User login
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

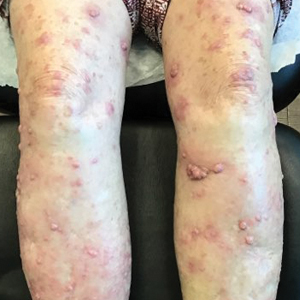
The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Pediatric Obesity Specialists Struggle to Get GLP-1s
While adults, many of whom don’t meet the clinical definition of obesity, scramble to procure glucagon-like peptide 1 (GLP-1) agonists for weight loss, pediatric obesity specialists said their young patients who could benefit more over the long term often are unable to access the potentially life-altering medications.
The US Food and Drug Administration (FDA) approved two GLP-1 agonists — both marketed by Novo Nordisk — for use in adolescents aged ≥ 12 years: Wegovy (semaglutide) in December 2022 and Saxenda (liraglutide) in December 2020. Novo Nordisk and Eli Lilly — which makes the dual glucose-dependent insulinotropic polypetide/GLP-1 agonist tirzepatide (Zepbound) — are also investigating the drugs for obesity in children as young as age 6 years. The crushing demand for semaglutide in the last year — driving a thriving market in compounded versions and online prescriptions — has made it increasingly difficult to find pharmacies that can fill prescriptions, pediatricians told this news organization.
“It’s been more difficult to get people initiated now than it was a year ago,” said Brooke Sweeney, MD, medical director of weight management services at Children’s Mercy in Kansas City, Missouri. “Because of the supply issues, for the most part we›re not starting anyone new because I don›t have enough medication to keep my patients on it who are already on it,” she said.
Sarah Raatz, MD, a pediatrician at the University of Minnesota’s Center for Pediatric Obesity Medicine, said, “I actually haven’t really been prescribing many of these medications as of late.” Both liraglutide and semaglutide “are largely unavailable or quite hard to get a hold of,” Dr. Raatz told this news organization.
Susma Shanti Vaidya, MPH, MD, associate medical director of the IDEAL pediatric obesity clinic at Children›s National Hospital in Washington, DC, said that patients taking GLP-1 agonists in her practice have reduced their body mass index and have seen resolution of prediabetes, diabetes, and fatty liver disease. «I had one patient who had severe obstructive sleep apnea which resolved with semaglutide.»
But when they can’t find the medications, it can lead to a plateauing of weight loss and a reversal of hard-won victories, Dr. Vaidya said.
Insurance Denials Also Growing
In January 2023, the American Academy of Pediatrics urged aggressive treatment of childhood obesity, including using FDA-approved medications such as GLP-1 agonists combined with lifestyle and dietary modifications.
The US Preventive Services Task Force, however, has issued a draft proposal that recommends a variety of lifestyle and behavior modification interventions for children and adolescents but says the evidence does not yet support recommending bariatric surgery or medications.
Insurance coverage for children — even for FDA-approved indications and the age 12-and-over population — has become increasingly difficult, said the pediatric obesity specialists. Insurers are also creating hurdles that make getting coverage more difficult, they said.
Some insurers track an adolescent’s weight trajectory, “and if they’re not meeting a certain response threshold set by the insurance company, then they can pull coverage and then we have to try to advocate for why continued coverage might be beneficial and necessary,” Dr. Raatz said.
Insurers in the region around Children’s Mercy are erecting similar barriers, said Sweeney. Interim weight loss goals are challenging in pediatrics — given that adolescents are constantly changing and growing, she said.
Dr. Vaidya said she’s had success with commercial insurers but that the Washington, DC, and Maryland Medicaid programs have been stingier.
All the pediatricians said they expect greater restrictions in 2024.
Dr. Vaidya said some patients told her they had been notified that prior authorization will be required for new prescriptions for a GLP-1 agonist.
“We will just kind of be forced to see what happens when these medications are taken away from patients who have benefited from them,” Dr. Raatz said.
Some Parents Asking for GLP-1 Agonists
Pediatric obesity specialists said more parents are asking if a GLP-1 agonist might be appropriate for their children this year than in 2022.
Dr. Sweeney said parents ask for the medications when they feel they have exhausted all other options for their children. “These parents are not coming because they are concerned about the cosmetic effects of the weight,” she said. In most cases, children she sees have been struggling for years with extreme hunger and lack of satiety and may have prediabetes or diabetes. Many are being bullied in school because of their weight. They have only marginally been helped by interventions suggested by primary care or dietitians or other specialists, Dr. Sweeney said.
“Starting semaglutide really is life-changing for some of these patients,” Dr. Vaidya said. One patient said, “it just stopped the food chatter,” she added, noting that the adolescent no longer felt ruled by cravings.
In a recent poll by Morning Consult, 65% of parents of children with weight-related issues said they would be interested in GLP-1 agonists for their kids. A third of all parents said they would be interested in having their children use the drugs if they were available.
Lifelong Medication?
Parents — and adolescents — are generally counseled that obesity is a chronic disease and GLP-1 agonists are likely a lifelong treatment.
With the medications, “our first step is to get induction of weight loss and get your set point decreased enough that we can get you to a healthier weight for your body,” Dr. Sweeney said.
She tells patients and families, “I can’t tell you that you’re necessarily going to be on this medication at this dose for the rest of your life, but you will need treatment for life.”
Based on current knowledge, the risks for lifelong obesity outweigh the risk for the medications. Dr. Sweeney said she would like to see more data. “There absolutely is an evidence gap, and we need more information on the long-term effectiveness and safety.”
“When we start kids on this medication, I’m very clear that we are going to try to get to the lowest effective dose,” Dr. Vaidya said. She also emphasizes to parents that the medications must be used in conjunction with continued lifestyle modifications. She expressed hope that as clinicians gain more experience, and patients’ comorbidities resolve, perhaps it will be possible in some cases to take individuals “off for a period of time, with the understanding that they might have to go back on in a few months.”
“We’re weighing the pros and cons of being on a medication long term but we’re also weighing the pros and cons of weight-related health complications long term,” Dr. Raatz said.
Dr. Raatz also said clinicians have much to learn about the long-term safety of GLP-1 agonists in their pediatric patients.
She tells parents and families, “we expect that this is going to be a long-term medication, and this is going to be something that we’re going to continue to monitor.”
Dr. Sweeney reports that she is a speaker and unpaid consultant on Rhythm Pharmaceuticals’ Imcivree (setmelanotide) medication and that she consults for Eli Lilly. Dr. Raatz is a coprincipal investigator for a Novo Nordisk trial of semaglutide in young children and will be a co-PI for a similar trial for Eli Lilly’s tirzepatide but receives no consulting fees or honoraria. Dr. Vaidya reported no conflicts.
A version of this article appeared on Medscape.com.
While adults, many of whom don’t meet the clinical definition of obesity, scramble to procure glucagon-like peptide 1 (GLP-1) agonists for weight loss, pediatric obesity specialists said their young patients who could benefit more over the long term often are unable to access the potentially life-altering medications.
The US Food and Drug Administration (FDA) approved two GLP-1 agonists — both marketed by Novo Nordisk — for use in adolescents aged ≥ 12 years: Wegovy (semaglutide) in December 2022 and Saxenda (liraglutide) in December 2020. Novo Nordisk and Eli Lilly — which makes the dual glucose-dependent insulinotropic polypetide/GLP-1 agonist tirzepatide (Zepbound) — are also investigating the drugs for obesity in children as young as age 6 years. The crushing demand for semaglutide in the last year — driving a thriving market in compounded versions and online prescriptions — has made it increasingly difficult to find pharmacies that can fill prescriptions, pediatricians told this news organization.
“It’s been more difficult to get people initiated now than it was a year ago,” said Brooke Sweeney, MD, medical director of weight management services at Children’s Mercy in Kansas City, Missouri. “Because of the supply issues, for the most part we›re not starting anyone new because I don›t have enough medication to keep my patients on it who are already on it,” she said.
Sarah Raatz, MD, a pediatrician at the University of Minnesota’s Center for Pediatric Obesity Medicine, said, “I actually haven’t really been prescribing many of these medications as of late.” Both liraglutide and semaglutide “are largely unavailable or quite hard to get a hold of,” Dr. Raatz told this news organization.
Susma Shanti Vaidya, MPH, MD, associate medical director of the IDEAL pediatric obesity clinic at Children›s National Hospital in Washington, DC, said that patients taking GLP-1 agonists in her practice have reduced their body mass index and have seen resolution of prediabetes, diabetes, and fatty liver disease. «I had one patient who had severe obstructive sleep apnea which resolved with semaglutide.»
But when they can’t find the medications, it can lead to a plateauing of weight loss and a reversal of hard-won victories, Dr. Vaidya said.
Insurance Denials Also Growing
In January 2023, the American Academy of Pediatrics urged aggressive treatment of childhood obesity, including using FDA-approved medications such as GLP-1 agonists combined with lifestyle and dietary modifications.
