User login
The grocery store hug
I grew up in a family that was pretty much devoid of physical demonstrations of affection. I certainly felt that my folks loved me, but there was no hugging. I don’t recall ever seeing my parents kiss or touch each other. My dad would occasionally physically tease my mother. For example, I can remember one incident at the dinner table when he was playfully and gently laying a hand on my mother’s arm just as she was raising her fork to her mouth. After about three of these gentle holds, she lifted her water glass and tossed its contents in his face. This was the full extent of physicality in our family.
It wasn’t just my parents. I can’t remember aunts or uncles or cousins ever hugging us when we met. Grandmothers of course would request a hug. I never knew either of my grandfathers, but I suspect they would not have been the hugging kind.
I never felt I was missing out on anything, because in the generally WASPish atmosphere of the community in which I grew up I saw very few public displays of affection. But somewhere over time, hugging crept into the American repertoire of expression. This incursion may have been a ripple effect from the flower power, free love hippiedom of the ‘60s and ‘70s. Or it may have been a symptom of globalization as Americans became more familiar with other cultures in which physical expression was more common.
Whatever the reason for the more widespread adoption of hugging in our social vocabulary with my somewhat physically impoverished upbringing, it took me longer than most folks to comfortably include it in my greeting options. Although I may have come to the dance late, I have fully adopted hugging as a way to greet people with whom I have more than a passing acquaintance.
In fact, the ability to comfortably hug former coworkers, old friends I haven’t seen in years, and parents with whom I had shared a particularly troublesome child is what I miss most about the restrictions that have come with the COVID-19 pandemic. Now when I meet folks in the grocery store with whom I share a special affection that magnetic spark still leaps between our eyes, just visible over our face masks, but mentally and physically we take a step back and say to ourselves that this hug shouldn’t happen and it isn’t going to happen. And that makes me sad.
One of the great perks of practicing pediatrics in a small town and then remaining there in retirement is that nearly every week I encounter one or two people with whom I have a long and sometimes emotionally charged relationship. Nurses with whom I sweated over difficult delivery room resuscitations. Parents for whom their anxiety was getting in the way of their ability to parent. Parents and caregivers of complex multiply disabled children who are now adults. Peers who have lost a spouse or a child.
I can envision a day sometime in the relatively near future that I will be able to hug my two grandchildren whom I haven’t hugged even though they live a short 10-minute walk away. But I have trouble imagining when I will again be able to enjoy and be enriched by those special grocery store hugs that I have grown to savor.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
I grew up in a family that was pretty much devoid of physical demonstrations of affection. I certainly felt that my folks loved me, but there was no hugging. I don’t recall ever seeing my parents kiss or touch each other. My dad would occasionally physically tease my mother. For example, I can remember one incident at the dinner table when he was playfully and gently laying a hand on my mother’s arm just as she was raising her fork to her mouth. After about three of these gentle holds, she lifted her water glass and tossed its contents in his face. This was the full extent of physicality in our family.
It wasn’t just my parents. I can’t remember aunts or uncles or cousins ever hugging us when we met. Grandmothers of course would request a hug. I never knew either of my grandfathers, but I suspect they would not have been the hugging kind.
I never felt I was missing out on anything, because in the generally WASPish atmosphere of the community in which I grew up I saw very few public displays of affection. But somewhere over time, hugging crept into the American repertoire of expression. This incursion may have been a ripple effect from the flower power, free love hippiedom of the ‘60s and ‘70s. Or it may have been a symptom of globalization as Americans became more familiar with other cultures in which physical expression was more common.
Whatever the reason for the more widespread adoption of hugging in our social vocabulary with my somewhat physically impoverished upbringing, it took me longer than most folks to comfortably include it in my greeting options. Although I may have come to the dance late, I have fully adopted hugging as a way to greet people with whom I have more than a passing acquaintance.
In fact, the ability to comfortably hug former coworkers, old friends I haven’t seen in years, and parents with whom I had shared a particularly troublesome child is what I miss most about the restrictions that have come with the COVID-19 pandemic. Now when I meet folks in the grocery store with whom I share a special affection that magnetic spark still leaps between our eyes, just visible over our face masks, but mentally and physically we take a step back and say to ourselves that this hug shouldn’t happen and it isn’t going to happen. And that makes me sad.
One of the great perks of practicing pediatrics in a small town and then remaining there in retirement is that nearly every week I encounter one or two people with whom I have a long and sometimes emotionally charged relationship. Nurses with whom I sweated over difficult delivery room resuscitations. Parents for whom their anxiety was getting in the way of their ability to parent. Parents and caregivers of complex multiply disabled children who are now adults. Peers who have lost a spouse or a child.
I can envision a day sometime in the relatively near future that I will be able to hug my two grandchildren whom I haven’t hugged even though they live a short 10-minute walk away. But I have trouble imagining when I will again be able to enjoy and be enriched by those special grocery store hugs that I have grown to savor.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
I grew up in a family that was pretty much devoid of physical demonstrations of affection. I certainly felt that my folks loved me, but there was no hugging. I don’t recall ever seeing my parents kiss or touch each other. My dad would occasionally physically tease my mother. For example, I can remember one incident at the dinner table when he was playfully and gently laying a hand on my mother’s arm just as she was raising her fork to her mouth. After about three of these gentle holds, she lifted her water glass and tossed its contents in his face. This was the full extent of physicality in our family.
It wasn’t just my parents. I can’t remember aunts or uncles or cousins ever hugging us when we met. Grandmothers of course would request a hug. I never knew either of my grandfathers, but I suspect they would not have been the hugging kind.
I never felt I was missing out on anything, because in the generally WASPish atmosphere of the community in which I grew up I saw very few public displays of affection. But somewhere over time, hugging crept into the American repertoire of expression. This incursion may have been a ripple effect from the flower power, free love hippiedom of the ‘60s and ‘70s. Or it may have been a symptom of globalization as Americans became more familiar with other cultures in which physical expression was more common.
Whatever the reason for the more widespread adoption of hugging in our social vocabulary with my somewhat physically impoverished upbringing, it took me longer than most folks to comfortably include it in my greeting options. Although I may have come to the dance late, I have fully adopted hugging as a way to greet people with whom I have more than a passing acquaintance.
In fact, the ability to comfortably hug former coworkers, old friends I haven’t seen in years, and parents with whom I had shared a particularly troublesome child is what I miss most about the restrictions that have come with the COVID-19 pandemic. Now when I meet folks in the grocery store with whom I share a special affection that magnetic spark still leaps between our eyes, just visible over our face masks, but mentally and physically we take a step back and say to ourselves that this hug shouldn’t happen and it isn’t going to happen. And that makes me sad.
One of the great perks of practicing pediatrics in a small town and then remaining there in retirement is that nearly every week I encounter one or two people with whom I have a long and sometimes emotionally charged relationship. Nurses with whom I sweated over difficult delivery room resuscitations. Parents for whom their anxiety was getting in the way of their ability to parent. Parents and caregivers of complex multiply disabled children who are now adults. Peers who have lost a spouse or a child.
I can envision a day sometime in the relatively near future that I will be able to hug my two grandchildren whom I haven’t hugged even though they live a short 10-minute walk away. But I have trouble imagining when I will again be able to enjoy and be enriched by those special grocery store hugs that I have grown to savor.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
On being nonessential
I don’t need to tell you that the COVID-19 pandemic has leveled a major hit on outpatient pediatrics. Offices that once had waiting rooms overflowing with tantruming toddlers and anxious adolescents are empty. With income slowed to trickle, support staff has had to be furloughed. Student loans, mortgage loans, and car payments are stretching the budgets of even the most cautious spenders. In many parts of the country, it is an economic apocalypse for outpatient physicians who once saw their jobs as financially secure. Despite the persistent efforts of the American Academy of Pediatrics, pediatricians have been left off the list of recipients for financial support from the federal government.
The recent marketing initiative labeled “Call Your Pediatrician” sounds like an S.O.S. As I mentioned in a recent Letters from Maine column, I never envisioned a scenario in which I wouldn’t be busy and paying the bills if I continued to show up in the office at least 5 days a week. I guess I never thought of my work as a general pediatrician in terms of essentialness. The issue of being essential just wasn’t something anyone ever thought about. I guess if you had asked me, I would have admitted that, compared with some other health care providers, what I did was low on the essential scale. But I figured enough people thought what I provided was of sufficient value that they would pay to come see me.
