User login
Welcome to HM20 Virtual
Welcome to the HM20 virtual conference! We’re glad to have you join us to virtually experience sessions from our most popular SHM annual conference tracks including Rapid Fire, Clinical Updates, and High-Value Care! We also have added some new timely topics given our current times that you won’t want to miss. We encourage you to engage with the larger community via social media at #HM20Virtual.
HM20 in San Diego, scheduled originally for April 2020, was trending to be the highest in-person attended SHM annual conference with a fantastic line-up of offerings. Unfortunately, then came our pandemic, or pandemics. In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 in San Diego because of the continued spread of COVID-19. Canceling the in-person conference during this unprecedented time was the right thing to do. I have valued the SHM leadership team and the larger SHM community for their support in being even more engaged on the front lines and with each other across our world during this time.
The COVID-19 pandemic has created a systemic challenge for health care systems across the nation. As hospitalists continue to be on the front lines of care and also innovations, organizations have leveraged telemedicine to support their patients, protect their clinicians, and conserve scarce resources. It is hospital medicine that has been on the front lines of change and adaptations and have led in this pandemic in many organizations across the nation and the world.
Unfortunately, known health disparities have also been amplified and there came an acute worsening of the chronic issues in this nation. On March 13, 2020, 26-year-old Breonna Taylor was shot after police forcibly entered her home. Armaud Arbery was shot and killed by armed neighbors while running through a neighborhood in Brunswick, Ga. Then on May 25, 5 miles from where I call home here in the Twin Cities in Minnesota, George Floyd, a 46-year-old father arrested for suspected use of a counterfeit $20 bill, died after police kneeled on his neck for over 8 minutes. This pandemic has also shaken up the status quo and laid bare a lot of our country’s long and deep-seated issues – from massive economic inequities to ongoing racial disparities to immigration concerns. It’s woken a lot of our valued hospitalists to the fact that the old ways of doing things just don’t work.
I’m grateful our society has taken steps to speak into these timely topics, and to share via publications, Twitter chats, advocacy items, and more! I want to encourage all of us to use the immense network of our hospitalist communities to comfort each other, learn, grow, and engage. We have not achieved big changes by ourselves. We’ve created valued offerings and innovative changes, and we’ve led on the front lines, in policies and procedures, by doing it together. Meaningful change requires allies in a common cause. We stand with our black and brown brothers and sisters who are particularly attuned to injustice, inequality, and struggle. We in hospital medicine stand up with many others who are struggling, our African American, Latin American, Native American, immigrant, LGBTQ+ communities. This intersection of the crisis of the COVID-19 pandemic and the racism pandemic have led us to a pivotal point in the arc of change and justice. I invite you to comfort each other, learn from each other, and act together in this community. To this end we have included timely resources in our HM20 virtual offering on these topics.
This year has been a big transition year. Not only did 2020 usher in a new decade, along with COVID-19 and our double pandemic, SHM has also had important transitions within its senior leadership. We say farewell to Larry Wellikson, MD, who has been at the helm of SHM since the beginning. On behalf of this annual conference, we want to celebrate and thank you, Larry, for your years of dedication and service to SHM. You have taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team may gather under a bigger tent for education, community, and for the betterment of care for our patients.
We extend a welcome to Eric Howell, MD, who succeeds Dr. Wellikson as SHM’s CEO. We also welcome Danielle Scheurer, MD, as the new SHM president, succeeding the great leadership offered this past year by Christopher Frost, MD. In addition, Jerome C. Siy, MD, was voted president-elect, Dr. Rachel Thompson, MD, was elected treasurer, Kris Rehm, MD, was voted secretary, and Darlene Tad-y, MD, was elected to the board of directors. We welcome these new officers.
HM20 Virtual will consist of prerecorded on-demand sessions that can be viewed at your convenience as well as live Q&A and attendee networking that will take place during specific dates/times. A few of the top-rated sessions from our historically popular tracks include: Update in Clinical Practice Guidelines, Antibiotics Made Ridiculously Simple, Getting to Know Oncology Emergencies, Inpatient Pain Management in the Era of the Opioid Epidemic, Updates in Heart Failure, and Hyponatremia: Don’t Drink the Water. Additionally, we have some of our perennial favorites including the Update in Hospital Medicine and Top Pediatric Articles of 2019. There will be COVID-19 specific content from expertise throughout the nation focusing on care pathways, clinical updates, telemedicine, point-of-care ultrasound, and more! To view the HM20 Virtual Opening Session and discover what you can expect in this educational experience, click here.
The Journal of Hospital Medicine has had a large presence in our meetings for many years. We are grateful for Samir Shah, MD, and his leadership during this double pandemic, for identifying areas where we can advance the field responsibly in the face of relatively limited evidence, and rapidly evolving news. As part of his commitment, all JHM articles related to COVID-19 and published during the pandemic are open access. A pre-COVID goal that has been realized during the pandemic was to bring more of the journal into our annual conference and the conference contents into the journal. We are proud to say this has been a great collaboration, particularly during this pandemic, and much thanks to Dr. Shah’s leadership for highlighting timely pieces. Kimberly Manning, MD, had an especially powerful piece on the topic of racism and our double pandemic, and she is a featured speaker during our HM20 Virtual offering, under the same title as her article: “When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics.” Additionally, Manpreet Malik, MD, and I will be copresenting on a timely topic about the “Immigrant Hospitalist during COVID-19.”
Aside from these sessions for HM20 Virtual, the real can’t miss(es) for the conference are the Research, Innovations, and Clinical Vignette (RIV) posters sessions. I am grateful for the leadership of Stephanie Mueller, MD, who served as chair for this year’s RIV. This unique year has led to the hosting of a virtual poster competition with judging and the opening of a virtual gallery. We are so pleased to be able to share and highlight the work of many of learners and staff hospitalists! I love that a hospitalist on one side of the country can help provide pearls on a case, an innovation, or a research idea that can help improve diagnosis for a patient at the other side of the country. Keep an eye on SHM’s social media and the presentation by Dr. Mueller for announcements of the winners.
A favorite reason many of us attend the annual conference is for the people and community. We wanted to keep this value as we shifted to a virtual offering. Networking will occur through a variety of offerings including Simulive sessions and Special Interest Forums. Simulive sessions will run for 3 weeks from August 11 to August 27. For those of you new to the term, Simulive may sound like a made-up word, but it is an actual amalgamation of a prerecorded webinar and a live interaction (simulated + live = Simulive). Simulive allows the faculty to sit in on their prerecorded session and interact with the audience via the chat feature during the live scheduled recording and spend time afterwards for a live Q&A from the audience.
There will also be over 20 Special Interest Forums hosted in the evenings after these Simulive sessions have concluded to give you a chance to connect with individuals, share experiences, and have meaningful discussions that can directly impact your practice. Samples of the forums include: Diversity and Inclusion, Rural Hospital Medicine, Pediatrics, NP/PA, Perioperative and Comanagement, Health Information Technology, and Point of Care Ultrasound! Take a look at the HM20 registration page for further information. You will receive direct information on how to attend. We encourage you to join!
HM20 also features a virtual 5K! Whether you run on a treadmill or jog in your neighborhood or local park, you can participate in HM20’s Virtual Fun Run or Walk. To participate, simply run your 5K during the weeks of HM20 Virtual and when you’re done, fill out our form to log your time. We encourage you to post a picture on social media as well with #HM20Virtual. You’ll also receive a certificate of completion at the close of HM20 Virtual.
All HM20 Virtual sessions will be available as on-demand after August 31. HM20 virtual offers more than 60 CME hours and over 35 MOC hours that you can claim at your convenience! That’s the most amount of CME and MOC ever offered at SHM for an event! This conference would not be possible without the tireless and relentless effort of SHM staff and leadership, our terrific speakers and faculty, and all the volunteer committee members of SHM. A huge thanks to the Annual Conference Committee who had the charge to develop the content for the Annual Conference, including topics, speakers, and learning objectives. I am grateful to have had the opportunity to serve on this committee for the past 7 years and to lead HM20 this year. Thanks to Brittany Evans, Hayleigh Lawrence, and Michelle Kann for their valued support this past year from an SHM staff perspective; to my assistant course director, Dan Steinberg, MD; and to the immediate past course director, Dustin Smith, MD, for their support.
Once again, we are excited to have you join, and we hope this conference elevates your education in hospital medicine, advances your career, stimulates innovative thinking, and provides you with enduring networking opportunities. We sincerely thank you for attending HM20 Virtual. Welcome!
Dr. Mathews is chief of hospital medicine at Regions Hospital, HealthPartners in St. Paul, Minn., an associate professor at the University of Minnesota, Minneapolis, and course director of HM20.
Welcome to the HM20 virtual conference! We’re glad to have you join us to virtually experience sessions from our most popular SHM annual conference tracks including Rapid Fire, Clinical Updates, and High-Value Care! We also have added some new timely topics given our current times that you won’t want to miss. We encourage you to engage with the larger community via social media at #HM20Virtual.
HM20 in San Diego, scheduled originally for April 2020, was trending to be the highest in-person attended SHM annual conference with a fantastic line-up of offerings. Unfortunately, then came our pandemic, or pandemics. In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 in San Diego because of the continued spread of COVID-19. Canceling the in-person conference during this unprecedented time was the right thing to do. I have valued the SHM leadership team and the larger SHM community for their support in being even more engaged on the front lines and with each other across our world during this time.
