changes observed in the study were durable,” she concluded.
The study was supported by the National Institutes of Health, along with additional infrastructure funding from the American Academy of Pediatrics and the Health Resources and Services Administration of the Department of Health and Human Services. The researchers had no financial conflicts to disclose.
Dr. Hamdy and Dr. Katz had no financial conflicts to disclose, but Dr. Katz disclosed grant support through the Centers for Disease Control and Prevention as a recipient of the Leadership in Epidemiology, Antimicrobial Stewardship, and Public Health fellowship, sponsored by the Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, and Pediatric Infectious Diseases Society.
Dr. Bryant disclosed serving as an investigator on multicenter clinical vaccine trials funded by Pfizer (but not in the last year). She also serves as the current president of the Pediatric Infectious Diseases Society, but the opinions expressed here are her own and do not necessarily reflect the views of PIDS.
SOURCE: Kronman MP et al. Pediatrics. 2020 Aug 3. doi: 10.1542/peds.2020-0038.
A clinician education program significantly reduced overall antibiotic prescribing during pediatric visits for acute respiratory tract infections, according to data from 57 clinicians who participated in an intervention.
In a study published in Pediatrics, Matthew P. Kronman, MD, of the University of Washington, Seattle, and associates randomized 57 clinicians at 19 pediatric practices to a stepped-wedge clinical trial. The study included visits for acute otitis media, bronchitis, pharyngitis, sinusitis, and upper respiratory infections (defined as ARTI visits) for children aged 6 months to less than 11 years, for a total of 72,723 ARTI visits by 29,762 patients. The primary outcome was overall antibiotic prescribing for ARTI visits.
For the intervention, known as the Dialogue Around Respiratory Illness Treatment (DART) quality improvement (QI) program, clinicians received three program modules containing online tutorials and webinars. These professionally-produced modules included a combination of evidence-based communication strategies and antibiotic prescribing, booster video vignettes, and individualized antibiotic prescribing feedback reports over 11 months.
Overall, the probability of antibiotic prescribing for ARTI visits decreased by 7% (adjusted relative risk 0.93) from baseline to a 2- to 8-month postintervention in an adjusted intent-to-treat analysis.
Analysis of secondary outcomes revealed that prescribing any antibiotics for viral ARTI decreased by 40% during the postintervention period compared to baseline (aRR 0.60).
In addition, second-line antibiotic prescribing decreased from baseline by 34% for streptococcal pharyngitis (aRR 0.66), and by 41% for sinusitis (aRR 0.59); however there was no significant change in prescribing for acute otitis media, the researchers said.
The study findings were limited by several factors including the potential for biased results because of the randomization of clinicians from multiple practices and the potential for clinicians to change their prescribing habits after the start of the study, Dr. Kronman and colleagues noted.
In addition, the study did not include complete data on rapid streptococcal antigen testing, which might eliminate some children from the study population, and the relatively short postintervention period “may not represent the true long-term intervention durability may not represent the true long-term intervention durability,” they said.
However, the results support the potential of the DART program. “The 7% reduction in antibiotic prescribing for all ARTIs, if extrapolated to all ambulatory ARTI visits to pediatricians nationally, would represent 1.5 million fewer antibiotic prescriptions for children with ARTI annually,” they wrote.
“Providing online communication training and evidence-based antibiotic prescribing education in combination with individualized antibiotic prescribing feedback reports may help achieve national goals of reducing unnecessary outpatient antibiotic prescribing for children,” Dr. Kronman and associates concluded.
Combining interventions are key to reducing unnecessary antibiotics use in pediatric ambulatory care, Rana F. Hamdy, MD, MPH, of Children’s National Hospital, Washington, , and Sophie E. Katz, MD, of Vanderbilt University, Nashville, Tenn., wrote in an accompanying editorial (Pediatrics. 2020 Aug 3. doi: 10.1542/peds.2020-012922).
The researchers in the current study “seem to recognize that clinicians are adult learners, and they combine interventions to implement these adult learning theory tenets to improve appropriate antibiotic prescribing,” they wrote. The DART intervention combined best practices training, communications training, and individualized antibiotic prescribing feedback reports to improve communication between providers and families “especially when faced with a situation in which a parent or guardian might expect an antibiotic prescription but the provider does not think one is necessary,” Dr. Hamdy and Dr. Katz said.
Overall, the findings suggest that the interventions work best in combination vs. being used alone, although the study did not evaluate the separate contributions of each intervention, the editorialists wrote.
“In the current study, nonengaged physicians had an increase in second-line antibiotic prescribing, whereas the engaged physicians had a decrease in second-line antibiotic prescribing,” they noted. “This suggests that the addition of communications training could mitigate the undesirable effects that may result from solely using feedback reports.”
“Each year, U.S. children are prescribed as many as 10 million unnecessary antibiotic courses for acute respiratory tract infections,” Kristina A. Bryant, MD, of the University of Louisville, Ky., said in an interview. “Some of these prescriptions result in side effects or allergic reactions, and they contribute to growing antibiotic resistance. We need effective interventions to reduce antibiotic prescribing.”
Although the DART modules are free and available online, busy clinicians might struggle to find time to view them consistently, said Dr. Bryant.
“One advantage of the study design was that information was pushed to clinicians along with communication booster videos,” she said. “We know that education and reinforcement over time works better than a one and done approach.
“Study participants also received feedback over time about their prescribing habits, which can be a powerful motivator for change, although not all clinicians may have easy access to these reports,” she noted.
To overcome some of the barriers to using the modules, clinicians who are “interested in improving their prescribing could work with their office managers to develop antibiotic prescribing reports and schedule reminders to review them,” said Dr. Bryant.
“An individual could commit to education and review of his or her own prescribing patterns, but support from one’s partners and shared accountability is likely to be even more effective,” she said. “Sharing data within a practice and exploring differences in prescribing patterns can drive improvement.
“Spaced education and regular feedback about prescribing patterns can improve antibiotic prescribing for pharyngitis and sinusitis, and reduce antibiotic prescriptions for ARTIs,” Dr. Bryant said. The take-home from the study is that it should prompt anyone who prescribes antibiotics for children to ask themselves how they can improve their own prescribing habits.
“In this study, prescribing for viral ARTIs was reduced but not eliminated. We need additional studies to further reduce unnecessary antibiotic use,” Dr. Bryant said.
In addition, areas for future research could include longer-term follow-up. “Study participants were followed for 2 to 8 months after the intervention ended in June 2018. It would be interesting to know about their prescribing practices now, and if the changes observed in the study were durable,” she concluded.
The study was supported by the National Institutes of Health, along with additional infrastructure funding from the American Academy of Pediatrics and the Health Resources and Services Administration of the Department of Health and Human Services. The researchers had no financial conflicts to disclose.
Dr. Hamdy and Dr. Katz had no financial conflicts to disclose, but Dr. Katz disclosed grant support through the Centers for Disease Control and Prevention as a recipient of the Leadership in Epidemiology, Antimicrobial Stewardship, and Public Health fellowship, sponsored by the Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, and Pediatric Infectious Diseases Society.
Dr. Bryant disclosed serving as an investigator on multicenter clinical vaccine trials funded by Pfizer (but not in the last year). She also serves as the current president of the Pediatric Infectious Diseases Society, but the opinions expressed here are her own and do not necessarily reflect the views of PIDS.
SOURCE: Kronman MP et al. Pediatrics. 2020 Aug 3. doi: 10.1542/peds.2020-0038.
FROM PEDIATRICS
New road map for CRC screening: Use more stool tests, says AGA
where the default screening modality to date has been colonoscopy.
Instead, the American Gastroenterological Association is proposing new approaches that combine better risk assessment, more use of noninvasive testing (such as fecal occult blood tests), and more targeted referrals for colonoscopy. Such changes could increase patient compliance and “save countless lives.”
“We need to improve our strategies to curb the cancer that ranks second for deaths in the U.S.,” commented Srinadh Komanduri, MD, chair of the AGA Center for GI Innovation and Technology, in a statement.
“Approximately 67% of eligible Americans are screened for colorectal cancer,” he said, which means that a third (33%) are not.
During the COVID-19 pandemic, the proportion of individuals not being screened has increased. One report of medical claims data showed that colonoscopies dropped by 90% during April.
The proposed changes are outlined in an AGA white paper: “Roadmap for the Future of Colorectal Cancer Screening in the United States.”
The report, written following consultation with 60 gastroenterology and research experts, was published online in Clinical Gastroenterology and Hepatology.
It proposed that alternative testing modalities to colonoscopy will need to be integrated into organized screening programs.
Rather than offering colonoscopy as the default screening method for all patients at risk, the AGA advised that it be offered initially only to patients at high risk, which would increase access for those who need it most. For patients at lower risk, noninvasive screening methods, such as fecal occult blood testing, could be offered initially and then integrated with colonoscopy.
“If we offered tests that were convenient, accurate, and of lower cost, and we could help people choose the best option based on their individual cancer risks, we would save more lives,” Joshua E. Melson, MD, MPH, lead author of the AGA white paper and professor at Rush University Medical Center, Chicago, said in an interview.
Screening can reduce CRC mortality by more than 50%, he added.
“Screening should be thought of as a process over time, not a single test isolated in time,” Dr. Melson commented. A clinical practice that has historically used only colonoscopy will need an organized, structured program to incorporate noninvasive testing, he said.
To date, efforts to increase CRC screening uptake have met with limited success, the AGA says. In 2014, the National Colorectal Cancer Round Table set the bar high with a 2018 screening goal of 80% for adults 50 years of age and older. As of 2020, some states had almost reached this goal, but most had not.
“In the opportunistic screening environment in the U.S., where colonoscopy is the most prevalent method, CRC screening has not reached aspirational goals in terms of uptake, reduction in CRC incidence, and disease burden,” the AGA said. “It is questionable if 80% uptake is achievable in a primarily opportunistic screening environment.”
In the proposed revamping of the current CRC screening infrastructure, patients whose physicians recommend CRC screening would no longer be left to their own devices to follow up. Clinicians would initiate CRC screening and oversee follow-up testing at defined intervals and would employ ongoing surveillance.
Ensuring that appropriate screening is readily available to at-risk individuals with no social, racial, or economic disparities is crucial, the AGA says. Racial disparities in access to screening disproportionately burden Blacks and Latin Americans as well as people living in rural areas. Screening differences account for 42% of the disparity in CRC incidence between Black and White Americans and 19% of the disparity in CRC mortality.
Compared with colonoscopy, which requires bowel preparation, time off from work, and a hospital or clinic procedure, the fecal immunochemical test (FIT), for which a patient provides stool samples that are examined for the presence of blood, is much less stressful: it is noninvasive, and the patients collect the samples themselves in their own home. Studies show that, in diverse environments, patients prefer FIT over colonoscopy.
In a controlled trial that involved more than 55,000 patients who were randomly assigned to undergo either FIT or colonoscopy, the participation rate in the first cycle was greater for FIT than for colonoscopy (34.2% vs. 24.6%). This partially offset the lower single-application sensitivity for CRC of FIT, the researchers said.