If I step back and look at what of all the things I did as a pediatrician might be considered essential, it boils down to providing immunizations. If you remove my delivery room experience from the picture, there were very few instances when I might have saved a life. I hope that I calmed a lot of anxious parents and gave them some suggestions that made the job of parenting a bit easier. But while my efforts may have seemed valuable at the time, they certainly wouldn’t pass the straight-faced test of essentialness that is being applied during this pandemic. The young man or woman who stocks the toilet paper shelves at the grocery store and who accepts the risk of contagion working behind the cash register would certainly win more votes than I would garner.
So it is not surprising, given the scope of the pandemic and the anxiety compounded by what we don’t know about the virus, that office pediatrics has been left out in the cold when federal financial support is being handed out. I’m certainly not saying the oversight is warranted. It’s just not surprising. Outpatient pediatricians have always been there and it is unfortunately assumed that we will continue to be there when this whole thing blows over and we are needed again.
The failure to support pediatric offices is shortsighted because, even when we return to the new normal, pediatricians will again be valued. However, without financial support some offices will close and some support staff and physicians will leave the practice of pediatrics. It has been suggested that in the wake of the pandemic, the demand for mental health support for children may increase. The new normal may see our patient mix shift even further toward behavioral problems.
For whatever reason, COVID-19 appears to attack the older end of the age spectrum. It is very possible that the next pandemic targets children. If that happens, whether or not we are considered essential will not be one of our worries.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Updated on 6/10/2020
I don’t need to tell you that the COVID-19 pandemic has leveled a major hit on outpatient pediatrics. Offices that once had waiting rooms overflowing with tantruming toddlers and anxious adolescents are empty. With income slowed to trickle, support staff has had to be furloughed. Student loans, mortgage loans, and car payments are stretching the budgets of even the most cautious spenders. In many parts of the country, it is an economic apocalypse for outpatient physicians who once saw their jobs as financially secure. Despite the persistent efforts of the American Academy of Pediatrics, pediatricians have been left off the list of recipients for financial support from the federal government.
The recent marketing initiative labeled “Call Your Pediatrician” sounds like an S.O.S. As I mentioned in a recent Letters from Maine column, I never envisioned a scenario in which I wouldn’t be busy and paying the bills if I continued to show up in the office at least 5 days a week. I guess I never thought of my work as a general pediatrician in terms of essentialness. The issue of being essential just wasn’t something anyone ever thought about. I guess if you had asked me, I would have admitted that, compared with some other health care providers, what I did was low on the essential scale. But I figured enough people thought what I provided was of sufficient value that they would pay to come see me.
If I step back and look at what of all the things I did as a pediatrician might be considered essential, it boils down to providing immunizations. If you remove my delivery room experience from the picture, there were very few instances when I might have saved a life. I hope that I calmed a lot of anxious parents and gave them some suggestions that made the job of parenting a bit easier. But while my efforts may have seemed valuable at the time, they certainly wouldn’t pass the straight-faced test of essentialness that is being applied during this pandemic. The young man or woman who stocks the toilet paper shelves at the grocery store and who accepts the risk of contagion working behind the cash register would certainly win more votes than I would garner.
So it is not surprising, given the scope of the pandemic and the anxiety compounded by what we don’t know about the virus, that office pediatrics has been left out in the cold when federal financial support is being handed out. I’m certainly not saying the oversight is warranted. It’s just not surprising. Outpatient pediatricians have always been there and it is unfortunately assumed that we will continue to be there when this whole thing blows over and we are needed again.
The failure to support pediatric offices is shortsighted because, even when we return to the new normal, pediatricians will again be valued. However, without financial support some offices will close and some support staff and physicians will leave the practice of pediatrics. It has been suggested that in the wake of the pandemic, the demand for mental health support for children may increase. The new normal may see our patient mix shift even further toward behavioral problems.
For whatever reason, COVID-19 appears to attack the older end of the age spectrum. It is very possible that the next pandemic targets children. If that happens, whether or not we are considered essential will not be one of our worries.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Updated on 6/10/2020
I don’t need to tell you that the COVID-19 pandemic has leveled a major hit on outpatient pediatrics. Offices that once had waiting rooms overflowing with tantruming toddlers and anxious adolescents are empty. With income slowed to trickle, support staff has had to be furloughed. Student loans, mortgage loans, and car payments are stretching the budgets of even the most cautious spenders. In many parts of the country, it is an economic apocalypse for outpatient physicians who once saw their jobs as financially secure. Despite the persistent efforts of the American Academy of Pediatrics, pediatricians have been left off the list of recipients for financial support from the federal government.
The recent marketing initiative labeled “Call Your Pediatrician” sounds like an S.O.S. As I mentioned in a recent Letters from Maine column, I never envisioned a scenario in which I wouldn’t be busy and paying the bills if I continued to show up in the office at least 5 days a week. I guess I never thought of my work as a general pediatrician in terms of essentialness. The issue of being essential just wasn’t something anyone ever thought about. I guess if you had asked me, I would have admitted that, compared with some other health care providers, what I did was low on the essential scale. But I figured enough people thought what I provided was of sufficient value that they would pay to come see me.
If I step back and look at what of all the things I did as a pediatrician might be considered essential, it boils down to providing immunizations. If you remove my delivery room experience from the picture, there were very few instances when I might have saved a life. I hope that I calmed a lot of anxious parents and gave them some suggestions that made the job of parenting a bit easier. But while my efforts may have seemed valuable at the time, they certainly wouldn’t pass the straight-faced test of essentialness that is being applied during this pandemic. The young man or woman who stocks the toilet paper shelves at the grocery store and who accepts the risk of contagion working behind the cash register would certainly win more votes than I would garner.
So it is not surprising, given the scope of the pandemic and the anxiety compounded by what we don’t know about the virus, that office pediatrics has been left out in the cold when federal financial support is being handed out. I’m certainly not saying the oversight is warranted. It’s just not surprising. Outpatient pediatricians have always been there and it is unfortunately assumed that we will continue to be there when this whole thing blows over and we are needed again.
The failure to support pediatric offices is shortsighted because, even when we return to the new normal, pediatricians will again be valued. However, without financial support some offices will close and some support staff and physicians will leave the practice of pediatrics. It has been suggested that in the wake of the pandemic, the demand for mental health support for children may increase. The new normal may see our patient mix shift even further toward behavioral problems.
For whatever reason, COVID-19 appears to attack the older end of the age spectrum. It is very possible that the next pandemic targets children. If that happens, whether or not we are considered essential will not be one of our worries.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Updated on 6/10/2020
Reducing low-value preop care for cataract surgery patients
Background: Although multiple randomized, controlled trials have shown that routine preoperative testing prior to cataract surgery has low yield, most Medicare beneficiaries continue to undergo this testing. The American Board of Internal Medicine started the Choosing Wisely campaign to help educate patients and providers about a crisis of unnecessary testing and procedures. This prompted multiple centers to create quality improvement (QI) projects to decrease low-value care.
_
Study design: Observational study of a health system quality improvement initiative.
Setting: Two academic, safety-net hospitals in Los Angeles.
Synopsis: The intervention hospital’s QI nurse underwent an extensive formal QI training program, followed by educating all health care team members involved in preoperative care for cataract patients. New guidelines were created and circulated, with a stated goal of eliminating routine preoperative visits and testing. The control hospital continued their usual preoperative care.
Preoperative visits decreased from 93% to 24% in the intervention group and increased from 89% to 91% in the control group (between-group difference, −71%; 95% confidence interval, –80% to –62%). Chest x-rays, laboratory tests, and electrocardiograms also had a similar decrease in the intervention group.
The intervention hospital lost $42,241 the first year because of training costs but 3-year projections estimated $67,241 in savings. The authors estimated $217,322 savings in 3 years from a societal perspective. Interestingly, the decrease in utilization would lead to financial loss in fee-for-service payment ($88,151 loss in 3 years).
No causal relationship can be established since this was an observational study. Several assumptions were made for the cost analysis. Results are less generalizable since the study was at hospitals in a single city and health system. It is unclear which component of the QI initiative was most effective.
Bottom line: A multidisciplinary, multicomponent initiative can be successful in decreasing low-value preoperative testing of patients undergoing cataract surgery. Although this results in cost savings overall and for capitated payment systems, it would actually cause revenue loss in fee-for-service systems. This emphasizes a potential barrier to eradicate low-value care.
Citation: Mafi JN et al. Evaluation of an intervention to reduce low-value preoperative care for patients undergoing cataract surgery at a safety-net health system. JAMA Intern Med. Published online 2019 Mar 25. doi: 10.1001/jamainternmed.2018.8358.