The US Preventive Services Task Force, however, has issued a draft proposal that recommends a variety of lifestyle and behavior modification interventions for children and adolescents but says the evidence does not yet support recommending bariatric surgery or medications.
Insurance coverage for children — even for FDA-approved indications and the age 12-and-over population — has become increasingly difficult, said the pediatric obesity specialists. Insurers are also creating hurdles that make getting coverage more difficult, they said.
Some insurers track an adolescent’s weight trajectory, “and if they’re not meeting a certain response threshold set by the insurance company, then they can pull coverage and then we have to try to advocate for why continued coverage might be beneficial and necessary,” Dr. Raatz said.
Insurers in the region around Children’s Mercy are erecting similar barriers, said Sweeney. Interim weight loss goals are challenging in pediatrics — given that adolescents are constantly changing and growing, she said.
Dr. Vaidya said she’s had success with commercial insurers but that the Washington, DC, and Maryland Medicaid programs have been stingier.
All the pediatricians said they expect greater restrictions in 2024.
Dr. Vaidya said some patients told her they had been notified that prior authorization will be required for new prescriptions for a GLP-1 agonist.
“We will just kind of be forced to see what happens when these medications are taken away from patients who have benefited from them,” Dr. Raatz said.
Some Parents Asking for GLP-1 Agonists
Pediatric obesity specialists said more parents are asking if a GLP-1 agonist might be appropriate for their children this year than in 2022.
Dr. Sweeney said parents ask for the medications when they feel they have exhausted all other options for their children. “These parents are not coming because they are concerned about the cosmetic effects of the weight,” she said. In most cases, children she sees have been struggling for years with extreme hunger and lack of satiety and may have prediabetes or diabetes. Many are being bullied in school because of their weight. They have only marginally been helped by interventions suggested by primary care or dietitians or other specialists, Dr. Sweeney said.
“Starting semaglutide really is life-changing for some of these patients,” Dr. Vaidya said. One patient said, “it just stopped the food chatter,” she added, noting that the adolescent no longer felt ruled by cravings.
In a recent poll by Morning Consult, 65% of parents of children with weight-related issues said they would be interested in GLP-1 agonists for their kids. A third of all parents said they would be interested in having their children use the drugs if they were available.
Lifelong Medication?
Parents — and adolescents — are generally counseled that obesity is a chronic disease and GLP-1 agonists are likely a lifelong treatment.
With the medications, “our first step is to get induction of weight loss and get your set point decreased enough that we can get you to a healthier weight for your body,” Dr. Sweeney said.
She tells patients and families, “I can’t tell you that you’re necessarily going to be on this medication at this dose for the rest of your life, but you will need treatment for life.”
Based on current knowledge, the risks for lifelong obesity outweigh the risk for the medications. Dr. Sweeney said she would like to see more data. “There absolutely is an evidence gap, and we need more information on the long-term effectiveness and safety.”
“When we start kids on this medication, I’m very clear that we are going to try to get to the lowest effective dose,” Dr. Vaidya said. She also emphasizes to parents that the medications must be used in conjunction with continued lifestyle modifications. She expressed hope that as clinicians gain more experience, and patients’ comorbidities resolve, perhaps it will be possible in some cases to take individuals “off for a period of time, with the understanding that they might have to go back on in a few months.”
“We’re weighing the pros and cons of being on a medication long term but we’re also weighing the pros and cons of weight-related health complications long term,” Dr. Raatz said.
Dr. Raatz also said clinicians have much to learn about the long-term safety of GLP-1 agonists in their pediatric patients.
She tells parents and families, “we expect that this is going to be a long-term medication, and this is going to be something that we’re going to continue to monitor.”
Dr. Sweeney reports that she is a speaker and unpaid consultant on Rhythm Pharmaceuticals’ Imcivree (setmelanotide) medication and that she consults for Eli Lilly. Dr. Raatz is a coprincipal investigator for a Novo Nordisk trial of semaglutide in young children and will be a co-PI for a similar trial for Eli Lilly’s tirzepatide but receives no consulting fees or honoraria. Dr. Vaidya reported no conflicts.
A version of this article appeared on Medscape.com.
While adults, many of whom don’t meet the clinical definition of obesity, scramble to procure glucagon-like peptide 1 (GLP-1) agonists for weight loss, pediatric obesity specialists said their young patients who could benefit more over the long term often are unable to access the potentially life-altering medications.
The US Food and Drug Administration (FDA) approved two GLP-1 agonists — both marketed by Novo Nordisk — for use in adolescents aged ≥ 12 years: Wegovy (semaglutide) in December 2022 and Saxenda (liraglutide) in December 2020. Novo Nordisk and Eli Lilly — which makes the dual glucose-dependent insulinotropic polypetide/GLP-1 agonist tirzepatide (Zepbound) — are also investigating the drugs for obesity in children as young as age 6 years. The crushing demand for semaglutide in the last year — driving a thriving market in compounded versions and online prescriptions — has made it increasingly difficult to find pharmacies that can fill prescriptions, pediatricians told this news organization.
“It’s been more difficult to get people initiated now than it was a year ago,” said Brooke Sweeney, MD, medical director of weight management services at Children’s Mercy in Kansas City, Missouri. “Because of the supply issues, for the most part we›re not starting anyone new because I don›t have enough medication to keep my patients on it who are already on it,” she said.
Sarah Raatz, MD, a pediatrician at the University of Minnesota’s Center for Pediatric Obesity Medicine, said, “I actually haven’t really been prescribing many of these medications as of late.” Both liraglutide and semaglutide “are largely unavailable or quite hard to get a hold of,” Dr. Raatz told this news organization.
Susma Shanti Vaidya, MPH, MD, associate medical director of the IDEAL pediatric obesity clinic at Children›s National Hospital in Washington, DC, said that patients taking GLP-1 agonists in her practice have reduced their body mass index and have seen resolution of prediabetes, diabetes, and fatty liver disease. «I had one patient who had severe obstructive sleep apnea which resolved with semaglutide.»
But when they can’t find the medications, it can lead to a plateauing of weight loss and a reversal of hard-won victories, Dr. Vaidya said.
Insurance Denials Also Growing
In January 2023, the American Academy of Pediatrics urged aggressive treatment of childhood obesity, including using FDA-approved medications such as GLP-1 agonists combined with lifestyle and dietary modifications.
The US Preventive Services Task Force, however, has issued a draft proposal that recommends a variety of lifestyle and behavior modification interventions for children and adolescents but says the evidence does not yet support recommending bariatric surgery or medications.
Insurance coverage for children — even for FDA-approved indications and the age 12-and-over population — has become increasingly difficult, said the pediatric obesity specialists. Insurers are also creating hurdles that make getting coverage more difficult, they said.
Some insurers track an adolescent’s weight trajectory, “and if they’re not meeting a certain response threshold set by the insurance company, then they can pull coverage and then we have to try to advocate for why continued coverage might be beneficial and necessary,” Dr. Raatz said.
Insurers in the region around Children’s Mercy are erecting similar barriers, said Sweeney. Interim weight loss goals are challenging in pediatrics — given that adolescents are constantly changing and growing, she said.
Dr. Vaidya said she’s had success with commercial insurers but that the Washington, DC, and Maryland Medicaid programs have been stingier.
All the pediatricians said they expect greater restrictions in 2024.
Dr. Vaidya said some patients told her they had been notified that prior authorization will be required for new prescriptions for a GLP-1 agonist.
“We will just kind of be forced to see what happens when these medications are taken away from patients who have benefited from them,” Dr. Raatz said.
Some Parents Asking for GLP-1 Agonists
Pediatric obesity specialists said more parents are asking if a GLP-1 agonist might be appropriate for their children this year than in 2022.
Dr. Sweeney said parents ask for the medications when they feel they have exhausted all other options for their children. “These parents are not coming because they are concerned about the cosmetic effects of the weight,” she said. In most cases, children she sees have been struggling for years with extreme hunger and lack of satiety and may have prediabetes or diabetes. Many are being bullied in school because of their weight. They have only marginally been helped by interventions suggested by primary care or dietitians or other specialists, Dr. Sweeney said.
“Starting semaglutide really is life-changing for some of these patients,” Dr. Vaidya said. One patient said, “it just stopped the food chatter,” she added, noting that the adolescent no longer felt ruled by cravings.
In a recent poll by Morning Consult, 65% of parents of children with weight-related issues said they would be interested in GLP-1 agonists for their kids. A third of all parents said they would be interested in having their children use the drugs if they were available.
Lifelong Medication?
Parents — and adolescents — are generally counseled that obesity is a chronic disease and GLP-1 agonists are likely a lifelong treatment.
With the medications, “our first step is to get induction of weight loss and get your set point decreased enough that we can get you to a healthier weight for your body,” Dr. Sweeney said.
She tells patients and families, “I can’t tell you that you’re necessarily going to be on this medication at this dose for the rest of your life, but you will need treatment for life.”