The COVID-19 pandemic has created a systemic challenge for health care systems across the nation. As hospitalists continue to be on the front lines of care and also innovations, organizations have leveraged telemedicine to support their patients, protect their clinicians, and conserve scarce resources. It is hospital medicine that has been on the front lines of change and adaptations and have led in this pandemic in many organizations across the nation and the world.
Unfortunately, known health disparities have also been amplified and there came an acute worsening of the chronic issues in this nation. On March 13, 2020, 26-year-old Breonna Taylor was shot after police forcibly entered her home. Armaud Arbery was shot and killed by armed neighbors while running through a neighborhood in Brunswick, Ga. Then on May 25, 5 miles from where I call home here in the Twin Cities in Minnesota, George Floyd, a 46-year-old father arrested for suspected use of a counterfeit $20 bill, died after police kneeled on his neck for over 8 minutes. This pandemic has also shaken up the status quo and laid bare a lot of our country’s long and deep-seated issues – from massive economic inequities to ongoing racial disparities to immigration concerns. It’s woken a lot of our valued hospitalists to the fact that the old ways of doing things just don’t work.
I’m grateful our society has taken steps to speak into these timely topics, and to share via publications, Twitter chats, advocacy items, and more! I want to encourage all of us to use the immense network of our hospitalist communities to comfort each other, learn, grow, and engage. We have not achieved big changes by ourselves. We’ve created valued offerings and innovative changes, and we’ve led on the front lines, in policies and procedures, by doing it together. Meaningful change requires allies in a common cause. We stand with our black and brown brothers and sisters who are particularly attuned to injustice, inequality, and struggle. We in hospital medicine stand up with many others who are struggling, our African American, Latin American, Native American, immigrant, LGBTQ+ communities. This intersection of the crisis of the COVID-19 pandemic and the racism pandemic have led us to a pivotal point in the arc of change and justice. I invite you to comfort each other, learn from each other, and act together in this community. To this end we have included timely resources in our HM20 virtual offering on these topics.
This year has been a big transition year. Not only did 2020 usher in a new decade, along with COVID-19 and our double pandemic, SHM has also had important transitions within its senior leadership. We say farewell to Larry Wellikson, MD, who has been at the helm of SHM since the beginning. On behalf of this annual conference, we want to celebrate and thank you, Larry, for your years of dedication and service to SHM. You have taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team may gather under a bigger tent for education, community, and for the betterment of care for our patients.
We extend a welcome to Eric Howell, MD, who succeeds Dr. Wellikson as SHM’s CEO. We also welcome Danielle Scheurer, MD, as the new SHM president, succeeding the great leadership offered this past year by Christopher Frost, MD. In addition, Jerome C. Siy, MD, was voted president-elect, Dr. Rachel Thompson, MD, was elected treasurer, Kris Rehm, MD, was voted secretary, and Darlene Tad-y, MD, was elected to the board of directors. We welcome these new officers.
HM20 Virtual will consist of prerecorded on-demand sessions that can be viewed at your convenience as well as live Q&A and attendee networking that will take place during specific dates/times. A few of the top-rated sessions from our historically popular tracks include: Update in Clinical Practice Guidelines, Antibiotics Made Ridiculously Simple, Getting to Know Oncology Emergencies, Inpatient Pain Management in the Era of the Opioid Epidemic, Updates in Heart Failure, and Hyponatremia: Don’t Drink the Water. Additionally, we have some of our perennial favorites including the Update in Hospital Medicine and Top Pediatric Articles of 2019. There will be COVID-19 specific content from expertise throughout the nation focusing on care pathways, clinical updates, telemedicine, point-of-care ultrasound, and more! To view the HM20 Virtual Opening Session and discover what you can expect in this educational experience, click here.
The Journal of Hospital Medicine has had a large presence in our meetings for many years. We are grateful for Samir Shah, MD, and his leadership during this double pandemic, for identifying areas where we can advance the field responsibly in the face of relatively limited evidence, and rapidly evolving news. As part of his commitment, all JHM articles related to COVID-19 and published during the pandemic are open access. A pre-COVID goal that has been realized during the pandemic was to bring more of the journal into our annual conference and the conference contents into the journal. We are proud to say this has been a great collaboration, particularly during this pandemic, and much thanks to Dr. Shah’s leadership for highlighting timely pieces. Kimberly Manning, MD, had an especially powerful piece on the topic of racism and our double pandemic, and she is a featured speaker during our HM20 Virtual offering, under the same title as her article: “When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics.” Additionally, Manpreet Malik, MD, and I will be copresenting on a timely topic about the “Immigrant Hospitalist during COVID-19.”
Aside from these sessions for HM20 Virtual, the real can’t miss(es) for the conference are the Research, Innovations, and Clinical Vignette (RIV) posters sessions. I am grateful for the leadership of Stephanie Mueller, MD, who served as chair for this year’s RIV. This unique year has led to the hosting of a virtual poster competition with judging and the opening of a virtual gallery. We are so pleased to be able to share and highlight the work of many of learners and staff hospitalists! I love that a hospitalist on one side of the country can help provide pearls on a case, an innovation, or a research idea that can help improve diagnosis for a patient at the other side of the country. Keep an eye on SHM’s social media and the presentation by Dr. Mueller for announcements of the winners.
A favorite reason many of us attend the annual conference is for the people and community. We wanted to keep this value as we shifted to a virtual offering. Networking will occur through a variety of offerings including Simulive sessions and Special Interest Forums. Simulive sessions will run for 3 weeks from August 11 to August 27. For those of you new to the term, Simulive may sound like a made-up word, but it is an actual amalgamation of a prerecorded webinar and a live interaction (simulated + live = Simulive). Simulive allows the faculty to sit in on their prerecorded session and interact with the audience via the chat feature during the live scheduled recording and spend time afterwards for a live Q&A from the audience.
There will also be over 20 Special Interest Forums hosted in the evenings after these Simulive sessions have concluded to give you a chance to connect with individuals, share experiences, and have meaningful discussions that can directly impact your practice. Samples of the forums include: Diversity and Inclusion, Rural Hospital Medicine, Pediatrics, NP/PA, Perioperative and Comanagement, Health Information Technology, and Point of Care Ultrasound! Take a look at the HM20 registration page for further information. You will receive direct information on how to attend. We encourage you to join!
HM20 also features a virtual 5K! Whether you run on a treadmill or jog in your neighborhood or local park, you can participate in HM20’s Virtual Fun Run or Walk. To participate, simply run your 5K during the weeks of HM20 Virtual and when you’re done, fill out our form to log your time. We encourage you to post a picture on social media as well with #HM20Virtual. You’ll also receive a certificate of completion at the close of HM20 Virtual.
All HM20 Virtual sessions will be available as on-demand after August 31. HM20 virtual offers more than 60 CME hours and over 35 MOC hours that you can claim at your convenience! That’s the most amount of CME and MOC ever offered at SHM for an event! This conference would not be possible without the tireless and relentless effort of SHM staff and leadership, our terrific speakers and faculty, and all the volunteer committee members of SHM. A huge thanks to the Annual Conference Committee who had the charge to develop the content for the Annual Conference, including topics, speakers, and learning objectives. I am grateful to have had the opportunity to serve on this committee for the past 7 years and to lead HM20 this year. Thanks to Brittany Evans, Hayleigh Lawrence, and Michelle Kann for their valued support this past year from an SHM staff perspective; to my assistant course director, Dan Steinberg, MD; and to the immediate past course director, Dustin Smith, MD, for their support.
Once again, we are excited to have you join, and we hope this conference elevates your education in hospital medicine, advances your career, stimulates innovative thinking, and provides you with enduring networking opportunities. We sincerely thank you for attending HM20 Virtual. Welcome!
Dr. Mathews is chief of hospital medicine at Regions Hospital, HealthPartners in St. Paul, Minn., an associate professor at the University of Minnesota, Minneapolis, and course director of HM20.
Welcome to the HM20 virtual conference! We’re glad to have you join us to virtually experience sessions from our most popular SHM annual conference tracks including Rapid Fire, Clinical Updates, and High-Value Care! We also have added some new timely topics given our current times that you won’t want to miss. We encourage you to engage with the larger community via social media at #HM20Virtual.
HM20 in San Diego, scheduled originally for April 2020, was trending to be the highest in-person attended SHM annual conference with a fantastic line-up of offerings. Unfortunately, then came our pandemic, or pandemics. In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 in San Diego because of the continued spread of COVID-19. Canceling the in-person conference during this unprecedented time was the right thing to do. I have valued the SHM leadership team and the larger SHM community for their support in being even more engaged on the front lines and with each other across our world during this time.
The COVID-19 pandemic has created a systemic challenge for health care systems across the nation. As hospitalists continue to be on the front lines of care and also innovations, organizations have leveraged telemedicine to support their patients, protect their clinicians, and conserve scarce resources. It is hospital medicine that has been on the front lines of change and adaptations and have led in this pandemic in many organizations across the nation and the world.