Dr. Menon is a hospitalist at Duke University Health System.
Background: Although multiple randomized, controlled trials have shown that routine preoperative testing prior to cataract surgery has low yield, most Medicare beneficiaries continue to undergo this testing. The American Board of Internal Medicine started the Choosing Wisely campaign to help educate patients and providers about a crisis of unnecessary testing and procedures. This prompted multiple centers to create quality improvement (QI) projects to decrease low-value care.
_
Study design: Observational study of a health system quality improvement initiative.
Setting: Two academic, safety-net hospitals in Los Angeles.
Synopsis: The intervention hospital’s QI nurse underwent an extensive formal QI training program, followed by educating all health care team members involved in preoperative care for cataract patients. New guidelines were created and circulated, with a stated goal of eliminating routine preoperative visits and testing. The control hospital continued their usual preoperative care.
Preoperative visits decreased from 93% to 24% in the intervention group and increased from 89% to 91% in the control group (between-group difference, −71%; 95% confidence interval, –80% to –62%). Chest x-rays, laboratory tests, and electrocardiograms also had a similar decrease in the intervention group.
The intervention hospital lost $42,241 the first year because of training costs but 3-year projections estimated $67,241 in savings. The authors estimated $217,322 savings in 3 years from a societal perspective. Interestingly, the decrease in utilization would lead to financial loss in fee-for-service payment ($88,151 loss in 3 years).
No causal relationship can be established since this was an observational study. Several assumptions were made for the cost analysis. Results are less generalizable since the study was at hospitals in a single city and health system. It is unclear which component of the QI initiative was most effective.
Bottom line: A multidisciplinary, multicomponent initiative can be successful in decreasing low-value preoperative testing of patients undergoing cataract surgery. Although this results in cost savings overall and for capitated payment systems, it would actually cause revenue loss in fee-for-service systems. This emphasizes a potential barrier to eradicate low-value care.
Citation: Mafi JN et al. Evaluation of an intervention to reduce low-value preoperative care for patients undergoing cataract surgery at a safety-net health system. JAMA Intern Med. Published online 2019 Mar 25. doi: 10.1001/jamainternmed.2018.8358.
Dr. Menon is a hospitalist at Duke University Health System.
Background: Although multiple randomized, controlled trials have shown that routine preoperative testing prior to cataract surgery has low yield, most Medicare beneficiaries continue to undergo this testing. The American Board of Internal Medicine started the Choosing Wisely campaign to help educate patients and providers about a crisis of unnecessary testing and procedures. This prompted multiple centers to create quality improvement (QI) projects to decrease low-value care.
_
Study design: Observational study of a health system quality improvement initiative.
Setting: Two academic, safety-net hospitals in Los Angeles.
Synopsis: The intervention hospital’s QI nurse underwent an extensive formal QI training program, followed by educating all health care team members involved in preoperative care for cataract patients. New guidelines were created and circulated, with a stated goal of eliminating routine preoperative visits and testing. The control hospital continued their usual preoperative care.
Preoperative visits decreased from 93% to 24% in the intervention group and increased from 89% to 91% in the control group (between-group difference, −71%; 95% confidence interval, –80% to –62%). Chest x-rays, laboratory tests, and electrocardiograms also had a similar decrease in the intervention group.
The intervention hospital lost $42,241 the first year because of training costs but 3-year projections estimated $67,241 in savings. The authors estimated $217,322 savings in 3 years from a societal perspective. Interestingly, the decrease in utilization would lead to financial loss in fee-for-service payment ($88,151 loss in 3 years).
No causal relationship can be established since this was an observational study. Several assumptions were made for the cost analysis. Results are less generalizable since the study was at hospitals in a single city and health system. It is unclear which component of the QI initiative was most effective.
Bottom line: A multidisciplinary, multicomponent initiative can be successful in decreasing low-value preoperative testing of patients undergoing cataract surgery. Although this results in cost savings overall and for capitated payment systems, it would actually cause revenue loss in fee-for-service systems. This emphasizes a potential barrier to eradicate low-value care.
Citation: Mafi JN et al. Evaluation of an intervention to reduce low-value preoperative care for patients undergoing cataract surgery at a safety-net health system. JAMA Intern Med. Published online 2019 Mar 25. doi: 10.1001/jamainternmed.2018.8358.
Dr. Menon is a hospitalist at Duke University Health System.
Acute lymphoblastic leukemia can be successfully treated in the frail elderly
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Compounding Topicals in Dermatology
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
Compounding is a way of mixing or combining medications in formulations that are not widely available. Because dermatology is a field that includes a variety of topical treatments, compounding topicals is a way to create unique and customized treatment options for patients.
Advantages
Custom compounding topical medications has many benefits in comparison to traditional topical formulations. Compounding is a way of personalizing prescriptions to best suit the individual needs of each patient. Multiple ingredients with different mechanisms of action can be combined in a single medication for patients to use, which ultimately can simplify their treatment regimen.1 For rare conditions with uncommon treatments, compounding pharmacies can provide medications that are not widely available in retail pharmacies. Compounding topical medications also can be an efficient way of prescribing medications without dealing with the uncertainty of prior authorizations or how much the co-pay will be.
Disadvantages
One of the major disadvantages of compounding topical medications is the lack of safety data. Although most active drugs have been tested independently, there is little data on the safety of compounding 2 or more active drugs. Furthermore, the vehicle used may change the permeability of the topical formulation, and systemic absorption may be possible. Two deaths were reported with the application of compounded topical lidocaine and tetracaine gel due to systemic absorption. In these cases, the gel was used before laser hair removal, and it was applied under occlusion to greater than 50% of the body surface area, leading to fatal systemic absorption.1,2
One of the hypothetical benefits of compounding topicals is being able to avoid side effects of systemic medications. However, depending on the skin intactness and the strength of the medication used, systemic adverse effects have been reported.1 In a case series of 2 patients detailing the use of amitriptyline cream 5% and 10% for neuropathic pain, the patient using 10% cream experienced systemic effects of drowsiness and discontinued treatment.3
Another major disadvantage of compounding topicals is a lack of published data about the efficacy, especially given the unique nature of what is being compounded. When combining multiple medications, there are little to no published data about the efficacy of these formulations and how they compare to monotherapy. Although there may be data about the efficacy of an oral agent, it does not translate to the topical form being safe and efficacious. Much of the published data of topical formulations is limited to case reports and case series.
Finally, many compounded medications are not covered by insurance, and the out-of-pocket cost may be prohibitive for some patients. Compounding pharmacies typically will give patients a price estimate before the prescription is filled. When compounding topicals for patient use, it is important to counsel patients about the following:the unknown safety profile; lack of data regarding efficacy; and cost, as the medication likely will not be covered by insurance.
Pharmaceutical Regulations
After a contaminated product at a compounding pharmacy in New England led to an outbreak of fungal meningitis, there has been increased regulation by the US Food and Drug Administration.4 To meet safety regulations, compounding pharmacies must adhere to the standards set by the US Pharmacopeia. The US Food and Drug Administration says that physicians are not to prescribe compounded medications that are “unapproved, adulterated, or misbranded drugs,” which has been interpreted to mean that compounded medications should not mimic a branded medication but should instead be a unique formulation or strength.4,5 Thus, while compounding topicals may provide an alternative when a specific medication is not covered by insurance, it cannot be the same as a branded medication.
Pharmaceutical Options
Most major cities have custom compounding pharmacies or apothecaries. One of the benefits of using a local compounding pharmacy is that you typically can speak directly with the pharmacist about your patient’s diagnosis and his/her specific needs. The pharmacist can guide you through which formulations to compound, which strength to choose, and the best vehicle to use as a base. This expertise is invaluable in the compounding process. There also are online compounding pharmacies available.
Options for Bases
Dermatologists can request for their medications to be compounded in traditional over-the-counter emollients or petrolatum-based products, which work by passively diffusing through the stratum corneum into the superficial epidermis to treat skin conditions.1 For a topical drug to be absorbed effectively through the skin and into the general circulation, the vehicle needs to have affinity for both lipid and aqueous environments. Lipophilic drugs will absorb better through the stratum corneum, while hydrophilic drugs will absorb better through the aqueous layer of the epidermis. For a topical formulation to be both hydrophobic and hydrophilic, components such as viscosity enhancers and permeation enhancers can be added.1 Many compounding pharmacies also have proprietary bases that can be used.