Based on current knowledge, the risks for lifelong obesity outweigh the risk for the medications. Dr. Sweeney said she would like to see more data. “There absolutely is an evidence gap, and we need more information on the long-term effectiveness and safety.”
“When we start kids on this medication, I’m very clear that we are going to try to get to the lowest effective dose,” Dr. Vaidya said. She also emphasizes to parents that the medications must be used in conjunction with continued lifestyle modifications. She expressed hope that as clinicians gain more experience, and patients’ comorbidities resolve, perhaps it will be possible in some cases to take individuals “off for a period of time, with the understanding that they might have to go back on in a few months.”
“We’re weighing the pros and cons of being on a medication long term but we’re also weighing the pros and cons of weight-related health complications long term,” Dr. Raatz said.
Dr. Raatz also said clinicians have much to learn about the long-term safety of GLP-1 agonists in their pediatric patients.
She tells parents and families, “we expect that this is going to be a long-term medication, and this is going to be something that we’re going to continue to monitor.”
Dr. Sweeney reports that she is a speaker and unpaid consultant on Rhythm Pharmaceuticals’ Imcivree (setmelanotide) medication and that she consults for Eli Lilly. Dr. Raatz is a coprincipal investigator for a Novo Nordisk trial of semaglutide in young children and will be a co-PI for a similar trial for Eli Lilly’s tirzepatide but receives no consulting fees or honoraria. Dr. Vaidya reported no conflicts.
A version of this article appeared on Medscape.com.
Hospital Adverse Events Rise After Private Equity Acquisition
Hospital-acquired adverse events or conditions including falls and infections increased by approximately 25% after hospitals’ acquisition by private equity compared with control hospitals, on the basis of a study of Medicare claims for more than 4,500,000 hospitalizations.
“Prior research on private equity in health care showed that acquisition is associated with higher charges, prices, and spending; however, the implications for quality of care and patient outcomes remained less understood,” corresponding author Zirui Song, MD, of Harvard Medical School, Boston, said in an interview. “This was particularly true for measures of clinical quality that were less susceptible to changes in patient mix or coding behavior, such as hospital-acquired adverse events.”
In the study, published in JAMA, the researchers compared data from 100% Medicare Part A claims for 662,095 hospitalizations at 51 hospitals acquired by private equities and 4,160,720 hospitalizations at 259 control hospitals. The hospitalizations occurred between 2009 and 2019. The researchers also used a difference-in-differences design to evaluate hospitalizations from 3 years before to 3 years after acquisition, controlling for patient and hospital attributes.
Hospital-acquired adverse events as defined by the US Centers for Medicare & Medicaid Services included falls, infections, stage III or IV pressure ulcers, foreign objects retained after surgery, air embolism, and blood incompatibility.
Overall, Medicare patients in private equity hospitals experienced a 25.4% increase in hospital-acquired conditions compared with those in control hospitals through a period of up to 3 years after acquisition, with a difference of 4.6 additional hospital-acquired conditions per 10,000 hospitalizations (P = .004). Central line-associated bloodstream infections accounted for 37.7% of the increase (P = .04), despite a 16.2% decrease in placement of central lines, and falls accounted for 27.3% (P = .02).
Notably, the incidence of surgical site infections increased from 10.8 per 10,000 hospitalizations before acquisition to 21.6 per 10,000 hospitalizations after acquisition, despite a reduction of 8.1% in surgical volume. By contrast, surgical site infections decreased at control hospitals over the study period.
In-hospital mortality decreased slightly at private equity hospitals compared with the control hospitals, but there was no differential change in mortality by 30 days after hospital discharge. The slight difference might be caused by the trend in slightly younger Medicare beneficiaries treated at private equity hospitals; these patients were less likely to be eligible for both Medicaid and Medicare and were more likely to be transferred to other hospitals, the researchers noted.
The findings were limited by several factors including the lack of generalizability to all private equity-acquired hospitals and to non-Medicare patients, the researchers noted. Other limitations include the use of the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes that might have failed to capture all hospital-acquired conditions and the inability to account for all confounding factors.
However, the results suggest that private equity acquisition was associated with increased hospital-acquired adverse events and highlight concerns about the impact of private equity ownership on healthcare delivery, the researchers concluded.
In a related story published in July 2023, this news organization described a report showing an association between private equity ownership of medical practices and increased consumer prices for multiple medical specialties.
“Medicare patients admitted to private equity-owned hospitals experienced, on average, an 25% increase in hospital-acquired adverse events after the hospital was bought compared to similar patients at hospitals not acquired by private equity firms. We were surprised by the extent of this change relative to the comparison (non-private equity) hospitals, including the sizable increase in central line-associated bloodstream infections and the doubling of surgical site infections at private equity hospitals — both of which went down at the comparison hospitals during the same period,” Dr. Song said in an interview.
“A key implication is that patients, providers, and policymakers might be more attuned to the potential clinical impact of private equity ownership in the delivery system. Given that a plausible explanation for these findings is reductions in clinician staffing, clinical organizations and policymakers might also be more aware of cost-cutting strategies after acquisition,” Dr. Song said. “Prior research has shown that hospitals, nursing homes, and physician practices experience staffing cuts after private equity acquisition, which is a common way to reduce operating costs and boost the profitability of acquired entities,” he noted.
“More research is needed to understand the impact of private equity acquisitions across health care settings and the potential effects of policy levers that aim to protect patients and societal resources,” said Dr. Song, who coauthored an article outlining a policy framework for addressing private equity in healthcare, published in JAMA in April 2023. “Potential regulatory remedies include minimum staffing ratios, antitrust enforcement, mitigating the financial risk of such acquisitions, increasing the transparency of these acquisitions, and protecting patients and society from the higher prices of care attributed to this model of provider ownership,” he said.
Patients Pay the Price of Private Equity Acquisition
“The exponential growth in private equity ownership in hospital and physician practices in the past few decades has left a majority of health care providers disillusioned with cost-cutting practices resulting in staffing reductions and ratios that sacrifice patient care as part of their approach to running clinical operations ‘lean,’ ” Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, NY, said in an interview.
“While private equity companies argue that such practices are essential to meet their bottom line and increase operating margins, it doesn’t translate into ideal care for patients; lean practices in staffing which focus on profits at the expense of patient safety and quality of care.
“When you look at patient outcomes, it is the patients who ultimately pay the price — not the shareholders,” Dr. Glatter said. “This translates to higher risks of hospital-acquired complications including falls and blood-borne infections, including surgical site infections, as noted by the authors of the current study when private equity took over operations in hospitals.
Dr. Glatter said he was not surprised by the findings. “In my world, patient care and safety come first. Period,” he said. “Would you want your family’s health and well-being sacrificed in the name of company profits? I think it’s a rhetorical question, but one that every health care provider who works in a hospital or practice run by private equity must consider.”
Despite a decline in utilization at private equity hospitals as noted in the current study, hospital-acquired infections and adverse outcomes still increased, illustrating a decline in quality of care, said Dr. Glatter. “While these disparities were not evident when looking at 30-day outcomes, they demonstrate how operational changes impact patient outcomes in the near term. Having younger and healthier patients, and fewer Medicare and Medicaid patients combined with more hospital transfers to non–private equity run hospitals, resulted in lower in-hospital mortality in the near term, which was not apparent at 30 days post discharge,” he said.
“The explosion of hospital mergers and consolidation in the past several decades has led to skyrocketing health care costs at the expense of patient satisfaction, but also health care providers’ autonomy to manage and maintain quality care for their patients,” Dr. Glatter said.
“It’s important to understand that private equity’s interests are primarily aligned with their shareholder’s interests, as opposed to patients’ outcomes and interests,” Dr. Glatter told this news organization. “Within 5-7 years, the goal is to increase operating margins and profits and then sell a practice or hospital, which is ultimately part of a ‘health care portfolio,’ ” he said.
Additional research is needed to examine whether other hospital-acquired conditions including pressure sores, catheter-associated UTIs, methicillin-resistant Staphylococcus aureus infections, Clostridium difficile infections, and nosocomial pneumonia have increased in hospitals following private equity acquisition, given the overall national decline in these events, he said.
“At the same time, it is vital to also look at management and readmission rates for patients with strokes, heart attacks, and congestive heart failure in hospitals that are run by private equity,” Dr. Glatter noted. “These are important benchmarks of care monitored by CMS that reflect the quality of care that payers ultimately factor into reimbursement.”
Examining the metrics associated with these diagnoses will help in understanding whether private equity-managed facilities are leading to adverse outcomes and mortality, increased length of stay, hospital readmissions, and increased nosocomial infections, apart from other aspects of patient experience, Dr. Glatter added.
The study was supported by the National Heart, Lung, and Blood Institute, the National Institute on Aging, and Arnold Ventures. The researchers had no financial conflicts to disclose. Dr. Glatter had no financial conflicts to disclose and serves on the Medscape Emergency Medicine Editorial Board.
A version of this article appeared on Medscape.com.