Results from a study with a cluster randomized design showed that offering up-front stool testing as an option in addition to colonoscopy increased screening uptake. Of patients offered fecal occult blood testing or colonoscopy, 69% completed the noninvasive screening, compared with 38% of those offered colonoscopy alone. Notably, non-White participants were more adherent to stool testing.
The success of the AGA’s new initiative hinges largely upon the development of affordable, highly accurate, easy-to-use, noninvasive tests. In this regard, the organization has challenged scientists and industry partners with an aspirational target that is “far superior to current methodologies in terms of sensitivity and specificity,” said Dr. Melson, who is associate professor at Rush Medical College, Chicago, and a member of the AGA Center for GI Innovation and Technology.
The AGA wants new CRC screening tests that are capable of detecting advanced adenomas and advanced serrated lesions with a one-time sensitivity and specificity of 90% or higher, which is comparable with colonoscopy.
The FIT test has a sensitivity of less than 50% for detecting an advanced polyp of 10 mm or larger, said Dr. Melson.
The multitarget stool DNA (MT-sDNA) test may offer some improvement.
In a 2014 pivotal trial that compared FIT with the MT-sDNA in patients at average risk, the MT-sDNA test had higher sensitivity for detecting nonadvanced CRC lesions than FIT (92% vs. 74%) but less specificity (87% vs. 95%). The rate of detection of polyps with high-grade dysplasia was 69.2% with DNA testing and 46.2% with FIT.
However, the MT-sDNA test costs more than $500, compared with $25 for the FIT test, Dr. Melson pointed out.
To help identify the most appropriate screening for individual patients, better understanding and more thorough identification of risk factors are needed. “Risk assessment is definitely not where it could be,” Dr. Melson said.
The accuracy of risk assessment can be improved by sharing information from electronic health records on past colonoscopy polyp data, the presence of molecular markers, and family history, the AGA said. “With clearer risk assessment, shared decision-making on the most appropriate test becomes more clear and screening rates would benefit from patient buy-in and from easier access.”
The AGA recommended that research focus on the cost-effectiveness of screening younger patients, because the proportion of CRC cases in adults aged younger than 50 years has doubled since 1990.
This has raised the question as to whether the age for initial CRC screening should be lowered to 45 years (it already has been by the American Cancer Society), but there is much debate over this move.
Dr. Melson has received consulting fees from Clinical Genomics and research support from Boston Scientific Corporation and holds stocks in Virgo Imaging. A number of AGA white paper coauthors have disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
where the default screening modality to date has been colonoscopy.
Instead, the American Gastroenterological Association is proposing new approaches that combine better risk assessment, more use of noninvasive testing (such as fecal occult blood tests), and more targeted referrals for colonoscopy. Such changes could increase patient compliance and “save countless lives.”
“We need to improve our strategies to curb the cancer that ranks second for deaths in the U.S.,” commented Srinadh Komanduri, MD, chair of the AGA Center for GI Innovation and Technology, in a statement.
“Approximately 67% of eligible Americans are screened for colorectal cancer,” he said, which means that a third (33%) are not.
During the COVID-19 pandemic, the proportion of individuals not being screened has increased. One report of medical claims data showed that colonoscopies dropped by 90% during April.
The proposed changes are outlined in an AGA white paper: “Roadmap for the Future of Colorectal Cancer Screening in the United States.”
The report, written following consultation with 60 gastroenterology and research experts, was published online in Clinical Gastroenterology and Hepatology.
It proposed that alternative testing modalities to colonoscopy will need to be integrated into organized screening programs.
Rather than offering colonoscopy as the default screening method for all patients at risk, the AGA advised that it be offered initially only to patients at high risk, which would increase access for those who need it most. For patients at lower risk, noninvasive screening methods, such as fecal occult blood testing, could be offered initially and then integrated with colonoscopy.
“If we offered tests that were convenient, accurate, and of lower cost, and we could help people choose the best option based on their individual cancer risks, we would save more lives,” Joshua E. Melson, MD, MPH, lead author of the AGA white paper and professor at Rush University Medical Center, Chicago, said in an interview.
Screening can reduce CRC mortality by more than 50%, he added.
“Screening should be thought of as a process over time, not a single test isolated in time,” Dr. Melson commented. A clinical practice that has historically used only colonoscopy will need an organized, structured program to incorporate noninvasive testing, he said.
To date, efforts to increase CRC screening uptake have met with limited success, the AGA says. In 2014, the National Colorectal Cancer Round Table set the bar high with a 2018 screening goal of 80% for adults 50 years of age and older. As of 2020, some states had almost reached this goal, but most had not.
“In the opportunistic screening environment in the U.S., where colonoscopy is the most prevalent method, CRC screening has not reached aspirational goals in terms of uptake, reduction in CRC incidence, and disease burden,” the AGA said. “It is questionable if 80% uptake is achievable in a primarily opportunistic screening environment.”
In the proposed revamping of the current CRC screening infrastructure, patients whose physicians recommend CRC screening would no longer be left to their own devices to follow up. Clinicians would initiate CRC screening and oversee follow-up testing at defined intervals and would employ ongoing surveillance.
Ensuring that appropriate screening is readily available to at-risk individuals with no social, racial, or economic disparities is crucial, the AGA says. Racial disparities in access to screening disproportionately burden Blacks and Latin Americans as well as people living in rural areas. Screening differences account for 42% of the disparity in CRC incidence between Black and White Americans and 19% of the disparity in CRC mortality.
Compared with colonoscopy, which requires bowel preparation, time off from work, and a hospital or clinic procedure, the fecal immunochemical test (FIT), for which a patient provides stool samples that are examined for the presence of blood, is much less stressful: it is noninvasive, and the patients collect the samples themselves in their own home. Studies show that, in diverse environments, patients prefer FIT over colonoscopy.
In a controlled trial that involved more than 55,000 patients who were randomly assigned to undergo either FIT or colonoscopy, the participation rate in the first cycle was greater for FIT than for colonoscopy (34.2% vs. 24.6%). This partially offset the lower single-application sensitivity for CRC of FIT, the researchers said.
Results from a study with a cluster randomized design showed that offering up-front stool testing as an option in addition to colonoscopy increased screening uptake. Of patients offered fecal occult blood testing or colonoscopy, 69% completed the noninvasive screening, compared with 38% of those offered colonoscopy alone. Notably, non-White participants were more adherent to stool testing.
The success of the AGA’s new initiative hinges largely upon the development of affordable, highly accurate, easy-to-use, noninvasive tests. In this regard, the organization has challenged scientists and industry partners with an aspirational target that is “far superior to current methodologies in terms of sensitivity and specificity,” said Dr. Melson, who is associate professor at Rush Medical College, Chicago, and a member of the AGA Center for GI Innovation and Technology.
The AGA wants new CRC screening tests that are capable of detecting advanced adenomas and advanced serrated lesions with a one-time sensitivity and specificity of 90% or higher, which is comparable with colonoscopy.
The FIT test has a sensitivity of less than 50% for detecting an advanced polyp of 10 mm or larger, said Dr. Melson.
The multitarget stool DNA (MT-sDNA) test may offer some improvement.
In a 2014 pivotal trial that compared FIT with the MT-sDNA in patients at average risk, the MT-sDNA test had higher sensitivity for detecting nonadvanced CRC lesions than FIT (92% vs. 74%) but less specificity (87% vs. 95%). The rate of detection of polyps with high-grade dysplasia was 69.2% with DNA testing and 46.2% with FIT.
However, the MT-sDNA test costs more than $500, compared with $25 for the FIT test, Dr. Melson pointed out.
To help identify the most appropriate screening for individual patients, better understanding and more thorough identification of risk factors are needed. “Risk assessment is definitely not where it could be,” Dr. Melson said.
The accuracy of risk assessment can be improved by sharing information from electronic health records on past colonoscopy polyp data, the presence of molecular markers, and family history, the AGA said. “With clearer risk assessment, shared decision-making on the most appropriate test becomes more clear and screening rates would benefit from patient buy-in and from easier access.”
The AGA recommended that research focus on the cost-effectiveness of screening younger patients, because the proportion of CRC cases in adults aged younger than 50 years has doubled since 1990.
This has raised the question as to whether the age for initial CRC screening should be lowered to 45 years (it already has been by the American Cancer Society), but there is much debate over this move.
Dr. Melson has received consulting fees from Clinical Genomics and research support from Boston Scientific Corporation and holds stocks in Virgo Imaging. A number of AGA white paper coauthors have disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
where the default screening modality to date has been colonoscopy.
Instead, the American Gastroenterological Association is proposing new approaches that combine better risk assessment, more use of noninvasive testing (such as fecal occult blood tests), and more targeted referrals for colonoscopy. Such changes could increase patient compliance and “save countless lives.”
“We need to improve our strategies to curb the cancer that ranks second for deaths in the U.S.,” commented Srinadh Komanduri, MD, chair of the AGA Center for GI Innovation and Technology, in a statement.
“Approximately 67% of eligible Americans are screened for colorectal cancer,” he said, which means that a third (33%) are not.
During the COVID-19 pandemic, the proportion of individuals not being screened has increased. One report of medical claims data showed that colonoscopies dropped by 90% during April.
The proposed changes are outlined in an AGA white paper: “Roadmap for the Future of Colorectal Cancer Screening in the United States.”
The report, written following consultation with 60 gastroenterology and research experts, was published online in Clinical Gastroenterology and Hepatology.
It proposed that alternative testing modalities to colonoscopy will need to be integrated into organized screening programs.
Rather than offering colonoscopy as the default screening method for all patients at risk, the AGA advised that it be offered initially only to patients at high risk, which would increase access for those who need it most. For patients at lower risk, noninvasive screening methods, such as fecal occult blood testing, could be offered initially and then integrated with colonoscopy.
“If we offered tests that were convenient, accurate, and of lower cost, and we could help people choose the best option based on their individual cancer risks, we would save more lives,” Joshua E. Melson, MD, MPH, lead author of the AGA white paper and professor at Rush University Medical Center, Chicago, said in an interview.
Screening can reduce CRC mortality by more than 50%, he added.
“Screening should be thought of as a process over time, not a single test isolated in time,” Dr. Melson commented. A clinical practice that has historically used only colonoscopy will need an organized, structured program to incorporate noninvasive testing, he said.
To date, efforts to increase CRC screening uptake have met with limited success, the AGA says. In 2014, the National Colorectal Cancer Round Table set the bar high with a 2018 screening goal of 80% for adults 50 years of age and older. As of 2020, some states had almost reached this goal, but most had not.
“In the opportunistic screening environment in the U.S., where colonoscopy is the most prevalent method, CRC screening has not reached aspirational goals in terms of uptake, reduction in CRC incidence, and disease burden,” the AGA said. “It is questionable if 80% uptake is achievable in a primarily opportunistic screening environment.”
