User login
How Accurately Do Current Criteria Identify CBD and PSP?
LOS ANGELES—Current criteria are comparatively insensitive and nonspecific for distinguishing between corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), according to research presented at the 70th Annual Meeting of the American Academy of Neurology. Including adjunctive biomarkers with the criteria might improve their sensitivity.
It is reasonable to ask whether CBD and PSP should be considered one entity instead of two, said Jessica Weinstein, MD, clinical fellow at the University of California, San Francisco, School of Medicine. Such an approach could enrich clinical trials, given that the diseases are uncommon, she added.
Comparing Pathologic and Clinical Diagnoses
Although CBD and PSP have been considered distinct disorders, each is heterogeneous. The two disorders share symptoms such as limb rigidity, akinesia, postural instability, and behavioral changes. They also share symptoms with other clinical syndromes such as behavioral variant frontotemporal dementia. Furthermore, the Armstrong criteria for CBD include criteria for PSP, and the Höglinger criteria for PSP include criteria for CBD.
Dr. Weinstein and colleagues examined data for patients with autopsy-confirmed four-repeat tauopathies to evaluate the sensitivity and specificity of the Armstrong and Höglinger criteria for diagnosing CBD and PSP, respectively. Information was extracted from the Penn Integrated Neurodegenerative Disease Database. Neuropathologic diagnosis for participants in this database was determined using established criteria.
A researcher blinded to pathologic diagnosis coded each patient for the presence or absence of clinical features using data from his or her first clinical visit. The researcher assessed subjects for 34 features associated with CBD, PSP, behavioral-variant frontotemporal dementia, or primary progressive aphasia. Patients with absent or insufficient data were excluded.
Criteria May Need Refinement
The population included 107 autopsy subjects, 37 of whom had a pathologic diagnosis of CBD and 70 of whom had a pathologic diagnosis of PSP. The investigators found no significant differences between the groups in age at death, age at onset, or disease duration. The percentage of females was higher in the CBD group. The percentage of patients evaluated in movement clinics, rather than cognitive clinics, was 8% for patients with CBD and 40% for patients with PSP.
Almost all clinical features were more prevalent in the PSP group than the CBD group, except limb dystonia, myoclonus, and alien limb syndrome. Language impairment (ie, speech apraxia; agrammatism; and impaired naming, single-word comprehension, and grammatical comprehension) was more prevalent in the CBD group, but this difference was not statistically significant. The PSP group had a significantly higher prevalence of falls, being chair bound, postural instability, and vertical saccades. The PSP group had more bradyphrenia than the CBD group, and the CBD group had more executive impairment than the PSP group.
The Armstrong criteria identified probable CBD with a sensitivity of 11% and a specificity of 100%. The specificity result “should be taken with a grain of salt because only four patients met criteria,” said Dr. Weinstein. Armstrong criteria identified possible CBD with 35% sensitivity and 34% specificity.
The Höglinger criteria identified probable PSP with 66% sensitivity and 70% specificity. They identified possible PSP with 63% sensitivity and 65% specificity. A post hoc analysis suggested that including grammar comprehension in the Höglinger criteria improved their sensitivity.
The study’s limitations include its retrospective design, potential for selection bias, and the uncertain generalizability of its results. Only one researcher coded patients’ clinical features, and many data were missing. Nevertheless, “it is studies like these that use the gold standard autopsy data and look backward that provide the heart of clinical diagnostic criteria,” said Dr. Weinstein.
Longitudinal, prospective studies could validate these results and potentially improve the Armstrong and Höglinger criteria.
—Erik Greb
Suggested Reading
Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503.
Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853-864.
LOS ANGELES—Current criteria are comparatively insensitive and nonspecific for distinguishing between corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), according to research presented at the 70th Annual Meeting of the American Academy of Neurology. Including adjunctive biomarkers with the criteria might improve their sensitivity.
It is reasonable to ask whether CBD and PSP should be considered one entity instead of two, said Jessica Weinstein, MD, clinical fellow at the University of California, San Francisco, School of Medicine. Such an approach could enrich clinical trials, given that the diseases are uncommon, she added.
Comparing Pathologic and Clinical Diagnoses
Although CBD and PSP have been considered distinct disorders, each is heterogeneous. The two disorders share symptoms such as limb rigidity, akinesia, postural instability, and behavioral changes. They also share symptoms with other clinical syndromes such as behavioral variant frontotemporal dementia. Furthermore, the Armstrong criteria for CBD include criteria for PSP, and the Höglinger criteria for PSP include criteria for CBD.
Dr. Weinstein and colleagues examined data for patients with autopsy-confirmed four-repeat tauopathies to evaluate the sensitivity and specificity of the Armstrong and Höglinger criteria for diagnosing CBD and PSP, respectively. Information was extracted from the Penn Integrated Neurodegenerative Disease Database. Neuropathologic diagnosis for participants in this database was determined using established criteria.
A researcher blinded to pathologic diagnosis coded each patient for the presence or absence of clinical features using data from his or her first clinical visit. The researcher assessed subjects for 34 features associated with CBD, PSP, behavioral-variant frontotemporal dementia, or primary progressive aphasia. Patients with absent or insufficient data were excluded.
Criteria May Need Refinement
The population included 107 autopsy subjects, 37 of whom had a pathologic diagnosis of CBD and 70 of whom had a pathologic diagnosis of PSP. The investigators found no significant differences between the groups in age at death, age at onset, or disease duration. The percentage of females was higher in the CBD group. The percentage of patients evaluated in movement clinics, rather than cognitive clinics, was 8% for patients with CBD and 40% for patients with PSP.
Almost all clinical features were more prevalent in the PSP group than the CBD group, except limb dystonia, myoclonus, and alien limb syndrome. Language impairment (ie, speech apraxia; agrammatism; and impaired naming, single-word comprehension, and grammatical comprehension) was more prevalent in the CBD group, but this difference was not statistically significant. The PSP group had a significantly higher prevalence of falls, being chair bound, postural instability, and vertical saccades. The PSP group had more bradyphrenia than the CBD group, and the CBD group had more executive impairment than the PSP group.
The Armstrong criteria identified probable CBD with a sensitivity of 11% and a specificity of 100%. The specificity result “should be taken with a grain of salt because only four patients met criteria,” said Dr. Weinstein. Armstrong criteria identified possible CBD with 35% sensitivity and 34% specificity.
The Höglinger criteria identified probable PSP with 66% sensitivity and 70% specificity. They identified possible PSP with 63% sensitivity and 65% specificity. A post hoc analysis suggested that including grammar comprehension in the Höglinger criteria improved their sensitivity.
The study’s limitations include its retrospective design, potential for selection bias, and the uncertain generalizability of its results. Only one researcher coded patients’ clinical features, and many data were missing. Nevertheless, “it is studies like these that use the gold standard autopsy data and look backward that provide the heart of clinical diagnostic criteria,” said Dr. Weinstein.
Longitudinal, prospective studies could validate these results and potentially improve the Armstrong and Höglinger criteria.
—Erik Greb
Suggested Reading
Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503.
Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853-864.
LOS ANGELES—Current criteria are comparatively insensitive and nonspecific for distinguishing between corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), according to research presented at the 70th Annual Meeting of the American Academy of Neurology. Including adjunctive biomarkers with the criteria might improve their sensitivity.
It is reasonable to ask whether CBD and PSP should be considered one entity instead of two, said Jessica Weinstein, MD, clinical fellow at the University of California, San Francisco, School of Medicine. Such an approach could enrich clinical trials, given that the diseases are uncommon, she added.
Comparing Pathologic and Clinical Diagnoses
Although CBD and PSP have been considered distinct disorders, each is heterogeneous. The two disorders share symptoms such as limb rigidity, akinesia, postural instability, and behavioral changes. They also share symptoms with other clinical syndromes such as behavioral variant frontotemporal dementia. Furthermore, the Armstrong criteria for CBD include criteria for PSP, and the Höglinger criteria for PSP include criteria for CBD.
Dr. Weinstein and colleagues examined data for patients with autopsy-confirmed four-repeat tauopathies to evaluate the sensitivity and specificity of the Armstrong and Höglinger criteria for diagnosing CBD and PSP, respectively. Information was extracted from the Penn Integrated Neurodegenerative Disease Database. Neuropathologic diagnosis for participants in this database was determined using established criteria.
A researcher blinded to pathologic diagnosis coded each patient for the presence or absence of clinical features using data from his or her first clinical visit. The researcher assessed subjects for 34 features associated with CBD, PSP, behavioral-variant frontotemporal dementia, or primary progressive aphasia. Patients with absent or insufficient data were excluded.
Criteria May Need Refinement
The population included 107 autopsy subjects, 37 of whom had a pathologic diagnosis of CBD and 70 of whom had a pathologic diagnosis of PSP. The investigators found no significant differences between the groups in age at death, age at onset, or disease duration. The percentage of females was higher in the CBD group. The percentage of patients evaluated in movement clinics, rather than cognitive clinics, was 8% for patients with CBD and 40% for patients with PSP.
Almost all clinical features were more prevalent in the PSP group than the CBD group, except limb dystonia, myoclonus, and alien limb syndrome. Language impairment (ie, speech apraxia; agrammatism; and impaired naming, single-word comprehension, and grammatical comprehension) was more prevalent in the CBD group, but this difference was not statistically significant. The PSP group had a significantly higher prevalence of falls, being chair bound, postural instability, and vertical saccades. The PSP group had more bradyphrenia than the CBD group, and the CBD group had more executive impairment than the PSP group.
The Armstrong criteria identified probable CBD with a sensitivity of 11% and a specificity of 100%. The specificity result “should be taken with a grain of salt because only four patients met criteria,” said Dr. Weinstein. Armstrong criteria identified possible CBD with 35% sensitivity and 34% specificity.
The Höglinger criteria identified probable PSP with 66% sensitivity and 70% specificity. They identified possible PSP with 63% sensitivity and 65% specificity. A post hoc analysis suggested that including grammar comprehension in the Höglinger criteria improved their sensitivity.
The study’s limitations include its retrospective design, potential for selection bias, and the uncertain generalizability of its results. Only one researcher coded patients’ clinical features, and many data were missing. Nevertheless, “it is studies like these that use the gold standard autopsy data and look backward that provide the heart of clinical diagnostic criteria,” said Dr. Weinstein.
Longitudinal, prospective studies could validate these results and potentially improve the Armstrong and Höglinger criteria.
—Erik Greb
Suggested Reading
Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503.
Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853-864.
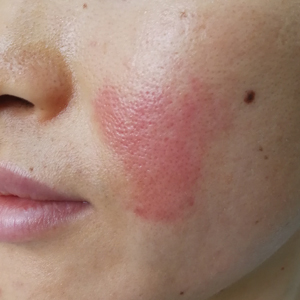
Going Digital With Dermoscopy
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
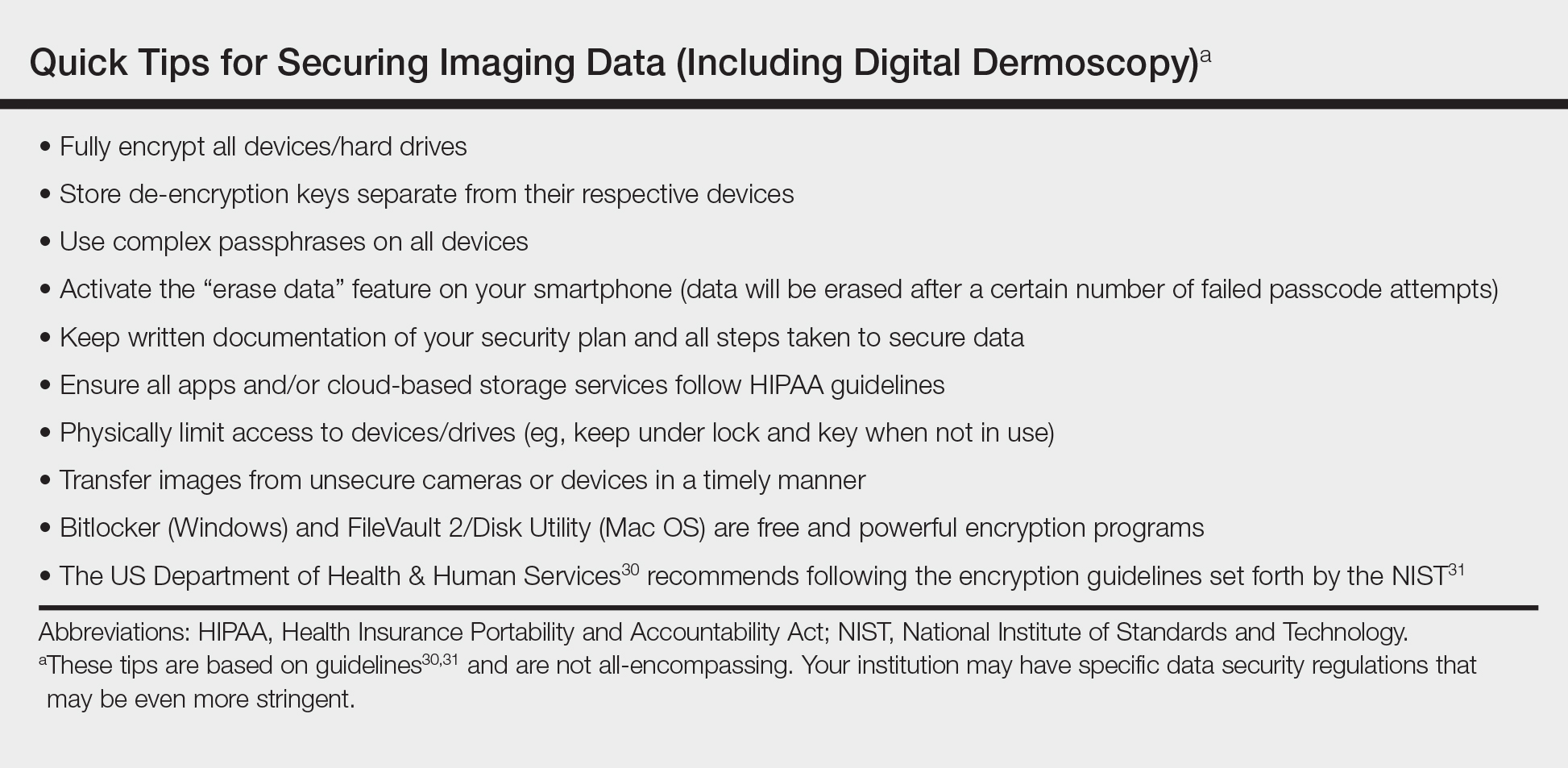
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Expanded approval for daratumumab in multiple myeloma
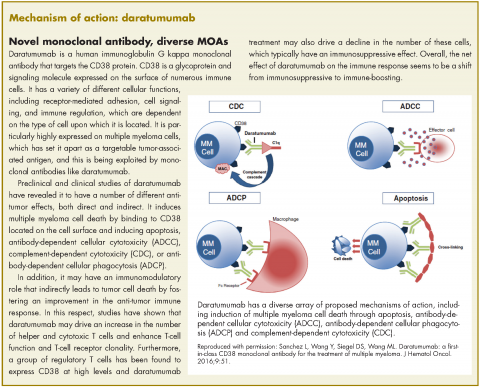
In November 2016, the US Food and Drug Administration expanded the approval of daratumumab for patients with multiple myeloma. The monoclonal antibody, which targets CD38, a protein that is highly expressed on the surface of multiple myeloma cells, was previously granted approval by the agency as a single agent for the treatment of patients who had received at least three previous therapies.