Final Thoughts
Compounding topical medications in dermatology provides dermatologists with the ability to provide unique formulations to best suit their patients’ individual needs. However, dermatologists must keep in mind the limitations of compounding topicals, including a lack of data on efficacy and safety.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
- Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27:263-271.
- Ukens C. Coed death tied to compounded drug. Drug Topics. March 7, 2005. https://www.drugtopics.com/community-pharmacy/coed-death-tied-compounded-drug. Accessed May 31, 2020.
- Kopsky DJ, Hesselink JM. High doses of topical amitriptyline in neuropathic pain: 2 cases and literature review. Pain Pract. 2012;12:148-153.
- Campbell EH, Elston DM, Straughan CL, et al. Regulations, liability, safety, and economics related to compounding [published online December 9, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.11.061.
- US Food and Drug Administration. Administrative Destruction of Certain Drugs Refused Admission to the United States; Final Rule: Docket No. FDA-2014-N-0504. https://www.fda.gov/media/93525/download. Accessed May 31, 2020.
Resident Pearls
- Compounding topical medications provides dermatologists with the ability to create custom formulations that cater to the individual needs of each patient.
- Dermatologists must keep in mind that data are limited regarding both safety and efficacy of compounded medications.
Follicular Traction Urticaria Induced by Electric Epilation
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
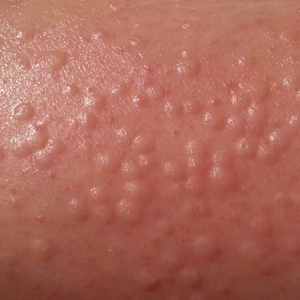
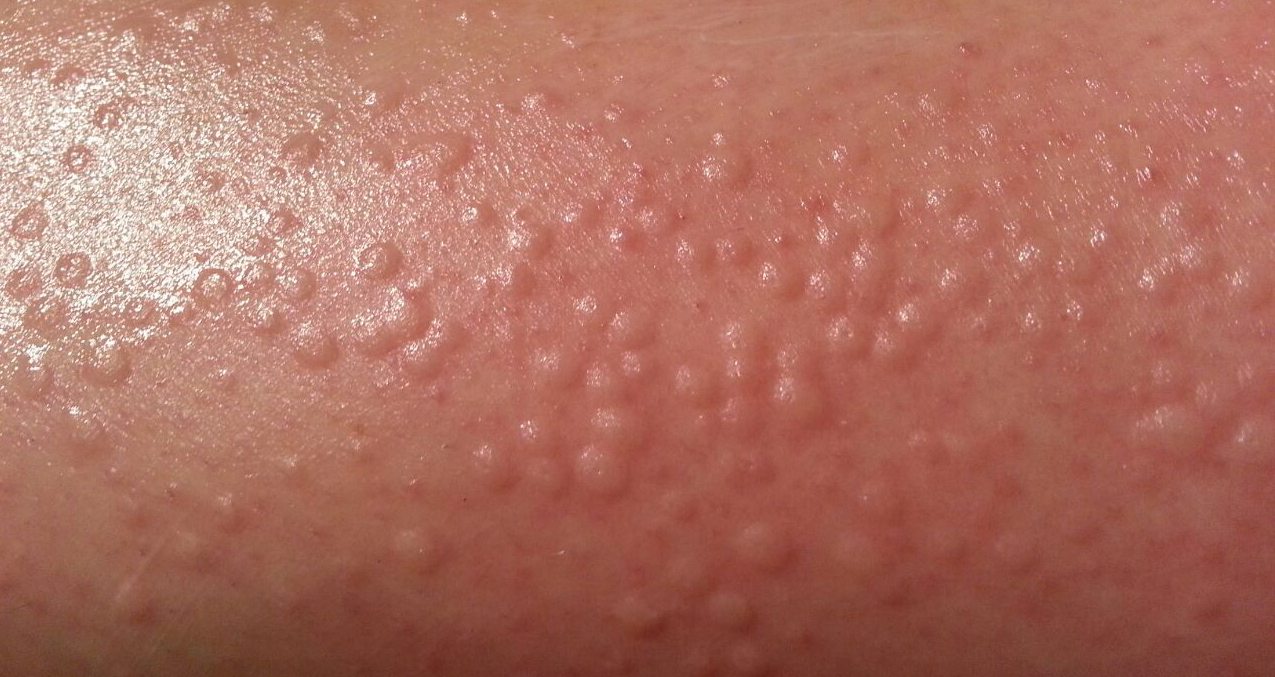
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.
Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.

Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.

Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
Practice Points
- Follicular traction urticaria is an unusual form of chronic inducible urticaria.
- Follicular traction urticaria consists of follicular hives that develop after being triggered by hair traction.
Secondary surgery extends OS in recurrent ovarian cancer
The trial, AGO DESKTOP III/ENGOT ov20, is the first prospective, randomized study showing an overall survival benefit for debulking surgery in patients with recurrent ovarian cancer.
Among 406 patients in first relapse, the median overall survival was 53.7 months for those randomized to cytoreductive surgery plus chemotherapy and 46 months for patients randomized to chemotherapy alone (P = .02).
“The overall survival benefit was highest and exclusively seen in the cohort with complete resection, indicating the importance of a thorough selection process of both the right patient and the right center,” said investigator Andreas du Bois, MD, of the Kliniken Essen-Mitte (Germany).
The median survival gain for patients with platinum-free intervals of more than 6 months who undergo complete resection is nearly 16 months “and is worth going for,” he added.
Dr. du Bois presented these results as part of the American Society of Clinical Oncology virtual scientific program (Abstract 6000).
In another trial, SOC-1, that was also presented in the virtual program, investigators reported a progression-free survival advantage of 5.5 months for patients with recurrent ovarian cancer who underwent debulking surgery, compared with those who did not (Abstract 6001).
Different trials, different results
The invited discussant for Dr. du Bois’s presentation was Robert L. Coleman, MD, chief scientific officer of the U.S. Oncology Network in The Woodlands, Tex., who was the principal investigator of the GOG-0213 trial (N Engl J Med. 2019;381:1929-39).
That trial did not show an overall survival advantage to secondary surgical cytoreduction followed by chemotherapy, compared with chemotherapy alone, among 485 women with platinum-sensitive recurrent ovarian cancer.
Referring to both AGO DESKTOP III and SOC-1, Dr. Coleman noted that, “while only DESKTOP III met its primary endpoint of improving overall survival, both demonstrated a benefit on PFS [progression-free survival].” Both trials also support a triage algorithm for selecting the approximately 75% of patients who are likely to benefit from secondary cytoreductive surgery.
“However, the price paid for being wrong is substantial, with no benefit seen in progression-free survival and possibly a detriment in overall survival. Because of these observations, both [presenters of SOC-1 and AGO DESKTOP III data] recommended that procedures be limited to select women having surgery performed at sites of excellence,” Dr. Coleman said.
Potential explanations for the differential findings of a secondary surgery benefit in DESKTOP III and SOC-1 versus GOG-0213 include the use of a selection algorithm in the former versus investigator selection based on clinical parameters and imaging in the latter.
In addition, “while platinum-based therapy was the rule in all trials, the use of concomitant and maintenance bevacizumab, a regimen found to improve overall survival in GOG-0213, was used in substantially higher numbers of patients in that trial relative to the two current trials,” Dr. Coleman said.
The GOG-0213 trial also demonstrated an advantage for adjuvant therapy with platinum-based chemotherapy and bevacizumab, which was given to 84% of patients in GOG-0213. That trial had a median overall survival for patients who did not undergo surgery of 65.7 months, compared with 46 months in AGO DESKTOP III and 53.9 months in SOC-1, Dr. Coleman said.
Third time’s a charm
As its name implies, the AGO DESKTOP III trial is the third in a series. AGO DESKTOP I developed the hypothesis that a positive AGO score – consisting of an Eastern Cooperative Oncology Group performance status score of 0, complete resection during first-line therapy, and ascites less than 500 mL – could be predictive of favorable outcomes with debulking surgery (Ann Surg Oncol. 2006 Dec;13[12]:1702-10).
AGO DESKTOP II was a prospective, multicenter trial testing the score in patients with platinum-free intervals of more than 6 months (Int J Gynecol Cancer. 2011 Feb;21[2]:289-95). In this trial, 51% of patients had a positive AGO score, and the score was shown to predict, with 95% probability, complete resectability in two-thirds of these patients, Dr. Du Bois said.
In AGO DESKTOP III, 407 patients were prospectively randomized to second-line chemotherapy alone (n = 201) or to cytoreductive surgery (n = 206) followed by the same chemotherapy, with platinum-containing regimens highly recommended.