In the proposed revamping of the current CRC screening infrastructure, patients whose physicians recommend CRC screening would no longer be left to their own devices to follow up. Clinicians would initiate CRC screening and oversee follow-up testing at defined intervals and would employ ongoing surveillance.
Ensuring that appropriate screening is readily available to at-risk individuals with no social, racial, or economic disparities is crucial, the AGA says. Racial disparities in access to screening disproportionately burden Blacks and Latin Americans as well as people living in rural areas. Screening differences account for 42% of the disparity in CRC incidence between Black and White Americans and 19% of the disparity in CRC mortality.
Compared with colonoscopy, which requires bowel preparation, time off from work, and a hospital or clinic procedure, the fecal immunochemical test (FIT), for which a patient provides stool samples that are examined for the presence of blood, is much less stressful: it is noninvasive, and the patients collect the samples themselves in their own home. Studies show that, in diverse environments, patients prefer FIT over colonoscopy.
In a controlled trial that involved more than 55,000 patients who were randomly assigned to undergo either FIT or colonoscopy, the participation rate in the first cycle was greater for FIT than for colonoscopy (34.2% vs. 24.6%). This partially offset the lower single-application sensitivity for CRC of FIT, the researchers said.
Results from a study with a cluster randomized design showed that offering up-front stool testing as an option in addition to colonoscopy increased screening uptake. Of patients offered fecal occult blood testing or colonoscopy, 69% completed the noninvasive screening, compared with 38% of those offered colonoscopy alone. Notably, non-White participants were more adherent to stool testing.
The success of the AGA’s new initiative hinges largely upon the development of affordable, highly accurate, easy-to-use, noninvasive tests. In this regard, the organization has challenged scientists and industry partners with an aspirational target that is “far superior to current methodologies in terms of sensitivity and specificity,” said Dr. Melson, who is associate professor at Rush Medical College, Chicago, and a member of the AGA Center for GI Innovation and Technology.
The AGA wants new CRC screening tests that are capable of detecting advanced adenomas and advanced serrated lesions with a one-time sensitivity and specificity of 90% or higher, which is comparable with colonoscopy.
The FIT test has a sensitivity of less than 50% for detecting an advanced polyp of 10 mm or larger, said Dr. Melson.
The multitarget stool DNA (MT-sDNA) test may offer some improvement.
In a 2014 pivotal trial that compared FIT with the MT-sDNA in patients at average risk, the MT-sDNA test had higher sensitivity for detecting nonadvanced CRC lesions than FIT (92% vs. 74%) but less specificity (87% vs. 95%). The rate of detection of polyps with high-grade dysplasia was 69.2% with DNA testing and 46.2% with FIT.
However, the MT-sDNA test costs more than $500, compared with $25 for the FIT test, Dr. Melson pointed out.
To help identify the most appropriate screening for individual patients, better understanding and more thorough identification of risk factors are needed. “Risk assessment is definitely not where it could be,” Dr. Melson said.
The accuracy of risk assessment can be improved by sharing information from electronic health records on past colonoscopy polyp data, the presence of molecular markers, and family history, the AGA said. “With clearer risk assessment, shared decision-making on the most appropriate test becomes more clear and screening rates would benefit from patient buy-in and from easier access.”
The AGA recommended that research focus on the cost-effectiveness of screening younger patients, because the proportion of CRC cases in adults aged younger than 50 years has doubled since 1990.
This has raised the question as to whether the age for initial CRC screening should be lowered to 45 years (it already has been by the American Cancer Society), but there is much debate over this move.
Dr. Melson has received consulting fees from Clinical Genomics and research support from Boston Scientific Corporation and holds stocks in Virgo Imaging. A number of AGA white paper coauthors have disclosed relevant financial relationships.
A version of this article originally appeared on Medscape.com.
PVR reassessed as predictor of heart failure
A study of patients with pulmonary hypertension suggests a reconsideration of the accepted benchmark for pulmonary vascular hypertension as a predictor of heart failure may be warranted.
An elevated pulmonary vascular resistance of 3.0 Wood units or greater has been used as a prognostic marker for death and heart failure in pulmonary hypertension subgroups. But a large, multiyear study of a veterans population suggests that shifting that threshold to 2.2 Wood units in patients with right-heart catheterization may be justified.
Bradley A. Maron, MD, of the Veterans Affairs Boston Healthcare System and Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues evaluated 40,082 veterans in the VA Clinical Assessment, Reporting and Tracking (CART) program who had right-heart catheterization (RHC) in the VA system from Oct. 1, 2007, to Sept. 30, 2016.
“To our knowledge, these data provide the first evidence-based information on the continuum of clinical risk related to PVR in patients with elevated pulmonary artery pressure,” the researchers wrote. Their report was published online in Lancet Respiratory Medicine (2020 Jul 27. doi: 10.1016/S2213-2600(20)30317-9).
The retrospective cohort study found that all-cause mortality hazard ratio (HR), when adjusted for clinical variables, and mean pulmonary artery pressure (mPAP) increased progressively beginning at around 2.0 Wood units (WU). Clinically significant mortality HR emerged at 2.2 WU, with an adjusted risk 9% greater than a PVR of 2.1 Wood units (P < .0034), which the study considered the upper limit of normal PVR in health adults of a similar age range (61.5 to 73.5 years) as the study cohort. The researchers noted that a PVR of 3.0 WU has been the standard for forecasting outcomes in pulmonary hypertension (PH) (Eur Heart J. 2010;31:2915-57).
“Overall, these results suggest that reconsidering the hemodynamic parameters that define pulmonary hypertension in patients with cardiopulmonary disease is warranted, and they identify a need for early detection strategies to capture this large and vulnerable population,” the researchers wrote.
A subsequent analysis focused on patients with an mPAP of >19 mm HG (n = 32,725) and found that all-cause death when adjusted over a wide range of clinical variables that included PVR of 2.2 WU increased to a 25% HR. “However,” the researchers added, “a median cardiac output of < 4.0 L/min, which has been shown to be independently associated with adverse outcome, was present only when PVR was more than 4.0 Wood units.”
For a PVR of 2.2-3.0 WU, the median cardiac output was 4.87 L/min; for > 3.0 WU, it was 4.13 L/min. Among the patients with PVR > 2.2 WU (n = 15,780), 13.6% (n = 2,147) had an mPAP of 19-24 mm Hg.
In all patients with mPAP > 19 mm HG, pulmonary artery wedge pressure (PAWP) became a determining risk factor, with 15 mm HG the demarcation between low and high PAWP. At PVR of 2.2 WU, low-PAWP patients had a 52% greater adjusted risk of death and high-PAWP a 23% greater risk. At 4.0 WU, those adjusted risks rose dramatically – to 272% and 58%, for the low- and high-PAWP subgroups, respectively (P < .0001).
“Stratification of patients by PAWP had a major effect on outcome estimates in our study, illustrating the limitations of using the same PVR level to define clinical risk between precapillary and postcapillary pulmonary hypertension,” the researchers wrote.
They called for further study into how these findings impact people with PH but lower levels of cardiopulmonary disease than the cohort. “Overall, these findings support reconsidering the combination of hemodynamic variables used to identify patients with pulmonary hypertension,” the researchers stated.
The analyses of the VA CART database makes this “an interesting study,” said G. Hossein Almassi, MD, FCCP, of the Medical College of Wisconsin and Zablocki VA Medical Center in Milwaukee. “Within its limitation as a retrospective cohort study, the findings of a lower PVR and a lower mean PAP of > 19 mm being associated with increased risk of all-cause mortality and HF hospitalization are significant.”
He added: “Time will tell whether this will be an impetus for the clinicians to consider earlier therapeutic interventions in addition to lifestyle modification such as smoking cessation in this group of patients.”
Dr. Maron disclosed a financial relationship with Actelion.
SOURCE: Maron BA et al. Lancet Respir Med. 2020 Jul 27. doi: 10.1016/S2213-2600(20)30317-9.
A study of patients with pulmonary hypertension suggests a reconsideration of the accepted benchmark for pulmonary vascular hypertension as a predictor of heart failure may be warranted.
An elevated pulmonary vascular resistance of 3.0 Wood units or greater has been used as a prognostic marker for death and heart failure in pulmonary hypertension subgroups. But a large, multiyear study of a veterans population suggests that shifting that threshold to 2.2 Wood units in patients with right-heart catheterization may be justified.
Bradley A. Maron, MD, of the Veterans Affairs Boston Healthcare System and Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues evaluated 40,082 veterans in the VA Clinical Assessment, Reporting and Tracking (CART) program who had right-heart catheterization (RHC) in the VA system from Oct. 1, 2007, to Sept. 30, 2016.
“To our knowledge, these data provide the first evidence-based information on the continuum of clinical risk related to PVR in patients with elevated pulmonary artery pressure,” the researchers wrote. Their report was published online in Lancet Respiratory Medicine (2020 Jul 27. doi: 10.1016/S2213-2600(20)30317-9).
The retrospective cohort study found that all-cause mortality hazard ratio (HR), when adjusted for clinical variables, and mean pulmonary artery pressure (mPAP) increased progressively beginning at around 2.0 Wood units (WU). Clinically significant mortality HR emerged at 2.2 WU, with an adjusted risk 9% greater than a PVR of 2.1 Wood units (P < .0034), which the study considered the upper limit of normal PVR in health adults of a similar age range (61.5 to 73.5 years) as the study cohort. The researchers noted that a PVR of 3.0 WU has been the standard for forecasting outcomes in pulmonary hypertension (PH) (Eur Heart J. 2010;31:2915-57).
“Overall, these results suggest that reconsidering the hemodynamic parameters that define pulmonary hypertension in patients with cardiopulmonary disease is warranted, and they identify a need for early detection strategies to capture this large and vulnerable population,” the researchers wrote.
A subsequent analysis focused on patients with an mPAP of >19 mm HG (n = 32,725) and found that all-cause death when adjusted over a wide range of clinical variables that included PVR of 2.2 WU increased to a 25% HR. “However,” the researchers added, “a median cardiac output of < 4.0 L/min, which has been shown to be independently associated with adverse outcome, was present only when PVR was more than 4.0 Wood units.”
For a PVR of 2.2-3.0 WU, the median cardiac output was 4.87 L/min; for > 3.0 WU, it was 4.13 L/min. Among the patients with PVR > 2.2 WU (n = 15,780), 13.6% (n = 2,147) had an mPAP of 19-24 mm Hg.
In all patients with mPAP > 19 mm HG, pulmonary artery wedge pressure (PAWP) became a determining risk factor, with 15 mm HG the demarcation between low and high PAWP. At PVR of 2.2 WU, low-PAWP patients had a 52% greater adjusted risk of death and high-PAWP a 23% greater risk. At 4.0 WU, those adjusted risks rose dramatically – to 272% and 58%, for the low- and high-PAWP subgroups, respectively (P < .0001).
“Stratification of patients by PAWP had a major effect on outcome estimates in our study, illustrating the limitations of using the same PVR level to define clinical risk between precapillary and postcapillary pulmonary hypertension,” the researchers wrote.