The current approval was for the use of daratumumab in two different combination regimens for the treatment of patients who have received one previous line of treatment. On the basis of improved progression-free survival (PFS), demonstrated in two randomized, open-label, phase 3 trials, daratumumab can now be used in combination with the immunomodulatory agent lenalidomide and dexamethasone, or the proteasome inhibitor bortezomib and dexamethasone, both standard therapies for the treatment of multiple myeloma.
In the POLLUX trial, 569 patients with relapsed/refractory multiple myeloma were randomized 1:1 to receive daratumumab in combination with lenalidomide-dexamethasone or lenalidomide-dexamethasone alone. The CASTOR trial randomized 498 patients with relapsed/refractory multiple myeloma 1:1 to daratumumab in combination with bortezomib-dexamethasone, or bortezomib-dexamethasone alone.
The eligibility and exclusion criteria for both trials were similar; patients had received at least one previous line of therapy, had documented progressive disease according to International Myeloma Working Group criteria, and had measurable disease on the basis of urine and/or serum assessments or serum-free, light-chain assay.
Patients with a neutrophil count of ≤1,000 cells/mm3, hemoglobin level of ≤7.5 g/dL, platelet count of <75,000 cells/mm3, creatinine clearance of ≤20 mL/min per 1.73m2 body surface area (or <30 mL/min in the POLLUX trial), alanine aminotransferase or aspartate aminotransferase level ≥2.5 times the upper limit of normal (ULN) range, bilirubin level of ≥1.5 or more times the ULN range, disease refractory to bortezomib or lenalidomide, and unacceptable side effects from bortezomib or lenalidomide, were ineligible for these studies. In addition, patients with grade 2 or higher peripheral neuropathy or neuropathic pain, were excluded from the CASTOR study.
Randomization was stratified according to International Staging System disease stage at the time of screening (stage I, II or III, with higher stage indicating more severe disease), number of previous lines of therapy (1 vs 2, or 3 vs >3), and previous receipt of lenalidomide or bortezomib.
In the CASTOR trial, patients received up to eight 21-day cycles of bortezomib, administered subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8, and dexamethasone, administered orally or intravenously at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 for a total dose of 160 mg per cycle. Daratumumab was administered at a dose of 16 mg/kg intravenously once weekly on days 1, 8, and 15 during cycles 1 to 3, once every 3 weeks on day 1 of cycles 4-8, and once every 4 weeks thereafter.
In the POLLUX trial, patients were treated in 28-day cycles. Daratumumab was administered at the same dose as in the CASTOR trial, but on days 1, 8, 15 and 22 for 8 weeks during cycles 1 and 2, every 2 weeks on days 1 and 15 for 16 weeks during cycles 3 through 7, and every 4 weeks from then onwards. Lenalidomide was administered at a dose of 25 mg orally on days 1-21 of each cycle, and dexamethasone at a dose of 20 mg before infusion and 20 mg the following day.
The combination of daratumumab with lenalidomide-dexamethasone demonstrated a substantial improvement in PFS, compared with lenalidomide-dexamethasone alone (estimated PFS not yet reached vs 18.4 months, respectively; HR, 0.37; P < .0001), representing a 63% reduction in the risk of disease progression or death. Meanwhile, there was a 61% reduction in the risk of disease progression or death for the combination of daratumumab with bortezomib-dexamethasone in the CASTOR trial (estimated PFS not yet reached vs 7.2 months; HR: 0.39; P < .0001). The PFS benefit was observed across all prespecified subgroups in both studies.
In the CASTOR trial, over a median follow-up of 7.4 months, the overall response rate (ORR) was 82.9% for the combination arm, compared with 63.2% for the bortezomib-dexamethasone arm (P < .001), with a very good partial response (VGPR) or better rate of 59.2% compared with 29.1%, and a complete response (CR) rate of 19.2% compared with 9%. In the POLLUX trial, over a median follow-up of 13.5 months, ORR was 92.9% for the combination arm, compared with 76.4% for lenalidomide-dexamethasone, with a VGPR or better rate of 75.8% versus 44% and a CR rate of 43.1% versus 19.2%.
Overall, the safety profile for both combinations was consistent with what is usually observed with daratumumab monotherapy and lenalidomide-dexamethasone or bortezomib-dexamethasone combinations. The most frequently reported adverse events (AEs) were similar in both studies and included infusion reactions, diarrhea, and upper respiratory tract infection. In the POLLUX trial they also included nausea, fatigue, pyrexia, muscle spasm, cough, and dyspnea, whereas in the CASTOR trial patients also frequently experienced peripheral edema.
The most common grade 3/4 AEs in both trials were neutropenia (51.9% vs 37% in the POLLUX trial and 12.8 vs 4.2% in the CASTOR trial), thrombocytopenia (12.7% vs 13.5% and 45.3% vs 32.9%, respectively), and anemia (12.4% vs 19.6% and 14.4% vs 16%, respectively). The percentage of patients who discontinued treatment due to AEs was similar in both groups across the two studies; in the CASTOR trial discontinuations resulted most commonly from peripheral sensory neuropathy and pneumonia, while in the POLLUX trial, from pneumonia, pulmonary embolism and deterioration in general physical health.
The recommended dose for daratumumab in both combination regimens is 16 mg/kg intravenously, calculated on actual body weight. The dosing schedules begin with weekly administration during weeks 1-8 (when used in combination with lenalidomide-dexamethasone) and weeks 1-9 (for use with the bortezomib-dexamethasone combination), decreasing to every 2 weeks between weeks 9 and 24 or 10 and 24, respectively, and progressing to every 4 weeks from week 25 onward until disease progression and unacceptable toxicity.
Daratumumab is marketed as Darzalex by Janssen Biotech Inc. Neutropenia and thrombocytopenia have been added to the list of warnings and precautions for the prescribing information for these new indications. Complete blood cell count should be monitored periodically during treatment and daratumumab administration delayed to allow recovery of neutrophils or platelets. Supportive care with growth factors or transfusion should be considered in the event of neutropenia or thrombocytopenia, respectively.
1. Darzalex (daratumumab) injection, for intravenous use. Prescribing information. Janssen Biotech Inc. https://www.darzalexhcp.com/shared/product/darzalex/darzalex-prescribing-information.pdf. Released November 2016. Accessed January 8, 2017.
2. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
3. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
In November 2016, the US Food and Drug Administration expanded the approval of daratumumab for patients with multiple myeloma. The monoclonal antibody, which targets CD38, a protein that is highly expressed on the surface of multiple myeloma cells, was previously granted approval by the agency as a single agent for the treatment of patients who had received at least three previous therapies.
The current approval was for the use of daratumumab in two different combination regimens for the treatment of patients who have received one previous line of treatment. On the basis of improved progression-free survival (PFS), demonstrated in two randomized, open-label, phase 3 trials, daratumumab can now be used in combination with the immunomodulatory agent lenalidomide and dexamethasone, or the proteasome inhibitor bortezomib and dexamethasone, both standard therapies for the treatment of multiple myeloma.
In the POLLUX trial, 569 patients with relapsed/refractory multiple myeloma were randomized 1:1 to receive daratumumab in combination with lenalidomide-dexamethasone or lenalidomide-dexamethasone alone. The CASTOR trial randomized 498 patients with relapsed/refractory multiple myeloma 1:1 to daratumumab in combination with bortezomib-dexamethasone, or bortezomib-dexamethasone alone.
The eligibility and exclusion criteria for both trials were similar; patients had received at least one previous line of therapy, had documented progressive disease according to International Myeloma Working Group criteria, and had measurable disease on the basis of urine and/or serum assessments or serum-free, light-chain assay.
Patients with a neutrophil count of ≤1,000 cells/mm3, hemoglobin level of ≤7.5 g/dL, platelet count of <75,000 cells/mm3, creatinine clearance of ≤20 mL/min per 1.73m2 body surface area (or <30 mL/min in the POLLUX trial), alanine aminotransferase or aspartate aminotransferase level ≥2.5 times the upper limit of normal (ULN) range, bilirubin level of ≥1.5 or more times the ULN range, disease refractory to bortezomib or lenalidomide, and unacceptable side effects from bortezomib or lenalidomide, were ineligible for these studies. In addition, patients with grade 2 or higher peripheral neuropathy or neuropathic pain, were excluded from the CASTOR study.
Randomization was stratified according to International Staging System disease stage at the time of screening (stage I, II or III, with higher stage indicating more severe disease), number of previous lines of therapy (1 vs 2, or 3 vs >3), and previous receipt of lenalidomide or bortezomib.
In the CASTOR trial, patients received up to eight 21-day cycles of bortezomib, administered subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8, and dexamethasone, administered orally or intravenously at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 for a total dose of 160 mg per cycle. Daratumumab was administered at a dose of 16 mg/kg intravenously once weekly on days 1, 8, and 15 during cycles 1 to 3, once every 3 weeks on day 1 of cycles 4-8, and once every 4 weeks thereafter.
In the POLLUX trial, patients were treated in 28-day cycles. Daratumumab was administered at the same dose as in the CASTOR trial, but on days 1, 8, 15 and 22 for 8 weeks during cycles 1 and 2, every 2 weeks on days 1 and 15 for 16 weeks during cycles 3 through 7, and every 4 weeks from then onwards. Lenalidomide was administered at a dose of 25 mg orally on days 1-21 of each cycle, and dexamethasone at a dose of 20 mg before infusion and 20 mg the following day.
The combination of daratumumab with lenalidomide-dexamethasone demonstrated a substantial improvement in PFS, compared with lenalidomide-dexamethasone alone (estimated PFS not yet reached vs 18.4 months, respectively; HR, 0.37; P < .0001), representing a 63% reduction in the risk of disease progression or death. Meanwhile, there was a 61% reduction in the risk of disease progression or death for the combination of daratumumab with bortezomib-dexamethasone in the CASTOR trial (estimated PFS not yet reached vs 7.2 months; HR: 0.39; P < .0001). The PFS benefit was observed across all prespecified subgroups in both studies.
In the CASTOR trial, over a median follow-up of 7.4 months, the overall response rate (ORR) was 82.9% for the combination arm, compared with 63.2% for the bortezomib-dexamethasone arm (P < .001), with a very good partial response (VGPR) or better rate of 59.2% compared with 29.1%, and a complete response (CR) rate of 19.2% compared with 9%. In the POLLUX trial, over a median follow-up of 13.5 months, ORR was 92.9% for the combination arm, compared with 76.4% for lenalidomide-dexamethasone, with a VGPR or better rate of 75.8% versus 44% and a CR rate of 43.1% versus 19.2%.
Overall, the safety profile for both combinations was consistent with what is usually observed with daratumumab monotherapy and lenalidomide-dexamethasone or bortezomib-dexamethasone combinations. The most frequently reported adverse events (AEs) were similar in both studies and included infusion reactions, diarrhea, and upper respiratory tract infection. In the POLLUX trial they also included nausea, fatigue, pyrexia, muscle spasm, cough, and dyspnea, whereas in the CASTOR trial patients also frequently experienced peripheral edema.
The most common grade 3/4 AEs in both trials were neutropenia (51.9% vs 37% in the POLLUX trial and 12.8 vs 4.2% in the CASTOR trial), thrombocytopenia (12.7% vs 13.5% and 45.3% vs 32.9%, respectively), and anemia (12.4% vs 19.6% and 14.4% vs 16%, respectively). The percentage of patients who discontinued treatment due to AEs was similar in both groups across the two studies; in the CASTOR trial discontinuations resulted most commonly from peripheral sensory neuropathy and pneumonia, while in the POLLUX trial, from pneumonia, pulmonary embolism and deterioration in general physical health.
The recommended dose for daratumumab in both combination regimens is 16 mg/kg intravenously, calculated on actual body weight. The dosing schedules begin with weekly administration during weeks 1-8 (when used in combination with lenalidomide-dexamethasone) and weeks 1-9 (for use with the bortezomib-dexamethasone combination), decreasing to every 2 weeks between weeks 9 and 24 or 10 and 24, respectively, and progressing to every 4 weeks from week 25 onward until disease progression and unacceptable toxicity.
Daratumumab is marketed as Darzalex by Janssen Biotech Inc. Neutropenia and thrombocytopenia have been added to the list of warnings and precautions for the prescribing information for these new indications. Complete blood cell count should be monitored periodically during treatment and daratumumab administration delayed to allow recovery of neutrophils or platelets. Supportive care with growth factors or transfusion should be considered in the event of neutropenia or thrombocytopenia, respectively.