Patient characteristics were well balanced between the arms. Nearly all patients (99%) in each arm had prior platinum exposure, and 75% had a platinum-free interval of more than 12 months (a median of 21.1 months in the surgery arm versus 18.7 months in the no-surgery arm).
Complete resections extend OS
There were 8 patients (4%) in the chemotherapy-only arm and 14 (6.8%) in the surgery arm who were noncompliant with randomization. The complete resection rate was 74.2%.
Following randomization, 88.8% of patients in the surgery arm and 90% in the no-surgery arm received platinum-containing chemotherapy; 22.8% and 23.4%, respectively, received bevacizumab; and 3.9% and 6.0% received a poly (ADP-ribose) polymerase inhibitor.
The median overall survival in the intention-to-treat population was 53.7 months in the surgery arm and 46 months in the no-surgery arm, an absolute difference of 7.7 months (hazard ratio, 0.75; P = .02).
The median progression-free survival, assessed in the intention-to-treat population after database closure in January 2020, was 18.4 months with surgery and 14 months without (HR, 0.66; P < .001).
A post hoc analysis showed the importance of complete versus partial resection. The median overall survival was 61.9 months in patients with complete resections and 28.8 months for patients with residual tumor after cytoreductive surgery, an absolute difference of nearly 3 years (HR, 0.40; P < .001).
Comparing only those patients with complete resections with patients who did not undergo surgery, the respective median overall survival was 61.9 months and 46 months (HR, 0.57; P < .001).
AGO DESKTOP III was sponsored by the AGO study group in collaboration with other oncology groups, Medac, and GlaxoSmithKline. Dr. du Bois and Dr. Coleman disclosed relationships with many companies.
SOURCE: du Bois A et al. ASCO 2020, Abstract 6000.
The trial, AGO DESKTOP III/ENGOT ov20, is the first prospective, randomized study showing an overall survival benefit for debulking surgery in patients with recurrent ovarian cancer.
Among 406 patients in first relapse, the median overall survival was 53.7 months for those randomized to cytoreductive surgery plus chemotherapy and 46 months for patients randomized to chemotherapy alone (P = .02).
“The overall survival benefit was highest and exclusively seen in the cohort with complete resection, indicating the importance of a thorough selection process of both the right patient and the right center,” said investigator Andreas du Bois, MD, of the Kliniken Essen-Mitte (Germany).
The median survival gain for patients with platinum-free intervals of more than 6 months who undergo complete resection is nearly 16 months “and is worth going for,” he added.
Dr. du Bois presented these results as part of the American Society of Clinical Oncology virtual scientific program (Abstract 6000).
In another trial, SOC-1, that was also presented in the virtual program, investigators reported a progression-free survival advantage of 5.5 months for patients with recurrent ovarian cancer who underwent debulking surgery, compared with those who did not (Abstract 6001).
Different trials, different results
The invited discussant for Dr. du Bois’s presentation was Robert L. Coleman, MD, chief scientific officer of the U.S. Oncology Network in The Woodlands, Tex., who was the principal investigator of the GOG-0213 trial (N Engl J Med. 2019;381:1929-39).
That trial did not show an overall survival advantage to secondary surgical cytoreduction followed by chemotherapy, compared with chemotherapy alone, among 485 women with platinum-sensitive recurrent ovarian cancer.
Referring to both AGO DESKTOP III and SOC-1, Dr. Coleman noted that, “while only DESKTOP III met its primary endpoint of improving overall survival, both demonstrated a benefit on PFS [progression-free survival].” Both trials also support a triage algorithm for selecting the approximately 75% of patients who are likely to benefit from secondary cytoreductive surgery.
“However, the price paid for being wrong is substantial, with no benefit seen in progression-free survival and possibly a detriment in overall survival. Because of these observations, both [presenters of SOC-1 and AGO DESKTOP III data] recommended that procedures be limited to select women having surgery performed at sites of excellence,” Dr. Coleman said.
Potential explanations for the differential findings of a secondary surgery benefit in DESKTOP III and SOC-1 versus GOG-0213 include the use of a selection algorithm in the former versus investigator selection based on clinical parameters and imaging in the latter.
In addition, “while platinum-based therapy was the rule in all trials, the use of concomitant and maintenance bevacizumab, a regimen found to improve overall survival in GOG-0213, was used in substantially higher numbers of patients in that trial relative to the two current trials,” Dr. Coleman said.
The GOG-0213 trial also demonstrated an advantage for adjuvant therapy with platinum-based chemotherapy and bevacizumab, which was given to 84% of patients in GOG-0213. That trial had a median overall survival for patients who did not undergo surgery of 65.7 months, compared with 46 months in AGO DESKTOP III and 53.9 months in SOC-1, Dr. Coleman said.
Third time’s a charm
As its name implies, the AGO DESKTOP III trial is the third in a series. AGO DESKTOP I developed the hypothesis that a positive AGO score – consisting of an Eastern Cooperative Oncology Group performance status score of 0, complete resection during first-line therapy, and ascites less than 500 mL – could be predictive of favorable outcomes with debulking surgery (Ann Surg Oncol. 2006 Dec;13[12]:1702-10).
AGO DESKTOP II was a prospective, multicenter trial testing the score in patients with platinum-free intervals of more than 6 months (Int J Gynecol Cancer. 2011 Feb;21[2]:289-95). In this trial, 51% of patients had a positive AGO score, and the score was shown to predict, with 95% probability, complete resectability in two-thirds of these patients, Dr. Du Bois said.
In AGO DESKTOP III, 407 patients were prospectively randomized to second-line chemotherapy alone (n = 201) or to cytoreductive surgery (n = 206) followed by the same chemotherapy, with platinum-containing regimens highly recommended.
Patient characteristics were well balanced between the arms. Nearly all patients (99%) in each arm had prior platinum exposure, and 75% had a platinum-free interval of more than 12 months (a median of 21.1 months in the surgery arm versus 18.7 months in the no-surgery arm).
Complete resections extend OS
There were 8 patients (4%) in the chemotherapy-only arm and 14 (6.8%) in the surgery arm who were noncompliant with randomization. The complete resection rate was 74.2%.
Following randomization, 88.8% of patients in the surgery arm and 90% in the no-surgery arm received platinum-containing chemotherapy; 22.8% and 23.4%, respectively, received bevacizumab; and 3.9% and 6.0% received a poly (ADP-ribose) polymerase inhibitor.
The median overall survival in the intention-to-treat population was 53.7 months in the surgery arm and 46 months in the no-surgery arm, an absolute difference of 7.7 months (hazard ratio, 0.75; P = .02).
The median progression-free survival, assessed in the intention-to-treat population after database closure in January 2020, was 18.4 months with surgery and 14 months without (HR, 0.66; P < .001).
A post hoc analysis showed the importance of complete versus partial resection. The median overall survival was 61.9 months in patients with complete resections and 28.8 months for patients with residual tumor after cytoreductive surgery, an absolute difference of nearly 3 years (HR, 0.40; P < .001).
Comparing only those patients with complete resections with patients who did not undergo surgery, the respective median overall survival was 61.9 months and 46 months (HR, 0.57; P < .001).
AGO DESKTOP III was sponsored by the AGO study group in collaboration with other oncology groups, Medac, and GlaxoSmithKline. Dr. du Bois and Dr. Coleman disclosed relationships with many companies.
SOURCE: du Bois A et al. ASCO 2020, Abstract 6000.
The trial, AGO DESKTOP III/ENGOT ov20, is the first prospective, randomized study showing an overall survival benefit for debulking surgery in patients with recurrent ovarian cancer.
Among 406 patients in first relapse, the median overall survival was 53.7 months for those randomized to cytoreductive surgery plus chemotherapy and 46 months for patients randomized to chemotherapy alone (P = .02).
“The overall survival benefit was highest and exclusively seen in the cohort with complete resection, indicating the importance of a thorough selection process of both the right patient and the right center,” said investigator Andreas du Bois, MD, of the Kliniken Essen-Mitte (Germany).
The median survival gain for patients with platinum-free intervals of more than 6 months who undergo complete resection is nearly 16 months “and is worth going for,” he added.
Dr. du Bois presented these results as part of the American Society of Clinical Oncology virtual scientific program (Abstract 6000).
In another trial, SOC-1, that was also presented in the virtual program, investigators reported a progression-free survival advantage of 5.5 months for patients with recurrent ovarian cancer who underwent debulking surgery, compared with those who did not (Abstract 6001).