Unfortunately, known health disparities have also been amplified and there came an acute worsening of the chronic issues in this nation. On March 13, 2020, 26-year-old Breonna Taylor was shot after police forcibly entered her home. Armaud Arbery was shot and killed by armed neighbors while running through a neighborhood in Brunswick, Ga. Then on May 25, 5 miles from where I call home here in the Twin Cities in Minnesota, George Floyd, a 46-year-old father arrested for suspected use of a counterfeit $20 bill, died after police kneeled on his neck for over 8 minutes. This pandemic has also shaken up the status quo and laid bare a lot of our country’s long and deep-seated issues – from massive economic inequities to ongoing racial disparities to immigration concerns. It’s woken a lot of our valued hospitalists to the fact that the old ways of doing things just don’t work.
I’m grateful our society has taken steps to speak into these timely topics, and to share via publications, Twitter chats, advocacy items, and more! I want to encourage all of us to use the immense network of our hospitalist communities to comfort each other, learn, grow, and engage. We have not achieved big changes by ourselves. We’ve created valued offerings and innovative changes, and we’ve led on the front lines, in policies and procedures, by doing it together. Meaningful change requires allies in a common cause. We stand with our black and brown brothers and sisters who are particularly attuned to injustice, inequality, and struggle. We in hospital medicine stand up with many others who are struggling, our African American, Latin American, Native American, immigrant, LGBTQ+ communities. This intersection of the crisis of the COVID-19 pandemic and the racism pandemic have led us to a pivotal point in the arc of change and justice. I invite you to comfort each other, learn from each other, and act together in this community. To this end we have included timely resources in our HM20 virtual offering on these topics.
This year has been a big transition year. Not only did 2020 usher in a new decade, along with COVID-19 and our double pandemic, SHM has also had important transitions within its senior leadership. We say farewell to Larry Wellikson, MD, who has been at the helm of SHM since the beginning. On behalf of this annual conference, we want to celebrate and thank you, Larry, for your years of dedication and service to SHM. You have taken the specialty of hospital medicine and created a movement in SHM, where the entire hospital medicine team may gather under a bigger tent for education, community, and for the betterment of care for our patients.
We extend a welcome to Eric Howell, MD, who succeeds Dr. Wellikson as SHM’s CEO. We also welcome Danielle Scheurer, MD, as the new SHM president, succeeding the great leadership offered this past year by Christopher Frost, MD. In addition, Jerome C. Siy, MD, was voted president-elect, Dr. Rachel Thompson, MD, was elected treasurer, Kris Rehm, MD, was voted secretary, and Darlene Tad-y, MD, was elected to the board of directors. We welcome these new officers.
HM20 Virtual will consist of prerecorded on-demand sessions that can be viewed at your convenience as well as live Q&A and attendee networking that will take place during specific dates/times. A few of the top-rated sessions from our historically popular tracks include: Update in Clinical Practice Guidelines, Antibiotics Made Ridiculously Simple, Getting to Know Oncology Emergencies, Inpatient Pain Management in the Era of the Opioid Epidemic, Updates in Heart Failure, and Hyponatremia: Don’t Drink the Water. Additionally, we have some of our perennial favorites including the Update in Hospital Medicine and Top Pediatric Articles of 2019. There will be COVID-19 specific content from expertise throughout the nation focusing on care pathways, clinical updates, telemedicine, point-of-care ultrasound, and more! To view the HM20 Virtual Opening Session and discover what you can expect in this educational experience, click here.
The Journal of Hospital Medicine has had a large presence in our meetings for many years. We are grateful for Samir Shah, MD, and his leadership during this double pandemic, for identifying areas where we can advance the field responsibly in the face of relatively limited evidence, and rapidly evolving news. As part of his commitment, all JHM articles related to COVID-19 and published during the pandemic are open access. A pre-COVID goal that has been realized during the pandemic was to bring more of the journal into our annual conference and the conference contents into the journal. We are proud to say this has been a great collaboration, particularly during this pandemic, and much thanks to Dr. Shah’s leadership for highlighting timely pieces. Kimberly Manning, MD, had an especially powerful piece on the topic of racism and our double pandemic, and she is a featured speaker during our HM20 Virtual offering, under the same title as her article: “When Grief and Crises Intersect: Perspectives of a Black Physician in the Time of Two Pandemics.” Additionally, Manpreet Malik, MD, and I will be copresenting on a timely topic about the “Immigrant Hospitalist during COVID-19.”
Aside from these sessions for HM20 Virtual, the real can’t miss(es) for the conference are the Research, Innovations, and Clinical Vignette (RIV) posters sessions. I am grateful for the leadership of Stephanie Mueller, MD, who served as chair for this year’s RIV. This unique year has led to the hosting of a virtual poster competition with judging and the opening of a virtual gallery. We are so pleased to be able to share and highlight the work of many of learners and staff hospitalists! I love that a hospitalist on one side of the country can help provide pearls on a case, an innovation, or a research idea that can help improve diagnosis for a patient at the other side of the country. Keep an eye on SHM’s social media and the presentation by Dr. Mueller for announcements of the winners.
A favorite reason many of us attend the annual conference is for the people and community. We wanted to keep this value as we shifted to a virtual offering. Networking will occur through a variety of offerings including Simulive sessions and Special Interest Forums. Simulive sessions will run for 3 weeks from August 11 to August 27. For those of you new to the term, Simulive may sound like a made-up word, but it is an actual amalgamation of a prerecorded webinar and a live interaction (simulated + live = Simulive). Simulive allows the faculty to sit in on their prerecorded session and interact with the audience via the chat feature during the live scheduled recording and spend time afterwards for a live Q&A from the audience.
There will also be over 20 Special Interest Forums hosted in the evenings after these Simulive sessions have concluded to give you a chance to connect with individuals, share experiences, and have meaningful discussions that can directly impact your practice. Samples of the forums include: Diversity and Inclusion, Rural Hospital Medicine, Pediatrics, NP/PA, Perioperative and Comanagement, Health Information Technology, and Point of Care Ultrasound! Take a look at the HM20 registration page for further information. You will receive direct information on how to attend. We encourage you to join!
HM20 also features a virtual 5K! Whether you run on a treadmill or jog in your neighborhood or local park, you can participate in HM20’s Virtual Fun Run or Walk. To participate, simply run your 5K during the weeks of HM20 Virtual and when you’re done, fill out our form to log your time. We encourage you to post a picture on social media as well with #HM20Virtual. You’ll also receive a certificate of completion at the close of HM20 Virtual.
All HM20 Virtual sessions will be available as on-demand after August 31. HM20 virtual offers more than 60 CME hours and over 35 MOC hours that you can claim at your convenience! That’s the most amount of CME and MOC ever offered at SHM for an event! This conference would not be possible without the tireless and relentless effort of SHM staff and leadership, our terrific speakers and faculty, and all the volunteer committee members of SHM. A huge thanks to the Annual Conference Committee who had the charge to develop the content for the Annual Conference, including topics, speakers, and learning objectives. I am grateful to have had the opportunity to serve on this committee for the past 7 years and to lead HM20 this year. Thanks to Brittany Evans, Hayleigh Lawrence, and Michelle Kann for their valued support this past year from an SHM staff perspective; to my assistant course director, Dan Steinberg, MD; and to the immediate past course director, Dustin Smith, MD, for their support.
Once again, we are excited to have you join, and we hope this conference elevates your education in hospital medicine, advances your career, stimulates innovative thinking, and provides you with enduring networking opportunities. We sincerely thank you for attending HM20 Virtual. Welcome!
Dr. Mathews is chief of hospital medicine at Regions Hospital, HealthPartners in St. Paul, Minn., an associate professor at the University of Minnesota, Minneapolis, and course director of HM20.
Cognitive impairment in 9/11 responders tied to brain atrophy
, suggest results from the first structural neuroimaging study conducted in this population. The study clarifies that a neurodegenerative condition is present in first responders who experience cognitive impairment in midlife, which “is incredibly important to know,” said lead author Sean Clouston, PhD, of Stony Brook (N.Y.) University.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were published online in Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring.
Brain atrophy in midlife
During the 9/11 attack and in its aftermath, WTC responders were exposed to a range of inhaled neurotoxicants, as well as extreme psychosocial stressors. A growing number of WTC responders who are now in their 50s and early 60s are experiencing early cognitive impairment.
Using MRI, the investigators examined cortical thickness (CTX), a surrogate marker for neurodegeneration, in 99 mostly male WTC responders; 48 had cognitive impairment, and 51 did not. The age range of the participants was 45 to 65 years, a range during which cortical atrophy is uncommon in the general population, the researchers noted.
Compared with cognitively normal responders, those with cognitive impairment were found to have reductions in CTX across the whole brain and across 21 of 34 cortical regions, including frontal, temporal, and occipital lobes.
In both cognitively impaired and cognitively unimpaired WTC responders, CTX was reduced in the entorhinal and temporal cortices compared with normative data, but reductions were greater with cognitive impairment. Posttraumatic distress disorder (PTSD) status was not predictive of a reduction in CTX across groups.
Dr. Clouston said the level of reduction in CTX in many responders is similar to that commonly found in patients with dementia and may reflect early-stage dementia occurring in midlife.
Limitations of the study include the small sample size, the cross-sectional design, the unique nature of the exposure, and a lack of a non-WTC external control group.
‘Illuminating’ study
Keith N. Fargo, PhD, director of scientific engagement for the Alzheimer’s Association, called the findings “interesting and illuminating” but cautioned that it is not possible to show cause and effect with this type of study.