Hospital-acquired adverse events or conditions including falls and infections increased by approximately 25% after hospitals’ acquisition by private equity compared with control hospitals, on the basis of a study of Medicare claims for more than 4,500,000 hospitalizations.
“Prior research on private equity in health care showed that acquisition is associated with higher charges, prices, and spending; however, the implications for quality of care and patient outcomes remained less understood,” corresponding author Zirui Song, MD, of Harvard Medical School, Boston, said in an interview. “This was particularly true for measures of clinical quality that were less susceptible to changes in patient mix or coding behavior, such as hospital-acquired adverse events.”
In the study, published in JAMA, the researchers compared data from 100% Medicare Part A claims for 662,095 hospitalizations at 51 hospitals acquired by private equities and 4,160,720 hospitalizations at 259 control hospitals. The hospitalizations occurred between 2009 and 2019. The researchers also used a difference-in-differences design to evaluate hospitalizations from 3 years before to 3 years after acquisition, controlling for patient and hospital attributes.
Hospital-acquired adverse events as defined by the US Centers for Medicare & Medicaid Services included falls, infections, stage III or IV pressure ulcers, foreign objects retained after surgery, air embolism, and blood incompatibility.
Overall, Medicare patients in private equity hospitals experienced a 25.4% increase in hospital-acquired conditions compared with those in control hospitals through a period of up to 3 years after acquisition, with a difference of 4.6 additional hospital-acquired conditions per 10,000 hospitalizations (P = .004). Central line-associated bloodstream infections accounted for 37.7% of the increase (P = .04), despite a 16.2% decrease in placement of central lines, and falls accounted for 27.3% (P = .02).
Notably, the incidence of surgical site infections increased from 10.8 per 10,000 hospitalizations before acquisition to 21.6 per 10,000 hospitalizations after acquisition, despite a reduction of 8.1% in surgical volume. By contrast, surgical site infections decreased at control hospitals over the study period.
In-hospital mortality decreased slightly at private equity hospitals compared with the control hospitals, but there was no differential change in mortality by 30 days after hospital discharge. The slight difference might be caused by the trend in slightly younger Medicare beneficiaries treated at private equity hospitals; these patients were less likely to be eligible for both Medicaid and Medicare and were more likely to be transferred to other hospitals, the researchers noted.
The findings were limited by several factors including the lack of generalizability to all private equity-acquired hospitals and to non-Medicare patients, the researchers noted. Other limitations include the use of the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes that might have failed to capture all hospital-acquired conditions and the inability to account for all confounding factors.
However, the results suggest that private equity acquisition was associated with increased hospital-acquired adverse events and highlight concerns about the impact of private equity ownership on healthcare delivery, the researchers concluded.
In a related story published in July 2023, this news organization described a report showing an association between private equity ownership of medical practices and increased consumer prices for multiple medical specialties.
“Medicare patients admitted to private equity-owned hospitals experienced, on average, an 25% increase in hospital-acquired adverse events after the hospital was bought compared to similar patients at hospitals not acquired by private equity firms. We were surprised by the extent of this change relative to the comparison (non-private equity) hospitals, including the sizable increase in central line-associated bloodstream infections and the doubling of surgical site infections at private equity hospitals — both of which went down at the comparison hospitals during the same period,” Dr. Song said in an interview.
“A key implication is that patients, providers, and policymakers might be more attuned to the potential clinical impact of private equity ownership in the delivery system. Given that a plausible explanation for these findings is reductions in clinician staffing, clinical organizations and policymakers might also be more aware of cost-cutting strategies after acquisition,” Dr. Song said. “Prior research has shown that hospitals, nursing homes, and physician practices experience staffing cuts after private equity acquisition, which is a common way to reduce operating costs and boost the profitability of acquired entities,” he noted.
“More research is needed to understand the impact of private equity acquisitions across health care settings and the potential effects of policy levers that aim to protect patients and societal resources,” said Dr. Song, who coauthored an article outlining a policy framework for addressing private equity in healthcare, published in JAMA in April 2023. “Potential regulatory remedies include minimum staffing ratios, antitrust enforcement, mitigating the financial risk of such acquisitions, increasing the transparency of these acquisitions, and protecting patients and society from the higher prices of care attributed to this model of provider ownership,” he said.
Patients Pay the Price of Private Equity Acquisition
“The exponential growth in private equity ownership in hospital and physician practices in the past few decades has left a majority of health care providers disillusioned with cost-cutting practices resulting in staffing reductions and ratios that sacrifice patient care as part of their approach to running clinical operations ‘lean,’ ” Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, NY, said in an interview.
“While private equity companies argue that such practices are essential to meet their bottom line and increase operating margins, it doesn’t translate into ideal care for patients; lean practices in staffing which focus on profits at the expense of patient safety and quality of care.
“When you look at patient outcomes, it is the patients who ultimately pay the price — not the shareholders,” Dr. Glatter said. “This translates to higher risks of hospital-acquired complications including falls and blood-borne infections, including surgical site infections, as noted by the authors of the current study when private equity took over operations in hospitals.
Dr. Glatter said he was not surprised by the findings. “In my world, patient care and safety come first. Period,” he said. “Would you want your family’s health and well-being sacrificed in the name of company profits? I think it’s a rhetorical question, but one that every health care provider who works in a hospital or practice run by private equity must consider.”
Despite a decline in utilization at private equity hospitals as noted in the current study, hospital-acquired infections and adverse outcomes still increased, illustrating a decline in quality of care, said Dr. Glatter. “While these disparities were not evident when looking at 30-day outcomes, they demonstrate how operational changes impact patient outcomes in the near term. Having younger and healthier patients, and fewer Medicare and Medicaid patients combined with more hospital transfers to non–private equity run hospitals, resulted in lower in-hospital mortality in the near term, which was not apparent at 30 days post discharge,” he said.
“The explosion of hospital mergers and consolidation in the past several decades has led to skyrocketing health care costs at the expense of patient satisfaction, but also health care providers’ autonomy to manage and maintain quality care for their patients,” Dr. Glatter said.
“It’s important to understand that private equity’s interests are primarily aligned with their shareholder’s interests, as opposed to patients’ outcomes and interests,” Dr. Glatter told this news organization. “Within 5-7 years, the goal is to increase operating margins and profits and then sell a practice or hospital, which is ultimately part of a ‘health care portfolio,’ ” he said.
Additional research is needed to examine whether other hospital-acquired conditions including pressure sores, catheter-associated UTIs, methicillin-resistant Staphylococcus aureus infections, Clostridium difficile infections, and nosocomial pneumonia have increased in hospitals following private equity acquisition, given the overall national decline in these events, he said.
“At the same time, it is vital to also look at management and readmission rates for patients with strokes, heart attacks, and congestive heart failure in hospitals that are run by private equity,” Dr. Glatter noted. “These are important benchmarks of care monitored by CMS that reflect the quality of care that payers ultimately factor into reimbursement.”
Examining the metrics associated with these diagnoses will help in understanding whether private equity-managed facilities are leading to adverse outcomes and mortality, increased length of stay, hospital readmissions, and increased nosocomial infections, apart from other aspects of patient experience, Dr. Glatter added.
The study was supported by the National Heart, Lung, and Blood Institute, the National Institute on Aging, and Arnold Ventures. The researchers had no financial conflicts to disclose. Dr. Glatter had no financial conflicts to disclose and serves on the Medscape Emergency Medicine Editorial Board.
A version of this article appeared on Medscape.com.
Hospital-acquired adverse events or conditions including falls and infections increased by approximately 25% after hospitals’ acquisition by private equity compared with control hospitals, on the basis of a study of Medicare claims for more than 4,500,000 hospitalizations.
“Prior research on private equity in health care showed that acquisition is associated with higher charges, prices, and spending; however, the implications for quality of care and patient outcomes remained less understood,” corresponding author Zirui Song, MD, of Harvard Medical School, Boston, said in an interview. “This was particularly true for measures of clinical quality that were less susceptible to changes in patient mix or coding behavior, such as hospital-acquired adverse events.”
In the study, published in JAMA, the researchers compared data from 100% Medicare Part A claims for 662,095 hospitalizations at 51 hospitals acquired by private equities and 4,160,720 hospitalizations at 259 control hospitals. The hospitalizations occurred between 2009 and 2019. The researchers also used a difference-in-differences design to evaluate hospitalizations from 3 years before to 3 years after acquisition, controlling for patient and hospital attributes.
Hospital-acquired adverse events as defined by the US Centers for Medicare & Medicaid Services included falls, infections, stage III or IV pressure ulcers, foreign objects retained after surgery, air embolism, and blood incompatibility.
Overall, Medicare patients in private equity hospitals experienced a 25.4% increase in hospital-acquired conditions compared with those in control hospitals through a period of up to 3 years after acquisition, with a difference of 4.6 additional hospital-acquired conditions per 10,000 hospitalizations (P = .004). Central line-associated bloodstream infections accounted for 37.7% of the increase (P = .04), despite a 16.2% decrease in placement of central lines, and falls accounted for 27.3% (P = .02).