In November 2016, the US Food and Drug Administration expanded the approval of daratumumab for patients with multiple myeloma. The monoclonal antibody, which targets CD38, a protein that is highly expressed on the surface of multiple myeloma cells, was previously granted approval by the agency as a single agent for the treatment of patients who had received at least three previous therapies.
The current approval was for the use of daratumumab in two different combination regimens for the treatment of patients who have received one previous line of treatment. On the basis of improved progression-free survival (PFS), demonstrated in two randomized, open-label, phase 3 trials, daratumumab can now be used in combination with the immunomodulatory agent lenalidomide and dexamethasone, or the proteasome inhibitor bortezomib and dexamethasone, both standard therapies for the treatment of multiple myeloma.
In the POLLUX trial, 569 patients with relapsed/refractory multiple myeloma were randomized 1:1 to receive daratumumab in combination with lenalidomide-dexamethasone or lenalidomide-dexamethasone alone. The CASTOR trial randomized 498 patients with relapsed/refractory multiple myeloma 1:1 to daratumumab in combination with bortezomib-dexamethasone, or bortezomib-dexamethasone alone.
The eligibility and exclusion criteria for both trials were similar; patients had received at least one previous line of therapy, had documented progressive disease according to International Myeloma Working Group criteria, and had measurable disease on the basis of urine and/or serum assessments or serum-free, light-chain assay.
Patients with a neutrophil count of ≤1,000 cells/mm3, hemoglobin level of ≤7.5 g/dL, platelet count of <75,000 cells/mm3, creatinine clearance of ≤20 mL/min per 1.73m2 body surface area (or <30 mL/min in the POLLUX trial), alanine aminotransferase or aspartate aminotransferase level ≥2.5 times the upper limit of normal (ULN) range, bilirubin level of ≥1.5 or more times the ULN range, disease refractory to bortezomib or lenalidomide, and unacceptable side effects from bortezomib or lenalidomide, were ineligible for these studies. In addition, patients with grade 2 or higher peripheral neuropathy or neuropathic pain, were excluded from the CASTOR study.
Randomization was stratified according to International Staging System disease stage at the time of screening (stage I, II or III, with higher stage indicating more severe disease), number of previous lines of therapy (1 vs 2, or 3 vs >3), and previous receipt of lenalidomide or bortezomib.
In the CASTOR trial, patients received up to eight 21-day cycles of bortezomib, administered subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of cycles 1-8, and dexamethasone, administered orally or intravenously at a dose of 20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 for a total dose of 160 mg per cycle. Daratumumab was administered at a dose of 16 mg/kg intravenously once weekly on days 1, 8, and 15 during cycles 1 to 3, once every 3 weeks on day 1 of cycles 4-8, and once every 4 weeks thereafter.
In the POLLUX trial, patients were treated in 28-day cycles. Daratumumab was administered at the same dose as in the CASTOR trial, but on days 1, 8, 15 and 22 for 8 weeks during cycles 1 and 2, every 2 weeks on days 1 and 15 for 16 weeks during cycles 3 through 7, and every 4 weeks from then onwards. Lenalidomide was administered at a dose of 25 mg orally on days 1-21 of each cycle, and dexamethasone at a dose of 20 mg before infusion and 20 mg the following day.
The combination of daratumumab with lenalidomide-dexamethasone demonstrated a substantial improvement in PFS, compared with lenalidomide-dexamethasone alone (estimated PFS not yet reached vs 18.4 months, respectively; HR, 0.37; P < .0001), representing a 63% reduction in the risk of disease progression or death. Meanwhile, there was a 61% reduction in the risk of disease progression or death for the combination of daratumumab with bortezomib-dexamethasone in the CASTOR trial (estimated PFS not yet reached vs 7.2 months; HR: 0.39; P < .0001). The PFS benefit was observed across all prespecified subgroups in both studies.
In the CASTOR trial, over a median follow-up of 7.4 months, the overall response rate (ORR) was 82.9% for the combination arm, compared with 63.2% for the bortezomib-dexamethasone arm (P < .001), with a very good partial response (VGPR) or better rate of 59.2% compared with 29.1%, and a complete response (CR) rate of 19.2% compared with 9%. In the POLLUX trial, over a median follow-up of 13.5 months, ORR was 92.9% for the combination arm, compared with 76.4% for lenalidomide-dexamethasone, with a VGPR or better rate of 75.8% versus 44% and a CR rate of 43.1% versus 19.2%.
Overall, the safety profile for both combinations was consistent with what is usually observed with daratumumab monotherapy and lenalidomide-dexamethasone or bortezomib-dexamethasone combinations. The most frequently reported adverse events (AEs) were similar in both studies and included infusion reactions, diarrhea, and upper respiratory tract infection. In the POLLUX trial they also included nausea, fatigue, pyrexia, muscle spasm, cough, and dyspnea, whereas in the CASTOR trial patients also frequently experienced peripheral edema.