“We also don’t know when cortical thinning might have started or how quickly it might be progressing,” Dr. Fargo said in an interview.
He noted that the pattern of cortical thinning is “somewhat consistent with what we see among people who live with high levels of air pollution, which is an emerging risk factor for Alzheimer’s disease and other dementias.”
The Lancet Commission on Dementia Prevention, Intervention, and Care added air pollution to its list of modifiable risk factors for dementia, which was recently updated.
Clinicians “need to be aware that their middle-aged 9/11 first responders are at a higher risk level for cognitive impairment, as well as PTSD and depression,” Dr. Fargo said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Aging. Dr. Clouston and Dr. Fargo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, suggest results from the first structural neuroimaging study conducted in this population. The study clarifies that a neurodegenerative condition is present in first responders who experience cognitive impairment in midlife, which “is incredibly important to know,” said lead author Sean Clouston, PhD, of Stony Brook (N.Y.) University.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were published online in Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring.
Brain atrophy in midlife
During the 9/11 attack and in its aftermath, WTC responders were exposed to a range of inhaled neurotoxicants, as well as extreme psychosocial stressors. A growing number of WTC responders who are now in their 50s and early 60s are experiencing early cognitive impairment.
Using MRI, the investigators examined cortical thickness (CTX), a surrogate marker for neurodegeneration, in 99 mostly male WTC responders; 48 had cognitive impairment, and 51 did not. The age range of the participants was 45 to 65 years, a range during which cortical atrophy is uncommon in the general population, the researchers noted.
Compared with cognitively normal responders, those with cognitive impairment were found to have reductions in CTX across the whole brain and across 21 of 34 cortical regions, including frontal, temporal, and occipital lobes.
In both cognitively impaired and cognitively unimpaired WTC responders, CTX was reduced in the entorhinal and temporal cortices compared with normative data, but reductions were greater with cognitive impairment. Posttraumatic distress disorder (PTSD) status was not predictive of a reduction in CTX across groups.
Dr. Clouston said the level of reduction in CTX in many responders is similar to that commonly found in patients with dementia and may reflect early-stage dementia occurring in midlife.
Limitations of the study include the small sample size, the cross-sectional design, the unique nature of the exposure, and a lack of a non-WTC external control group.
‘Illuminating’ study
Keith N. Fargo, PhD, director of scientific engagement for the Alzheimer’s Association, called the findings “interesting and illuminating” but cautioned that it is not possible to show cause and effect with this type of study.
“We also don’t know when cortical thinning might have started or how quickly it might be progressing,” Dr. Fargo said in an interview.
He noted that the pattern of cortical thinning is “somewhat consistent with what we see among people who live with high levels of air pollution, which is an emerging risk factor for Alzheimer’s disease and other dementias.”
The Lancet Commission on Dementia Prevention, Intervention, and Care added air pollution to its list of modifiable risk factors for dementia, which was recently updated.
Clinicians “need to be aware that their middle-aged 9/11 first responders are at a higher risk level for cognitive impairment, as well as PTSD and depression,” Dr. Fargo said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Aging. Dr. Clouston and Dr. Fargo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, suggest results from the first structural neuroimaging study conducted in this population. The study clarifies that a neurodegenerative condition is present in first responders who experience cognitive impairment in midlife, which “is incredibly important to know,” said lead author Sean Clouston, PhD, of Stony Brook (N.Y.) University.
The findings were presented at the virtual annual meeting of the Alzheimer’s Association International Conference and were published online in Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring.
Brain atrophy in midlife
During the 9/11 attack and in its aftermath, WTC responders were exposed to a range of inhaled neurotoxicants, as well as extreme psychosocial stressors. A growing number of WTC responders who are now in their 50s and early 60s are experiencing early cognitive impairment.
Using MRI, the investigators examined cortical thickness (CTX), a surrogate marker for neurodegeneration, in 99 mostly male WTC responders; 48 had cognitive impairment, and 51 did not. The age range of the participants was 45 to 65 years, a range during which cortical atrophy is uncommon in the general population, the researchers noted.
Compared with cognitively normal responders, those with cognitive impairment were found to have reductions in CTX across the whole brain and across 21 of 34 cortical regions, including frontal, temporal, and occipital lobes.
In both cognitively impaired and cognitively unimpaired WTC responders, CTX was reduced in the entorhinal and temporal cortices compared with normative data, but reductions were greater with cognitive impairment. Posttraumatic distress disorder (PTSD) status was not predictive of a reduction in CTX across groups.
Dr. Clouston said the level of reduction in CTX in many responders is similar to that commonly found in patients with dementia and may reflect early-stage dementia occurring in midlife.
Limitations of the study include the small sample size, the cross-sectional design, the unique nature of the exposure, and a lack of a non-WTC external control group.
‘Illuminating’ study
Keith N. Fargo, PhD, director of scientific engagement for the Alzheimer’s Association, called the findings “interesting and illuminating” but cautioned that it is not possible to show cause and effect with this type of study.
“We also don’t know when cortical thinning might have started or how quickly it might be progressing,” Dr. Fargo said in an interview.
He noted that the pattern of cortical thinning is “somewhat consistent with what we see among people who live with high levels of air pollution, which is an emerging risk factor for Alzheimer’s disease and other dementias.”
The Lancet Commission on Dementia Prevention, Intervention, and Care added air pollution to its list of modifiable risk factors for dementia, which was recently updated.
Clinicians “need to be aware that their middle-aged 9/11 first responders are at a higher risk level for cognitive impairment, as well as PTSD and depression,” Dr. Fargo said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Aging. Dr. Clouston and Dr. Fargo have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From AAIC 2020
Patent foramen ovale linked with increased risk of ischemic stroke in PE
Background: Studies have demonstrated the increased risk for ischemic stroke in patients diagnosed with acute PE, and data support the mechanism of paradoxical embolism via PFO. However, the frequency of this phenomenon is unknown and the strength of the association between PFO and ischemic stroke in patients with PE is unclear.
Study design: Prospective cohort study.
Setting: Four French hospitals.
Synopsis: 315 patients aged 18 years and older presenting with acute symptomatic PE were evaluated at the time of diagnosis for PFO with contrast transthoracic echocardiography and for ischemic stroke with cerebral magnetic resonance imaging. The overall frequency of ischemic stroke at the time of PE diagnosis was high (7.6%), and was nearly four times higher in the PFO group than the non-PFO group (21.4% vs. 5.5%; difference in proportions, 15.9 percentage points; 95% confidence interval, 4.7-30.7).
This study adds to the growing body of data which supports the association of ischemic stroke with PFO and PE. Given the moderate indication for indefinite anticoagulation in patients at high risk for recurrent PE and stroke, there may be a role for screening for PFO in patients with acute PE so that they can be appropriately risk stratified.
Bottom line: The presence of ischemic stroke in patients with acute pulmonary embolism is high, and there is a strong association with PFO.
Citation: Le Moigne E et al. Patent Foramen Ovale and Ischemic Stroke in Patients With Pulmonary Embolism: A Prospective Cohort Study. Ann Intern Med. 2019;170:756-63.
Dr. McIntyre is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have demonstrated the increased risk for ischemic stroke in patients diagnosed with acute PE, and data support the mechanism of paradoxical embolism via PFO. However, the frequency of this phenomenon is unknown and the strength of the association between PFO and ischemic stroke in patients with PE is unclear.
Study design: Prospective cohort study.
Setting: Four French hospitals.
Synopsis: 315 patients aged 18 years and older presenting with acute symptomatic PE were evaluated at the time of diagnosis for PFO with contrast transthoracic echocardiography and for ischemic stroke with cerebral magnetic resonance imaging. The overall frequency of ischemic stroke at the time of PE diagnosis was high (7.6%), and was nearly four times higher in the PFO group than the non-PFO group (21.4% vs. 5.5%; difference in proportions, 15.9 percentage points; 95% confidence interval, 4.7-30.7).
This study adds to the growing body of data which supports the association of ischemic stroke with PFO and PE. Given the moderate indication for indefinite anticoagulation in patients at high risk for recurrent PE and stroke, there may be a role for screening for PFO in patients with acute PE so that they can be appropriately risk stratified.
Bottom line: The presence of ischemic stroke in patients with acute pulmonary embolism is high, and there is a strong association with PFO.
Citation: Le Moigne E et al. Patent Foramen Ovale and Ischemic Stroke in Patients With Pulmonary Embolism: A Prospective Cohort Study. Ann Intern Med. 2019;170:756-63.
Dr. McIntyre is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have demonstrated the increased risk for ischemic stroke in patients diagnosed with acute PE, and data support the mechanism of paradoxical embolism via PFO. However, the frequency of this phenomenon is unknown and the strength of the association between PFO and ischemic stroke in patients with PE is unclear.
Study design: Prospective cohort study.
Setting: Four French hospitals.
Synopsis: 315 patients aged 18 years and older presenting with acute symptomatic PE were evaluated at the time of diagnosis for PFO with contrast transthoracic echocardiography and for ischemic stroke with cerebral magnetic resonance imaging. The overall frequency of ischemic stroke at the time of PE diagnosis was high (7.6%), and was nearly four times higher in the PFO group than the non-PFO group (21.4% vs. 5.5%; difference in proportions, 15.9 percentage points; 95% confidence interval, 4.7-30.7).