Different trials, different results
The invited discussant for Dr. du Bois’s presentation was Robert L. Coleman, MD, chief scientific officer of the U.S. Oncology Network in The Woodlands, Tex., who was the principal investigator of the GOG-0213 trial (N Engl J Med. 2019;381:1929-39).
That trial did not show an overall survival advantage to secondary surgical cytoreduction followed by chemotherapy, compared with chemotherapy alone, among 485 women with platinum-sensitive recurrent ovarian cancer.
Referring to both AGO DESKTOP III and SOC-1, Dr. Coleman noted that, “while only DESKTOP III met its primary endpoint of improving overall survival, both demonstrated a benefit on PFS [progression-free survival].” Both trials also support a triage algorithm for selecting the approximately 75% of patients who are likely to benefit from secondary cytoreductive surgery.
“However, the price paid for being wrong is substantial, with no benefit seen in progression-free survival and possibly a detriment in overall survival. Because of these observations, both [presenters of SOC-1 and AGO DESKTOP III data] recommended that procedures be limited to select women having surgery performed at sites of excellence,” Dr. Coleman said.
Potential explanations for the differential findings of a secondary surgery benefit in DESKTOP III and SOC-1 versus GOG-0213 include the use of a selection algorithm in the former versus investigator selection based on clinical parameters and imaging in the latter.
In addition, “while platinum-based therapy was the rule in all trials, the use of concomitant and maintenance bevacizumab, a regimen found to improve overall survival in GOG-0213, was used in substantially higher numbers of patients in that trial relative to the two current trials,” Dr. Coleman said.
The GOG-0213 trial also demonstrated an advantage for adjuvant therapy with platinum-based chemotherapy and bevacizumab, which was given to 84% of patients in GOG-0213. That trial had a median overall survival for patients who did not undergo surgery of 65.7 months, compared with 46 months in AGO DESKTOP III and 53.9 months in SOC-1, Dr. Coleman said.
Third time’s a charm
As its name implies, the AGO DESKTOP III trial is the third in a series. AGO DESKTOP I developed the hypothesis that a positive AGO score – consisting of an Eastern Cooperative Oncology Group performance status score of 0, complete resection during first-line therapy, and ascites less than 500 mL – could be predictive of favorable outcomes with debulking surgery (Ann Surg Oncol. 2006 Dec;13[12]:1702-10).
AGO DESKTOP II was a prospective, multicenter trial testing the score in patients with platinum-free intervals of more than 6 months (Int J Gynecol Cancer. 2011 Feb;21[2]:289-95). In this trial, 51% of patients had a positive AGO score, and the score was shown to predict, with 95% probability, complete resectability in two-thirds of these patients, Dr. Du Bois said.
In AGO DESKTOP III, 407 patients were prospectively randomized to second-line chemotherapy alone (n = 201) or to cytoreductive surgery (n = 206) followed by the same chemotherapy, with platinum-containing regimens highly recommended.
Patient characteristics were well balanced between the arms. Nearly all patients (99%) in each arm had prior platinum exposure, and 75% had a platinum-free interval of more than 12 months (a median of 21.1 months in the surgery arm versus 18.7 months in the no-surgery arm).
Complete resections extend OS
There were 8 patients (4%) in the chemotherapy-only arm and 14 (6.8%) in the surgery arm who were noncompliant with randomization. The complete resection rate was 74.2%.
Following randomization, 88.8% of patients in the surgery arm and 90% in the no-surgery arm received platinum-containing chemotherapy; 22.8% and 23.4%, respectively, received bevacizumab; and 3.9% and 6.0% received a poly (ADP-ribose) polymerase inhibitor.
The median overall survival in the intention-to-treat population was 53.7 months in the surgery arm and 46 months in the no-surgery arm, an absolute difference of 7.7 months (hazard ratio, 0.75; P = .02).
The median progression-free survival, assessed in the intention-to-treat population after database closure in January 2020, was 18.4 months with surgery and 14 months without (HR, 0.66; P < .001).
A post hoc analysis showed the importance of complete versus partial resection. The median overall survival was 61.9 months in patients with complete resections and 28.8 months for patients with residual tumor after cytoreductive surgery, an absolute difference of nearly 3 years (HR, 0.40; P < .001).
Comparing only those patients with complete resections with patients who did not undergo surgery, the respective median overall survival was 61.9 months and 46 months (HR, 0.57; P < .001).
AGO DESKTOP III was sponsored by the AGO study group in collaboration with other oncology groups, Medac, and GlaxoSmithKline. Dr. du Bois and Dr. Coleman disclosed relationships with many companies.
SOURCE: du Bois A et al. ASCO 2020, Abstract 6000.
FROM ASCO 2020
Evaluating complications of midline catheters
Background: Midline catheters have gained popularity in inpatient medical settings as a convenient alternative to PICC lines. This is primarily because of the ability to avoid central line–associated bloodstream infections (CLABSI) since these catheters terminate in the peripheral veins and cannot be reported as such. Additionally, they are potentially able to dwell longer than are traditional peripheral intravenous catheters. However, insufficient data exist to accurately describe the rate of complications in these catheters, as prior studies are based on single-center experiences.
Study design: Multicenter prospective cohort study.
Setting: Hospital medicine ward or medical ICU.
Synopsis: With use of a large database of adult patients from a quality initiative supported by Blue Cross Blue Shield of Michigan and Blue Care Network, this study identified 1,161 patients who had midline catheters placed and showed a 10.3% complication rate, of which 66.7% were minor (dislodgment, leaking, infiltration, or superficial thrombophlebitis) rather than major complications (occlusion, symptomatic upper-extremity deep venous thrombosis, or bloodstream infection). However, a similar rate of removal of the catheters was reported for major and minor complications (53.8% vs. 52.5%; P = .90). Across sites, there was substantial variation in utilization rates (0.97%-12.92%; P less than .001), dwell time and indication for use, and complication rates (3.4%-16.7%; P = .07).
The article does not provide guidance on when and how midline catheters should be used in order to minimize risk; nor does it include a comparison with traditional peripheral intravenous catheters or with PICC lines. Further studies are needed to guide indications and practices for catheter placement in order to minimize risk. Providers should continue to carefully consider the risks and benefits of midline catheter placement in individual cases.
Bottom line: Midline catheter placement more commonly leads to minor rather than major complications, though patterns of use and outcomes vary substantially across sites.
Citation: Chopra V et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019 Mar 18. doi: 10.1136/bmjqs-2018-008554.
Dr. Marcantonio is a Med-Peds hospitalist at Duke University Health System.
Background: Midline catheters have gained popularity in inpatient medical settings as a convenient alternative to PICC lines. This is primarily because of the ability to avoid central line–associated bloodstream infections (CLABSI) since these catheters terminate in the peripheral veins and cannot be reported as such. Additionally, they are potentially able to dwell longer than are traditional peripheral intravenous catheters. However, insufficient data exist to accurately describe the rate of complications in these catheters, as prior studies are based on single-center experiences.
Study design: Multicenter prospective cohort study.
Setting: Hospital medicine ward or medical ICU.
Synopsis: With use of a large database of adult patients from a quality initiative supported by Blue Cross Blue Shield of Michigan and Blue Care Network, this study identified 1,161 patients who had midline catheters placed and showed a 10.3% complication rate, of which 66.7% were minor (dislodgment, leaking, infiltration, or superficial thrombophlebitis) rather than major complications (occlusion, symptomatic upper-extremity deep venous thrombosis, or bloodstream infection). However, a similar rate of removal of the catheters was reported for major and minor complications (53.8% vs. 52.5%; P = .90). Across sites, there was substantial variation in utilization rates (0.97%-12.92%; P less than .001), dwell time and indication for use, and complication rates (3.4%-16.7%; P = .07).
The article does not provide guidance on when and how midline catheters should be used in order to minimize risk; nor does it include a comparison with traditional peripheral intravenous catheters or with PICC lines. Further studies are needed to guide indications and practices for catheter placement in order to minimize risk. Providers should continue to carefully consider the risks and benefits of midline catheter placement in individual cases.
Bottom line: Midline catheter placement more commonly leads to minor rather than major complications, though patterns of use and outcomes vary substantially across sites.
Citation: Chopra V et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019 Mar 18. doi: 10.1136/bmjqs-2018-008554.
Dr. Marcantonio is a Med-Peds hospitalist at Duke University Health System.