They called for further study into how these findings impact people with PH but lower levels of cardiopulmonary disease than the cohort. “Overall, these findings support reconsidering the combination of hemodynamic variables used to identify patients with pulmonary hypertension,” the researchers stated.
The analyses of the VA CART database makes this “an interesting study,” said G. Hossein Almassi, MD, FCCP, of the Medical College of Wisconsin and Zablocki VA Medical Center in Milwaukee. “Within its limitation as a retrospective cohort study, the findings of a lower PVR and a lower mean PAP of > 19 mm being associated with increased risk of all-cause mortality and HF hospitalization are significant.”
He added: “Time will tell whether this will be an impetus for the clinicians to consider earlier therapeutic interventions in addition to lifestyle modification such as smoking cessation in this group of patients.”
Dr. Maron disclosed a financial relationship with Actelion.
SOURCE: Maron BA et al. Lancet Respir Med. 2020 Jul 27. doi: 10.1016/S2213-2600(20)30317-9.
A study of patients with pulmonary hypertension suggests a reconsideration of the accepted benchmark for pulmonary vascular hypertension as a predictor of heart failure may be warranted.
An elevated pulmonary vascular resistance of 3.0 Wood units or greater has been used as a prognostic marker for death and heart failure in pulmonary hypertension subgroups. But a large, multiyear study of a veterans population suggests that shifting that threshold to 2.2 Wood units in patients with right-heart catheterization may be justified.
Bradley A. Maron, MD, of the Veterans Affairs Boston Healthcare System and Brigham and Women’s Hospital and Harvard Medical School, Boston, and colleagues evaluated 40,082 veterans in the VA Clinical Assessment, Reporting and Tracking (CART) program who had right-heart catheterization (RHC) in the VA system from Oct. 1, 2007, to Sept. 30, 2016.
“To our knowledge, these data provide the first evidence-based information on the continuum of clinical risk related to PVR in patients with elevated pulmonary artery pressure,” the researchers wrote. Their report was published online in Lancet Respiratory Medicine (2020 Jul 27. doi: 10.1016/S2213-2600(20)30317-9).
The retrospective cohort study found that all-cause mortality hazard ratio (HR), when adjusted for clinical variables, and mean pulmonary artery pressure (mPAP) increased progressively beginning at around 2.0 Wood units (WU). Clinically significant mortality HR emerged at 2.2 WU, with an adjusted risk 9% greater than a PVR of 2.1 Wood units (P < .0034), which the study considered the upper limit of normal PVR in health adults of a similar age range (61.5 to 73.5 years) as the study cohort. The researchers noted that a PVR of 3.0 WU has been the standard for forecasting outcomes in pulmonary hypertension (PH) (Eur Heart J. 2010;31:2915-57).
“Overall, these results suggest that reconsidering the hemodynamic parameters that define pulmonary hypertension in patients with cardiopulmonary disease is warranted, and they identify a need for early detection strategies to capture this large and vulnerable population,” the researchers wrote.
A subsequent analysis focused on patients with an mPAP of >19 mm HG (n = 32,725) and found that all-cause death when adjusted over a wide range of clinical variables that included PVR of 2.2 WU increased to a 25% HR. “However,” the researchers added, “a median cardiac output of < 4.0 L/min, which has been shown to be independently associated with adverse outcome, was present only when PVR was more than 4.0 Wood units.”
For a PVR of 2.2-3.0 WU, the median cardiac output was 4.87 L/min; for > 3.0 WU, it was 4.13 L/min. Among the patients with PVR > 2.2 WU (n = 15,780), 13.6% (n = 2,147) had an mPAP of 19-24 mm Hg.
In all patients with mPAP > 19 mm HG, pulmonary artery wedge pressure (PAWP) became a determining risk factor, with 15 mm HG the demarcation between low and high PAWP. At PVR of 2.2 WU, low-PAWP patients had a 52% greater adjusted risk of death and high-PAWP a 23% greater risk. At 4.0 WU, those adjusted risks rose dramatically – to 272% and 58%, for the low- and high-PAWP subgroups, respectively (P < .0001).
“Stratification of patients by PAWP had a major effect on outcome estimates in our study, illustrating the limitations of using the same PVR level to define clinical risk between precapillary and postcapillary pulmonary hypertension,” the researchers wrote.
They called for further study into how these findings impact people with PH but lower levels of cardiopulmonary disease than the cohort. “Overall, these findings support reconsidering the combination of hemodynamic variables used to identify patients with pulmonary hypertension,” the researchers stated.
The analyses of the VA CART database makes this “an interesting study,” said G. Hossein Almassi, MD, FCCP, of the Medical College of Wisconsin and Zablocki VA Medical Center in Milwaukee. “Within its limitation as a retrospective cohort study, the findings of a lower PVR and a lower mean PAP of > 19 mm being associated with increased risk of all-cause mortality and HF hospitalization are significant.”
He added: “Time will tell whether this will be an impetus for the clinicians to consider earlier therapeutic interventions in addition to lifestyle modification such as smoking cessation in this group of patients.”
Dr. Maron disclosed a financial relationship with Actelion.
SOURCE: Maron BA et al. Lancet Respir Med. 2020 Jul 27. doi: 10.1016/S2213-2600(20)30317-9.
FROM LANCET RESPIRATORY MEDICINE
Health disparity: Race, mortality, and infants of teenage mothers
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
according to a new analysis from the National Center for Health Statistics.

In 2017-2018, overall mortality rates were 12.5 per 100,000 live births for infants born to Black mothers aged 15-19 years, 8.4 per 100,000 for infants born to White teenagers, and 6.5 per 100,000 for those born to Hispanic teens, Ashley M. Woodall, MPH, and Anne K. Driscoll, PhD, of the NCHS said in a data brief.
Looking at the five leading causes of those deaths shows that deaths of Black infants were the highest by significant margins in four, although, when it comes to “disorders related to short gestation and low birth weight,” significant may be an understatement.
The rate of preterm/low-birth-weight deaths for white infants in 2017-2018 was 119 per 100,000 live births; for Hispanic infants it was 94 per 100,000. Among infants born to Black teenagers, however, it was 284 deaths per 100,000, they reported based on data from the National Vital Statistics System’s linked birth/infant death file.
The numbers for congenital malformations and accidents were closer but still significantly different, and with each of the three most common causes, the rates for infants of Hispanic mothers also were significantly lower than those of White infants, the researchers said.
The situation changes for mortality-cause No. 4, sudden infant death syndrome, which was significantly more common among infants born to White teenagers, with a rate of 91 deaths per 100,000 live births, compared with either black (77) or Hispanic (44) infants, Ms. Woodall and Dr. Driscoll said.
Infants born to Black teens had the highest death rate again (68 per 100,000) for maternal complications of pregnancy, the fifth-leading cause of mortality, but for the first time Hispanic infants had a higher rate (36) than did those of White teenagers (29), they reported.
Children’s doctors in the world of adults
Pediatric hospitalists venture into COVID-19 adult care
The memories I have from the few nights spent in the adult pop-up cardiac intensive care unit are pouring in as I sit down to tell this story. I am a pediatric hospitalist at Columbia University NewYork-Presbyterian Morgan Stanley Children’s Hospital. I usually take care of sick, hospitalized children. However, in these extraordinary times, I have joined an army of colleagues taking care of adult patients with COVID-19.
Almost all these patients had tracheostomies connected to ventilators, as well as acute-on-chronic cardiac issues. They were often delirious and unable to speak, and always alone. I was happy to help our adult colleagues, but I was also afraid. I was scared to make a mistake that could be detrimental to my patient, even though I knew well that ICU residents, fellows, and attendings were just a phone call away.
I felt like Alice in Wonderland, initially too small compared with her environment, and the next minute hunched, giant, and still clearly displaced. Except I was not dreaming or watching a movie. There was no white rabbit to chase. The situation was serious and emotionally challenging. I imagined that each patient was the dearest member of my family: my mother, my father, my aunt or uncle. I took pleasure in sharing smiles while asking the patients how they were feeling, and I touched their hands, even though much of my face was covered and there were gloves on my hands.
The year 2020 has been surreal. People have had to find their own way of pushing through the unknown and unexpected. For a start, I would never in a million years have imagined using phrases like pop-up ICU.1 I was signing an admission note for a 90-year-old lady with acute-on-chronic congestive heart failure and acute respiratory hypoxemic failure and there, at the bottom of the note, was my name, followed by an odd remark: “pediatric hospital medicine.” That is what happened in New York City in 2020: Many unexpected events took place.
This article represents a virtual conversation with three other pediatric hospitalists who, under different sets of circumstances, did the same thing: took care of adult patients. I hope that the answers to the questions I asked make you pause, reflect, and learn from the experiences described.
Would you describe the usual environment where you practice pediatric hospital medicine?
Julie Dunbar, MD: I am a full-time pediatric hospitalist at the Children’s Hospital at Montefiore, a tertiary care academic children’s hospital in the Bronx. A typical day on service involves staffing up to 14 patients, up to 21 years old, on a teaching service with residents and physician assistants. We normally staff the hospital in two shifts – day and evening – until 11:00 at night. We are situated at the heart of a medically underserved area, and our hospital system cares for about one-third of the total population of the Bronx.
L. Nell Hodo, MD: I work at Kravis Children’s Hospital at the Mount Sinai Hospital, in Manhattan at the juncture of the Upper East Side and Harlem. Our usual hospital medicine environment is the general ward/floor in a nested children’s hospital within an adult hospital. We have about 32 non-ICU beds, and the patients are managed by a combination of hospitalists, general pediatricians, and specialist attendings. All patients are on resident teams. We have a comanagement model in which the primary attending for surgical patients is always a pediatric attending (hospitalist or specialist).
Avital M. Fischer, MD: NewYork-Presbyterian Morgan Stanley Children’s Hospital is a quaternary care center – where children from the area receive subspecialty care – as well as, functionally, a community hospital for the Washington Heights area. Therefore, we always have an interesting mix of general pediatric inpatient medicine including patients with complex medical conditions, rare diseases, postoperative conditions, and undiagnosed illnesses on our wards. We are a children’s hospital, connected to a larger adult hospital system. Pediatric hospitalists cover two pediatric wards, team-staffed by residents, and a progressive care unit, staffed by nurse practitioners. There is usually evening coverage until 11 p.m.
How did this change when New York became the U.S. epicenter of the SARS-CoV-2 pandemic? Was the transition to taking care of adult patients gradual or sudden? Were you deployed to a different hospital or part of the hospital? How prepared did you feel?
Dr. Dunbar: We experienced the COVID-19 pandemic like much of the rest of New York City – it started as a slow and uncertain process, and then it hit us all at once. In initial conversations, like everyone else, we did not know exactly what was coming. We started with small changes like working from home on nonclinical days and canceling family-centered rounds to conserve personal protective equipment (PPE). In mid-March, we were still expecting that redeployment to adult floors was a highly unlikely scenario. We made work-from-home schedules and planned projects we would work on while social distancing. We planned journal clubs about emerging evidence on COVID-19. However, things happened fast, and many of these plans were scrapped.