This study adds to the growing body of data which supports the association of ischemic stroke with PFO and PE. Given the moderate indication for indefinite anticoagulation in patients at high risk for recurrent PE and stroke, there may be a role for screening for PFO in patients with acute PE so that they can be appropriately risk stratified.
Bottom line: The presence of ischemic stroke in patients with acute pulmonary embolism is high, and there is a strong association with PFO.
Citation: Le Moigne E et al. Patent Foramen Ovale and Ischemic Stroke in Patients With Pulmonary Embolism: A Prospective Cohort Study. Ann Intern Med. 2019;170:756-63.
Dr. McIntyre is a hospitalist at Ochsner Health System, New Orleans.
When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Medicare sticks with E/M pay plan over some groups’ objections
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests, in keeping with applicable state laws.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
“Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty,” the CMS said.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), general practice (8%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–11%), and thoracic surgery (–8%).
An umbrella group, the Surgical Care Coalition, on Aug. 3 had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
The Surgical Care Coalition already has been asking Congress to waive budget-neutrality requirements. Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said. “While APTA, AOTA, and ASHA do not oppose payment increases for primary care physicians, we believe these increases can be implemented without imposing payment reductions on other providers.”
A version of this article originally appeared on Medscape.com.
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests, in keeping with applicable state laws.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
“Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty,” the CMS said.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), general practice (8%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–11%), and thoracic surgery (–8%).
An umbrella group, the Surgical Care Coalition, on Aug. 3 had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
The Surgical Care Coalition already has been asking Congress to waive budget-neutrality requirements. Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said. “While APTA, AOTA, and ASHA do not oppose payment increases for primary care physicians, we believe these increases can be implemented without imposing payment reductions on other providers.”
A version of this article originally appeared on Medscape.com.
The Trump administration is sticking with a plan to boost certain Medicare pay for many primary care and other specialties focused heavily on office visits while lowering that for other groups to balance these increased costs.
On Aug. 4, the Centers for Medicare & Medicaid Services posted on the Federal Register draft versions of two of its major annual payment measures: the physician fee schedule and the payment rule for hospital outpatient services. On Aug. 3, the CMS informally posted a copy of the physician fee schedule on its own website, allowing medical groups to begin reading the more than 1,300-page rule.
Federal officials normally use annual Medicare payment rules to make many revisions to policies as well as adjust reimbursement.
The draft 2021 physician fee schedule, for example, calls for broadening the authority of clinicians other than physicians to authorize testing of people enrolled in Medicare.
The CMS intends to allow nurse practitioners, physician assistants, and certain other health care professionals to more widely supervise diagnostic psychological and neuropsychological tests, in keeping with applicable state laws.
The draft 2021 hospital outpatient rule proposes a gradual changeover to allow more procedures to be performed on an outpatient basis. This shift could save money for Medicare as well as for the people enrolled in the giant federal health program who need these services, the CMS explained.
Medicare would begin with a change in status for almost 300 musculoskeletal-related services, making them eligible for payment in the hospital outpatient setting when appropriate, CMS wrote in a fact sheet.
The initial reaction to Medicare’s proposed 2021 rules centered on its planned redistribution of funds among medical specialties. The CMS had outlined this plan last year. It is part of longstanding efforts to boost pay for primary care specialists and other physicians whose practice centers more around office visits than procedures.
There is broad support in health policy circles for raising pay for these specialties, but there also are strong objections to the cuts the CMS plans to offset the cost of rising pay for some fields.
Susan R. Bailey, MD, president of the American Medical Association, addressed both of these ideas in an AMA news release on the proposed 2021 physician fee schedule. The increase in pay for office visits, covered under evaluation and management services (E/M), stems from recommendations on resource costs from the AMA/Specialty Society RVS Update Committee, Dr. Bailey said.
“Unfortunately, these office visit payment increases, and a multitude of other new CMS proposed payment increases, are required by statute to be offset by payment reductions to other services, through an unsustainable reduction of nearly 11% to the Medicare conversion factor,” Dr. Bailey explained.
In the news release, Dr. Bailey asked Congress to waive Medicare’s budget-neutrality requirements to allow increases without the cuts.
“Physicians are already experiencing substantial economic hardships due to COVID-19, so these pay cuts could not come at a worse time,” she said.
Winners and losers
The CMS details the possible winners and losers in its payment reshuffle in Table 90 of the proposed 2021 physician fee schedule. In the proposed rule, CMS notes in the draft that these figures are based upon estimates of aggregate allowed charges across all services furnished by physicians and other clinicians.
“Therefore, they are averages, and may not necessarily be representative of what is happening to the particular services furnished by a single practitioner within any given specialty,” the CMS said.
Specialties in line for increases under the 2021 draft rule include allergy/immunology (9%), endocrinology (17%), family practice (13%), general practice (8%), geriatrics (4%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (8%), rheumatology (16%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–9%), emergency medicine (–6%), general surgery (–7%), infectious disease (–4%), neurosurgery (–7%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–11%), and thoracic surgery (–8%).
An umbrella group, the Surgical Care Coalition, on Aug. 3 had a quick statement ready about the CMS proposal. Writing on behalf of the group was David B. Hoyt, MD, executive director of the American College of Surgeons.
“Today’s proposed rule ignores both patients and the surgeons who care for them. The middle of a pandemic is no time for cuts to any form of health care, but today’s announcement moves ahead as if nothing has changed,” Hoyt said in the statement. “The Surgical Care Coalition believes no physician should see payment cuts that will reduce patients’ access to care.”
The Surgical Care Coalition already has been asking Congress to waive budget-neutrality requirements. Making a similar request Aug. 4 in a unified statement were the American Physical Therapy Association (APTA), the American Occupational Therapy Association (AOTA), and the American Speech-Language-Hearing Association (ASHA).
“Our organizations call on Congress and CMS to advance well-reasoned fee schedule payment policies and waive budget neutrality,” the groups said. “While APTA, AOTA, and ASHA do not oppose payment increases for primary care physicians, we believe these increases can be implemented without imposing payment reductions on other providers.”
A version of this article originally appeared on Medscape.com.
Septicemia first among hospital inpatient costs
according to a recent analysis from the Agency for Healthcare Research and Quality.
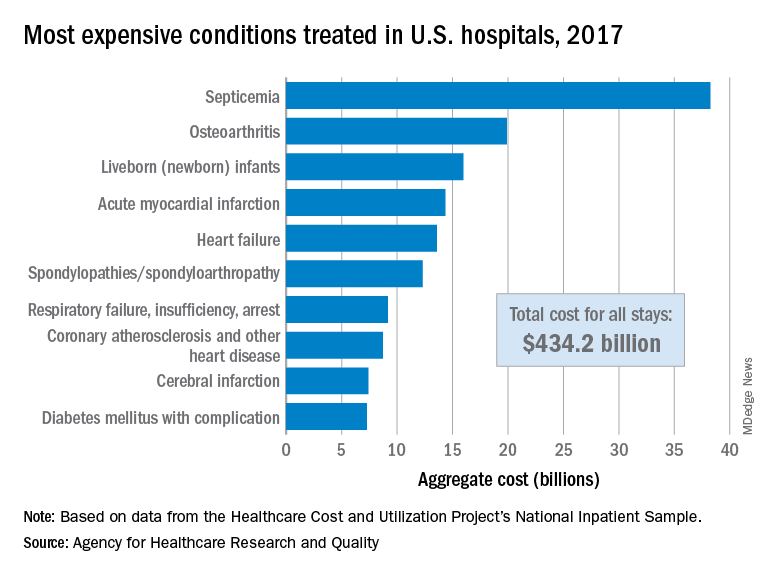
The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
according to a recent analysis from the Agency for Healthcare Research and Quality.

The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
according to a recent analysis from the Agency for Healthcare Research and Quality.

The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
FDA approves belantamab in relapsed/refractory multiple myeloma
The first-in-class drug belantamab mafodotin (Blenrep) has been granted an accelerated approval by the Food and Drug Administration for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
This follows a recommendation for approval on July 15 by an FDA advisory committee, which voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
The product has a novel mechanism of action: it targets B-cell maturation antigen (BCMA), a protein that is present on the surface of virtually all multiple myeloma cells but is absent from normal B cells.
The drug had already received an FDA breakthrough therapy designation, which facilitates the development of drugs that have shown clinical promise for conditions in which there is significant unmet need.
Belantamab mafodotin was recommended for conditional marketing approval in the European Union on July 24 and was accepted into the European Medicines Agency PRIME scheme for medicines that have potential to address unmet medical needs.
The new drug is indicated for patients with refractory or relapsed multiple myeloma who have already tried treatment with one of the three major classes of drugs, namely, an immunomolatory agent, a proteasome inhibitor, and a CD38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA comments. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
“While treatable, refractory multiple myeloma is a significant clinical challenge with poor outcomes for patients whose disease has become resistant to the current standard of care,” commented Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University, Atlanta, chair of the department of hematology and medical oncology at Emory, and a principal investigator for the clinical trial that led to the approval.
“Due to the limited options currently available, these patients are often retreated with drugs from the same classes after they relapse, which is why the approval of belantamab mafodotin, the first anti-BCMA therapy, is significant for both patients and physicians alike,” he said.