Notably, the incidence of surgical site infections increased from 10.8 per 10,000 hospitalizations before acquisition to 21.6 per 10,000 hospitalizations after acquisition, despite a reduction of 8.1% in surgical volume. By contrast, surgical site infections decreased at control hospitals over the study period.
In-hospital mortality decreased slightly at private equity hospitals compared with the control hospitals, but there was no differential change in mortality by 30 days after hospital discharge. The slight difference might be caused by the trend in slightly younger Medicare beneficiaries treated at private equity hospitals; these patients were less likely to be eligible for both Medicaid and Medicare and were more likely to be transferred to other hospitals, the researchers noted.
The findings were limited by several factors including the lack of generalizability to all private equity-acquired hospitals and to non-Medicare patients, the researchers noted. Other limitations include the use of the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes that might have failed to capture all hospital-acquired conditions and the inability to account for all confounding factors.
However, the results suggest that private equity acquisition was associated with increased hospital-acquired adverse events and highlight concerns about the impact of private equity ownership on healthcare delivery, the researchers concluded.
In a related story published in July 2023, this news organization described a report showing an association between private equity ownership of medical practices and increased consumer prices for multiple medical specialties.
“Medicare patients admitted to private equity-owned hospitals experienced, on average, an 25% increase in hospital-acquired adverse events after the hospital was bought compared to similar patients at hospitals not acquired by private equity firms. We were surprised by the extent of this change relative to the comparison (non-private equity) hospitals, including the sizable increase in central line-associated bloodstream infections and the doubling of surgical site infections at private equity hospitals — both of which went down at the comparison hospitals during the same period,” Dr. Song said in an interview.
“A key implication is that patients, providers, and policymakers might be more attuned to the potential clinical impact of private equity ownership in the delivery system. Given that a plausible explanation for these findings is reductions in clinician staffing, clinical organizations and policymakers might also be more aware of cost-cutting strategies after acquisition,” Dr. Song said. “Prior research has shown that hospitals, nursing homes, and physician practices experience staffing cuts after private equity acquisition, which is a common way to reduce operating costs and boost the profitability of acquired entities,” he noted.
“More research is needed to understand the impact of private equity acquisitions across health care settings and the potential effects of policy levers that aim to protect patients and societal resources,” said Dr. Song, who coauthored an article outlining a policy framework for addressing private equity in healthcare, published in JAMA in April 2023. “Potential regulatory remedies include minimum staffing ratios, antitrust enforcement, mitigating the financial risk of such acquisitions, increasing the transparency of these acquisitions, and protecting patients and society from the higher prices of care attributed to this model of provider ownership,” he said.
Patients Pay the Price of Private Equity Acquisition
“The exponential growth in private equity ownership in hospital and physician practices in the past few decades has left a majority of health care providers disillusioned with cost-cutting practices resulting in staffing reductions and ratios that sacrifice patient care as part of their approach to running clinical operations ‘lean,’ ” Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, NY, said in an interview.
“While private equity companies argue that such practices are essential to meet their bottom line and increase operating margins, it doesn’t translate into ideal care for patients; lean practices in staffing which focus on profits at the expense of patient safety and quality of care.
“When you look at patient outcomes, it is the patients who ultimately pay the price — not the shareholders,” Dr. Glatter said. “This translates to higher risks of hospital-acquired complications including falls and blood-borne infections, including surgical site infections, as noted by the authors of the current study when private equity took over operations in hospitals.
Dr. Glatter said he was not surprised by the findings. “In my world, patient care and safety come first. Period,” he said. “Would you want your family’s health and well-being sacrificed in the name of company profits? I think it’s a rhetorical question, but one that every health care provider who works in a hospital or practice run by private equity must consider.”
Despite a decline in utilization at private equity hospitals as noted in the current study, hospital-acquired infections and adverse outcomes still increased, illustrating a decline in quality of care, said Dr. Glatter. “While these disparities were not evident when looking at 30-day outcomes, they demonstrate how operational changes impact patient outcomes in the near term. Having younger and healthier patients, and fewer Medicare and Medicaid patients combined with more hospital transfers to non–private equity run hospitals, resulted in lower in-hospital mortality in the near term, which was not apparent at 30 days post discharge,” he said.
“The explosion of hospital mergers and consolidation in the past several decades has led to skyrocketing health care costs at the expense of patient satisfaction, but also health care providers’ autonomy to manage and maintain quality care for their patients,” Dr. Glatter said.
“It’s important to understand that private equity’s interests are primarily aligned with their shareholder’s interests, as opposed to patients’ outcomes and interests,” Dr. Glatter told this news organization. “Within 5-7 years, the goal is to increase operating margins and profits and then sell a practice or hospital, which is ultimately part of a ‘health care portfolio,’ ” he said.
Additional research is needed to examine whether other hospital-acquired conditions including pressure sores, catheter-associated UTIs, methicillin-resistant Staphylococcus aureus infections, Clostridium difficile infections, and nosocomial pneumonia have increased in hospitals following private equity acquisition, given the overall national decline in these events, he said.
“At the same time, it is vital to also look at management and readmission rates for patients with strokes, heart attacks, and congestive heart failure in hospitals that are run by private equity,” Dr. Glatter noted. “These are important benchmarks of care monitored by CMS that reflect the quality of care that payers ultimately factor into reimbursement.”
Examining the metrics associated with these diagnoses will help in understanding whether private equity-managed facilities are leading to adverse outcomes and mortality, increased length of stay, hospital readmissions, and increased nosocomial infections, apart from other aspects of patient experience, Dr. Glatter added.
The study was supported by the National Heart, Lung, and Blood Institute, the National Institute on Aging, and Arnold Ventures. The researchers had no financial conflicts to disclose. Dr. Glatter had no financial conflicts to disclose and serves on the Medscape Emergency Medicine Editorial Board.
A version of this article appeared on Medscape.com.
Perinatal Psychiatry in 2024: Helping More Patients Access Care
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at obnews@mdedge.com.
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
Catch and Treat a Stealth Diagnosis: Obsessive-Compulsive Disorder
“Allie” is a 16-year-old African American female, presenting to her primary care provider for a routine well-child visit. She gets straight As in school, has a boyfriend, and works as a lifeguard. She is always on her phone using Snapchat, TikTok, and Instagram. Over the past year, it’s been taking her longer to turn off the phone and electronics at night. She needs to close the apps one by one and check the power sources a number of times. In the past few months, this ritual has become longer, includes more checks, and is interfering with sleep. She reports knowing this is abnormal and thinking she is “just kind of crazy” but she cannot stop. Her parents reassure her each evening. They now help her doublecheck that her devices are plugged in at least twice.
Unlike its depiction in the movies, many symptoms of obsessive-compulsive disorder (OCD) happen internally. Often patients are aware that these are “not normal” and cover up their experiences. It can be hard for treaters to learn about these challenges. Children spend years suffering from OCD and even regularly attend nonspecific therapy without being diagnosed. However,
OCD impacts 2.3% of the population in their lifetime but more than 28% of people report symptoms consistent with OCD traits.1 OCD symptoms have increased since the pandemic2 so it is showing up in primary care more frequently. Younger patients meet criteria when their symptoms on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) are sufficiently present, and impact the ability to function. The youngest patients with OCD are more likely to be male1 and children are most likely to be identified between ages 8-12 and during the later teenage years,3 although symptoms can occur at any time in life.
Usually, symptom onset happens gradually and then waxes and wanes. Often OCD has been present over months to years but not identified until they reach a functional tipping point. Alternatively, symptoms caused by PANDAS/PANS occur out of the blue and should be treated according to infectious disease/autoimmune workup protocols. Other differential diagnosis for OCD include other anxiety disorders, mood disorders, eating disorders, psychotic disorders, and other compulsive behaviors. OCD, tics, and ADHD are a combination seen more frequently in younger patients.4 Comorbidities frequently occur, including anxiety disorders, mood disorders, impulse control disorders, and substance use disorders.1 PTSD frequently presents with comorbid OCD symptoms.1 Finding the underlying cause is key to effective treatment.
How do I identify OCD in primary care?
Administer the CY-BOCS if these symptoms cause inability to function. The cut off for moderate symptoms is a score of 16 or above. Like all mental health screening, clinical judgment should be used to interpret the score. Many therapists do not screen for OCD.
How do I treat OCD in primary care?
Exposure Therapy with Response Prevention (ERP) is the gold-standard therapy and medication management is most effective when paired with ERP. ERP helps patients list their obsessions and compulsions in order of how much anxiety they cause, then work on gradual exposure starting with those that cause the least amount of anxiety. Picking up on any sneaky internal or external “responses” is important. An example response could include externally checking the rearview mirror to make sure the patient didn’t run over a puppy after they hit a pothole, or internally reassuring themselves. This “response prevention” can be the trickiest part of the therapy and is key to efficacy.
How to access ERP?