Background: Midline catheters have gained popularity in inpatient medical settings as a convenient alternative to PICC lines. This is primarily because of the ability to avoid central line–associated bloodstream infections (CLABSI) since these catheters terminate in the peripheral veins and cannot be reported as such. Additionally, they are potentially able to dwell longer than are traditional peripheral intravenous catheters. However, insufficient data exist to accurately describe the rate of complications in these catheters, as prior studies are based on single-center experiences.
Study design: Multicenter prospective cohort study.
Setting: Hospital medicine ward or medical ICU.
Synopsis: With use of a large database of adult patients from a quality initiative supported by Blue Cross Blue Shield of Michigan and Blue Care Network, this study identified 1,161 patients who had midline catheters placed and showed a 10.3% complication rate, of which 66.7% were minor (dislodgment, leaking, infiltration, or superficial thrombophlebitis) rather than major complications (occlusion, symptomatic upper-extremity deep venous thrombosis, or bloodstream infection). However, a similar rate of removal of the catheters was reported for major and minor complications (53.8% vs. 52.5%; P = .90). Across sites, there was substantial variation in utilization rates (0.97%-12.92%; P less than .001), dwell time and indication for use, and complication rates (3.4%-16.7%; P = .07).
The article does not provide guidance on when and how midline catheters should be used in order to minimize risk; nor does it include a comparison with traditional peripheral intravenous catheters or with PICC lines. Further studies are needed to guide indications and practices for catheter placement in order to minimize risk. Providers should continue to carefully consider the risks and benefits of midline catheter placement in individual cases.
Bottom line: Midline catheter placement more commonly leads to minor rather than major complications, though patterns of use and outcomes vary substantially across sites.
Citation: Chopra V et al. Variation in use and outcomes related to midline catheters: results from a multicentre pilot study. BMJ Qual Saf. 2019 Mar 18. doi: 10.1136/bmjqs-2018-008554.
Dr. Marcantonio is a Med-Peds hospitalist at Duke University Health System.
Updated BEACON: Doublet as good as triplet in metastatic CRC
The BEACON study tested both a doublet and a triplet of drugs for patients with previously treated BRAF V600E–mutated metastatic colorectal cancer (CRC).
New results from that trial, with an updated survival analysis, show that the doublet is sufficient and that patients can safely be treated with a combination of the BRAF inhibitor encorafenib (Braftovi, Array BioPharma) and the EGFR inhibitor monoclonal antibody cetuximab (Erbitux, Eli Lilly).
This means that the increased toxicity from the addition of the MEK inhibitor binimetinib (Mektovi, Array BioPharma) can be avoided and that good outcomes can be maintained.
The new results were presented as part of the virtual scientific program of the 2020 American Society of Clinical Oncology annual meeting.
Study discussant Michael S. Lee, MD, from the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said he “would consider encorafenib and cetuximab now the standard of care in the second line and beyond in BRAF-mutant colorectal cancer.
“I would give the doublet rather than the triplet because of the comparable survival and better side effect profile,” he said.
“This is immediately practice changing, given the FDA approval of encorafenib and cetuximab,” commented Autumn J. McRee, MD, UNC Lineberger Comprehensive Cancer Center.
This is a “clinically relevant” trial, and it demonstrates that the “poorest prognostic group in colorectal cancer now has viable options,” she said at a Highlights of the Day session.
“We will await the results of the ANCHOR-CRC trial to see if we should be using this targeted approach in earlier lines of therapy,” she added.
New survival data
The primary analysis of BEACON CRC, presented at the 2019 World Conference on Gastrointestinal Cancer, showed that use of the triplet therapy was associated with a 48% improvement in overall survival in comparison with standard chemotherapy.
Although that was also linked to improved quality of life, questions have been raised as to whether the benefits are worth the cost, and one commentator singled the study out for harsh criticism.
Scott Kopetz, MD, PhD, from the University of Texas MD Anderson Cancer Center, Houston, presented the updated survival results from BEACON CRC, which included data from an additional 6 months of follow-up.
“We know that the V600E mutation in BRAF occurs in about 10%-15% of patients and is associated with a poor prognosis,” Dr. Kopetz commented.
“BRAF inhibitors alone are not effective,” he noted. He said preclinical models point toward an approach that targets multiple pathways, including BRAF, MEK, and EGFR.
The BEACON study had three arms, each with 205 patients, as follows:
- Encorafenib plus binimetinib plus cetuximab
- Encorafenib plus cetixumab
- A control arm of FOLFIRI plus cetuximab or irinotecan plus cetuximab.
The three arms of the trial were relatively well balanced with respect to patient characteristics, Dr. Kopetz commented. The median age of the patients ranged from 60 to 62 years, and 48%-57% of the patients were women.
Of note, between 5% and 10% of the cases were characterized by high microsatellite instability, and 34%-35% of patients had received more than one prior line of therapy.
The data now presented at ASCO represent a post hoc updated analysis that includes 6 months of additional follow-up since the primary analysis was presented, Dr. Kopetz explained.
After a median follow-up of 12.8 months, 13% of triplet therapy patients, 14% of those who received doublet therapy, and 3% of control patients were still on treatment.
The updated analysis shows that median overall survival with the doublet therapy was 9.3 months versus 5.9 months for the control group (hazard ratio, 0.61). The benefit was seen across all subgroups, including those based on the number of prior regimens.
Dr. Kopetz pointed out that overall survival was numerically superior to that seen in the primary analysis, which was 8.4 months.
The updated survival analysis for the triplet therapy indicated that the median overall survival was 9.3 months versus 5.9 months in the control arm (HR, 0.60). Again, this was consistent across subgroups.
In the primary analysis, the median overall survival with the triplet therapy was 9.0 months.
The team also compared the triplet and doublet therapies, finding that, “in contrast to what was seen in the primary analysis ... we see numerically identical median overall survival with the two arms.” The result was sustained across subgroups.
However, there were indications that there may be survival benefits with the triplet in certain patient subgroups, including those with Eastern Cooperative Oncology Group performance status 1, those in whom three or more organs are involved, those with higher baseline levels of C-reactive protein, and those with partially resected or unresected disease.
Progression-free survival was comparable between the two treatment groups, at 4.5 months with the triplet therapy and 4.3 months with the doublet, compared with just 1.5 months in the control arm.
The updated analysis also showed that the objective response rate was 27% with triplet therapy versus 20% with the doublet and 2% in the control arm. In both the doublet and triplet arms, the majority of responses were partial.
With regard to safety, Dr. Kopetz said that the updated data regarding adverse events were in general “consistent with the previously reported safety profile.”
He added it is “notable” that the triplet therapy “had slightly higher rates of GI toxicity and anemia,” which was not seen with the doublet, something that is “again associated with the known safety profile of a MEK inhibitor.”
The study was funded by Pfizer. Dr. Kopetz has stock and other ownership interests with numerous pharmaceutical companies. Other authors, as well as Dr. Lee and Dr. McRee, have also reported relevant financial relationships.
This article first appeared on Medscape.com.
The BEACON study tested both a doublet and a triplet of drugs for patients with previously treated BRAF V600E–mutated metastatic colorectal cancer (CRC).
New results from that trial, with an updated survival analysis, show that the doublet is sufficient and that patients can safely be treated with a combination of the BRAF inhibitor encorafenib (Braftovi, Array BioPharma) and the EGFR inhibitor monoclonal antibody cetuximab (Erbitux, Eli Lilly).
This means that the increased toxicity from the addition of the MEK inhibitor binimetinib (Mektovi, Array BioPharma) can be avoided and that good outcomes can be maintained.
The new results were presented as part of the virtual scientific program of the 2020 American Society of Clinical Oncology annual meeting.
Study discussant Michael S. Lee, MD, from the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said he “would consider encorafenib and cetuximab now the standard of care in the second line and beyond in BRAF-mutant colorectal cancer.
“I would give the doublet rather than the triplet because of the comparable survival and better side effect profile,” he said.
“This is immediately practice changing, given the FDA approval of encorafenib and cetuximab,” commented Autumn J. McRee, MD, UNC Lineberger Comprehensive Cancer Center.
This is a “clinically relevant” trial, and it demonstrates that the “poorest prognostic group in colorectal cancer now has viable options,” she said at a Highlights of the Day session.
“We will await the results of the ANCHOR-CRC trial to see if we should be using this targeted approach in earlier lines of therapy,” she added.
New survival data
The primary analysis of BEACON CRC, presented at the 2019 World Conference on Gastrointestinal Cancer, showed that use of the triplet therapy was associated with a 48% improvement in overall survival in comparison with standard chemotherapy.