The product is an antibody-drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. It homes in on BCMA on myeloma cell surfaces. Once inside the myeloma cell, the cytotoxic agent is released, leading to apoptosis, the programmed death of the cancerous plasma cells.
Approval based on response rates
The accelerated approval from the FDA and the recommendation for conditional approval from the EMA are based on results for overall response rate and duration of response from a phase 2, open-label, randomized, two-arm study known as DREAMM-2. Both agencies said that they are waiting for further data on clinical benefit from ongoing trials.
The DREAMM-2 study investigated the efficacy and safety of two doses of belantamab mafodotin in multiple myeloma patients whose disease was still active after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results from this study were published in The Lancet Oncology in December. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The median duration of response had not been reached at the 6-month analysis, but for 73% of responders, DoR was ≥6 months.
The most commonly reported adverse events (≥20%) were keratopathy (changes in the cornea), decreased visual acuity, nausea, blurred vision, pyrexia, infusion-related reactions, and fatigue, the manufacturer notes.
Ocular toxicity
One of the most common adverse events with this product affects the eyes.
Ocular adverse reactions occurred in 77% of the 218 patients in the pooled safety population and included keratopathy (76%), changes in visual acuity (55%), blurred vision (27%), and dry eye (19%).
Corneal adverse events were monitored with eye exams prior to each dose, allowing dose reductions or interruptions as appropriate, the manufacturer noted. Patients also used preservative-free eyedrops. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the 2.5-mg/kg cohort.
Because of this ocular toxicity, the company has set up a risk evaluation and mitigation strategy (REMS) for the product. This requires education for all physicians who prescribe the product as well as their patients regarding the ocular risks associated with treatment. It also requires monitoring that includes regular ophthalmic examinations. Information about the scheme can be found at www.blenreprems.com.
At the FDA advisory committee meeting last month, one of the panelists, Gita Thanarajasingam, MD, an assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated but for ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some people than current treatments.
“It’s reasonable to leave open the option for decision making. Patients can express their values and preferences,” Dr. Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk-benefit discussion in a REMS program.”
Another panelist, Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it to them” with belantamab.
This article first appeared on Medscape.com.
The first-in-class drug belantamab mafodotin (Blenrep) has been granted an accelerated approval by the Food and Drug Administration for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
This follows a recommendation for approval on July 15 by an FDA advisory committee, which voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
The product has a novel mechanism of action: it targets B-cell maturation antigen (BCMA), a protein that is present on the surface of virtually all multiple myeloma cells but is absent from normal B cells.
The drug had already received an FDA breakthrough therapy designation, which facilitates the development of drugs that have shown clinical promise for conditions in which there is significant unmet need.
Belantamab mafodotin was recommended for conditional marketing approval in the European Union on July 24 and was accepted into the European Medicines Agency PRIME scheme for medicines that have potential to address unmet medical needs.
The new drug is indicated for patients with refractory or relapsed multiple myeloma who have already tried treatment with one of the three major classes of drugs, namely, an immunomolatory agent, a proteasome inhibitor, and a CD38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA comments. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
“While treatable, refractory multiple myeloma is a significant clinical challenge with poor outcomes for patients whose disease has become resistant to the current standard of care,” commented Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University, Atlanta, chair of the department of hematology and medical oncology at Emory, and a principal investigator for the clinical trial that led to the approval.
“Due to the limited options currently available, these patients are often retreated with drugs from the same classes after they relapse, which is why the approval of belantamab mafodotin, the first anti-BCMA therapy, is significant for both patients and physicians alike,” he said.
The product is an antibody-drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. It homes in on BCMA on myeloma cell surfaces. Once inside the myeloma cell, the cytotoxic agent is released, leading to apoptosis, the programmed death of the cancerous plasma cells.
Approval based on response rates
The accelerated approval from the FDA and the recommendation for conditional approval from the EMA are based on results for overall response rate and duration of response from a phase 2, open-label, randomized, two-arm study known as DREAMM-2. Both agencies said that they are waiting for further data on clinical benefit from ongoing trials.
The DREAMM-2 study investigated the efficacy and safety of two doses of belantamab mafodotin in multiple myeloma patients whose disease was still active after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results from this study were published in The Lancet Oncology in December. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The median duration of response had not been reached at the 6-month analysis, but for 73% of responders, DoR was ≥6 months.
The most commonly reported adverse events (≥20%) were keratopathy (changes in the cornea), decreased visual acuity, nausea, blurred vision, pyrexia, infusion-related reactions, and fatigue, the manufacturer notes.
Ocular toxicity
One of the most common adverse events with this product affects the eyes.
Ocular adverse reactions occurred in 77% of the 218 patients in the pooled safety population and included keratopathy (76%), changes in visual acuity (55%), blurred vision (27%), and dry eye (19%).
Corneal adverse events were monitored with eye exams prior to each dose, allowing dose reductions or interruptions as appropriate, the manufacturer noted. Patients also used preservative-free eyedrops. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the 2.5-mg/kg cohort.
Because of this ocular toxicity, the company has set up a risk evaluation and mitigation strategy (REMS) for the product. This requires education for all physicians who prescribe the product as well as their patients regarding the ocular risks associated with treatment. It also requires monitoring that includes regular ophthalmic examinations. Information about the scheme can be found at www.blenreprems.com.
At the FDA advisory committee meeting last month, one of the panelists, Gita Thanarajasingam, MD, an assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated but for ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some people than current treatments.
“It’s reasonable to leave open the option for decision making. Patients can express their values and preferences,” Dr. Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk-benefit discussion in a REMS program.”
Another panelist, Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it to them” with belantamab.
This article first appeared on Medscape.com.
The first-in-class drug belantamab mafodotin (Blenrep) has been granted an accelerated approval by the Food and Drug Administration for use in the treatment of relapsed and refractory multiple myeloma in patients who have already tried other therapies.
This follows a recommendation for approval on July 15 by an FDA advisory committee, which voted 12-0 in favor of the drug’s benefits outweighing risks in this patient population.
The product has a novel mechanism of action: it targets B-cell maturation antigen (BCMA), a protein that is present on the surface of virtually all multiple myeloma cells but is absent from normal B cells.
The drug had already received an FDA breakthrough therapy designation, which facilitates the development of drugs that have shown clinical promise for conditions in which there is significant unmet need.
Belantamab mafodotin was recommended for conditional marketing approval in the European Union on July 24 and was accepted into the European Medicines Agency PRIME scheme for medicines that have potential to address unmet medical needs.
The new drug is indicated for patients with refractory or relapsed multiple myeloma who have already tried treatment with one of the three major classes of drugs, namely, an immunomolatory agent, a proteasome inhibitor, and a CD38 monoclonal antibody.
For patients who no longer respond to these drugs, the outlook is bleak, the EMA comments. There is an unmet medical need for new treatments that improve survival of these patients beyond the currently observed 3 months or less.
“While treatable, refractory multiple myeloma is a significant clinical challenge with poor outcomes for patients whose disease has become resistant to the current standard of care,” commented Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University, Atlanta, chair of the department of hematology and medical oncology at Emory, and a principal investigator for the clinical trial that led to the approval.
“Due to the limited options currently available, these patients are often retreated with drugs from the same classes after they relapse, which is why the approval of belantamab mafodotin, the first anti-BCMA therapy, is significant for both patients and physicians alike,” he said.
The product is an antibody-drug conjugate that combines a monoclonal antibody that targets BCMA with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. It homes in on BCMA on myeloma cell surfaces. Once inside the myeloma cell, the cytotoxic agent is released, leading to apoptosis, the programmed death of the cancerous plasma cells.
Approval based on response rates
The accelerated approval from the FDA and the recommendation for conditional approval from the EMA are based on results for overall response rate and duration of response from a phase 2, open-label, randomized, two-arm study known as DREAMM-2. Both agencies said that they are waiting for further data on clinical benefit from ongoing trials.
The DREAMM-2 study investigated the efficacy and safety of two doses of belantamab mafodotin in multiple myeloma patients whose disease was still active after three or more lines of therapy and who no longer responded to treatment with immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 monoclonal antibody.
Six-month results from this study were published in The Lancet Oncology in December. The overall response rate was 31% in the cohort given a 2.5-mg/kg dose of the drug; 30 of 97 patients had outcomes that met the study’s positive threshold.
Another 99 patients in DREAMM-2 received a dose of 3.4 mg/kg, which was judged to have a less favorable safety profile.
The median duration of response had not been reached at the 6-month analysis, but for 73% of responders, DoR was ≥6 months.
The most commonly reported adverse events (≥20%) were keratopathy (changes in the cornea), decreased visual acuity, nausea, blurred vision, pyrexia, infusion-related reactions, and fatigue, the manufacturer notes.
Ocular toxicity
One of the most common adverse events with this product affects the eyes.
Ocular adverse reactions occurred in 77% of the 218 patients in the pooled safety population and included keratopathy (76%), changes in visual acuity (55%), blurred vision (27%), and dry eye (19%).
Corneal adverse events were monitored with eye exams prior to each dose, allowing dose reductions or interruptions as appropriate, the manufacturer noted. Patients also used preservative-free eyedrops. Keratopathy leading to treatment discontinuation affected 2.1% of patients in the 2.5-mg/kg cohort.