The International OCD Foundation offers a list of therapists trained in ERP, and most states’ psychiatry access lines can help primary care providers find available targeted resources. Despite these resources, it can be frustrating to help a family try find any available therapist who takes insurance, let alone a specialist. A recent JAMA article review found that IInternet-based treatment with both therapist- and non-therapist–guided interventions resulted in symptom improvements.2 Interventions that include parents are most helpful for children.
Other therapy options include:
- MGH/McLean/ (iocd.org) hosts an online, low cost ($65 per family) OCD camp for those age 6-17 and caregivers found here.
- Many workbooks are available, Standing Up to OCD Workbook for Kids by Tyson Reuter, PhD, is one good option.
- A book for parents about how not to accidentally reinforce anxiety is Anxious Kids, Anxious Parents: 7 Ways to Stop the Worry Cycle by Lynn Lyons and Reid Wilson.
- Sometimes a therapist without expertise can work with families using workbooks and other supports to help with ERP.
Medication options
Medications alone do not cure OCD, but can help patients better participate in ERP therapy. When the most likely cause of OCD symptoms is OCD (ruling out family history of bipolar or other psychiatric illness), using SSRIs to treat symptoms is the gold standard for medications. There is FDA approval for sertraline (≥ age 6) and fluoxetine (≥ age 7) as first-line options. If tolerated, up-titrate to efficacy. Clomipramine and fluvoxamine also have FDA approval but have more side effects so are not first line. Citalopram has randomized clinical trial support.5
Allie’s primary care provider administered and scored the CY-BOCS, started her on an SSRI, and up-titrated to efficacy over 4 months. The family signed up for an online OCD camp and learned more about OCD at iocdf.org. They talked with her therapist and worked through an OCD workbook together as no specialist was available. Her parents decreased their reassurances. Because of her primary care provider’s intervention, Allie got the care she required and was better prepared to face future exacerbations.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. Ruscio AM et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010 Jan;15(1):53-63. doi: 10.1038/mp.2008.94.
2. Lattie EG, Stamatis CA. Focusing on accessibility of evidence-based treatments for obsessive-compulsive disorder. JAMA Netw Open. 2022;5(3):e221978. doi: 10.1001/jamanetworkopen.2022.1978.
3. International OCD Foundation pediatric OCD for professionals. https://kids.iocdf.org/professionals/md/pediatric-ocd/. Accessed December 27, 2023.
4. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 2013. https://doi.org/10.1176/appi.books.9780890425596. Accessed December 27, 2023.5. Hilt RJ, Nussbaum AM. DSM-5 pocket guide to child and adolescent mental health. Arlington, Virginia: American Psychiatric Association Publishing, 2015.
“Allie” is a 16-year-old African American female, presenting to her primary care provider for a routine well-child visit. She gets straight As in school, has a boyfriend, and works as a lifeguard. She is always on her phone using Snapchat, TikTok, and Instagram. Over the past year, it’s been taking her longer to turn off the phone and electronics at night. She needs to close the apps one by one and check the power sources a number of times. In the past few months, this ritual has become longer, includes more checks, and is interfering with sleep. She reports knowing this is abnormal and thinking she is “just kind of crazy” but she cannot stop. Her parents reassure her each evening. They now help her doublecheck that her devices are plugged in at least twice.
Unlike its depiction in the movies, many symptoms of obsessive-compulsive disorder (OCD) happen internally. Often patients are aware that these are “not normal” and cover up their experiences. It can be hard for treaters to learn about these challenges. Children spend years suffering from OCD and even regularly attend nonspecific therapy without being diagnosed. However,
OCD impacts 2.3% of the population in their lifetime but more than 28% of people report symptoms consistent with OCD traits.1 OCD symptoms have increased since the pandemic2 so it is showing up in primary care more frequently. Younger patients meet criteria when their symptoms on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) are sufficiently present, and impact the ability to function. The youngest patients with OCD are more likely to be male1 and children are most likely to be identified between ages 8-12 and during the later teenage years,3 although symptoms can occur at any time in life.
Usually, symptom onset happens gradually and then waxes and wanes. Often OCD has been present over months to years but not identified until they reach a functional tipping point. Alternatively, symptoms caused by PANDAS/PANS occur out of the blue and should be treated according to infectious disease/autoimmune workup protocols. Other differential diagnosis for OCD include other anxiety disorders, mood disorders, eating disorders, psychotic disorders, and other compulsive behaviors. OCD, tics, and ADHD are a combination seen more frequently in younger patients.4 Comorbidities frequently occur, including anxiety disorders, mood disorders, impulse control disorders, and substance use disorders.1 PTSD frequently presents with comorbid OCD symptoms.1 Finding the underlying cause is key to effective treatment.
How do I identify OCD in primary care?
Administer the CY-BOCS if these symptoms cause inability to function. The cut off for moderate symptoms is a score of 16 or above. Like all mental health screening, clinical judgment should be used to interpret the score. Many therapists do not screen for OCD.
How do I treat OCD in primary care?
Exposure Therapy with Response Prevention (ERP) is the gold-standard therapy and medication management is most effective when paired with ERP. ERP helps patients list their obsessions and compulsions in order of how much anxiety they cause, then work on gradual exposure starting with those that cause the least amount of anxiety. Picking up on any sneaky internal or external “responses” is important. An example response could include externally checking the rearview mirror to make sure the patient didn’t run over a puppy after they hit a pothole, or internally reassuring themselves. This “response prevention” can be the trickiest part of the therapy and is key to efficacy.
How to access ERP?
The International OCD Foundation offers a list of therapists trained in ERP, and most states’ psychiatry access lines can help primary care providers find available targeted resources. Despite these resources, it can be frustrating to help a family try find any available therapist who takes insurance, let alone a specialist. A recent JAMA article review found that IInternet-based treatment with both therapist- and non-therapist–guided interventions resulted in symptom improvements.2 Interventions that include parents are most helpful for children.
Other therapy options include:
- MGH/McLean/ (iocd.org) hosts an online, low cost ($65 per family) OCD camp for those age 6-17 and caregivers found here.
- Many workbooks are available, Standing Up to OCD Workbook for Kids by Tyson Reuter, PhD, is one good option.
- A book for parents about how not to accidentally reinforce anxiety is Anxious Kids, Anxious Parents: 7 Ways to Stop the Worry Cycle by Lynn Lyons and Reid Wilson.
- Sometimes a therapist without expertise can work with families using workbooks and other supports to help with ERP.
Medication options
Medications alone do not cure OCD, but can help patients better participate in ERP therapy. When the most likely cause of OCD symptoms is OCD (ruling out family history of bipolar or other psychiatric illness), using SSRIs to treat symptoms is the gold standard for medications. There is FDA approval for sertraline (≥ age 6) and fluoxetine (≥ age 7) as first-line options. If tolerated, up-titrate to efficacy. Clomipramine and fluvoxamine also have FDA approval but have more side effects so are not first line. Citalopram has randomized clinical trial support.5
Allie’s primary care provider administered and scored the CY-BOCS, started her on an SSRI, and up-titrated to efficacy over 4 months. The family signed up for an online OCD camp and learned more about OCD at iocdf.org. They talked with her therapist and worked through an OCD workbook together as no specialist was available. Her parents decreased their reassurances. Because of her primary care provider’s intervention, Allie got the care she required and was better prepared to face future exacerbations.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. Ruscio AM et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010 Jan;15(1):53-63. doi: 10.1038/mp.2008.94.
2. Lattie EG, Stamatis CA. Focusing on accessibility of evidence-based treatments for obsessive-compulsive disorder. JAMA Netw Open. 2022;5(3):e221978. doi: 10.1001/jamanetworkopen.2022.1978.
3. International OCD Foundation pediatric OCD for professionals. https://kids.iocdf.org/professionals/md/pediatric-ocd/. Accessed December 27, 2023.
4. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 2013. https://doi.org/10.1176/appi.books.9780890425596. Accessed December 27, 2023.5. Hilt RJ, Nussbaum AM. DSM-5 pocket guide to child and adolescent mental health. Arlington, Virginia: American Psychiatric Association Publishing, 2015.
“Allie” is a 16-year-old African American female, presenting to her primary care provider for a routine well-child visit. She gets straight As in school, has a boyfriend, and works as a lifeguard. She is always on her phone using Snapchat, TikTok, and Instagram. Over the past year, it’s been taking her longer to turn off the phone and electronics at night. She needs to close the apps one by one and check the power sources a number of times. In the past few months, this ritual has become longer, includes more checks, and is interfering with sleep. She reports knowing this is abnormal and thinking she is “just kind of crazy” but she cannot stop. Her parents reassure her each evening. They now help her doublecheck that her devices are plugged in at least twice.