Although that was also linked to improved quality of life, questions have been raised as to whether the benefits are worth the cost, and one commentator singled the study out for harsh criticism.
Scott Kopetz, MD, PhD, from the University of Texas MD Anderson Cancer Center, Houston, presented the updated survival results from BEACON CRC, which included data from an additional 6 months of follow-up.
“We know that the V600E mutation in BRAF occurs in about 10%-15% of patients and is associated with a poor prognosis,” Dr. Kopetz commented.
“BRAF inhibitors alone are not effective,” he noted. He said preclinical models point toward an approach that targets multiple pathways, including BRAF, MEK, and EGFR.
The BEACON study had three arms, each with 205 patients, as follows:
- Encorafenib plus binimetinib plus cetuximab
- Encorafenib plus cetixumab
- A control arm of FOLFIRI plus cetuximab or irinotecan plus cetuximab.
The three arms of the trial were relatively well balanced with respect to patient characteristics, Dr. Kopetz commented. The median age of the patients ranged from 60 to 62 years, and 48%-57% of the patients were women.
Of note, between 5% and 10% of the cases were characterized by high microsatellite instability, and 34%-35% of patients had received more than one prior line of therapy.
The data now presented at ASCO represent a post hoc updated analysis that includes 6 months of additional follow-up since the primary analysis was presented, Dr. Kopetz explained.
After a median follow-up of 12.8 months, 13% of triplet therapy patients, 14% of those who received doublet therapy, and 3% of control patients were still on treatment.
The updated analysis shows that median overall survival with the doublet therapy was 9.3 months versus 5.9 months for the control group (hazard ratio, 0.61). The benefit was seen across all subgroups, including those based on the number of prior regimens.
Dr. Kopetz pointed out that overall survival was numerically superior to that seen in the primary analysis, which was 8.4 months.
The updated survival analysis for the triplet therapy indicated that the median overall survival was 9.3 months versus 5.9 months in the control arm (HR, 0.60). Again, this was consistent across subgroups.
In the primary analysis, the median overall survival with the triplet therapy was 9.0 months.
The team also compared the triplet and doublet therapies, finding that, “in contrast to what was seen in the primary analysis ... we see numerically identical median overall survival with the two arms.” The result was sustained across subgroups.
However, there were indications that there may be survival benefits with the triplet in certain patient subgroups, including those with Eastern Cooperative Oncology Group performance status 1, those in whom three or more organs are involved, those with higher baseline levels of C-reactive protein, and those with partially resected or unresected disease.
Progression-free survival was comparable between the two treatment groups, at 4.5 months with the triplet therapy and 4.3 months with the doublet, compared with just 1.5 months in the control arm.
The updated analysis also showed that the objective response rate was 27% with triplet therapy versus 20% with the doublet and 2% in the control arm. In both the doublet and triplet arms, the majority of responses were partial.
With regard to safety, Dr. Kopetz said that the updated data regarding adverse events were in general “consistent with the previously reported safety profile.”
He added it is “notable” that the triplet therapy “had slightly higher rates of GI toxicity and anemia,” which was not seen with the doublet, something that is “again associated with the known safety profile of a MEK inhibitor.”
The study was funded by Pfizer. Dr. Kopetz has stock and other ownership interests with numerous pharmaceutical companies. Other authors, as well as Dr. Lee and Dr. McRee, have also reported relevant financial relationships.
This article first appeared on Medscape.com.
The BEACON study tested both a doublet and a triplet of drugs for patients with previously treated BRAF V600E–mutated metastatic colorectal cancer (CRC).
New results from that trial, with an updated survival analysis, show that the doublet is sufficient and that patients can safely be treated with a combination of the BRAF inhibitor encorafenib (Braftovi, Array BioPharma) and the EGFR inhibitor monoclonal antibody cetuximab (Erbitux, Eli Lilly).
This means that the increased toxicity from the addition of the MEK inhibitor binimetinib (Mektovi, Array BioPharma) can be avoided and that good outcomes can be maintained.
The new results were presented as part of the virtual scientific program of the 2020 American Society of Clinical Oncology annual meeting.
Study discussant Michael S. Lee, MD, from the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, said he “would consider encorafenib and cetuximab now the standard of care in the second line and beyond in BRAF-mutant colorectal cancer.
“I would give the doublet rather than the triplet because of the comparable survival and better side effect profile,” he said.
“This is immediately practice changing, given the FDA approval of encorafenib and cetuximab,” commented Autumn J. McRee, MD, UNC Lineberger Comprehensive Cancer Center.
This is a “clinically relevant” trial, and it demonstrates that the “poorest prognostic group in colorectal cancer now has viable options,” she said at a Highlights of the Day session.
“We will await the results of the ANCHOR-CRC trial to see if we should be using this targeted approach in earlier lines of therapy,” she added.
New survival data
The primary analysis of BEACON CRC, presented at the 2019 World Conference on Gastrointestinal Cancer, showed that use of the triplet therapy was associated with a 48% improvement in overall survival in comparison with standard chemotherapy.
Although that was also linked to improved quality of life, questions have been raised as to whether the benefits are worth the cost, and one commentator singled the study out for harsh criticism.
Scott Kopetz, MD, PhD, from the University of Texas MD Anderson Cancer Center, Houston, presented the updated survival results from BEACON CRC, which included data from an additional 6 months of follow-up.
“We know that the V600E mutation in BRAF occurs in about 10%-15% of patients and is associated with a poor prognosis,” Dr. Kopetz commented.
“BRAF inhibitors alone are not effective,” he noted. He said preclinical models point toward an approach that targets multiple pathways, including BRAF, MEK, and EGFR.
The BEACON study had three arms, each with 205 patients, as follows:
- Encorafenib plus binimetinib plus cetuximab
- Encorafenib plus cetixumab
- A control arm of FOLFIRI plus cetuximab or irinotecan plus cetuximab.
The three arms of the trial were relatively well balanced with respect to patient characteristics, Dr. Kopetz commented. The median age of the patients ranged from 60 to 62 years, and 48%-57% of the patients were women.
Of note, between 5% and 10% of the cases were characterized by high microsatellite instability, and 34%-35% of patients had received more than one prior line of therapy.
The data now presented at ASCO represent a post hoc updated analysis that includes 6 months of additional follow-up since the primary analysis was presented, Dr. Kopetz explained.
After a median follow-up of 12.8 months, 13% of triplet therapy patients, 14% of those who received doublet therapy, and 3% of control patients were still on treatment.
The updated analysis shows that median overall survival with the doublet therapy was 9.3 months versus 5.9 months for the control group (hazard ratio, 0.61). The benefit was seen across all subgroups, including those based on the number of prior regimens.
Dr. Kopetz pointed out that overall survival was numerically superior to that seen in the primary analysis, which was 8.4 months.
The updated survival analysis for the triplet therapy indicated that the median overall survival was 9.3 months versus 5.9 months in the control arm (HR, 0.60). Again, this was consistent across subgroups.
In the primary analysis, the median overall survival with the triplet therapy was 9.0 months.
The team also compared the triplet and doublet therapies, finding that, “in contrast to what was seen in the primary analysis ... we see numerically identical median overall survival with the two arms.” The result was sustained across subgroups.
However, there were indications that there may be survival benefits with the triplet in certain patient subgroups, including those with Eastern Cooperative Oncology Group performance status 1, those in whom three or more organs are involved, those with higher baseline levels of C-reactive protein, and those with partially resected or unresected disease.
Progression-free survival was comparable between the two treatment groups, at 4.5 months with the triplet therapy and 4.3 months with the doublet, compared with just 1.5 months in the control arm.
The updated analysis also showed that the objective response rate was 27% with triplet therapy versus 20% with the doublet and 2% in the control arm. In both the doublet and triplet arms, the majority of responses were partial.
With regard to safety, Dr. Kopetz said that the updated data regarding adverse events were in general “consistent with the previously reported safety profile.”
He added it is “notable” that the triplet therapy “had slightly higher rates of GI toxicity and anemia,” which was not seen with the doublet, something that is “again associated with the known safety profile of a MEK inhibitor.”
The study was funded by Pfizer. Dr. Kopetz has stock and other ownership interests with numerous pharmaceutical companies. Other authors, as well as Dr. Lee and Dr. McRee, have also reported relevant financial relationships.
This article first appeared on Medscape.com.
FROM ASCO 2020
Germline testing in advanced cancer can lead to targeted treatment
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ASCO 2020