The most common grade 3/4 AEs in both trials were neutropenia (51.9% vs 37% in the POLLUX trial and 12.8 vs 4.2% in the CASTOR trial), thrombocytopenia (12.7% vs 13.5% and 45.3% vs 32.9%, respectively), and anemia (12.4% vs 19.6% and 14.4% vs 16%, respectively). The percentage of patients who discontinued treatment due to AEs was similar in both groups across the two studies; in the CASTOR trial discontinuations resulted most commonly from peripheral sensory neuropathy and pneumonia, while in the POLLUX trial, from pneumonia, pulmonary embolism and deterioration in general physical health.
The recommended dose for daratumumab in both combination regimens is 16 mg/kg intravenously, calculated on actual body weight. The dosing schedules begin with weekly administration during weeks 1-8 (when used in combination with lenalidomide-dexamethasone) and weeks 1-9 (for use with the bortezomib-dexamethasone combination), decreasing to every 2 weeks between weeks 9 and 24 or 10 and 24, respectively, and progressing to every 4 weeks from week 25 onward until disease progression and unacceptable toxicity.
Daratumumab is marketed as Darzalex by Janssen Biotech Inc. Neutropenia and thrombocytopenia have been added to the list of warnings and precautions for the prescribing information for these new indications. Complete blood cell count should be monitored periodically during treatment and daratumumab administration delayed to allow recovery of neutrophils or platelets. Supportive care with growth factors or transfusion should be considered in the event of neutropenia or thrombocytopenia, respectively.
1. Darzalex (daratumumab) injection, for intravenous use. Prescribing information. Janssen Biotech Inc. https://www.darzalexhcp.com/shared/product/darzalex/darzalex-prescribing-information.pdf. Released November 2016. Accessed January 8, 2017.
2. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
3. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
1. Darzalex (daratumumab) injection, for intravenous use. Prescribing information. Janssen Biotech Inc. https://www.darzalexhcp.com/shared/product/darzalex/darzalex-prescribing-information.pdf. Released November 2016. Accessed January 8, 2017.
2. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
3. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
Laser Scar Management: Focused and High-Intensity Medical Exchange in Vietnam
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.
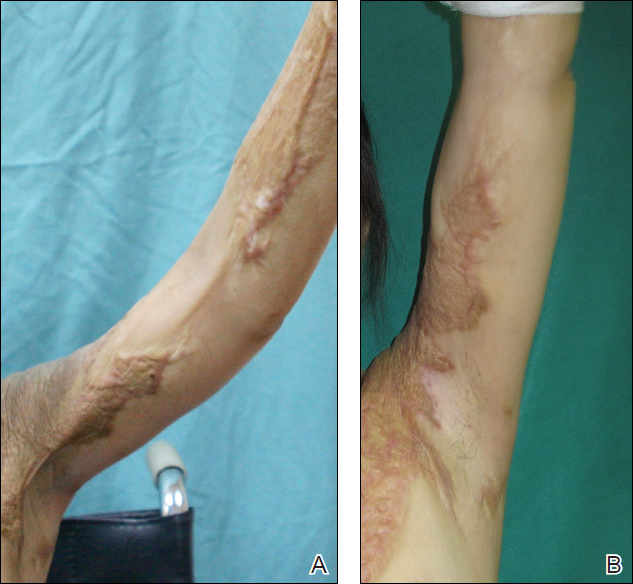
Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Pigmented Lesion on the Forearm
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3
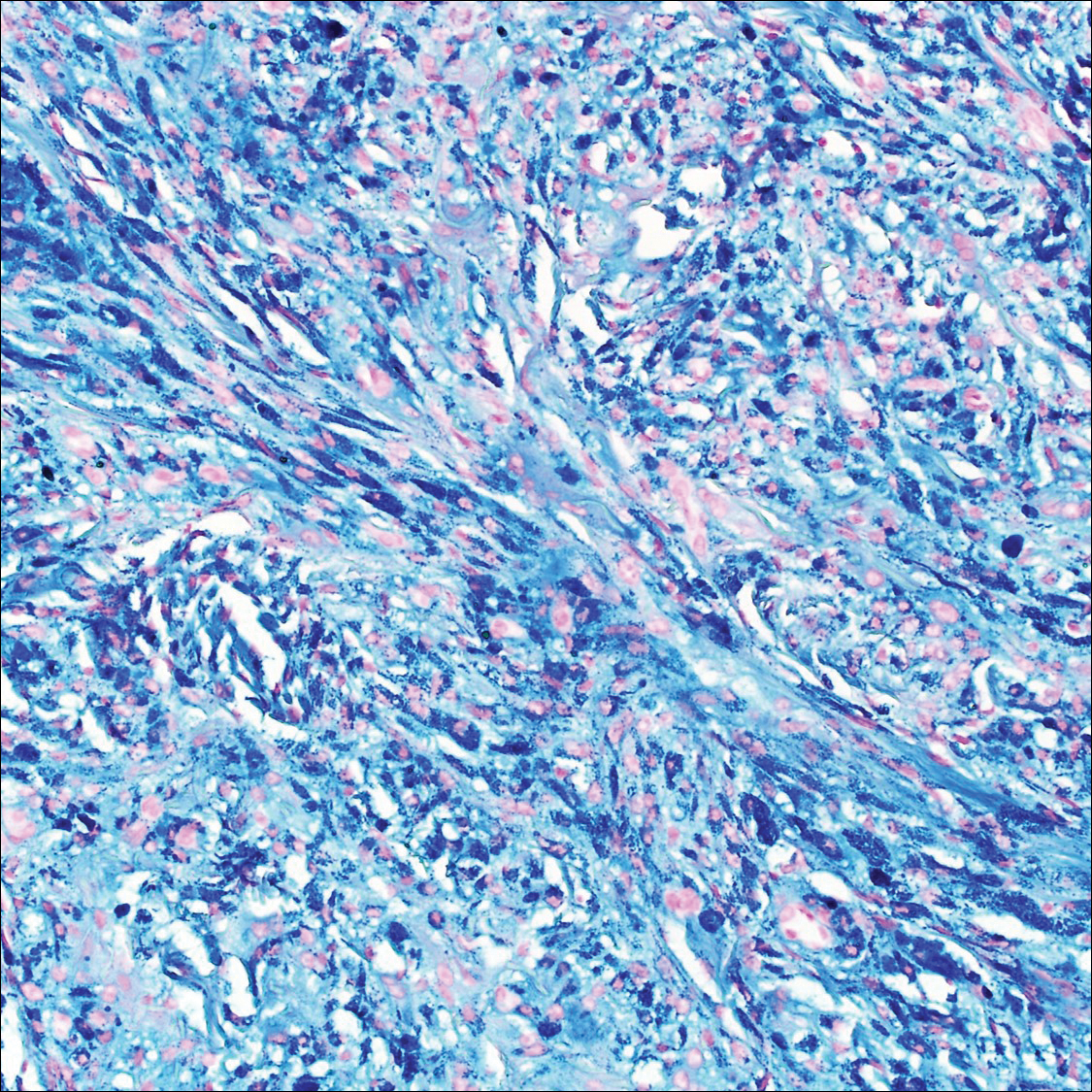
Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4
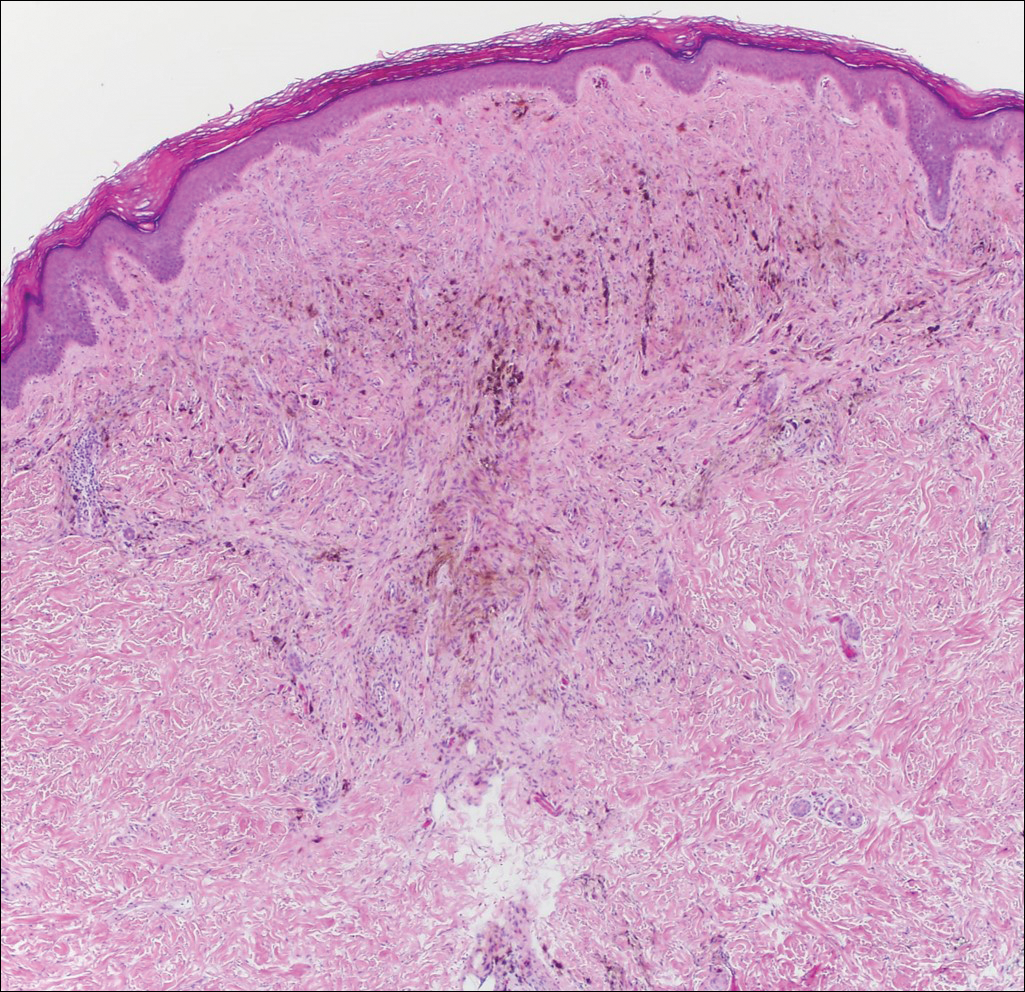
Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8
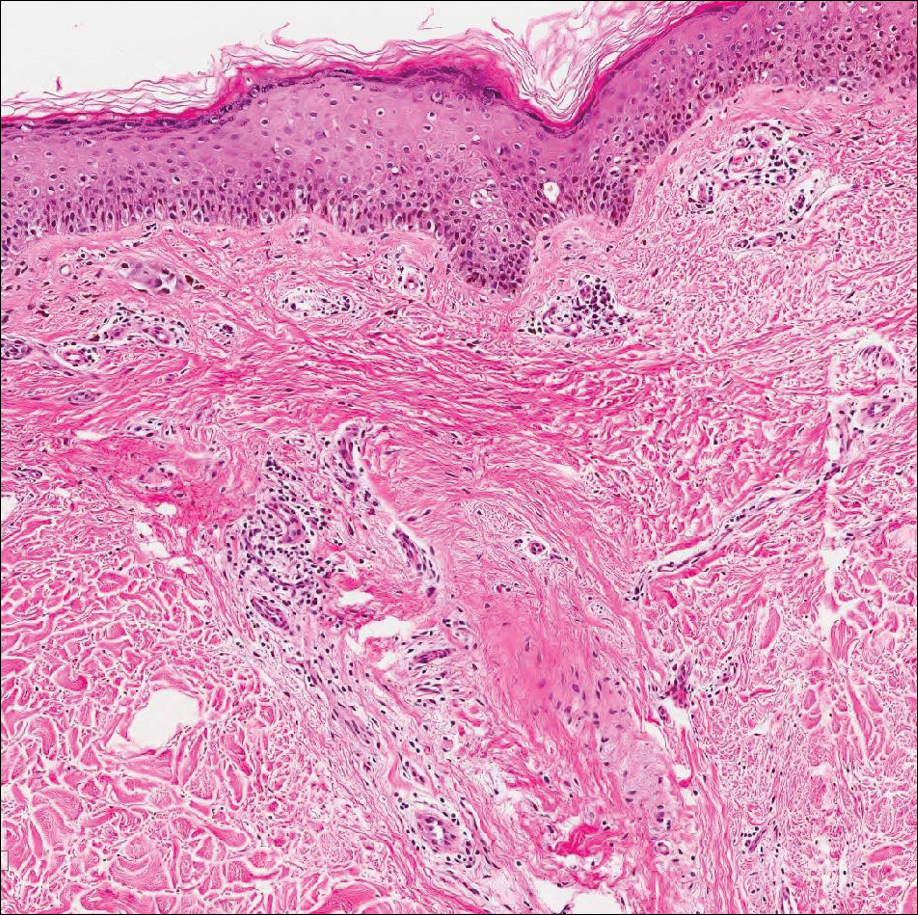
Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3

Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4

Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8

Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3

Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4

Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8

Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
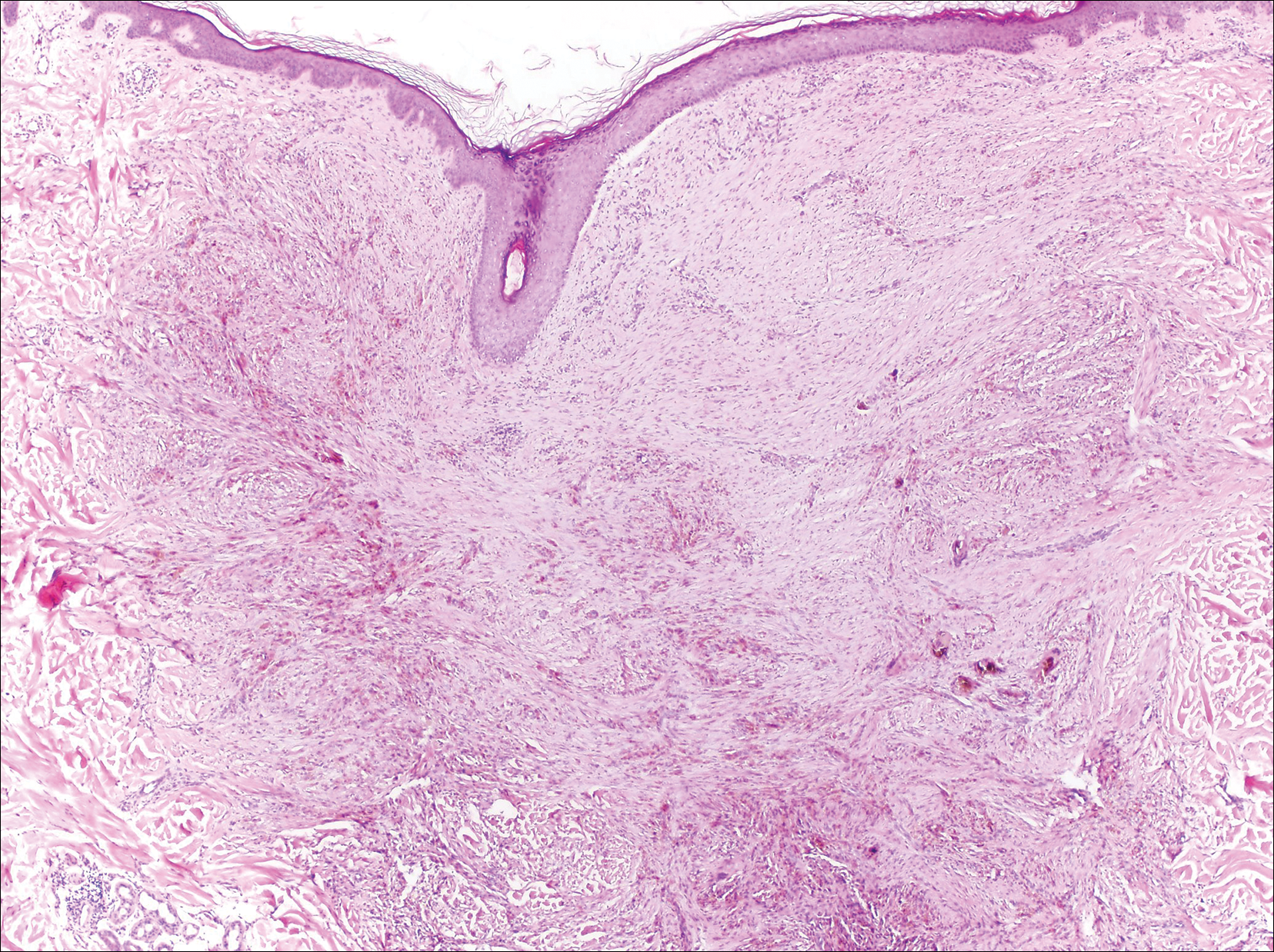
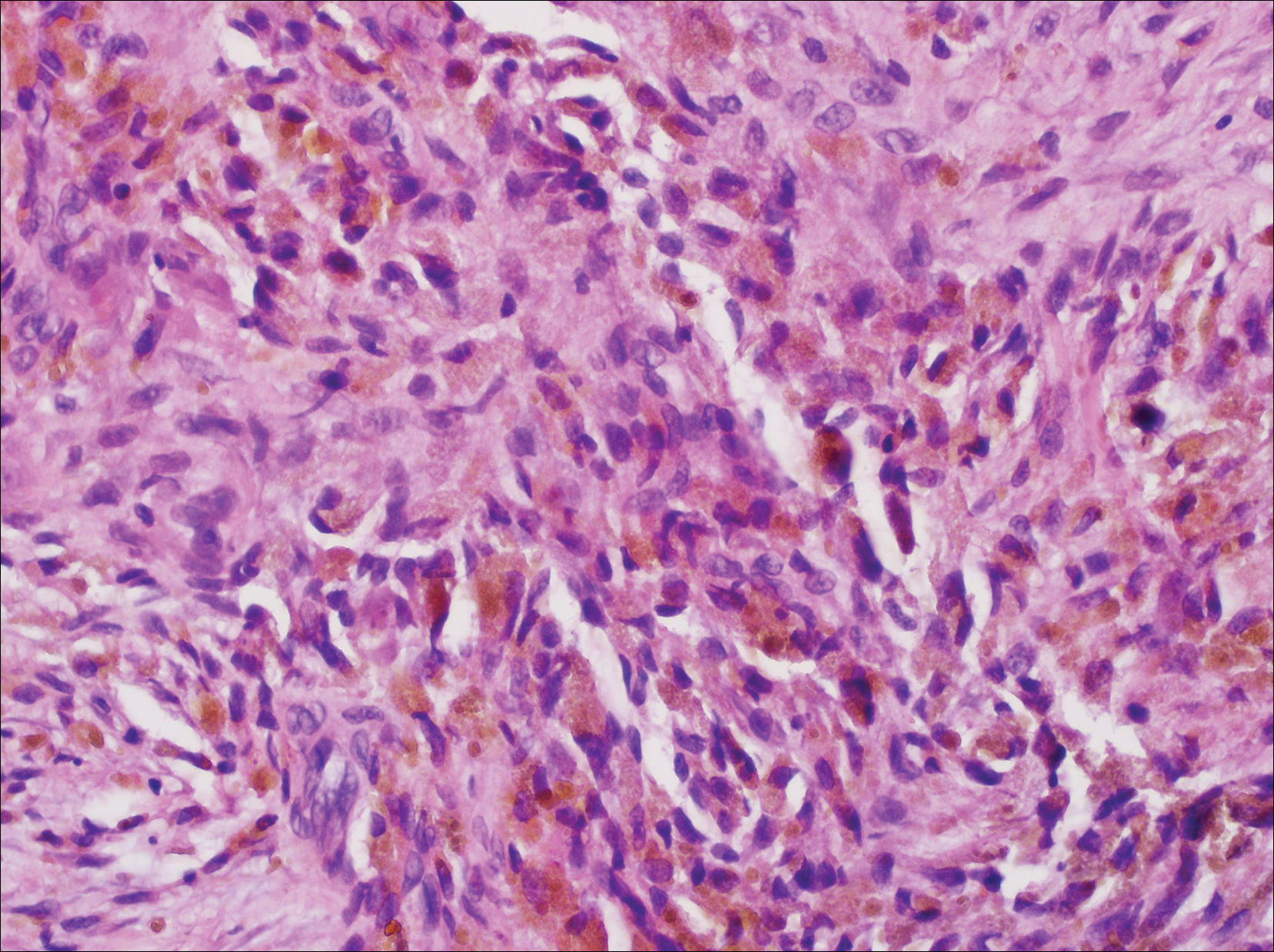
A 67-year-old man presented to the dermatology clinic with a 2-cm pigmented lesion on the forearm. An excisional biopsy was obtained.
Psoriasis pipeline is full of biologics
CHICAGO – Most psoriasis therapies in the pipeline are biologics, including several interleukin (IL)-23 inhibitors and a promising dual inhibitor of IL-17A and IL-17F, so dermatologists are likely to gain a few more options for treating psoriasis patients who have not responded well to or tolerated existing therapies.
“The IL-23 blockers are ideal for patients who want a few injections,” Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and chair of the department of dermatology of the Mount Sinai Health System, said after the American Academy of Dermatology summer meeting. He discussed clinical trial results for risankizumab, mirikizumab, certolizumab pegol (which was recently approved for psoriasis), bimekizumab, as well as tildrakizumab, which has been approved by the Food and Drug Administration, but has not yet been released.
Tildrakizumab: The FDA approved tildrakizumab (Ilumya), a selective IL-23p19 inhibitor, for treatment of moderate to severe plaque psoriasis in March 2018 based on data from the reSURFACE 1 and reSURFACE 2 trials. After initial doses at weeks 0 and 4, patients received a 100-mg subcutaneous injection dose every 12 weeks. Results of reSURFACE 1 showed that 14% of trial participants achieved a Psoriasis Area and Severity Index (PASI) score of 100 with tildrakizumab at week 12, compared with 1% of those receiving a placebo. Similarly, 35% achieved a PASI 90 and 64% achieved a PASI 75 with the drug, compared with 3% and 6%, respectively, in the placebo group. Findings for reSURFACE 2 were similar; in a pooled analysis, a quarter of the 371 patients reached PASI 100 by week 28, 36% reached PASI 90-PASI 99, 24% reached PASI 75-PASI 89, and 10% reached PASI 50-PASI 74. Efficacy remained high 2 years after treatment, although body weight affected the efficacy. “The authors concluded that PASI and PGA [Physician Global Assessment] responses were numerically greater in patients with lower versus higher body weight,” Dr. Lebwohl said at the meeting.