On Saturday, March 28, we closed the main floor of the children’s hospital because so few pediatric patients were being admitted. Two days later, we admitted our first cohort of adult COVID-19 patients, all more than 30 years old. They were transferred en masse from an outside hospital emergency department that desperately needed our beds. They arrived all at once, and they all required respiratory support. At the last hospitalist division meeting before the adults arrived, we had time for only one priority set of information, and so we chose end-of-life care. We reviewed scripts for advance care planning and logistics of death certificates. As fast as things changed for us, they changed even faster for the patients. Most were relatively healthy people who rather suddenly found themselves isolated, on oxygen, dictating their final wishes to pediatricians in full protective gear. Many, many patients got better, and of course, several spent their last moments with us. One physician assistant, who works closely with the hospitalists, spent the last 5 hours of an elderly patient’s life holding her hand and helping her FaceTime with family.
For the most part, the patients came to us. We worked with our own colleagues and our own nurses, on our own territory. A few of my colleagues were briefly redeployed to a series of conference rooms that were used for several weeks as overflow space for more stable COVID-19 patients. Staffing by the pediatrics teams was so robust, with willing volunteers from every corner of the children’s hospital, that we were not needed for long.
During the early days, there was no clinical pathway to follow to care for COVID-19 patients – it didn’t exist for this novel and variable disease. We created a platform to share documents and resources in real time as they became available to us. We used group texts and emails to learn from our experiences and encourage one another. Importantly, no one was afraid to ask for help, and we relied on our adult colleagues when patients started to decompensate. Adult critical care came to our aid for all rapid responses for patients older than 30. Pediatric critical care, in their infinite flexibility, was responsible for anyone younger.
Dr. Hodo: We had a variety of changes. The first thing was the deployment of many of our attendings (hospital medicine, ICU, outpatient, and subspecialists) and residents to the adult side to work on medical COVID-19 units or in the many ICUs (some new “pop-up” units in former medical units, postanesthesia care units, and so on).2 On the adult floor we had “COVID teams,” which had an attending and two frontline providers; one of these three people was an internal medicine faculty member or resident. Residents from other specialties (emergency medicine, family medicine) were pulled off pediatric assignments in pediatric wards, PICUs, and EDs, so pediatric residents not originally assigned to inpatient rotations were sent to cover these core pediatric areas. The remaining pediatric faculty backfilled the pediatric services – so the remaining ICU docs did more shifts to cover ICU; the undeployed specialists took more inpatient service or clinic time, and so on. Outpatient pediatrics covered the inpatient pediatric service for the 3 weeks when most of the hospitalists were deployed.
We had one pediatric unit, which was a unit with equipment that made it capable of having ICU patients or floor patients, that was designated a COVID-19 unit. Most COVID-19 patients were there. Some were also in negative-pressure rooms on other floors or in the unit directly above the COVID-19 unit. Some adult patients came to the unit in the pediatric hospital but not as many as initially expected, and most were young adults in their 20s. So rather than adult patients coming to pediatrics, our experience was more that pediatricians went to the adult side.
The transition to adult care for physicians was variable in its suddenness. Most people had at least 48 hours’ notice, whereas some had as much as a week. Most of our department members deployed within the hospital complex of which we are a part, though a few went to other sites in the health system. Some were deployed into administrative or support roles in the system, rather than patient-facing roles. I felt, I would say, reasonably prepared. I trained in family medicine, though I have been exclusively in pediatrics for the past 7 years. I felt rusty, for sure, but perhaps not quite as out of my element as others. In preparation, I read a lot about COVID, reviewed some adult medicine topics provided by the medicine department, used the resources on the Pediatric Overflow Planning Contingency Response Network (POPCoRN), including an Advanced Cardiac Life Support review, and was able to shadow on a COVID-19 unit before I actually started – that was incredibly helpful. I also had the opportunity to speak about that shadowing experience in a department meeting, which I hope was helpful for others.
Dr. Fischer: Our whole focus for a relatively short time shifted to how to take care of adults within the children’s hospital. Although we had some time to prepare – the ICU was the first unit to take adults, so we knew they would come to the floor – it still felt quick. We took adult patients onto the general pediatrics floor from both the emergency department and the ICU. We took adults mostly with COVID-19, but we did have some young adults admitted for other reasons too. Those of us who were on service during this time collaborated closely, sharing what we learned and even joining one another on rounds to provide support. We basically would “teach it forward” as we learned. We also had adult providers available by phone for questions, and our pediatric subspecialists were readily available for consults and would reach out to their adult counterparts for support. Some of the hospitalists were reaching out to POPCoRN, and some were attending an ACLS crash course prior to getting on service.
What was hardest about this experience for you?
Dr. Dunbar: For me, one of the hardest aspects of dealing with COVID-19 was the unknown. In every aspect of professional life and clinical care, there were unanswered questions. What’s the best way to care for these patients? What prognoses can we give their loved ones? How can I help when it seems like there’s so little I can offer? Will we run out of PPE? As doctors, what behaviors most endanger our friends and family when we go home after work? When will things start to get better?
Dr. Hodo: For me, the week or two before being notified of the deployment was the worst and hardest time. The uncertainty about if I would be called or no, and to do what? And where? I was trying to read everything there was on management, what little was known about treatment, and so on. Once I received notification of a start date, that allowed me to focus on very clear endpoints and knowledge items (for example, reviewing ACLS algorithms) and to do things I knew would help me settle and be more effective (like shadowing).
Dr. Fischer: It was a lot of new. Not only were we taking care of a population that we hadn’t cared for since medical school (adults), but we were facing a disease process that was also new to everyone. We were learning on our feet, while at the same time providing guidance to our house staff.
What have you learned about yourself that you did not know before?
Dr. Dunbar: I was surprised to learn how much I liked caring for adult patients. The fear I felt immediately before they arrived dissipated fairly quickly after they arrived. The opportunity to address their chronic conditions while supporting them in an acute illness took me back to many of the fundamentals of medicine that I hadn’t thought much about since medical school. I liked that they could speak up to tell us how they were feeling, both physically and emotionally, so that we could address their needs and allow them to participate in their own care. Some of my favorite patients kept detailed histories of their own C-reactive protein values and oxygen levels to show they were active participants in their own recovery.
I was worried that these adult patients would be offended or scared to learn that they were being cared for by pediatricians, but at no point did anyone ask me why they were not assigned to an adult hospitalist. They saw us only as doctors and nurses, and they were grateful for our care. One 65-year-old U.S. Army veteran told me that his nurse had told him to take a shower and make his bed. “She treated me just like a 5-year-old kid. And I loved it!” he said.
Dr. Hodo: I don’t know that I was totally unaware of these things, but I will say that I had partially forgotten them: I really like adult medicine, and I love geriatrics. I like high-energy and high-stress situations … at least occasionally! I feel very comfortable discussing end-of-life decisions and death. I cope with personal stress by helping and supporting others – patients, team members, colleagues, neighbors. I risk not taking enough time for myself and have to remind myself to do so.
Dr. Fischer: I actually loved taking care of adults. It felt like there was a different kind of patient-doctor relationship to be had, and it was interesting to get to know people who had jobs and families of their own – essentially a different type of story than you typically hear taking care of children.
Were there any silver linings in this situation? How did you grow personally through this experience? What do we need to do better going forward as a profession and a community?
Dr. Dunbar: The part that I hope will stay with me is the memory of how we came together as clinicians to fight a common invisible enemy. The teamwork was unprecedented. Our day-to-day goals were simple and straightforward: do what needed to be done to help as many New Yorkers as possible. Our team made themselves available for last-minute meetings and shift changes without complaint. We practiced a type of medicine that prioritized patient comfort, flexibility, and compassionate care. We ordered methadone and insulin and antihypertensives – brand new experiences for us, but we figured it out. We worked through novel clinical problems together because there was no textbook to read.
Our colleagues from other specialties and different levels of experience stepped up to join us on overnight shifts, and we welcomed them. With the help of an ad hoc palliative care team, we improved how we listened to patients’ own self-directed needs. We reached across the aisle to our internal medicine and adult hospitalist colleagues to refresh our memories on chronic conditions, and they always answered the phone. I hope we always remember who we were during this crisis, because we were ourselves at our most generous.
Dr. Hodo: This was an unexpected but great opportunity to meet physicians, nurses, and staff in different departments and sections of the hospital from my own. I am hopeful that this experience will help us in the future with multidisciplinary work and breaking down silos that isolate specialties and units in the hospital.
I feel (and this is probably weird) invigorated by this experience. It feels good to have been able to help when I was needed. Even though there are a lot of things in adult hospital medicine I do not know, I know I did my best, asked for help when I needed it, and asked for feedback regularly from the medicine residents and nurses I worked with. I know I supported my team and my colleagues to the best of my ability through stressful and sometimes upsetting and emotionally draining times.
As a profession, we can continue to remember the value of the multidisciplinary team and the value of listening to, and making space for, different voices to be heard. We can reconsider the traditional, rigid hierarchy in medicine and medical education that can stifle creative thought and innovative ideas. We can remember that the people “at the top” of the pyramid can always learn something from those “at the bottom.” We can see the ways that department and discipline and specialty can help us but also sometimes hinder, and seek involvement in programs and discussions that unite and pool resources and skills. And, most of all, we can try, every day we are at work, to put the patients’ and families’ needs first – and when we leave work, to turn that around, and put ourselves and our loved ones in that prime position.
As a community, we also can work on thinking communally – that, after all, is the entire point of the wearing of masks in public and social distancing. It is as much about you as about me! We can try to hold on to some of this perspective of the greater good and appreciation for the work others do that makes our lives better and easier. It is not only health care workers who deserve a round of applause every day; it is every person who did something today that benefited someone else, be that giving extra space in a line, wearing a mask in a store, delivering food to an elder, teaching a class over Zoom, or simply minimizing time outside the house. It is every person who thought about the community at or near the same level of priority that they thought about themselves.
Dr. Fischer: It was a very challenging situation, but because our adult patients in the children’s hospital were relatively young with fewer comorbidities, we got to see people get well. I took care of one man with renal failure who we thought would be on dialysis for the rest of his life. By the end of my first week on service, he had begun to regain kidney function. It was amazing. I think most frontline providers caring for adults in this pandemic have had to face significant morbidity and mortality. I felt lucky that we were able to care for patients who generally got better.
I recently read the article published in the Journal of Pediatrics laying out how the Children’s Hospital at Montefiore adapted an entire pediatric floor to caring for adults.3 This example of recognition of need, quick preparation, and collaboration both within the children’s hospital and with the adult hospital was admirable. I also feel that at the beginning of this pandemic, there was a glimmer that the failure of our health care system to cover everyone and the repercussions of this failure would be drawn into sharp relief. I hope that this understanding of the importance of universal coverage persists beyond the pandemic.