Unlike its depiction in the movies, many symptoms of obsessive-compulsive disorder (OCD) happen internally. Often patients are aware that these are “not normal” and cover up their experiences. It can be hard for treaters to learn about these challenges. Children spend years suffering from OCD and even regularly attend nonspecific therapy without being diagnosed. However,
OCD impacts 2.3% of the population in their lifetime but more than 28% of people report symptoms consistent with OCD traits.1 OCD symptoms have increased since the pandemic2 so it is showing up in primary care more frequently. Younger patients meet criteria when their symptoms on the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) are sufficiently present, and impact the ability to function. The youngest patients with OCD are more likely to be male1 and children are most likely to be identified between ages 8-12 and during the later teenage years,3 although symptoms can occur at any time in life.
Usually, symptom onset happens gradually and then waxes and wanes. Often OCD has been present over months to years but not identified until they reach a functional tipping point. Alternatively, symptoms caused by PANDAS/PANS occur out of the blue and should be treated according to infectious disease/autoimmune workup protocols. Other differential diagnosis for OCD include other anxiety disorders, mood disorders, eating disorders, psychotic disorders, and other compulsive behaviors. OCD, tics, and ADHD are a combination seen more frequently in younger patients.4 Comorbidities frequently occur, including anxiety disorders, mood disorders, impulse control disorders, and substance use disorders.1 PTSD frequently presents with comorbid OCD symptoms.1 Finding the underlying cause is key to effective treatment.
How do I identify OCD in primary care?
Administer the CY-BOCS if these symptoms cause inability to function. The cut off for moderate symptoms is a score of 16 or above. Like all mental health screening, clinical judgment should be used to interpret the score. Many therapists do not screen for OCD.
How do I treat OCD in primary care?
Exposure Therapy with Response Prevention (ERP) is the gold-standard therapy and medication management is most effective when paired with ERP. ERP helps patients list their obsessions and compulsions in order of how much anxiety they cause, then work on gradual exposure starting with those that cause the least amount of anxiety. Picking up on any sneaky internal or external “responses” is important. An example response could include externally checking the rearview mirror to make sure the patient didn’t run over a puppy after they hit a pothole, or internally reassuring themselves. This “response prevention” can be the trickiest part of the therapy and is key to efficacy.
How to access ERP?
The International OCD Foundation offers a list of therapists trained in ERP, and most states’ psychiatry access lines can help primary care providers find available targeted resources. Despite these resources, it can be frustrating to help a family try find any available therapist who takes insurance, let alone a specialist. A recent JAMA article review found that IInternet-based treatment with both therapist- and non-therapist–guided interventions resulted in symptom improvements.2 Interventions that include parents are most helpful for children.
Other therapy options include:
- MGH/McLean/ (iocd.org) hosts an online, low cost ($65 per family) OCD camp for those age 6-17 and caregivers found here.
- Many workbooks are available, Standing Up to OCD Workbook for Kids by Tyson Reuter, PhD, is one good option.
- A book for parents about how not to accidentally reinforce anxiety is Anxious Kids, Anxious Parents: 7 Ways to Stop the Worry Cycle by Lynn Lyons and Reid Wilson.
- Sometimes a therapist without expertise can work with families using workbooks and other supports to help with ERP.
Medication options
Medications alone do not cure OCD, but can help patients better participate in ERP therapy. When the most likely cause of OCD symptoms is OCD (ruling out family history of bipolar or other psychiatric illness), using SSRIs to treat symptoms is the gold standard for medications. There is FDA approval for sertraline (≥ age 6) and fluoxetine (≥ age 7) as first-line options. If tolerated, up-titrate to efficacy. Clomipramine and fluvoxamine also have FDA approval but have more side effects so are not first line. Citalopram has randomized clinical trial support.5
Allie’s primary care provider administered and scored the CY-BOCS, started her on an SSRI, and up-titrated to efficacy over 4 months. The family signed up for an online OCD camp and learned more about OCD at iocdf.org. They talked with her therapist and worked through an OCD workbook together as no specialist was available. Her parents decreased their reassurances. Because of her primary care provider’s intervention, Allie got the care she required and was better prepared to face future exacerbations.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. Ruscio AM et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010 Jan;15(1):53-63. doi: 10.1038/mp.2008.94.
2. Lattie EG, Stamatis CA. Focusing on accessibility of evidence-based treatments for obsessive-compulsive disorder. JAMA Netw Open. 2022;5(3):e221978. doi: 10.1001/jamanetworkopen.2022.1978.
3. International OCD Foundation pediatric OCD for professionals. https://kids.iocdf.org/professionals/md/pediatric-ocd/. Accessed December 27, 2023.
4. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 2013. https://doi.org/10.1176/appi.books.9780890425596. Accessed December 27, 2023.5. Hilt RJ, Nussbaum AM. DSM-5 pocket guide to child and adolescent mental health. Arlington, Virginia: American Psychiatric Association Publishing, 2015.
Nodal Radiation May Make BC Axillary Dissection Unnecessary
SAN ANTONIO — Axillary lymph node dissection may be unnecessary if breast cancer patients with one or two positive sentinel lymph nodes plan to have adjuvant nodal radiation, according to a major Scandinavian trial presented at the San Antonio Breast Cancer Symposium.
“It means that you don’t need to dissect the axilla if you” are going to “radiate the axilla.” “For the U.S., that’s the conclusion because there are still centers that do both, and that’s out,” lead investigator Jana de Boniface, MD, PhD, a breast cancer surgeon at the Karolinska Institutet, Stockholm, said in an interview.
Some even wondered if 5 years of endocrine therapy is necessary.
Dr. Boniface shared her thoughts after presenting the Scandinavian trial, SENOMAC, which she led.
SENOMAC randomized 1,204 patients with one or two positive sentinel lymph nodes to axillary dissection; 1,335 with the same finding were randomized to no dissection.
Subjects had clinically T1-3, N0 primary breast cancer. About 89% in both arms went on to adjuvant radiation, including nodal radiation, and almost all also went on to systemic therapy, which included endocrine therapy in over 90%. Only about 2% of subjects had neoadjuvant therapy.
At a median follow-up of nearly 4 years, recurrence-free survival was virtually identical in both groups, with 8% of patients in the dissection arm and 7.1% in the no-dissection group having recurrences. Estimated 5-year recurrence-free survival was just shy of 90% in both groups. Skipping dissection was strongly non-inferior to having one (P < .001).
SENOMAC “clearly shows that you don’t need to dissect the axilla if you have one to two positive sentinel lymph nodes” so long as patients have adjuvant nodal radiation. Recurrence-free survival “curves practically overlap, and we cannot see any difference between the two groups,” Dr. Boniface said.
Meanwhile, the dissection group fared worse on patient reported outcomes. Overall survival outcomes, the primary endpoint of the trial, are expected within 2 years.
The goal of the trial, the largest to date to look into the issue, was to fill gaps in the literature. Similar outcomes were reported around a decade ago in patients with low sentinel lymph node burdens, but the extensive exclusion criteria raised questions about general applicability.
In contrast, SENOMAC was widely inclusive. Over a third of patients had mastectomies, over a third had sentinel lymph node extracapsular extension, almost 6% had T3 disease, almost 20% had lobular carcinoma, 40% were 65 years or older, and tumors were as large as 15.5 cm.
The findings held regardless of those and other factors on subgroup analyses, including estrogen receptor and HER2 status and the number of additional positive nodes retrieved in the dissection group.
Andrea V. Barrio, MD, the study discussant and a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York, agreed with the message from SENOMAC.
“Based on this, ALND [axillary lymph node dissection] should not be considered standard in patients with clinical T1-3, N0 breast cancer with one to two positive sentinel nodes, with or without microscopic extracapsular extension, undergoing lumpectomy or mastectomy,” provided nodal adjuvant radiotherapy is indicated, she said.
Although adjuvant nodal radiation for patients with one to three positive sentinel nodes is standard of care in Denmark and Sweden, where most of the patients in SENOMAC were located, practices vary widely in the United States. If adjuvant radiation isn’t used, “then ALND [is still] indicated,” Dr. Barrio said, but in either case, “only one is needed.”
In keeping with the de-escalation theme at the 2023 symposium, both Dr. Boniface and Dr. Barrio noted that trials are now underway to find patients who can avoid any axillary treatment at all if they have just one or two positive sentinel lymph nodes.
Preoperative axillary ultrasound was mandatory in SENOMAC and patients with non-palpable suspicious axillary lymph nodes were enrolled.
Thirty-six were positive on fine needle aspiration and randomized into the study, but when asked, Dr. Boniface didn’t have the data immediately at hand on how they fared.
The work was funded by the Swedish Research Council, Nordic Cancer Union, and others. Dr. Boniface and Dr. Barrio didn’t have any disclosures.
SAN ANTONIO — Axillary lymph node dissection may be unnecessary if breast cancer patients with one or two positive sentinel lymph nodes plan to have adjuvant nodal radiation, according to a major Scandinavian trial presented at the San Antonio Breast Cancer Symposium.
“It means that you don’t need to dissect the axilla if you” are going to “radiate the axilla.” “For the U.S., that’s the conclusion because there are still centers that do both, and that’s out,” lead investigator Jana de Boniface, MD, PhD, a breast cancer surgeon at the Karolinska Institutet, Stockholm, said in an interview.