Tildrakizumab also has an “overall clean safety profile,” he said. Among all patients treated in the trials, the rate of severe infections, malignancies and major adverse cardiac events did not significantly differ from placebo.*
Risankizumab: Also an IL-23 inhibitor, risankizumab, under FDA review for treatment of moderate to severe plaque psoriasis, outperformed both ustekinumab and adalimumab in pivotal phase 3 trials reported in October 2017. In the two ultlMMa trials, 75% of 598 total patients achieved a PASI 90 score after 16 weeks of treatment, compared with 2%-5% of placebo participants and 42%-48% of those on ustekinumab. In ultlMMa-1, just over a third of patients treated with achieved PASI 100, and just over half did in ultlMMa-2, compared with 12% and 24% of those on ustekinumab, respectively.
At 1 year, the proportion of those with PASI 90 rose to 82% in ultlMMa-1 and 81% in ultlMMa-2, with over half the participants achieving PASI 100 in both studies. The risankizumab trial findings were “among highest efficacy results reported to date, with impressive durability of response on and off drug,” Dr. Lebwohl said. “Preliminary safety is encouraging,” but “long-term data are required.”
Mirikizumab: Although not as far along in clinical trials, mirikizumab is another IL-23 inhibitor with “interesting and impressive preliminary results,” Dr. Lebwohl said. In a phase 2 trial of 205 participants whose baseline demographics indicated more severe psoriasis, 67% achieved PASI 90 at week 16 with a 300-mg dose (administered every 8 weeks). Doses of 100 mg and 30 mg resulted in 59% and 29% of participants achieving PASI 90 at week 16.
“The most common treatment emergent adverse events included upper respiratory tract infection [including viral], injection site pain, hypertension and diarrhea,” Dr. Lebwohl said. Patients are now being recruited for two phase 3 studies of mirikizumab (OASIS-1 and OASIS-2).
Certolizumab pegol: Certolizumab pegol is a tumor necrosis factor blocker approved in 2013 for treatment of psoriatic arthritis, and for moderate to severe plaque psoriasis in May 2018. In a pooled data analysis of three phase 3 trials (CIMPASI-1, CIMPASI-2, and CIMPACT), 52.3% of participants taking 400 mg subcutaneously every 2 weeks and 44.5% of those taking 200 mg every 2 weeks achieved PASI 90 at week 16, compared with 1.6% of those on placebo. In addition, a trial evaluating maternal transfer of certolizumab to the fetus via placenta found minimal drug concentration levels in the umbilical cord and infant’s plasma. “Certolizumab is ideal for women of childbearing potential,” Dr. Lebwohl said after the meeting.
Bimekizumab: This is a dual inhibitor of IL-17A and IL-17F being studied for treatment of mild psoriasis but “is very promising for psoriatic arthritis, as well as psoriasis,” Dr. Lebwohl said. In the phase 2b BE ABLE 1 trial, up to 79% of patients receiving bimekizumab achieved PASI 90 at week 12, and up to 46% of psoriatic arthritis patients had at least a 50% improvement in joint symptoms, compared with 7% of those on placebo.
Dr. Lebwohl is a consultant for Allergan, Boehringer-Ingelheim, Leo and Promius Pharma, and is an employee of Mount Sinai, which receives research funds from Abbvie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen/Johnson & Johnson, Kadmon, Medimmune/AstraZeneca, Novartis, Pfizer, and ViDac Pharma.
*Correction, 8/6/18: An earlier version of this article misstated the adverse event data for tildrakizumab.
CHICAGO – Most psoriasis therapies in the pipeline are biologics, including several interleukin (IL)-23 inhibitors and a promising dual inhibitor of IL-17A and IL-17F, so dermatologists are likely to gain a few more options for treating psoriasis patients who have not responded well to or tolerated existing therapies.
“The IL-23 blockers are ideal for patients who want a few injections,” Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and chair of the department of dermatology of the Mount Sinai Health System, said after the American Academy of Dermatology summer meeting. He discussed clinical trial results for risankizumab, mirikizumab, certolizumab pegol (which was recently approved for psoriasis), bimekizumab, as well as tildrakizumab, which has been approved by the Food and Drug Administration, but has not yet been released.
Tildrakizumab: The FDA approved tildrakizumab (Ilumya), a selective IL-23p19 inhibitor, for treatment of moderate to severe plaque psoriasis in March 2018 based on data from the reSURFACE 1 and reSURFACE 2 trials. After initial doses at weeks 0 and 4, patients received a 100-mg subcutaneous injection dose every 12 weeks. Results of reSURFACE 1 showed that 14% of trial participants achieved a Psoriasis Area and Severity Index (PASI) score of 100 with tildrakizumab at week 12, compared with 1% of those receiving a placebo. Similarly, 35% achieved a PASI 90 and 64% achieved a PASI 75 with the drug, compared with 3% and 6%, respectively, in the placebo group. Findings for reSURFACE 2 were similar; in a pooled analysis, a quarter of the 371 patients reached PASI 100 by week 28, 36% reached PASI 90-PASI 99, 24% reached PASI 75-PASI 89, and 10% reached PASI 50-PASI 74. Efficacy remained high 2 years after treatment, although body weight affected the efficacy. “The authors concluded that PASI and PGA [Physician Global Assessment] responses were numerically greater in patients with lower versus higher body weight,” Dr. Lebwohl said at the meeting.
Tildrakizumab also has an “overall clean safety profile,” he said. Among all patients treated in the trials, the rate of severe infections, malignancies and major adverse cardiac events did not significantly differ from placebo.*
Risankizumab: Also an IL-23 inhibitor, risankizumab, under FDA review for treatment of moderate to severe plaque psoriasis, outperformed both ustekinumab and adalimumab in pivotal phase 3 trials reported in October 2017. In the two ultlMMa trials, 75% of 598 total patients achieved a PASI 90 score after 16 weeks of treatment, compared with 2%-5% of placebo participants and 42%-48% of those on ustekinumab. In ultlMMa-1, just over a third of patients treated with achieved PASI 100, and just over half did in ultlMMa-2, compared with 12% and 24% of those on ustekinumab, respectively.
At 1 year, the proportion of those with PASI 90 rose to 82% in ultlMMa-1 and 81% in ultlMMa-2, with over half the participants achieving PASI 100 in both studies. The risankizumab trial findings were “among highest efficacy results reported to date, with impressive durability of response on and off drug,” Dr. Lebwohl said. “Preliminary safety is encouraging,” but “long-term data are required.”
Mirikizumab: Although not as far along in clinical trials, mirikizumab is another IL-23 inhibitor with “interesting and impressive preliminary results,” Dr. Lebwohl said. In a phase 2 trial of 205 participants whose baseline demographics indicated more severe psoriasis, 67% achieved PASI 90 at week 16 with a 300-mg dose (administered every 8 weeks). Doses of 100 mg and 30 mg resulted in 59% and 29% of participants achieving PASI 90 at week 16.
“The most common treatment emergent adverse events included upper respiratory tract infection [including viral], injection site pain, hypertension and diarrhea,” Dr. Lebwohl said. Patients are now being recruited for two phase 3 studies of mirikizumab (OASIS-1 and OASIS-2).
Certolizumab pegol: Certolizumab pegol is a tumor necrosis factor blocker approved in 2013 for treatment of psoriatic arthritis, and for moderate to severe plaque psoriasis in May 2018. In a pooled data analysis of three phase 3 trials (CIMPASI-1, CIMPASI-2, and CIMPACT), 52.3% of participants taking 400 mg subcutaneously every 2 weeks and 44.5% of those taking 200 mg every 2 weeks achieved PASI 90 at week 16, compared with 1.6% of those on placebo. In addition, a trial evaluating maternal transfer of certolizumab to the fetus via placenta found minimal drug concentration levels in the umbilical cord and infant’s plasma. “Certolizumab is ideal for women of childbearing potential,” Dr. Lebwohl said after the meeting.
Bimekizumab: This is a dual inhibitor of IL-17A and IL-17F being studied for treatment of mild psoriasis but “is very promising for psoriatic arthritis, as well as psoriasis,” Dr. Lebwohl said. In the phase 2b BE ABLE 1 trial, up to 79% of patients receiving bimekizumab achieved PASI 90 at week 12, and up to 46% of psoriatic arthritis patients had at least a 50% improvement in joint symptoms, compared with 7% of those on placebo.
Dr. Lebwohl is a consultant for Allergan, Boehringer-Ingelheim, Leo and Promius Pharma, and is an employee of Mount Sinai, which receives research funds from Abbvie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen/Johnson & Johnson, Kadmon, Medimmune/AstraZeneca, Novartis, Pfizer, and ViDac Pharma.
*Correction, 8/6/18: An earlier version of this article misstated the adverse event data for tildrakizumab.
CHICAGO – Most psoriasis therapies in the pipeline are biologics, including several interleukin (IL)-23 inhibitors and a promising dual inhibitor of IL-17A and IL-17F, so dermatologists are likely to gain a few more options for treating psoriasis patients who have not responded well to or tolerated existing therapies.
“The IL-23 blockers are ideal for patients who want a few injections,” Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and chair of the department of dermatology of the Mount Sinai Health System, said after the American Academy of Dermatology summer meeting. He discussed clinical trial results for risankizumab, mirikizumab, certolizumab pegol (which was recently approved for psoriasis), bimekizumab, as well as tildrakizumab, which has been approved by the Food and Drug Administration, but has not yet been released.
Tildrakizumab: The FDA approved tildrakizumab (Ilumya), a selective IL-23p19 inhibitor, for treatment of moderate to severe plaque psoriasis in March 2018 based on data from the reSURFACE 1 and reSURFACE 2 trials. After initial doses at weeks 0 and 4, patients received a 100-mg subcutaneous injection dose every 12 weeks. Results of reSURFACE 1 showed that 14% of trial participants achieved a Psoriasis Area and Severity Index (PASI) score of 100 with tildrakizumab at week 12, compared with 1% of those receiving a placebo. Similarly, 35% achieved a PASI 90 and 64% achieved a PASI 75 with the drug, compared with 3% and 6%, respectively, in the placebo group. Findings for reSURFACE 2 were similar; in a pooled analysis, a quarter of the 371 patients reached PASI 100 by week 28, 36% reached PASI 90-PASI 99, 24% reached PASI 75-PASI 89, and 10% reached PASI 50-PASI 74. Efficacy remained high 2 years after treatment, although body weight affected the efficacy. “The authors concluded that PASI and PGA [Physician Global Assessment] responses were numerically greater in patients with lower versus higher body weight,” Dr. Lebwohl said at the meeting.
Tildrakizumab also has an “overall clean safety profile,” he said. Among all patients treated in the trials, the rate of severe infections, malignancies and major adverse cardiac events did not significantly differ from placebo.*
Risankizumab: Also an IL-23 inhibitor, risankizumab, under FDA review for treatment of moderate to severe plaque psoriasis, outperformed both ustekinumab and adalimumab in pivotal phase 3 trials reported in October 2017. In the two ultlMMa trials, 75% of 598 total patients achieved a PASI 90 score after 16 weeks of treatment, compared with 2%-5% of placebo participants and 42%-48% of those on ustekinumab. In ultlMMa-1, just over a third of patients treated with achieved PASI 100, and just over half did in ultlMMa-2, compared with 12% and 24% of those on ustekinumab, respectively.
At 1 year, the proportion of those with PASI 90 rose to 82% in ultlMMa-1 and 81% in ultlMMa-2, with over half the participants achieving PASI 100 in both studies. The risankizumab trial findings were “among highest efficacy results reported to date, with impressive durability of response on and off drug,” Dr. Lebwohl said. “Preliminary safety is encouraging,” but “long-term data are required.”
Mirikizumab: Although not as far along in clinical trials, mirikizumab is another IL-23 inhibitor with “interesting and impressive preliminary results,” Dr. Lebwohl said. In a phase 2 trial of 205 participants whose baseline demographics indicated more severe psoriasis, 67% achieved PASI 90 at week 16 with a 300-mg dose (administered every 8 weeks). Doses of 100 mg and 30 mg resulted in 59% and 29% of participants achieving PASI 90 at week 16.
“The most common treatment emergent adverse events included upper respiratory tract infection [including viral], injection site pain, hypertension and diarrhea,” Dr. Lebwohl said. Patients are now being recruited for two phase 3 studies of mirikizumab (OASIS-1 and OASIS-2).
Certolizumab pegol: Certolizumab pegol is a tumor necrosis factor blocker approved in 2013 for treatment of psoriatic arthritis, and for moderate to severe plaque psoriasis in May 2018. In a pooled data analysis of three phase 3 trials (CIMPASI-1, CIMPASI-2, and CIMPACT), 52.3% of participants taking 400 mg subcutaneously every 2 weeks and 44.5% of those taking 200 mg every 2 weeks achieved PASI 90 at week 16, compared with 1.6% of those on placebo. In addition, a trial evaluating maternal transfer of certolizumab to the fetus via placenta found minimal drug concentration levels in the umbilical cord and infant’s plasma. “Certolizumab is ideal for women of childbearing potential,” Dr. Lebwohl said after the meeting.
Bimekizumab: This is a dual inhibitor of IL-17A and IL-17F being studied for treatment of mild psoriasis but “is very promising for psoriatic arthritis, as well as psoriasis,” Dr. Lebwohl said. In the phase 2b BE ABLE 1 trial, up to 79% of patients receiving bimekizumab achieved PASI 90 at week 12, and up to 46% of psoriatic arthritis patients had at least a 50% improvement in joint symptoms, compared with 7% of those on placebo.
Dr. Lebwohl is a consultant for Allergan, Boehringer-Ingelheim, Leo and Promius Pharma, and is an employee of Mount Sinai, which receives research funds from Abbvie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen/Johnson & Johnson, Kadmon, Medimmune/AstraZeneca, Novartis, Pfizer, and ViDac Pharma.
*Correction, 8/6/18: An earlier version of this article misstated the adverse event data for tildrakizumab.
EXPERT ANALYSIS FROM SUMMER AAD 2018
Wound Closure Tips
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
Acute Painful Rash on the Cheek
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.