Dr. Giordano is assistant professor of pediatrics at Columbia University and a pediatric hospitalist at NewYork-Presbyterian Morgan Stanley Children’s Hospital with an interest in surgical comanagement. She serves on the Society of Hospital Medicine’s Pediatric Special Interest Group Executive Committee and is the chair of the Education Subcommittee. She is also an advisory board member for the New York/Westchester SHM Chapter.
References
1. Kumaraiah D et al. Innovative ICU physician care models: Covid-19 pandemic at NewYork-Presbyterian. NEJM Catal. 2020 Apr 28. doi: 10.1056/CAT.20.0158.
2. Kim MK et al. A primer for clinician deployment to the medicine floors from an epicenter of Covid-19. NEJM Catal. 2020 May 4. doi: 10.1056/CAT.20.0180.
3. Philips K, et al. Rapid Implementation of an Adult COVID-19 Unit in a Children’s Hospital. J Pediatr. 2020. doi: 10.1016/j.jpeds.2020.04.060.
Pediatric hospitalists venture into COVID-19 adult care
Pediatric hospitalists venture into COVID-19 adult care
The memories I have from the few nights spent in the adult pop-up cardiac intensive care unit are pouring in as I sit down to tell this story. I am a pediatric hospitalist at Columbia University NewYork-Presbyterian Morgan Stanley Children’s Hospital. I usually take care of sick, hospitalized children. However, in these extraordinary times, I have joined an army of colleagues taking care of adult patients with COVID-19.
Almost all these patients had tracheostomies connected to ventilators, as well as acute-on-chronic cardiac issues. They were often delirious and unable to speak, and always alone. I was happy to help our adult colleagues, but I was also afraid. I was scared to make a mistake that could be detrimental to my patient, even though I knew well that ICU residents, fellows, and attendings were just a phone call away.
I felt like Alice in Wonderland, initially too small compared with her environment, and the next minute hunched, giant, and still clearly displaced. Except I was not dreaming or watching a movie. There was no white rabbit to chase. The situation was serious and emotionally challenging. I imagined that each patient was the dearest member of my family: my mother, my father, my aunt or uncle. I took pleasure in sharing smiles while asking the patients how they were feeling, and I touched their hands, even though much of my face was covered and there were gloves on my hands.
The year 2020 has been surreal. People have had to find their own way of pushing through the unknown and unexpected. For a start, I would never in a million years have imagined using phrases like pop-up ICU.1 I was signing an admission note for a 90-year-old lady with acute-on-chronic congestive heart failure and acute respiratory hypoxemic failure and there, at the bottom of the note, was my name, followed by an odd remark: “pediatric hospital medicine.” That is what happened in New York City in 2020: Many unexpected events took place.
This article represents a virtual conversation with three other pediatric hospitalists who, under different sets of circumstances, did the same thing: took care of adult patients. I hope that the answers to the questions I asked make you pause, reflect, and learn from the experiences described.
Would you describe the usual environment where you practice pediatric hospital medicine?
Julie Dunbar, MD: I am a full-time pediatric hospitalist at the Children’s Hospital at Montefiore, a tertiary care academic children’s hospital in the Bronx. A typical day on service involves staffing up to 14 patients, up to 21 years old, on a teaching service with residents and physician assistants. We normally staff the hospital in two shifts – day and evening – until 11:00 at night. We are situated at the heart of a medically underserved area, and our hospital system cares for about one-third of the total population of the Bronx.
L. Nell Hodo, MD: I work at Kravis Children’s Hospital at the Mount Sinai Hospital, in Manhattan at the juncture of the Upper East Side and Harlem. Our usual hospital medicine environment is the general ward/floor in a nested children’s hospital within an adult hospital. We have about 32 non-ICU beds, and the patients are managed by a combination of hospitalists, general pediatricians, and specialist attendings. All patients are on resident teams. We have a comanagement model in which the primary attending for surgical patients is always a pediatric attending (hospitalist or specialist).
Avital M. Fischer, MD: NewYork-Presbyterian Morgan Stanley Children’s Hospital is a quaternary care center – where children from the area receive subspecialty care – as well as, functionally, a community hospital for the Washington Heights area. Therefore, we always have an interesting mix of general pediatric inpatient medicine including patients with complex medical conditions, rare diseases, postoperative conditions, and undiagnosed illnesses on our wards. We are a children’s hospital, connected to a larger adult hospital system. Pediatric hospitalists cover two pediatric wards, team-staffed by residents, and a progressive care unit, staffed by nurse practitioners. There is usually evening coverage until 11 p.m.
How did this change when New York became the U.S. epicenter of the SARS-CoV-2 pandemic? Was the transition to taking care of adult patients gradual or sudden? Were you deployed to a different hospital or part of the hospital? How prepared did you feel?
Dr. Dunbar: We experienced the COVID-19 pandemic like much of the rest of New York City – it started as a slow and uncertain process, and then it hit us all at once. In initial conversations, like everyone else, we did not know exactly what was coming. We started with small changes like working from home on nonclinical days and canceling family-centered rounds to conserve personal protective equipment (PPE). In mid-March, we were still expecting that redeployment to adult floors was a highly unlikely scenario. We made work-from-home schedules and planned projects we would work on while social distancing. We planned journal clubs about emerging evidence on COVID-19. However, things happened fast, and many of these plans were scrapped.
On Saturday, March 28, we closed the main floor of the children’s hospital because so few pediatric patients were being admitted. Two days later, we admitted our first cohort of adult COVID-19 patients, all more than 30 years old. They were transferred en masse from an outside hospital emergency department that desperately needed our beds. They arrived all at once, and they all required respiratory support. At the last hospitalist division meeting before the adults arrived, we had time for only one priority set of information, and so we chose end-of-life care. We reviewed scripts for advance care planning and logistics of death certificates. As fast as things changed for us, they changed even faster for the patients. Most were relatively healthy people who rather suddenly found themselves isolated, on oxygen, dictating their final wishes to pediatricians in full protective gear. Many, many patients got better, and of course, several spent their last moments with us. One physician assistant, who works closely with the hospitalists, spent the last 5 hours of an elderly patient’s life holding her hand and helping her FaceTime with family.
For the most part, the patients came to us. We worked with our own colleagues and our own nurses, on our own territory. A few of my colleagues were briefly redeployed to a series of conference rooms that were used for several weeks as overflow space for more stable COVID-19 patients. Staffing by the pediatrics teams was so robust, with willing volunteers from every corner of the children’s hospital, that we were not needed for long.
During the early days, there was no clinical pathway to follow to care for COVID-19 patients – it didn’t exist for this novel and variable disease. We created a platform to share documents and resources in real time as they became available to us. We used group texts and emails to learn from our experiences and encourage one another. Importantly, no one was afraid to ask for help, and we relied on our adult colleagues when patients started to decompensate. Adult critical care came to our aid for all rapid responses for patients older than 30. Pediatric critical care, in their infinite flexibility, was responsible for anyone younger.
Dr. Hodo: We had a variety of changes. The first thing was the deployment of many of our attendings (hospital medicine, ICU, outpatient, and subspecialists) and residents to the adult side to work on medical COVID-19 units or in the many ICUs (some new “pop-up” units in former medical units, postanesthesia care units, and so on).2 On the adult floor we had “COVID teams,” which had an attending and two frontline providers; one of these three people was an internal medicine faculty member or resident. Residents from other specialties (emergency medicine, family medicine) were pulled off pediatric assignments in pediatric wards, PICUs, and EDs, so pediatric residents not originally assigned to inpatient rotations were sent to cover these core pediatric areas. The remaining pediatric faculty backfilled the pediatric services – so the remaining ICU docs did more shifts to cover ICU; the undeployed specialists took more inpatient service or clinic time, and so on. Outpatient pediatrics covered the inpatient pediatric service for the 3 weeks when most of the hospitalists were deployed.
We had one pediatric unit, which was a unit with equipment that made it capable of having ICU patients or floor patients, that was designated a COVID-19 unit. Most COVID-19 patients were there. Some were also in negative-pressure rooms on other floors or in the unit directly above the COVID-19 unit. Some adult patients came to the unit in the pediatric hospital but not as many as initially expected, and most were young adults in their 20s. So rather than adult patients coming to pediatrics, our experience was more that pediatricians went to the adult side.
The transition to adult care for physicians was variable in its suddenness. Most people had at least 48 hours’ notice, whereas some had as much as a week. Most of our department members deployed within the hospital complex of which we are a part, though a few went to other sites in the health system. Some were deployed into administrative or support roles in the system, rather than patient-facing roles. I felt, I would say, reasonably prepared. I trained in family medicine, though I have been exclusively in pediatrics for the past 7 years. I felt rusty, for sure, but perhaps not quite as out of my element as others. In preparation, I read a lot about COVID, reviewed some adult medicine topics provided by the medicine department, used the resources on the Pediatric Overflow Planning Contingency Response Network (POPCoRN), including an Advanced Cardiac Life Support review, and was able to shadow on a COVID-19 unit before I actually started – that was incredibly helpful. I also had the opportunity to speak about that shadowing experience in a department meeting, which I hope was helpful for others.
Dr. Fischer: Our whole focus for a relatively short time shifted to how to take care of adults within the children’s hospital. Although we had some time to prepare – the ICU was the first unit to take adults, so we knew they would come to the floor – it still felt quick. We took adult patients onto the general pediatrics floor from both the emergency department and the ICU. We took adults mostly with COVID-19, but we did have some young adults admitted for other reasons too. Those of us who were on service during this time collaborated closely, sharing what we learned and even joining one another on rounds to provide support. We basically would “teach it forward” as we learned. We also had adult providers available by phone for questions, and our pediatric subspecialists were readily available for consults and would reach out to their adult counterparts for support. Some of the hospitalists were reaching out to POPCoRN, and some were attending an ACLS crash course prior to getting on service.
What was hardest about this experience for you?
Dr. Dunbar: For me, one of the hardest aspects of dealing with COVID-19 was the unknown. In every aspect of professional life and clinical care, there were unanswered questions. What’s the best way to care for these patients? What prognoses can we give their loved ones? How can I help when it seems like there’s so little I can offer? Will we run out of PPE? As doctors, what behaviors most endanger our friends and family when we go home after work? When will things start to get better?
Dr. Hodo: For me, the week or two before being notified of the deployment was the worst and hardest time. The uncertainty about if I would be called or no, and to do what? And where? I was trying to read everything there was on management, what little was known about treatment, and so on. Once I received notification of a start date, that allowed me to focus on very clear endpoints and knowledge items (for example, reviewing ACLS algorithms) and to do things I knew would help me settle and be more effective (like shadowing).
Dr. Fischer: It was a lot of new. Not only were we taking care of a population that we hadn’t cared for since medical school (adults), but we were facing a disease process that was also new to everyone. We were learning on our feet, while at the same time providing guidance to our house staff.
What have you learned about yourself that you did not know before?