Because of this ocular toxicity, the company has set up a risk evaluation and mitigation strategy (REMS) for the product. This requires education for all physicians who prescribe the product as well as their patients regarding the ocular risks associated with treatment. It also requires monitoring that includes regular ophthalmic examinations. Information about the scheme can be found at www.blenreprems.com.
At the FDA advisory committee meeting last month, one of the panelists, Gita Thanarajasingam, MD, an assistant professor of medicine at the Mayo Clinic, in Rochester, Minn., said belantamab appeared to be well tolerated but for ocular toxicity. Physicians need to acknowledge how severe this risk may be for patients while keeping in mind that belantamab still may be more tolerable for some people than current treatments.
“It’s reasonable to leave open the option for decision making. Patients can express their values and preferences,” Dr. Thanarajasingam said. “There’s adequate, albeit not complete, information to guide this risk-benefit discussion in a REMS program.”
Another panelist, Heidi D. Klepin, MD, a professor at Wake Forest University Health Sciences, Winston Salem, N.C., agreed that the informed consent process should allow patients “to choose whether the trade-off is worth it to them” with belantamab.
This article first appeared on Medscape.com.
Hepatitis screening now for all patients with cancer on therapy
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
All patients with cancer who are candidates for systemic anticancer therapy should be screened for hepatitis B virus (HBV) infection prior to or at the start of therapy, according to an updated provisional clinical opinion (PCO) from the American Society of Clinical Oncology.
“This is a new approach [that] will actively take system changes ... but it will ultimately be safer for patients – and that is crucial,” commented Jessica P. Hwang, MD, MPH, cochair of the American Society of Clinical Oncology HBV Screening Expert Panel and the first author of the PCO.
Uptake of this universal screening approach would streamline testing protocols and identify more patients at risk for HBV reactivation who should receive prophylactic antiviral therapy, Dr. Hwang said in an interview.
The PCO calls for antiviral prophylaxis during and for at least 12 months after therapy for those with chronic HBV infection who are receiving any systemic anticancer treatment and for those with have had HBV in the past and are receiving any therapies that pose a risk for HBV reactivation.
“Hepatitis B reactivation can cause really terrible outcomes, like organ failure and even death,” Dr. Hwang, who is also a professor at the University of Texas MD Anderson Cancer Center, Houston, commented in an interview.
“This whole [issue of] reactivation and adverse outcomes with anticancer therapies is completely preventable with good planning, good communication, comanagement with specialists, and antiviral therapy and monitoring,” she added.
The updated opinion was published online July 27 in the Journal of Clinical Oncology.
It was developed in response to new data that call into question the previously recommended risk-adaptive approach to HBV screening of cancer patients, say the authors.
ASCO PCOs are developed “to provide timely clinical guidance” on the basis of emerging practice-changing information. This is the second update to follow the initial HBV screening PCO, published in 2010. In the absence of clear consensus because of limited data, the original PCO called for a risk-based approach to screening. A 2015 update extended the recommendation for screening to patients starting anti-CD20 therapy or who are to undergo stem cell transplant and to those with risk factors for HBV exposure.
The current update provides “a clinically pragmatic approach to HBV screening and management” that is based on the latest findings, say the authors. These include findings from a multicenter prospective cohort study of more than 3000 patients. In that study, 21% of patients with chronic HBV had no known risk factors for the infection. In another large prospective observational cohort study, led by Dr. Hwang, which included more than 2100 patients with cancer, 90% had one or more significant risk factors for HBV infection, making selective screening “inefficient and impractical,” she said.
“The results of these two studies suggest that a universal screening approach, its potential harms (e.g., patient and clinician anxiety about management, financial burden associated with antiviral therapy) notwithstanding, is the most efficient, clinically pragmatic approach to HBV screening in persons anticipating systemic anticancer treatment,” the authors comment.
The screening recommended in the PCO requires three tests: hepatitis B surface antigen (HBsAg), core antibody total immunoglobulin or IgG, and antibody to HBsAg tests.
Anticancer therapy should not be delayed pending the results, they write.
Planning for monitoring and long-term prophylaxis for chronic HBV infection should involve a clinician experienced in HBV management, the authors write. Management of those with past infection should be individualized. Alternatively, patients with past infection can be carefully monitored rather than given prophylactic treatment, as long as frequent and consistent follow-up is possible to allow for rapid initiation of antiviral therapy in the event of reactivation, they say.
Hormonal therapy without systemic anticancer therapy is not likely to lead to HBV reactivation in patients with chronic or past infection; antiviral therapy and management of these patients should follow relevant national HBV guidelines, they note.
Challenges in implementing universal HBV screening
The expert panel acknowledges the challenges associated with implementation of universal HBV screening as recommended in their report and notes that electronic health record–based approaches that use alerts to prompt screening have demonstrated success. In one study of high-risk primary care patients, an EHR alert system significantly increased testing rates (odds ratio, 2.64 in comparison with a control group without alerts), and another study that used a simple “sticky-note” alert system to promote referral of HBsAg patients to hepatologists increased referrals from 28% to 73%.
In a cancer population, a “comprehensive set of multimodal interventions,” including pharmacy staff checks for screening prior to anti-CD20 therapy administration and electronic medication order reviews to assess for appropriate testing and treatment before anti-CD20 therapy, increased testing rates to greater than 90% and antiviral prophylaxis rates to more than 80%.
A study of 965 patients in Taiwan showed that a computer-assisted reminder system that prompted for testing prior to ordering anticancer therapy increased screening from 8% to 86% but was less effective for improving the rates of antiviral prophylaxis for those who tested positive for HBV, particularly among physicians treating patients with nonhematologic malignancies.
“Future studies will be needed to make universal HBV screening and linkage to care efficient and systematic, likely based in EHR systems,” the panel says. The authors note that “[o]ngoing studies of HBV tests such as ultrasensitive HBsAg, HBV RNA, and hepatitis B core antigen are being studied and may be useful in predicting risk of HBV reactivation.”
The panel also identified a research gap related to HBV reactivation risks “for the growing list of agents that deplete or modulate B cells.” It notes a need for additional research on the cost-effectiveness of HBV screening. The results of prior cost analyses have been inconsistent and vary with respect to the population studied. For example, universal screening and antiviral prophylaxis approaches have been shown to be cost-effective for patients with hematologic malignancies and high HBV reactivation risk but are less so for patients with solid tumors and lower reactivation risk, they explain.
Dr. Hwang said that not one of the more than 2100 patients in her HBV screening cohort study encountered problems with receiving insurance payment for their HBV screening.
“That’s a really strong statement that insurance payers are accepting of this kind of preventative service,” she said.
Expert panel cochair Andrew Artz, MD, commented that there is now greater acceptance of the need for HBV screening across medical specialties.
“There’s growing consensus among hepatologists, infectious disease specialists, oncologists, and HBV specialists that we need to do a better job of finding patients with hepatitis B [who are] about to receive immunocompromising treatment,” Dr. Artz said in an interview.
Dr. Artz is director of the Program for Aging and Blood Cancers and deputy director of the Center for Cancer and Aging at City of Hope Comprehensive Cancer Center, Duarte, California.
He suggested that the growing acceptance is due in part to the increasing number of anticancer therapies available and the resulting increase in the likelihood of patients receiving therapies that could cause reactivation.
More therapies – and more lines of therapy – could mean greater risk, he explained. He said that testing is easy and that universal screening is the simplest approach to determining who needs it. “There’s no question we will have to change practice,” Dr. Artz said in an interview. “But this is easier than the previous approach that essentially wasn’t being followed because it was too difficult to follow and patients were being missed.”
Most clinicians will appreciate having an approach that’s easier to follow, Dr. Artz predicted.
If there’s a challenge it will be in developing partnerships with HBV specialists, particularly in rural areas. In areas where there is a paucity of subspecialists, oncologists will have to “take some ownership of the issue,” as they often do in such settings, he said.
However, with support from pharmacists, administrators, and others in embracing this guidance, implementation can take place at a systems level rather than an individual clinician level, he added.
The recommendations in this updated PCO were all rated as “strong,” with the exception of the recommendation on hormonal therapy in the absence of systemic anticancer therapy, which was rated as “moderate.” All were based on “informal consensus,” with the exception of the key recommendation for universal HBV screening – use of three specific tests – which was “evidence based.”
The expert panel agreed that the benefits outweigh the harms for each recommendation in the update.
Dr. Hwang received research funding to her institution from Gilead Sciences and Merck Sharp & Dohme. She also has a relationship with the Asian Health Foundation. Dr. Artz received research funding from Miltenyi Biotec. All expert panel members’ disclosures are available in the PCO update.
This article first appeared on Medscape.com.
Novel probiotic shows promise in treating type 2 diabetes
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
A novel probiotic product (Pendulum Glucose Control) containing gut bacteria strains that are deficient in people with type 2 diabetes modestly improves blood glucose levels, new research suggests.
The findings were published in BMJ Open Diabetes Research & Care by Fanny Perraudeau, PhD, and colleagues, all employees of Pendulum Therapeutics.
The product, classified as a medical food, is currently available for purchase on the company’s website without a prescription.
It contains the oligosaccharide-consuming Akkermansia muciniphila and Bifidobacterium infantis, the butyrate producers Anaerobutyricum hallii, Clostridium beijerinckii, and Clostridium butyricum, along with the “prebiotic” dietary fiber inulin.