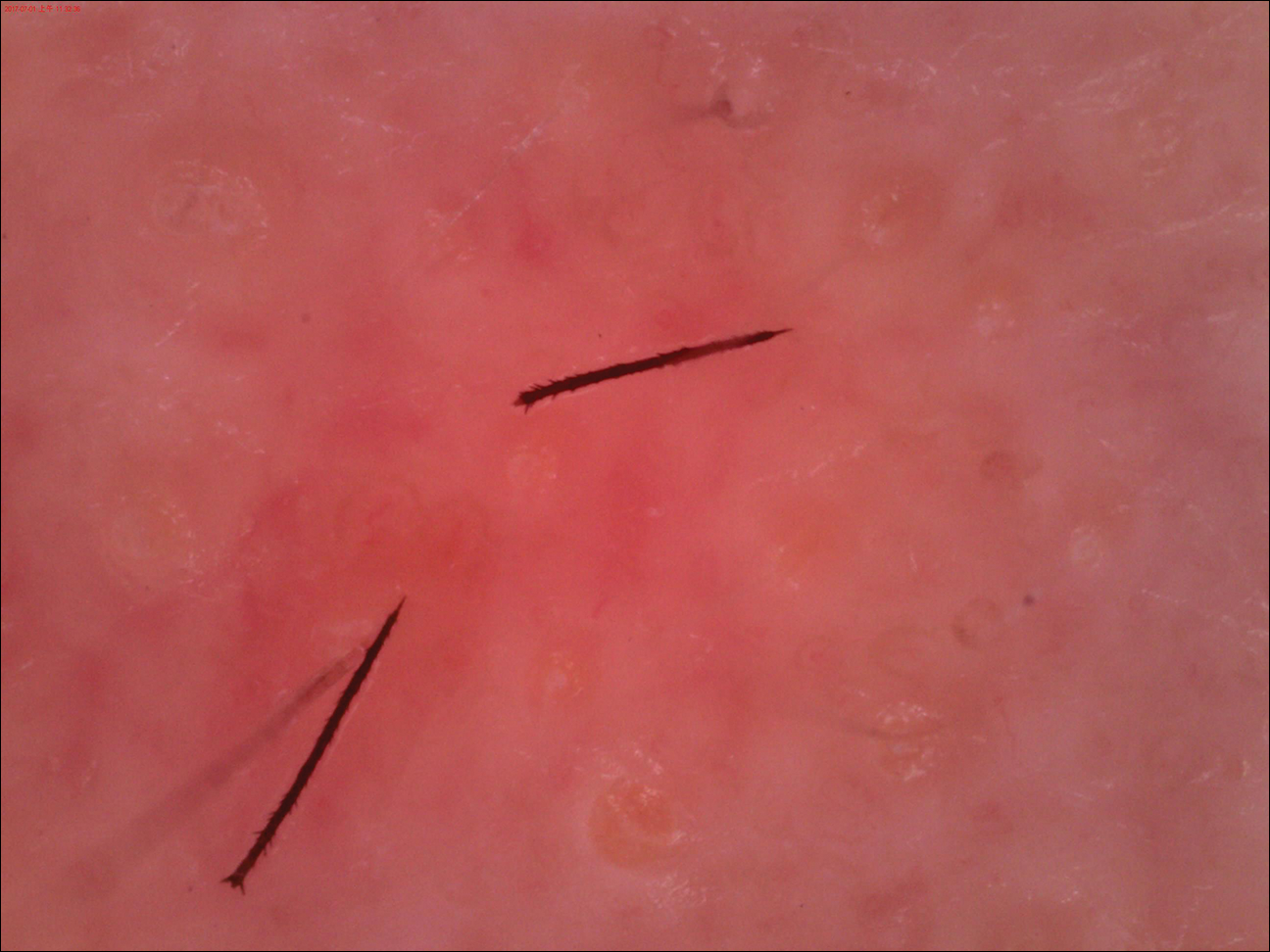
Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.

Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.

Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
A 31-year-old woman presented to an outpatient dermatology department with acute pruritus, burning, and moderate swelling of the left cheek of 10 minutes' duration that occurred while waiting to see a hematologist in the same building. The patient was diagnosed with aplastic anemia 11 years prior and was awaiting bone marrow transplantation. Physical examination showed an edematous erythematous wheal with a relatively distinct border measuring 3 cm in diameter. No foreign material could be identified on the surface with the naked eye. Dermoscopy was performed.
Electronic Medical Records in Dermatology: The Good, the Bad, and the Ugly
The American Recovery and Reinvestment Act of 2009 introduced the Health Information Technology for Economic and Clinical Health (HITECH) Act and allocated $19.2 billion to promote the implementation of an electronic medical record (EMR) by hospitals as well as physicians in private practices.1 The EMR stores longitudinal health information and constructs a comprehensive picture of a patient's medical history.2,3 Following its debut, the US Department of Health & Human Services set forth meaningful use (MU) criteria,1 which aimed to increase quality of care, safety, and efficiency. Meaningful use criteria also sought to decrease health disparities; improve coordination of care; engage patients in their care; refine population and public health measures; and finally ensure accessibility, privacy, and security of patient data.1,2
The EMR offers potential gains at multiple levels of the patient-physician-public health hierarchy: a decrease in medical errors and duplicate services, timely access to test results and records, timely notification of patients in need for preventive services, and preservation of medical records in the event of an environmental disaster.1-3 Furthermore, physicians can take advantage of informational reciprocity with other providers, gain remote access to medical records, be reminded of need for service, e-prescribe, monitor for drug interactions, and utilize clinical information for research purposes.1,3 Lastly, public health organizations can use EMRs to improve outcomes by employing surveillance measures and creating patient registries that serve to protect the society at large.1
Although it seems that the broad-scale implementation of EMRs will undoubtedly enhance the quality of patient care in the years to come, many obstacles must be overcome to reach this potential. Certification of EMR systems and implementation of confidentiality measures that are compatible with the Health Insurance Portability and Accountability Act are the forerunning concerns, and the interconnectivity of EMR systems becomes more important as MU enters its later stages (eg, increased electronic transmission of patient data during phases of care, reliance on e-prescribing, population-level analysis of patient data to improve health outcomes). Additionally, the cost to implement and maintain an EMR deters many physicians in smaller practices from shouldering the charges, as they do not necessarily see increased productivity with this technology.2 Last but not least, unintended consequences of EMR implementation can involve the dangers of upcoding and overdocumentation.1
Dermatology is a visually dominant field serving a high volume of patients that require both medical and surgical care. These factors do not preclude the implementation of EMRs in our specialty but rather necessitate the utilization of a system specifically designed to cater to the needs of specialists in dermatology. This editorial will address some fundamental considerations in implementing an EMR in dermatology; discuss what platforms and patient interfaces are most practical; reiterate the importance of interoperability; and highlight the implications of this powerful tool in education, research, training, and monitoring quality of care.
Implementation and Specific Considerations in Dermatology
One of the biggest areas of concern in adopting an EMR is the associated financial burden.2-5 Although government incentives of MU cover a part of the initial cost of purchasing an EMR, expert maintenance costs, changes in workflow dynamics, and a steep learning curve can translate into lost productivity and revenue, discouraging many dermatologists from implementing an EMR.3,5 Furthermore, physicians who are nearing the end of their careers may not realize the longer-term benefits of implementing an EMR and therefore may decline to do so despite the disincentives of lower Medicare reimbursements.2 In fact, when juxtaposing the upfront cost of implementing an EMR against the expected increase in revenue after its implementation, there are general concerns that there will be a net loss on the part of the provider, which is a barrier to adoption of EMRs.3
Beyond the cost-benefit analysis, dermatologists often report multiple lesions in different anatomic sites or identify multiple biopsy sites that have been conveniently recorded on body templates included in their paper-based examination forms. Converting that information into words to be entered into an EMR can be excruciatingly time consuming and not easily comprehensible upon follow-up visits with the patient, which again results in decreased efficiency and productivity.4 Therefore, selecting a dermatology-compatible EMR that aims to make this transition easier is of utmost importance. One developer introduced an EMR with a touch interface containing a human body in all its facets that can be rotated, zoomed, and marked multiple times for accurate and convenient recording of lesions and intended procedure sites. This system automatically produces codes for examinations and procedures; facilitates e-prescription and ordering of laboratory results; prints pathology requests and consent forms; and includes information for patient education regarding their care. For example, the physical examination section of an H&P (history and physical examination) can be generated with a few taps on the screen, translating into increased efficiency and productivity.
The visual nature of dermatology demands the use of images, and as such, photographs have become integral in the diagnosis and follow-up of dermatology patients. Digital and dermatoscopic images not only help to eliminate unnecessary biopsies but also can promote early detection and management of malignancies.6 Thus, the capability to link photographs to a patient's medical record using an EMR is an invaluable gain. However, employing this feature in clinical practice has ethical implications that must be addressed, given that taking photographs can evoke an avoidable fear in patients regarding unlawful dissemination or unnecessary exposure of these images to physicians who do not need to access them.6 Thus, guidelines must be set for uploading, de-identifying, and annotating patient photographs, and only physicians involved in the care of the patient should be allocated access.4,6
EMR Platforms
When computers were first introduced into examination rooms, many physicians were reluctant, as computers were thought to disconnect physicians from patients, arousing a sense of remoteness and further depersonalizing the encounter as physicians spent more time typing and less time making meaningful eye contact with patients.5 The practice of dermatology requires patient-centered communication that serves to enhance the quality of care while at the same time allowing physicians to fulfill professional competencies and reduce medical errors, which may ultimately translate into patient satisfaction.7 In fact, when the patient-physician relationship is interrupted, patients are more likely to pursue legal action in the wake of a bad treatment outcome.5
Employing a tablet-friendly EMR can help circumvent (or at least minimize) this problem by providing the physician with a light, user-friendly device that eliminates the need for laptops or desktop computers.4 Going one step further, the utilization of tablets eliminates the need for accessory digital cameras, as most tablets come with built-in high-resolution cameras for capturing clinical photographs and immediately linking them to the patient's medical record. Lastly, tablet technology allows physicians to access consent forms while in the examination room with the patient to more readily obtain a signature for procedural or research consent.4,8
Interoperability, MU, and Quality of Care
The real goal in nationwide implementation of EMR technology is to accomplish MU criteria. Different EMRs should not only allow data to be imported and exported but ideally should be compatible and interoperable with one another.2 The myriad of different EMR platforms available impedes maximal functionality as MU moves into its final stage. For example, if an academic dermatology program in the setting of a larger hospital is required to use the generic hospital EMR, the dermatologist's specific needs may not be effectively met; on the other hand, if a dermatology-specific EMR is implemented, access to the hospital's larger database of patient information may be sacrificed. Optimal EMR systems should be designed to allow specificity for a given specialty while being able to receive and integrate laboratory values, dermatopathology and radiology results, and notes from consultations by other physicians. Such integration may reduce duplicate services, increase patient satisfaction, and fulfill MU criteria. In fact, the fear of many physicians, especially those in a field such as dermatology, are the unwanted costs that come with implementation of an EMR system that will soon become impractical due to compatibility issues.2 As a result, until a system that can meet the needs of multiple specialties is developed, dermatologists and other physicians upgrading to an EMR should consider implementing a system that is compatible with nearby hospitals, other specialists' offices, and diagnostic centers to maximize interoperability at the local level.2,3
Electronic medical record systems that interoperate also provide the ability to set forth performance measures for physicians aimed at improving quality of care. As our health care system moves toward a pay-for-performance model, EMRs will become a tool to determine if standards of care have been met, unnecessary diagnostic tests have been avoided, and unwanted outcomes have been minimized. These measures will usher in a new era of medicine in which physicians strive to improve the care provided to their patients and receive increases in their reimbursements, while patient outcomes and satisfaction are improved.9
Academics, Education, Research, and Residency
The practicality of EMRs in dermatology may best be appreciated in academic settings. Electronic medical records serve as a repository of coded information that is neatly organized and can be rapidly searched, allowing for use as a powerful research tool. As an example, physicians can use EMR systems to identify patients with specific qualifications and study outcome variables over time.2,3 Additionally, with the rise of interoperable systems, we can expect a new dawn in medical research as more information becomes available to clinical investigators, opening doors to endless possibilities for evidence-based care.2,3,8
Another advantage of EMRs is their utility in residency programs that are charged with the task of ensuring resident competency via exposure to a comprehensive host of clinical encounters. An EMR system uniquely allows residents and attending dermatologists to monitor adequate exposure to general, pediatric, complex medical, procedural, and dermatopathologic cases, and to track the number of procedures performed by the residents by directly linking the information into the Accreditation Council for Graduate Medical Education procedure log.
Furthermore, due to the dominance of digital and dermatoscopic images in the field, interoperable EMRs could be used to construct a database of clinical and dermatopathologic specimens that not only can be used in educating residents but also may serve as a powerful reference tool in the diagnosis of complex and rare cases.8 Another often unrecognized advantage of EMRs is their utility in teledermatology. With the interoperable EMRs within academic institutions, teledermatology can be used locally and nationally for rapid consultation with high diagnostic validity,10 which has the burgeoning potential of providing patients with quicker time to diagnosis considering the dermatologist shortage in various parts of the country.
Lastly, the implementation of EMRs in residency programs has the additional benefit of exposing residents and medical students to emerging technology early on in their careers and fosters a degree of familiarity and comfort that may lead to implementation of EMRs in their future practices.2 For dermatologists in-training, early exposure to these technologies also may serve as a way to develop an interactive interview style and adapt to the presence of EMRs in examination rooms without sacrificing quality of care and meaningful patient interaction.7
Conclusion
Electronic medical records are already becoming an integral part of many hospitals and private dermatology practices. Although EMRs provide potential benefits that can be expected to ultimately outweigh the associated costs in larger settings such as hospitals, residency programs, and multidisciplinary practices, EMRs may not be immediately beneficial to physicians in private practices or those approaching the end of their careers. Although a perfect system may be unattainable, development of interoperable systems designed to meet the needs of specialties such as dermatology are essential in attaining a comprehensive patient medical profile, improving quality of care, minimizing costs, reducing medical errors, and maximizing patient satisfaction.