Dr. Dunbar: I was surprised to learn how much I liked caring for adult patients. The fear I felt immediately before they arrived dissipated fairly quickly after they arrived. The opportunity to address their chronic conditions while supporting them in an acute illness took me back to many of the fundamentals of medicine that I hadn’t thought much about since medical school. I liked that they could speak up to tell us how they were feeling, both physically and emotionally, so that we could address their needs and allow them to participate in their own care. Some of my favorite patients kept detailed histories of their own C-reactive protein values and oxygen levels to show they were active participants in their own recovery.
I was worried that these adult patients would be offended or scared to learn that they were being cared for by pediatricians, but at no point did anyone ask me why they were not assigned to an adult hospitalist. They saw us only as doctors and nurses, and they were grateful for our care. One 65-year-old U.S. Army veteran told me that his nurse had told him to take a shower and make his bed. “She treated me just like a 5-year-old kid. And I loved it!” he said.
Dr. Hodo: I don’t know that I was totally unaware of these things, but I will say that I had partially forgotten them: I really like adult medicine, and I love geriatrics. I like high-energy and high-stress situations … at least occasionally! I feel very comfortable discussing end-of-life decisions and death. I cope with personal stress by helping and supporting others – patients, team members, colleagues, neighbors. I risk not taking enough time for myself and have to remind myself to do so.
Dr. Fischer: I actually loved taking care of adults. It felt like there was a different kind of patient-doctor relationship to be had, and it was interesting to get to know people who had jobs and families of their own – essentially a different type of story than you typically hear taking care of children.
Were there any silver linings in this situation? How did you grow personally through this experience? What do we need to do better going forward as a profession and a community?
Dr. Dunbar: The part that I hope will stay with me is the memory of how we came together as clinicians to fight a common invisible enemy. The teamwork was unprecedented. Our day-to-day goals were simple and straightforward: do what needed to be done to help as many New Yorkers as possible. Our team made themselves available for last-minute meetings and shift changes without complaint. We practiced a type of medicine that prioritized patient comfort, flexibility, and compassionate care. We ordered methadone and insulin and antihypertensives – brand new experiences for us, but we figured it out. We worked through novel clinical problems together because there was no textbook to read.
Our colleagues from other specialties and different levels of experience stepped up to join us on overnight shifts, and we welcomed them. With the help of an ad hoc palliative care team, we improved how we listened to patients’ own self-directed needs. We reached across the aisle to our internal medicine and adult hospitalist colleagues to refresh our memories on chronic conditions, and they always answered the phone. I hope we always remember who we were during this crisis, because we were ourselves at our most generous.
Dr. Hodo: This was an unexpected but great opportunity to meet physicians, nurses, and staff in different departments and sections of the hospital from my own. I am hopeful that this experience will help us in the future with multidisciplinary work and breaking down silos that isolate specialties and units in the hospital.
I feel (and this is probably weird) invigorated by this experience. It feels good to have been able to help when I was needed. Even though there are a lot of things in adult hospital medicine I do not know, I know I did my best, asked for help when I needed it, and asked for feedback regularly from the medicine residents and nurses I worked with. I know I supported my team and my colleagues to the best of my ability through stressful and sometimes upsetting and emotionally draining times.
As a profession, we can continue to remember the value of the multidisciplinary team and the value of listening to, and making space for, different voices to be heard. We can reconsider the traditional, rigid hierarchy in medicine and medical education that can stifle creative thought and innovative ideas. We can remember that the people “at the top” of the pyramid can always learn something from those “at the bottom.” We can see the ways that department and discipline and specialty can help us but also sometimes hinder, and seek involvement in programs and discussions that unite and pool resources and skills. And, most of all, we can try, every day we are at work, to put the patients’ and families’ needs first – and when we leave work, to turn that around, and put ourselves and our loved ones in that prime position.
As a community, we also can work on thinking communally – that, after all, is the entire point of the wearing of masks in public and social distancing. It is as much about you as about me! We can try to hold on to some of this perspective of the greater good and appreciation for the work others do that makes our lives better and easier. It is not only health care workers who deserve a round of applause every day; it is every person who did something today that benefited someone else, be that giving extra space in a line, wearing a mask in a store, delivering food to an elder, teaching a class over Zoom, or simply minimizing time outside the house. It is every person who thought about the community at or near the same level of priority that they thought about themselves.
Dr. Fischer: It was a very challenging situation, but because our adult patients in the children’s hospital were relatively young with fewer comorbidities, we got to see people get well. I took care of one man with renal failure who we thought would be on dialysis for the rest of his life. By the end of my first week on service, he had begun to regain kidney function. It was amazing. I think most frontline providers caring for adults in this pandemic have had to face significant morbidity and mortality. I felt lucky that we were able to care for patients who generally got better.
I recently read the article published in the Journal of Pediatrics laying out how the Children’s Hospital at Montefiore adapted an entire pediatric floor to caring for adults.3 This example of recognition of need, quick preparation, and collaboration both within the children’s hospital and with the adult hospital was admirable. I also feel that at the beginning of this pandemic, there was a glimmer that the failure of our health care system to cover everyone and the repercussions of this failure would be drawn into sharp relief. I hope that this understanding of the importance of universal coverage persists beyond the pandemic.
Dr. Giordano is assistant professor of pediatrics at Columbia University and a pediatric hospitalist at NewYork-Presbyterian Morgan Stanley Children’s Hospital with an interest in surgical comanagement. She serves on the Society of Hospital Medicine’s Pediatric Special Interest Group Executive Committee and is the chair of the Education Subcommittee. She is also an advisory board member for the New York/Westchester SHM Chapter.
References
1. Kumaraiah D et al. Innovative ICU physician care models: Covid-19 pandemic at NewYork-Presbyterian. NEJM Catal. 2020 Apr 28. doi: 10.1056/CAT.20.0158.
2. Kim MK et al. A primer for clinician deployment to the medicine floors from an epicenter of Covid-19. NEJM Catal. 2020 May 4. doi: 10.1056/CAT.20.0180.
3. Philips K, et al. Rapid Implementation of an Adult COVID-19 Unit in a Children’s Hospital. J Pediatr. 2020. doi: 10.1016/j.jpeds.2020.04.060.
The memories I have from the few nights spent in the adult pop-up cardiac intensive care unit are pouring in as I sit down to tell this story. I am a pediatric hospitalist at Columbia University NewYork-Presbyterian Morgan Stanley Children’s Hospital. I usually take care of sick, hospitalized children. However, in these extraordinary times, I have joined an army of colleagues taking care of adult patients with COVID-19.
Almost all these patients had tracheostomies connected to ventilators, as well as acute-on-chronic cardiac issues. They were often delirious and unable to speak, and always alone. I was happy to help our adult colleagues, but I was also afraid. I was scared to make a mistake that could be detrimental to my patient, even though I knew well that ICU residents, fellows, and attendings were just a phone call away.
I felt like Alice in Wonderland, initially too small compared with her environment, and the next minute hunched, giant, and still clearly displaced. Except I was not dreaming or watching a movie. There was no white rabbit to chase. The situation was serious and emotionally challenging. I imagined that each patient was the dearest member of my family: my mother, my father, my aunt or uncle. I took pleasure in sharing smiles while asking the patients how they were feeling, and I touched their hands, even though much of my face was covered and there were gloves on my hands.
The year 2020 has been surreal. People have had to find their own way of pushing through the unknown and unexpected. For a start, I would never in a million years have imagined using phrases like pop-up ICU.1 I was signing an admission note for a 90-year-old lady with acute-on-chronic congestive heart failure and acute respiratory hypoxemic failure and there, at the bottom of the note, was my name, followed by an odd remark: “pediatric hospital medicine.” That is what happened in New York City in 2020: Many unexpected events took place.
This article represents a virtual conversation with three other pediatric hospitalists who, under different sets of circumstances, did the same thing: took care of adult patients. I hope that the answers to the questions I asked make you pause, reflect, and learn from the experiences described.
Would you describe the usual environment where you practice pediatric hospital medicine?
Julie Dunbar, MD: I am a full-time pediatric hospitalist at the Children’s Hospital at Montefiore, a tertiary care academic children’s hospital in the Bronx. A typical day on service involves staffing up to 14 patients, up to 21 years old, on a teaching service with residents and physician assistants. We normally staff the hospital in two shifts – day and evening – until 11:00 at night. We are situated at the heart of a medically underserved area, and our hospital system cares for about one-third of the total population of the Bronx.
L. Nell Hodo, MD: I work at Kravis Children’s Hospital at the Mount Sinai Hospital, in Manhattan at the juncture of the Upper East Side and Harlem. Our usual hospital medicine environment is the general ward/floor in a nested children’s hospital within an adult hospital. We have about 32 non-ICU beds, and the patients are managed by a combination of hospitalists, general pediatricians, and specialist attendings. All patients are on resident teams. We have a comanagement model in which the primary attending for surgical patients is always a pediatric attending (hospitalist or specialist).
Avital M. Fischer, MD: NewYork-Presbyterian Morgan Stanley Children’s Hospital is a quaternary care center – where children from the area receive subspecialty care – as well as, functionally, a community hospital for the Washington Heights area. Therefore, we always have an interesting mix of general pediatric inpatient medicine including patients with complex medical conditions, rare diseases, postoperative conditions, and undiagnosed illnesses on our wards. We are a children’s hospital, connected to a larger adult hospital system. Pediatric hospitalists cover two pediatric wards, team-staffed by residents, and a progressive care unit, staffed by nurse practitioners. There is usually evening coverage until 11 p.m.
How did this change when New York became the U.S. epicenter of the SARS-CoV-2 pandemic? Was the transition to taking care of adult patients gradual or sudden? Were you deployed to a different hospital or part of the hospital? How prepared did you feel?
Dr. Dunbar: We experienced the COVID-19 pandemic like much of the rest of New York City – it started as a slow and uncertain process, and then it hit us all at once. In initial conversations, like everyone else, we did not know exactly what was coming. We started with small changes like working from home on nonclinical days and canceling family-centered rounds to conserve personal protective equipment (PPE). In mid-March, we were still expecting that redeployment to adult floors was a highly unlikely scenario. We made work-from-home schedules and planned projects we would work on while social distancing. We planned journal clubs about emerging evidence on COVID-19. However, things happened fast, and many of these plans were scrapped.
On Saturday, March 28, we closed the main floor of the children’s hospital because so few pediatric patients were being admitted. Two days later, we admitted our first cohort of adult COVID-19 patients, all more than 30 years old. They were transferred en masse from an outside hospital emergency department that desperately needed our beds. They arrived all at once, and they all required respiratory support. At the last hospitalist division meeting before the adults arrived, we had time for only one priority set of information, and so we chose end-of-life care. We reviewed scripts for advance care planning and logistics of death certificates. As fast as things changed for us, they changed even faster for the patients. Most were relatively healthy people who rather suddenly found themselves isolated, on oxygen, dictating their final wishes to pediatricians in full protective gear. Many, many patients got better, and of course, several spent their last moments with us. One physician assistant, who works closely with the hospitalists, spent the last 5 hours of an elderly patient’s life holding her hand and helping her FaceTime with family.