In the 12-week trial of people with type 2 diabetes who were already taking metformin, with or without a sulfonylurea, 23 were randomized to the product and 26 received placebo capsules.
Participants in the active-treatment arm had significantly reduced glucose levels after a 3-hour standard meal-tolerance test, by 36.1 mg/dL (P = .05), and average A1c reduction of 0.6 percentage points (P = .054), compared with those taking placebo.
There were no major safety or tolerability issues, only transient gastrointestinal symptoms (nausea, diarrhea) lasting 3-5 days. No changes were seen in body weight, insulin sensitivity, or fasting blood glucose.
Asked to comment on the findings, Nanette I. Steinle, MD, an endocrinologist with expertise in nutrition who was not involved in the research, said that “to me it looks like the research was designed well and they didn’t overstate the results. ... I would say, for folks with mild to modest blood glucose elevations, it could be helpful to augment a healthy lifestyle.”
However, the product is not cheap, so cost could be a limiting factor for some patients, said Dr. Steinle, who is associate professor of medicine at the University of Maryland, Baltimore, and chief of the endocrine section, Maryland Veterans Affairs Health Care System.
Lead author Orville Kolterman, MD, chief medical officer at Pendulum, said in an interview that the formulation’s specificity distinguishes it from most commercially available probiotics.
“The ones sold in stores are reconfigurations of food probiotics, which are primarily aerobic organisms, whereas the abnormalities in the microbiome associated with type 2 diabetes reside in anaerobic organisms, which are more difficult to manufacture,” he explained.
The fiber component, inulin, is important as well, he said. “This product may make the dietary management of type 2 diabetes more effective, in that you need both the fiber and the microbes to ferment the fiber and produce short-chain fatty acids that appear to be very important for many reasons.”
The blood glucose-lowering effect is related in part to the three organisms’ production of butyrate, which binds to epithelial cells in the gut to secrete glucagonlike peptide–1, leading to inhibition of glucagon secretion among other actions.
And Akkermansia muciniphila protects the gut epithelium and has shown some evidence of improving insulin sensitivity and other beneficial metabolic effects in humans.
Dr. Kolterman, who was with Amylin Pharmaceuticals prior to moving to Pendulum, commented: “After doing this for 30 years or so, I’ve come to the strong appreciation that whenever you can do something to move back toward what Mother Nature set up, you’re doing a good thing.”
Clinically, Dr. Kolterman said, “I think perhaps the ideal place to try this would be shortly after diagnosis of type 2 diabetes, before patients go on to pharmacologic therapy.”
However, for practical reasons the study was done in patients who were already taking metformin, he said. “The results we have are that it’s beneficial above and beyond metformin, since [these] patients weren’t controlled with metformin.”
He also noted that it might benefit patients who can’t tolerate metformin or who have prediabetes; there’s an ongoing investigator-initiated study of the latter.
Dr. Steinle also endorsed the possibility that the product may benefit people with prediabetes. “I would suspect this could be very helpful to augment attempts to prevent diabetes. ... The group with prediabetes is huge.”
However, she cautioned, “if the blood glucose is over 200 [mg/dL], I wouldn’t think a probiotic would get them where they need to go.”
Overall, she pointed out that targeting the microbiome is a very active and potentially important field of medical research, and that it has received support from the National Institutes of Health. “I think we’re in the early stages of understanding how what grows in us, and on us, impacts our health and how we may be able to use these organisms to our benefit. I would expect we’ll see more of these probiotics being marketed in various forms.”
Dr. Kolterman is an employee of Pendulum. Dr. Steinle has reported receiving funding from the NIH, and she is conducting a study funded by Kowa through the VA.
A version of this article originally appeared on Medscape.com.
Cardiorespiratory fitness may alter AFib ablation outcomes
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.
Higher baseline cardiorespiratory fitness (CRF) is associated with better outcomes after atrial fibrillation (AFib) ablation, according to new research.
In a single-center, retrospective cohort study, patients with the highest level of baseline CRF had significantly lower rates of arrhythmia recurrence and death than did patients with lower levels of CRF.
“It is stunning how just a simple measure, in this case walking on a treadmill, can predict whether atrial fibrillation ablation will be a successful endeavor or if it will fail,” senior author Wael A. Jaber, MD, professor of medicine, Cleveland Clinic, said in an interview.
“We found that ablation was not successful in most patients who had poor functional class and, conversely, that it was successful in most patients who were in tip-top shape when they walked on the treadmill. Our results can help clinicians inform patients about what they can expect after the procedure, depending on the baseline fitness level,” Dr. Jaber said.
The study was published online Aug. 2 in Heart Rhythm.
Several studies have shown a reduction in AFib incidence among individuals who report a physically active lifestyle, but the extent to which baseline CRF influences arrhythmia rates after AFib ablation is unknown, the authors note.
For the study, Dr. Jaber and colleagues analyzed results in 591 consecutive patients (mean age, 66.5 years; 75% male) with symptomatic paroxysmal or persistent AFib who underwent de novo AFib ablation at their institution. Only patients who had undergone an exercise stress test in the 12 months before AFib ablation (average, 4.5 months) were included.
Age- and sex-specific predicted metabolic equivalents (METs) were calculated using the St. James model for women and the Veterans Affairs referral model for men. The number of METs achieved was then divided by the predicted METs, and the patients were categorized into low (<85% predicted; n = 152), adequate (85%-100% predicted; n = 115), and high (>100% predicted; n = 324) CRF groups. Functional capacity was characterized as poor in 56 patients (9.5%), fair in 94 (16.0%), average in 225 (38.1%), good in 169 (28.6%), and high in 47 (8.0%).
During a mean follow-up of 32 months, arrhythmia recurrence was observed in 79% of patients in the low-CRF group, 54% of patients in the adequate-CRF group, and 27.5% of patients in the high-CRF group (P < .0001). Rates of repeat arrhythmia-related hospitalization, repeat rhythm-control procedures, and the need for ongoing antiarrhythmic therapy (ATT) were significantly lower in the high-CRF group. Specifically, ATT was stopped in 56% of patients in the high-CRF group, compared with 24% in the adequate-CRF group and 11% in the low-CRF group (P < .0001). Rehospitalization for arrhythmia was required in 18.5%, 38.0%, and 60.5% of cases, respectively, and repeat direct-current cardioversion or ablation was performed in 26.0%, 49.0%, and 65.0%, respectively (P < .0001 for both).
Death occurred in 11% of the low-CRF group, compared with 4% in the adequate-CRF group and 2.5% in the high-CRF group. Most (70%) of the deaths were caused by cardiovascular events, including heart failure, cardiac arrest, and coronary artery disease. The most common cause of noncardiac death was respiratory failure (13%), followed by sepsis (10%), malignancy (3%), and complications of Parkinson’s disease (3%).
“Although there was a statistically significant association between higher CRF and lower mortality in this cohort, the findings are to be viewed through the prism of a small sample size and relatively low death rate,” the authors wrote.
Don’t “overpromise” results
“The important message for clinicians is that when, you are discussing what to expect after atrial fibrillation ablation with your patients, do not overpromise the results. You can inform them that the success of the procedure depends more on how they perform on the baseline exercise test, and less on the ablation itself,” Dr. Jaber said.
Clinicians might want to consider advising their patients to become more active and increase their fitness level before undergoing the procedure, but whether doing so will improve outcomes is still unknown.
“This is what we don’t know. It makes sense. Hopefully, our results will encourage people to be more active before they arrive here for the procedure,” he said. “Our study is retrospective and is hypothesis generating, but we are planning a prospective study where patients will be referred to cardiac rehab prior to having ablation to try to improve their functional class to see if this will improve outcomes.”
Survival of the fittest
In an accompanying editorial commentary, Eric Black-Maier, MD, and Jonathan P. Piccini Sr, MD, from Duke University Medical Center, Durham, N.C., wrote that the findings have “important implications for clinical practice and raise important additional questions.”
They note that catheter ablation as a first-line rhythm-control strategy, per current recommendations, “seems reasonable” in individuals with high baseline cardiorespiratory fitness, but that the benefit is less clear for patients with poor baseline CRF and uncontrolled risk factors.
“Significant limitations in functional status may be at least partially attributable to uncontrolled [AFib], and patients with limited exercise capacity may stand to gain most from successful catheter ablation,” the editorialists wrote.
“Furthermore, because shorter time from [AFib] diagnosis to catheter ablation has been associated with improved outcomes, the decision to postpone ablation in favor of lifestyle modification is not without potential adverse consequences,” they added.
Dr. Black-Maier and Dr. Piccini agree with the need for additional prospective randomized clinical trials to confirm that exercise training to improve cardiorespiratory fitness before AFib ablation is practical and effective for reducing arrhythmia recurrence.
“Over the past 50-plus years, our understanding of cardiorespiratory fitness, exercise capacity, and arrhythmia occurrence in patients with [AFib] continues to evolve,” the editorialists concluded. Data from the study “clearly demonstrate that arrhythmia-free survival is indeed survival of the fittest. Time will tell if exercise training and improvements in cardiorespiratory fitness can change outcomes after ablation.”
The study was sponsored by the Cleveland Clinic. Dr. Jaber and Dr. Black-Maier report no relevant financial relationships. Dr. Piccini receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and UpToDate.
A version of this story originally appeared on Medscape.com.