- Health IT and health information exchange basics. Office of the National Coordinator for Health Information Technology website. http://www.healthit.gov/providers-professionals/learn-ehr-basics. Reviewed January 8, 2018. Accessed July 10, 2018.
- Grosshandler JA, Tulbert B, Kaufmann MD, et al. The electronic medical record in dermatology. Arch Dermatol. 2010;146:1031-1036.
- Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
- Kaufmann MD, Desai S. Special requirements for electronic health records in dermatology. Semin Cutan Med Surg. 2012;31:160-162.
- Wheeland RG. Separating EMR implementation hype from fact. Dermatology Times. http://www.dermatologytimes.com/modern-medicine-now/separating-emr-implementation-hype-fact. Published July 1, 2012. Accessed July 16, 2018.
- Lakdawala N, Bercovitch L, Grant-Kels JM. A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J Am Acad Dermatol. 2013;69:473-475.
- Nguyen TV, Hong J, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part I: patient-centered communication. J Am Acad Dermatol. 2013;68:353.e1-8.
- Ratner D, Thomas CO, Bickers D. The uses of digital photography in dermatology. J Am Acad Dermatol. 1999;41(5, pt 1):749-756.
- Wilson RL, Feldman SR. Physician performance measures in dermatology. J Am Acad Dermatol. 2010;63:E29-E35.
- Vañó-Galván S, Hidalgo A, Aguayo-Leiva I, et al. Store-and-forward teledermatology: assessment of validity in a series of 2000 observations [in Spanish]. Actas Dermosifiliogr. 2011;102:277-283.
The American Recovery and Reinvestment Act of 2009 introduced the Health Information Technology for Economic and Clinical Health (HITECH) Act and allocated $19.2 billion to promote the implementation of an electronic medical record (EMR) by hospitals as well as physicians in private practices.1 The EMR stores longitudinal health information and constructs a comprehensive picture of a patient's medical history.2,3 Following its debut, the US Department of Health & Human Services set forth meaningful use (MU) criteria,1 which aimed to increase quality of care, safety, and efficiency. Meaningful use criteria also sought to decrease health disparities; improve coordination of care; engage patients in their care; refine population and public health measures; and finally ensure accessibility, privacy, and security of patient data.1,2
The EMR offers potential gains at multiple levels of the patient-physician-public health hierarchy: a decrease in medical errors and duplicate services, timely access to test results and records, timely notification of patients in need for preventive services, and preservation of medical records in the event of an environmental disaster.1-3 Furthermore, physicians can take advantage of informational reciprocity with other providers, gain remote access to medical records, be reminded of need for service, e-prescribe, monitor for drug interactions, and utilize clinical information for research purposes.1,3 Lastly, public health organizations can use EMRs to improve outcomes by employing surveillance measures and creating patient registries that serve to protect the society at large.1
Although it seems that the broad-scale implementation of EMRs will undoubtedly enhance the quality of patient care in the years to come, many obstacles must be overcome to reach this potential. Certification of EMR systems and implementation of confidentiality measures that are compatible with the Health Insurance Portability and Accountability Act are the forerunning concerns, and the interconnectivity of EMR systems becomes more important as MU enters its later stages (eg, increased electronic transmission of patient data during phases of care, reliance on e-prescribing, population-level analysis of patient data to improve health outcomes). Additionally, the cost to implement and maintain an EMR deters many physicians in smaller practices from shouldering the charges, as they do not necessarily see increased productivity with this technology.2 Last but not least, unintended consequences of EMR implementation can involve the dangers of upcoding and overdocumentation.1
Dermatology is a visually dominant field serving a high volume of patients that require both medical and surgical care. These factors do not preclude the implementation of EMRs in our specialty but rather necessitate the utilization of a system specifically designed to cater to the needs of specialists in dermatology. This editorial will address some fundamental considerations in implementing an EMR in dermatology; discuss what platforms and patient interfaces are most practical; reiterate the importance of interoperability; and highlight the implications of this powerful tool in education, research, training, and monitoring quality of care.
Implementation and Specific Considerations in Dermatology
One of the biggest areas of concern in adopting an EMR is the associated financial burden.2-5 Although government incentives of MU cover a part of the initial cost of purchasing an EMR, expert maintenance costs, changes in workflow dynamics, and a steep learning curve can translate into lost productivity and revenue, discouraging many dermatologists from implementing an EMR.3,5 Furthermore, physicians who are nearing the end of their careers may not realize the longer-term benefits of implementing an EMR and therefore may decline to do so despite the disincentives of lower Medicare reimbursements.2 In fact, when juxtaposing the upfront cost of implementing an EMR against the expected increase in revenue after its implementation, there are general concerns that there will be a net loss on the part of the provider, which is a barrier to adoption of EMRs.3
Beyond the cost-benefit analysis, dermatologists often report multiple lesions in different anatomic sites or identify multiple biopsy sites that have been conveniently recorded on body templates included in their paper-based examination forms. Converting that information into words to be entered into an EMR can be excruciatingly time consuming and not easily comprehensible upon follow-up visits with the patient, which again results in decreased efficiency and productivity.4 Therefore, selecting a dermatology-compatible EMR that aims to make this transition easier is of utmost importance. One developer introduced an EMR with a touch interface containing a human body in all its facets that can be rotated, zoomed, and marked multiple times for accurate and convenient recording of lesions and intended procedure sites. This system automatically produces codes for examinations and procedures; facilitates e-prescription and ordering of laboratory results; prints pathology requests and consent forms; and includes information for patient education regarding their care. For example, the physical examination section of an H&P (history and physical examination) can be generated with a few taps on the screen, translating into increased efficiency and productivity.
The visual nature of dermatology demands the use of images, and as such, photographs have become integral in the diagnosis and follow-up of dermatology patients. Digital and dermatoscopic images not only help to eliminate unnecessary biopsies but also can promote early detection and management of malignancies.6 Thus, the capability to link photographs to a patient's medical record using an EMR is an invaluable gain. However, employing this feature in clinical practice has ethical implications that must be addressed, given that taking photographs can evoke an avoidable fear in patients regarding unlawful dissemination or unnecessary exposure of these images to physicians who do not need to access them.6 Thus, guidelines must be set for uploading, de-identifying, and annotating patient photographs, and only physicians involved in the care of the patient should be allocated access.4,6
EMR Platforms
When computers were first introduced into examination rooms, many physicians were reluctant, as computers were thought to disconnect physicians from patients, arousing a sense of remoteness and further depersonalizing the encounter as physicians spent more time typing and less time making meaningful eye contact with patients.5 The practice of dermatology requires patient-centered communication that serves to enhance the quality of care while at the same time allowing physicians to fulfill professional competencies and reduce medical errors, which may ultimately translate into patient satisfaction.7 In fact, when the patient-physician relationship is interrupted, patients are more likely to pursue legal action in the wake of a bad treatment outcome.5
Employing a tablet-friendly EMR can help circumvent (or at least minimize) this problem by providing the physician with a light, user-friendly device that eliminates the need for laptops or desktop computers.4 Going one step further, the utilization of tablets eliminates the need for accessory digital cameras, as most tablets come with built-in high-resolution cameras for capturing clinical photographs and immediately linking them to the patient's medical record. Lastly, tablet technology allows physicians to access consent forms while in the examination room with the patient to more readily obtain a signature for procedural or research consent.4,8
Interoperability, MU, and Quality of Care
The real goal in nationwide implementation of EMR technology is to accomplish MU criteria. Different EMRs should not only allow data to be imported and exported but ideally should be compatible and interoperable with one another.2 The myriad of different EMR platforms available impedes maximal functionality as MU moves into its final stage. For example, if an academic dermatology program in the setting of a larger hospital is required to use the generic hospital EMR, the dermatologist's specific needs may not be effectively met; on the other hand, if a dermatology-specific EMR is implemented, access to the hospital's larger database of patient information may be sacrificed. Optimal EMR systems should be designed to allow specificity for a given specialty while being able to receive and integrate laboratory values, dermatopathology and radiology results, and notes from consultations by other physicians. Such integration may reduce duplicate services, increase patient satisfaction, and fulfill MU criteria. In fact, the fear of many physicians, especially those in a field such as dermatology, are the unwanted costs that come with implementation of an EMR system that will soon become impractical due to compatibility issues.2 As a result, until a system that can meet the needs of multiple specialties is developed, dermatologists and other physicians upgrading to an EMR should consider implementing a system that is compatible with nearby hospitals, other specialists' offices, and diagnostic centers to maximize interoperability at the local level.2,3
Electronic medical record systems that interoperate also provide the ability to set forth performance measures for physicians aimed at improving quality of care. As our health care system moves toward a pay-for-performance model, EMRs will become a tool to determine if standards of care have been met, unnecessary diagnostic tests have been avoided, and unwanted outcomes have been minimized. These measures will usher in a new era of medicine in which physicians strive to improve the care provided to their patients and receive increases in their reimbursements, while patient outcomes and satisfaction are improved.9
Academics, Education, Research, and Residency
The practicality of EMRs in dermatology may best be appreciated in academic settings. Electronic medical records serve as a repository of coded information that is neatly organized and can be rapidly searched, allowing for use as a powerful research tool. As an example, physicians can use EMR systems to identify patients with specific qualifications and study outcome variables over time.2,3 Additionally, with the rise of interoperable systems, we can expect a new dawn in medical research as more information becomes available to clinical investigators, opening doors to endless possibilities for evidence-based care.2,3,8
Another advantage of EMRs is their utility in residency programs that are charged with the task of ensuring resident competency via exposure to a comprehensive host of clinical encounters. An EMR system uniquely allows residents and attending dermatologists to monitor adequate exposure to general, pediatric, complex medical, procedural, and dermatopathologic cases, and to track the number of procedures performed by the residents by directly linking the information into the Accreditation Council for Graduate Medical Education procedure log.
Furthermore, due to the dominance of digital and dermatoscopic images in the field, interoperable EMRs could be used to construct a database of clinical and dermatopathologic specimens that not only can be used in educating residents but also may serve as a powerful reference tool in the diagnosis of complex and rare cases.8 Another often unrecognized advantage of EMRs is their utility in teledermatology. With the interoperable EMRs within academic institutions, teledermatology can be used locally and nationally for rapid consultation with high diagnostic validity,10 which has the burgeoning potential of providing patients with quicker time to diagnosis considering the dermatologist shortage in various parts of the country.
Lastly, the implementation of EMRs in residency programs has the additional benefit of exposing residents and medical students to emerging technology early on in their careers and fosters a degree of familiarity and comfort that may lead to implementation of EMRs in their future practices.2 For dermatologists in-training, early exposure to these technologies also may serve as a way to develop an interactive interview style and adapt to the presence of EMRs in examination rooms without sacrificing quality of care and meaningful patient interaction.7
Conclusion
Electronic medical records are already becoming an integral part of many hospitals and private dermatology practices. Although EMRs provide potential benefits that can be expected to ultimately outweigh the associated costs in larger settings such as hospitals, residency programs, and multidisciplinary practices, EMRs may not be immediately beneficial to physicians in private practices or those approaching the end of their careers. Although a perfect system may be unattainable, development of interoperable systems designed to meet the needs of specialties such as dermatology are essential in attaining a comprehensive patient medical profile, improving quality of care, minimizing costs, reducing medical errors, and maximizing patient satisfaction.
The American Recovery and Reinvestment Act of 2009 introduced the Health Information Technology for Economic and Clinical Health (HITECH) Act and allocated $19.2 billion to promote the implementation of an electronic medical record (EMR) by hospitals as well as physicians in private practices.1 The EMR stores longitudinal health information and constructs a comprehensive picture of a patient's medical history.2,3 Following its debut, the US Department of Health & Human Services set forth meaningful use (MU) criteria,1 which aimed to increase quality of care, safety, and efficiency. Meaningful use criteria also sought to decrease health disparities; improve coordination of care; engage patients in their care; refine population and public health measures; and finally ensure accessibility, privacy, and security of patient data.1,2
The EMR offers potential gains at multiple levels of the patient-physician-public health hierarchy: a decrease in medical errors and duplicate services, timely access to test results and records, timely notification of patients in need for preventive services, and preservation of medical records in the event of an environmental disaster.1-3 Furthermore, physicians can take advantage of informational reciprocity with other providers, gain remote access to medical records, be reminded of need for service, e-prescribe, monitor for drug interactions, and utilize clinical information for research purposes.1,3 Lastly, public health organizations can use EMRs to improve outcomes by employing surveillance measures and creating patient registries that serve to protect the society at large.1
Although it seems that the broad-scale implementation of EMRs will undoubtedly enhance the quality of patient care in the years to come, many obstacles must be overcome to reach this potential. Certification of EMR systems and implementation of confidentiality measures that are compatible with the Health Insurance Portability and Accountability Act are the forerunning concerns, and the interconnectivity of EMR systems becomes more important as MU enters its later stages (eg, increased electronic transmission of patient data during phases of care, reliance on e-prescribing, population-level analysis of patient data to improve health outcomes). Additionally, the cost to implement and maintain an EMR deters many physicians in smaller practices from shouldering the charges, as they do not necessarily see increased productivity with this technology.2 Last but not least, unintended consequences of EMR implementation can involve the dangers of upcoding and overdocumentation.1
Dermatology is a visually dominant field serving a high volume of patients that require both medical and surgical care. These factors do not preclude the implementation of EMRs in our specialty but rather necessitate the utilization of a system specifically designed to cater to the needs of specialists in dermatology. This editorial will address some fundamental considerations in implementing an EMR in dermatology; discuss what platforms and patient interfaces are most practical; reiterate the importance of interoperability; and highlight the implications of this powerful tool in education, research, training, and monitoring quality of care.