For the most part, the patients came to us. We worked with our own colleagues and our own nurses, on our own territory. A few of my colleagues were briefly redeployed to a series of conference rooms that were used for several weeks as overflow space for more stable COVID-19 patients. Staffing by the pediatrics teams was so robust, with willing volunteers from every corner of the children’s hospital, that we were not needed for long.
During the early days, there was no clinical pathway to follow to care for COVID-19 patients – it didn’t exist for this novel and variable disease. We created a platform to share documents and resources in real time as they became available to us. We used group texts and emails to learn from our experiences and encourage one another. Importantly, no one was afraid to ask for help, and we relied on our adult colleagues when patients started to decompensate. Adult critical care came to our aid for all rapid responses for patients older than 30. Pediatric critical care, in their infinite flexibility, was responsible for anyone younger.
Dr. Hodo: We had a variety of changes. The first thing was the deployment of many of our attendings (hospital medicine, ICU, outpatient, and subspecialists) and residents to the adult side to work on medical COVID-19 units or in the many ICUs (some new “pop-up” units in former medical units, postanesthesia care units, and so on).2 On the adult floor we had “COVID teams,” which had an attending and two frontline providers; one of these three people was an internal medicine faculty member or resident. Residents from other specialties (emergency medicine, family medicine) were pulled off pediatric assignments in pediatric wards, PICUs, and EDs, so pediatric residents not originally assigned to inpatient rotations were sent to cover these core pediatric areas. The remaining pediatric faculty backfilled the pediatric services – so the remaining ICU docs did more shifts to cover ICU; the undeployed specialists took more inpatient service or clinic time, and so on. Outpatient pediatrics covered the inpatient pediatric service for the 3 weeks when most of the hospitalists were deployed.
We had one pediatric unit, which was a unit with equipment that made it capable of having ICU patients or floor patients, that was designated a COVID-19 unit. Most COVID-19 patients were there. Some were also in negative-pressure rooms on other floors or in the unit directly above the COVID-19 unit. Some adult patients came to the unit in the pediatric hospital but not as many as initially expected, and most were young adults in their 20s. So rather than adult patients coming to pediatrics, our experience was more that pediatricians went to the adult side.
The transition to adult care for physicians was variable in its suddenness. Most people had at least 48 hours’ notice, whereas some had as much as a week. Most of our department members deployed within the hospital complex of which we are a part, though a few went to other sites in the health system. Some were deployed into administrative or support roles in the system, rather than patient-facing roles. I felt, I would say, reasonably prepared. I trained in family medicine, though I have been exclusively in pediatrics for the past 7 years. I felt rusty, for sure, but perhaps not quite as out of my element as others. In preparation, I read a lot about COVID, reviewed some adult medicine topics provided by the medicine department, used the resources on the Pediatric Overflow Planning Contingency Response Network (POPCoRN), including an Advanced Cardiac Life Support review, and was able to shadow on a COVID-19 unit before I actually started – that was incredibly helpful. I also had the opportunity to speak about that shadowing experience in a department meeting, which I hope was helpful for others.
Dr. Fischer: Our whole focus for a relatively short time shifted to how to take care of adults within the children’s hospital. Although we had some time to prepare – the ICU was the first unit to take adults, so we knew they would come to the floor – it still felt quick. We took adult patients onto the general pediatrics floor from both the emergency department and the ICU. We took adults mostly with COVID-19, but we did have some young adults admitted for other reasons too. Those of us who were on service during this time collaborated closely, sharing what we learned and even joining one another on rounds to provide support. We basically would “teach it forward” as we learned. We also had adult providers available by phone for questions, and our pediatric subspecialists were readily available for consults and would reach out to their adult counterparts for support. Some of the hospitalists were reaching out to POPCoRN, and some were attending an ACLS crash course prior to getting on service.
What was hardest about this experience for you?
Dr. Dunbar: For me, one of the hardest aspects of dealing with COVID-19 was the unknown. In every aspect of professional life and clinical care, there were unanswered questions. What’s the best way to care for these patients? What prognoses can we give their loved ones? How can I help when it seems like there’s so little I can offer? Will we run out of PPE? As doctors, what behaviors most endanger our friends and family when we go home after work? When will things start to get better?
Dr. Hodo: For me, the week or two before being notified of the deployment was the worst and hardest time. The uncertainty about if I would be called or no, and to do what? And where? I was trying to read everything there was on management, what little was known about treatment, and so on. Once I received notification of a start date, that allowed me to focus on very clear endpoints and knowledge items (for example, reviewing ACLS algorithms) and to do things I knew would help me settle and be more effective (like shadowing).
Dr. Fischer: It was a lot of new. Not only were we taking care of a population that we hadn’t cared for since medical school (adults), but we were facing a disease process that was also new to everyone. We were learning on our feet, while at the same time providing guidance to our house staff.
What have you learned about yourself that you did not know before?
Dr. Dunbar: I was surprised to learn how much I liked caring for adult patients. The fear I felt immediately before they arrived dissipated fairly quickly after they arrived. The opportunity to address their chronic conditions while supporting them in an acute illness took me back to many of the fundamentals of medicine that I hadn’t thought much about since medical school. I liked that they could speak up to tell us how they were feeling, both physically and emotionally, so that we could address their needs and allow them to participate in their own care. Some of my favorite patients kept detailed histories of their own C-reactive protein values and oxygen levels to show they were active participants in their own recovery.
I was worried that these adult patients would be offended or scared to learn that they were being cared for by pediatricians, but at no point did anyone ask me why they were not assigned to an adult hospitalist. They saw us only as doctors and nurses, and they were grateful for our care. One 65-year-old U.S. Army veteran told me that his nurse had told him to take a shower and make his bed. “She treated me just like a 5-year-old kid. And I loved it!” he said.
Dr. Hodo: I don’t know that I was totally unaware of these things, but I will say that I had partially forgotten them: I really like adult medicine, and I love geriatrics. I like high-energy and high-stress situations … at least occasionally! I feel very comfortable discussing end-of-life decisions and death. I cope with personal stress by helping and supporting others – patients, team members, colleagues, neighbors. I risk not taking enough time for myself and have to remind myself to do so.
Dr. Fischer: I actually loved taking care of adults. It felt like there was a different kind of patient-doctor relationship to be had, and it was interesting to get to know people who had jobs and families of their own – essentially a different type of story than you typically hear taking care of children.
Were there any silver linings in this situation? How did you grow personally through this experience? What do we need to do better going forward as a profession and a community?
Dr. Dunbar: The part that I hope will stay with me is the memory of how we came together as clinicians to fight a common invisible enemy. The teamwork was unprecedented. Our day-to-day goals were simple and straightforward: do what needed to be done to help as many New Yorkers as possible. Our team made themselves available for last-minute meetings and shift changes without complaint. We practiced a type of medicine that prioritized patient comfort, flexibility, and compassionate care. We ordered methadone and insulin and antihypertensives – brand new experiences for us, but we figured it out. We worked through novel clinical problems together because there was no textbook to read.
Our colleagues from other specialties and different levels of experience stepped up to join us on overnight shifts, and we welcomed them. With the help of an ad hoc palliative care team, we improved how we listened to patients’ own self-directed needs. We reached across the aisle to our internal medicine and adult hospitalist colleagues to refresh our memories on chronic conditions, and they always answered the phone. I hope we always remember who we were during this crisis, because we were ourselves at our most generous.
Dr. Hodo: This was an unexpected but great opportunity to meet physicians, nurses, and staff in different departments and sections of the hospital from my own. I am hopeful that this experience will help us in the future with multidisciplinary work and breaking down silos that isolate specialties and units in the hospital.
I feel (and this is probably weird) invigorated by this experience. It feels good to have been able to help when I was needed. Even though there are a lot of things in adult hospital medicine I do not know, I know I did my best, asked for help when I needed it, and asked for feedback regularly from the medicine residents and nurses I worked with. I know I supported my team and my colleagues to the best of my ability through stressful and sometimes upsetting and emotionally draining times.
As a profession, we can continue to remember the value of the multidisciplinary team and the value of listening to, and making space for, different voices to be heard. We can reconsider the traditional, rigid hierarchy in medicine and medical education that can stifle creative thought and innovative ideas. We can remember that the people “at the top” of the pyramid can always learn something from those “at the bottom.” We can see the ways that department and discipline and specialty can help us but also sometimes hinder, and seek involvement in programs and discussions that unite and pool resources and skills. And, most of all, we can try, every day we are at work, to put the patients’ and families’ needs first – and when we leave work, to turn that around, and put ourselves and our loved ones in that prime position.
As a community, we also can work on thinking communally – that, after all, is the entire point of the wearing of masks in public and social distancing. It is as much about you as about me! We can try to hold on to some of this perspective of the greater good and appreciation for the work others do that makes our lives better and easier. It is not only health care workers who deserve a round of applause every day; it is every person who did something today that benefited someone else, be that giving extra space in a line, wearing a mask in a store, delivering food to an elder, teaching a class over Zoom, or simply minimizing time outside the house. It is every person who thought about the community at or near the same level of priority that they thought about themselves.
Dr. Fischer: It was a very challenging situation, but because our adult patients in the children’s hospital were relatively young with fewer comorbidities, we got to see people get well. I took care of one man with renal failure who we thought would be on dialysis for the rest of his life. By the end of my first week on service, he had begun to regain kidney function. It was amazing. I think most frontline providers caring for adults in this pandemic have had to face significant morbidity and mortality. I felt lucky that we were able to care for patients who generally got better.
I recently read the article published in the Journal of Pediatrics laying out how the Children’s Hospital at Montefiore adapted an entire pediatric floor to caring for adults.3 This example of recognition of need, quick preparation, and collaboration both within the children’s hospital and with the adult hospital was admirable. I also feel that at the beginning of this pandemic, there was a glimmer that the failure of our health care system to cover everyone and the repercussions of this failure would be drawn into sharp relief. I hope that this understanding of the importance of universal coverage persists beyond the pandemic.
Dr. Giordano is assistant professor of pediatrics at Columbia University and a pediatric hospitalist at NewYork-Presbyterian Morgan Stanley Children’s Hospital with an interest in surgical comanagement. She serves on the Society of Hospital Medicine’s Pediatric Special Interest Group Executive Committee and is the chair of the Education Subcommittee. She is also an advisory board member for the New York/Westchester SHM Chapter.
References
1. Kumaraiah D et al. Innovative ICU physician care models: Covid-19 pandemic at NewYork-Presbyterian. NEJM Catal. 2020 Apr 28. doi: 10.1056/CAT.20.0158.
2. Kim MK et al. A primer for clinician deployment to the medicine floors from an epicenter of Covid-19. NEJM Catal. 2020 May 4. doi: 10.1056/CAT.20.0180.
3. Philips K, et al. Rapid Implementation of an Adult COVID-19 Unit in a Children’s Hospital. J Pediatr. 2020. doi: 10.1016/j.jpeds.2020.04.060.
Beyond PSA: New prostate cancer screening options
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.