Implementation and Specific Considerations in Dermatology
One of the biggest areas of concern in adopting an EMR is the associated financial burden.2-5 Although government incentives of MU cover a part of the initial cost of purchasing an EMR, expert maintenance costs, changes in workflow dynamics, and a steep learning curve can translate into lost productivity and revenue, discouraging many dermatologists from implementing an EMR.3,5 Furthermore, physicians who are nearing the end of their careers may not realize the longer-term benefits of implementing an EMR and therefore may decline to do so despite the disincentives of lower Medicare reimbursements.2 In fact, when juxtaposing the upfront cost of implementing an EMR against the expected increase in revenue after its implementation, there are general concerns that there will be a net loss on the part of the provider, which is a barrier to adoption of EMRs.3
Beyond the cost-benefit analysis, dermatologists often report multiple lesions in different anatomic sites or identify multiple biopsy sites that have been conveniently recorded on body templates included in their paper-based examination forms. Converting that information into words to be entered into an EMR can be excruciatingly time consuming and not easily comprehensible upon follow-up visits with the patient, which again results in decreased efficiency and productivity.4 Therefore, selecting a dermatology-compatible EMR that aims to make this transition easier is of utmost importance. One developer introduced an EMR with a touch interface containing a human body in all its facets that can be rotated, zoomed, and marked multiple times for accurate and convenient recording of lesions and intended procedure sites. This system automatically produces codes for examinations and procedures; facilitates e-prescription and ordering of laboratory results; prints pathology requests and consent forms; and includes information for patient education regarding their care. For example, the physical examination section of an H&P (history and physical examination) can be generated with a few taps on the screen, translating into increased efficiency and productivity.
The visual nature of dermatology demands the use of images, and as such, photographs have become integral in the diagnosis and follow-up of dermatology patients. Digital and dermatoscopic images not only help to eliminate unnecessary biopsies but also can promote early detection and management of malignancies.6 Thus, the capability to link photographs to a patient's medical record using an EMR is an invaluable gain. However, employing this feature in clinical practice has ethical implications that must be addressed, given that taking photographs can evoke an avoidable fear in patients regarding unlawful dissemination or unnecessary exposure of these images to physicians who do not need to access them.6 Thus, guidelines must be set for uploading, de-identifying, and annotating patient photographs, and only physicians involved in the care of the patient should be allocated access.4,6
EMR Platforms
When computers were first introduced into examination rooms, many physicians were reluctant, as computers were thought to disconnect physicians from patients, arousing a sense of remoteness and further depersonalizing the encounter as physicians spent more time typing and less time making meaningful eye contact with patients.5 The practice of dermatology requires patient-centered communication that serves to enhance the quality of care while at the same time allowing physicians to fulfill professional competencies and reduce medical errors, which may ultimately translate into patient satisfaction.7 In fact, when the patient-physician relationship is interrupted, patients are more likely to pursue legal action in the wake of a bad treatment outcome.5
Employing a tablet-friendly EMR can help circumvent (or at least minimize) this problem by providing the physician with a light, user-friendly device that eliminates the need for laptops or desktop computers.4 Going one step further, the utilization of tablets eliminates the need for accessory digital cameras, as most tablets come with built-in high-resolution cameras for capturing clinical photographs and immediately linking them to the patient's medical record. Lastly, tablet technology allows physicians to access consent forms while in the examination room with the patient to more readily obtain a signature for procedural or research consent.4,8
Interoperability, MU, and Quality of Care
The real goal in nationwide implementation of EMR technology is to accomplish MU criteria. Different EMRs should not only allow data to be imported and exported but ideally should be compatible and interoperable with one another.2 The myriad of different EMR platforms available impedes maximal functionality as MU moves into its final stage. For example, if an academic dermatology program in the setting of a larger hospital is required to use the generic hospital EMR, the dermatologist's specific needs may not be effectively met; on the other hand, if a dermatology-specific EMR is implemented, access to the hospital's larger database of patient information may be sacrificed. Optimal EMR systems should be designed to allow specificity for a given specialty while being able to receive and integrate laboratory values, dermatopathology and radiology results, and notes from consultations by other physicians. Such integration may reduce duplicate services, increase patient satisfaction, and fulfill MU criteria. In fact, the fear of many physicians, especially those in a field such as dermatology, are the unwanted costs that come with implementation of an EMR system that will soon become impractical due to compatibility issues.2 As a result, until a system that can meet the needs of multiple specialties is developed, dermatologists and other physicians upgrading to an EMR should consider implementing a system that is compatible with nearby hospitals, other specialists' offices, and diagnostic centers to maximize interoperability at the local level.2,3
Electronic medical record systems that interoperate also provide the ability to set forth performance measures for physicians aimed at improving quality of care. As our health care system moves toward a pay-for-performance model, EMRs will become a tool to determine if standards of care have been met, unnecessary diagnostic tests have been avoided, and unwanted outcomes have been minimized. These measures will usher in a new era of medicine in which physicians strive to improve the care provided to their patients and receive increases in their reimbursements, while patient outcomes and satisfaction are improved.9
Academics, Education, Research, and Residency
The practicality of EMRs in dermatology may best be appreciated in academic settings. Electronic medical records serve as a repository of coded information that is neatly organized and can be rapidly searched, allowing for use as a powerful research tool. As an example, physicians can use EMR systems to identify patients with specific qualifications and study outcome variables over time.2,3 Additionally, with the rise of interoperable systems, we can expect a new dawn in medical research as more information becomes available to clinical investigators, opening doors to endless possibilities for evidence-based care.2,3,8
Another advantage of EMRs is their utility in residency programs that are charged with the task of ensuring resident competency via exposure to a comprehensive host of clinical encounters. An EMR system uniquely allows residents and attending dermatologists to monitor adequate exposure to general, pediatric, complex medical, procedural, and dermatopathologic cases, and to track the number of procedures performed by the residents by directly linking the information into the Accreditation Council for Graduate Medical Education procedure log.
Furthermore, due to the dominance of digital and dermatoscopic images in the field, interoperable EMRs could be used to construct a database of clinical and dermatopathologic specimens that not only can be used in educating residents but also may serve as a powerful reference tool in the diagnosis of complex and rare cases.8 Another often unrecognized advantage of EMRs is their utility in teledermatology. With the interoperable EMRs within academic institutions, teledermatology can be used locally and nationally for rapid consultation with high diagnostic validity,10 which has the burgeoning potential of providing patients with quicker time to diagnosis considering the dermatologist shortage in various parts of the country.
Lastly, the implementation of EMRs in residency programs has the additional benefit of exposing residents and medical students to emerging technology early on in their careers and fosters a degree of familiarity and comfort that may lead to implementation of EMRs in their future practices.2 For dermatologists in-training, early exposure to these technologies also may serve as a way to develop an interactive interview style and adapt to the presence of EMRs in examination rooms without sacrificing quality of care and meaningful patient interaction.7
Conclusion
Electronic medical records are already becoming an integral part of many hospitals and private dermatology practices. Although EMRs provide potential benefits that can be expected to ultimately outweigh the associated costs in larger settings such as hospitals, residency programs, and multidisciplinary practices, EMRs may not be immediately beneficial to physicians in private practices or those approaching the end of their careers. Although a perfect system may be unattainable, development of interoperable systems designed to meet the needs of specialties such as dermatology are essential in attaining a comprehensive patient medical profile, improving quality of care, minimizing costs, reducing medical errors, and maximizing patient satisfaction.
- Health IT and health information exchange basics. Office of the National Coordinator for Health Information Technology website. http://www.healthit.gov/providers-professionals/learn-ehr-basics. Reviewed January 8, 2018. Accessed July 10, 2018.
- Grosshandler JA, Tulbert B, Kaufmann MD, et al. The electronic medical record in dermatology. Arch Dermatol. 2010;146:1031-1036.
- Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
- Kaufmann MD, Desai S. Special requirements for electronic health records in dermatology. Semin Cutan Med Surg. 2012;31:160-162.
- Wheeland RG. Separating EMR implementation hype from fact. Dermatology Times. http://www.dermatologytimes.com/modern-medicine-now/separating-emr-implementation-hype-fact. Published July 1, 2012. Accessed July 16, 2018.
- Lakdawala N, Bercovitch L, Grant-Kels JM. A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J Am Acad Dermatol. 2013;69:473-475.
- Nguyen TV, Hong J, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part I: patient-centered communication. J Am Acad Dermatol. 2013;68:353.e1-8.
- Ratner D, Thomas CO, Bickers D. The uses of digital photography in dermatology. J Am Acad Dermatol. 1999;41(5, pt 1):749-756.
- Wilson RL, Feldman SR. Physician performance measures in dermatology. J Am Acad Dermatol. 2010;63:E29-E35.
- Vañó-Galván S, Hidalgo A, Aguayo-Leiva I, et al. Store-and-forward teledermatology: assessment of validity in a series of 2000 observations [in Spanish]. Actas Dermosifiliogr. 2011;102:277-283.
- Health IT and health information exchange basics. Office of the National Coordinator for Health Information Technology website. http://www.healthit.gov/providers-professionals/learn-ehr-basics. Reviewed January 8, 2018. Accessed July 10, 2018.
- Grosshandler JA, Tulbert B, Kaufmann MD, et al. The electronic medical record in dermatology. Arch Dermatol. 2010;146:1031-1036.
- Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
- Kaufmann MD, Desai S. Special requirements for electronic health records in dermatology. Semin Cutan Med Surg. 2012;31:160-162.
- Wheeland RG. Separating EMR implementation hype from fact. Dermatology Times. http://www.dermatologytimes.com/modern-medicine-now/separating-emr-implementation-hype-fact. Published July 1, 2012. Accessed July 16, 2018.
- Lakdawala N, Bercovitch L, Grant-Kels JM. A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J Am Acad Dermatol. 2013;69:473-475.
- Nguyen TV, Hong J, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part I: patient-centered communication. J Am Acad Dermatol. 2013;68:353.e1-8.
- Ratner D, Thomas CO, Bickers D. The uses of digital photography in dermatology. J Am Acad Dermatol. 1999;41(5, pt 1):749-756.
- Wilson RL, Feldman SR. Physician performance measures in dermatology. J Am Acad Dermatol. 2010;63:E29-E35.
- Vañó-Galván S, Hidalgo A, Aguayo-Leiva I, et al. Store-and-forward teledermatology: assessment of validity in a series of 2000 observations [in Spanish]. Actas Dermosifiliogr. 2011;102:277-283.
Keeping the doctor-patient relationship at the office
I recently picked up my daughter from summer camp, and on the 5-hour drive home she kept texting people back and forth. I asked her if they were other campers or counselors she’d befriended.
She said yes, they were other campers she’d met, but was horrified that I thought some might be counselors. Counselors, understandably, aren’t allowed to have any contact with kids outside of camp. Not by text, Instagram, Facebook, or any other modern social contrivances.
That probably should have occurred to me before I even asked. It makes sense.
I keep a similar policy with patients.
Nothing against them: The majority are decent people, and there are a few I could easily see being social friends with – meeting for dinner, going to a basketball game ... but I won’t.
Like the kids and counselors at camp, I need to keep a distance between myself and patients. I don’t have any social media accounts, anyway, but I keep the relationship confined to my office.
Keeping an emotional distance with patients makes it easier to do this job. While we may genuinely care about them and are trying to help, it’s important to be objective. Seeing them through the lens of friendship might affect the decision-making process.
The divider of professionalism is there for a good reason, across many fields. It allows us to try and think clearly, to give good and bad news, and make diagnostic and treatment decisions as rationally as possible, based on scientific evidence and each individual’s circumstances.
It’s what makes good medicine possible. I wouldn’t want it to be any other way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I recently picked up my daughter from summer camp, and on the 5-hour drive home she kept texting people back and forth. I asked her if they were other campers or counselors she’d befriended.
She said yes, they were other campers she’d met, but was horrified that I thought some might be counselors. Counselors, understandably, aren’t allowed to have any contact with kids outside of camp. Not by text, Instagram, Facebook, or any other modern social contrivances.
That probably should have occurred to me before I even asked. It makes sense.
I keep a similar policy with patients.
Nothing against them: The majority are decent people, and there are a few I could easily see being social friends with – meeting for dinner, going to a basketball game ... but I won’t.
Like the kids and counselors at camp, I need to keep a distance between myself and patients. I don’t have any social media accounts, anyway, but I keep the relationship confined to my office.
Keeping an emotional distance with patients makes it easier to do this job. While we may genuinely care about them and are trying to help, it’s important to be objective. Seeing them through the lens of friendship might affect the decision-making process.
The divider of professionalism is there for a good reason, across many fields. It allows us to try and think clearly, to give good and bad news, and make diagnostic and treatment decisions as rationally as possible, based on scientific evidence and each individual’s circumstances.
It’s what makes good medicine possible. I wouldn’t want it to be any other way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I recently picked up my daughter from summer camp, and on the 5-hour drive home she kept texting people back and forth. I asked her if they were other campers or counselors she’d befriended.
She said yes, they were other campers she’d met, but was horrified that I thought some might be counselors. Counselors, understandably, aren’t allowed to have any contact with kids outside of camp. Not by text, Instagram, Facebook, or any other modern social contrivances.
That probably should have occurred to me before I even asked. It makes sense.
I keep a similar policy with patients.
Nothing against them: The majority are decent people, and there are a few I could easily see being social friends with – meeting for dinner, going to a basketball game ... but I won’t.
Like the kids and counselors at camp, I need to keep a distance between myself and patients. I don’t have any social media accounts, anyway, but I keep the relationship confined to my office.
Keeping an emotional distance with patients makes it easier to do this job. While we may genuinely care about them and are trying to help, it’s important to be objective. Seeing them through the lens of friendship might affect the decision-making process.
The divider of professionalism is there for a good reason, across many fields. It allows us to try and think clearly, to give good and bad news, and make diagnostic and treatment decisions as rationally as possible, based on scientific evidence and each individual’s circumstances.
It’s what makes good medicine possible. I wouldn’t want it to be any other way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.