Some even wondered if 5 years of endocrine therapy is necessary.
Dr. Boniface shared her thoughts after presenting the Scandinavian trial, SENOMAC, which she led.
SENOMAC randomized 1,204 patients with one or two positive sentinel lymph nodes to axillary dissection; 1,335 with the same finding were randomized to no dissection.
Subjects had clinically T1-3, N0 primary breast cancer. About 89% in both arms went on to adjuvant radiation, including nodal radiation, and almost all also went on to systemic therapy, which included endocrine therapy in over 90%. Only about 2% of subjects had neoadjuvant therapy.
At a median follow-up of nearly 4 years, recurrence-free survival was virtually identical in both groups, with 8% of patients in the dissection arm and 7.1% in the no-dissection group having recurrences. Estimated 5-year recurrence-free survival was just shy of 90% in both groups. Skipping dissection was strongly non-inferior to having one (P < .001).
SENOMAC “clearly shows that you don’t need to dissect the axilla if you have one to two positive sentinel lymph nodes” so long as patients have adjuvant nodal radiation. Recurrence-free survival “curves practically overlap, and we cannot see any difference between the two groups,” Dr. Boniface said.
Meanwhile, the dissection group fared worse on patient reported outcomes. Overall survival outcomes, the primary endpoint of the trial, are expected within 2 years.
The goal of the trial, the largest to date to look into the issue, was to fill gaps in the literature. Similar outcomes were reported around a decade ago in patients with low sentinel lymph node burdens, but the extensive exclusion criteria raised questions about general applicability.
In contrast, SENOMAC was widely inclusive. Over a third of patients had mastectomies, over a third had sentinel lymph node extracapsular extension, almost 6% had T3 disease, almost 20% had lobular carcinoma, 40% were 65 years or older, and tumors were as large as 15.5 cm.
The findings held regardless of those and other factors on subgroup analyses, including estrogen receptor and HER2 status and the number of additional positive nodes retrieved in the dissection group.
Andrea V. Barrio, MD, the study discussant and a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York, agreed with the message from SENOMAC.
“Based on this, ALND [axillary lymph node dissection] should not be considered standard in patients with clinical T1-3, N0 breast cancer with one to two positive sentinel nodes, with or without microscopic extracapsular extension, undergoing lumpectomy or mastectomy,” provided nodal adjuvant radiotherapy is indicated, she said.
Although adjuvant nodal radiation for patients with one to three positive sentinel nodes is standard of care in Denmark and Sweden, where most of the patients in SENOMAC were located, practices vary widely in the United States. If adjuvant radiation isn’t used, “then ALND [is still] indicated,” Dr. Barrio said, but in either case, “only one is needed.”
In keeping with the de-escalation theme at the 2023 symposium, both Dr. Boniface and Dr. Barrio noted that trials are now underway to find patients who can avoid any axillary treatment at all if they have just one or two positive sentinel lymph nodes.
Preoperative axillary ultrasound was mandatory in SENOMAC and patients with non-palpable suspicious axillary lymph nodes were enrolled.
Thirty-six were positive on fine needle aspiration and randomized into the study, but when asked, Dr. Boniface didn’t have the data immediately at hand on how they fared.
The work was funded by the Swedish Research Council, Nordic Cancer Union, and others. Dr. Boniface and Dr. Barrio didn’t have any disclosures.
SAN ANTONIO — Axillary lymph node dissection may be unnecessary if breast cancer patients with one or two positive sentinel lymph nodes plan to have adjuvant nodal radiation, according to a major Scandinavian trial presented at the San Antonio Breast Cancer Symposium.
“It means that you don’t need to dissect the axilla if you” are going to “radiate the axilla.” “For the U.S., that’s the conclusion because there are still centers that do both, and that’s out,” lead investigator Jana de Boniface, MD, PhD, a breast cancer surgeon at the Karolinska Institutet, Stockholm, said in an interview.
Some even wondered if 5 years of endocrine therapy is necessary.
Dr. Boniface shared her thoughts after presenting the Scandinavian trial, SENOMAC, which she led.
SENOMAC randomized 1,204 patients with one or two positive sentinel lymph nodes to axillary dissection; 1,335 with the same finding were randomized to no dissection.
Subjects had clinically T1-3, N0 primary breast cancer. About 89% in both arms went on to adjuvant radiation, including nodal radiation, and almost all also went on to systemic therapy, which included endocrine therapy in over 90%. Only about 2% of subjects had neoadjuvant therapy.
At a median follow-up of nearly 4 years, recurrence-free survival was virtually identical in both groups, with 8% of patients in the dissection arm and 7.1% in the no-dissection group having recurrences. Estimated 5-year recurrence-free survival was just shy of 90% in both groups. Skipping dissection was strongly non-inferior to having one (P < .001).
SENOMAC “clearly shows that you don’t need to dissect the axilla if you have one to two positive sentinel lymph nodes” so long as patients have adjuvant nodal radiation. Recurrence-free survival “curves practically overlap, and we cannot see any difference between the two groups,” Dr. Boniface said.
Meanwhile, the dissection group fared worse on patient reported outcomes. Overall survival outcomes, the primary endpoint of the trial, are expected within 2 years.
The goal of the trial, the largest to date to look into the issue, was to fill gaps in the literature. Similar outcomes were reported around a decade ago in patients with low sentinel lymph node burdens, but the extensive exclusion criteria raised questions about general applicability.
In contrast, SENOMAC was widely inclusive. Over a third of patients had mastectomies, over a third had sentinel lymph node extracapsular extension, almost 6% had T3 disease, almost 20% had lobular carcinoma, 40% were 65 years or older, and tumors were as large as 15.5 cm.
The findings held regardless of those and other factors on subgroup analyses, including estrogen receptor and HER2 status and the number of additional positive nodes retrieved in the dissection group.
Andrea V. Barrio, MD, the study discussant and a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York, agreed with the message from SENOMAC.
“Based on this, ALND [axillary lymph node dissection] should not be considered standard in patients with clinical T1-3, N0 breast cancer with one to two positive sentinel nodes, with or without microscopic extracapsular extension, undergoing lumpectomy or mastectomy,” provided nodal adjuvant radiotherapy is indicated, she said.
Although adjuvant nodal radiation for patients with one to three positive sentinel nodes is standard of care in Denmark and Sweden, where most of the patients in SENOMAC were located, practices vary widely in the United States. If adjuvant radiation isn’t used, “then ALND [is still] indicated,” Dr. Barrio said, but in either case, “only one is needed.”
In keeping with the de-escalation theme at the 2023 symposium, both Dr. Boniface and Dr. Barrio noted that trials are now underway to find patients who can avoid any axillary treatment at all if they have just one or two positive sentinel lymph nodes.
Preoperative axillary ultrasound was mandatory in SENOMAC and patients with non-palpable suspicious axillary lymph nodes were enrolled.
Thirty-six were positive on fine needle aspiration and randomized into the study, but when asked, Dr. Boniface didn’t have the data immediately at hand on how they fared.
The work was funded by the Swedish Research Council, Nordic Cancer Union, and others. Dr. Boniface and Dr. Barrio didn’t have any disclosures.
FROM SABCS 2023
Diagnosing Adrenal Insufficiency: The ‘Quick and Dirty’ Method
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Watto, here with America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about some adrenal insufficiency? We had a great conversation with Dr. Atil Kargi, and I’d like you to start us off.
Paul N. Williams, MD: How about thinking about it? It’s a good place to start.
That’s one of the ways this episode changed my approach a little bit. I never really thought about the fact that . It’s such a protean sort of nonspecific presentation. But if you have someone with chronic malaise and poor appetite and maybe unexplained weight loss, and your GI workup is not really leading you anywhere, it’s probably worth thinking about adrenal insufficiency. Even though primary adrenal insufficiency is pretty rare — we’re talking cases per millions — secondary adrenal insufficiency is actually fairly common. It’s probably worth thinking about and testing for more often than I have in the past. So for me, it’s having a lower threshold to start looking for it.
Dr. Watto: When it’s adrenal crisis, you probably think about it, but then it’s too late. Ideally, you would think about it before that happens. But the symptoms can be quite vague. The mineralocorticoid symptoms, like salt cravings, dizziness, near syncope, muscle cramps, might make me think of it because they sound more like something endocrine is going on. But if it’s just a little weight loss, a little fatigue, or a little nausea, that’s everybody.
Dr. Williams: Right. If a patient came to me saying, “I’m craving salt,” that might hasten the workup a little bit, but that’s not the typical presentation.
Dr. Watto: If you are going to check a cortisol level, you should really check it in the morning, between 7 AM and 9 AM. If you check it too early, it might not have peaked yet, so you might get a level that looks low. But if you had checked an hour or 2 later, it might have been above a threshold, and then you would know you could rule out the diagnosis. The cutoffs depend on your source: < 3-5 µg/dL that early in the morning is pretty much diagnostic of adrenal insufficiency. If it’s > 15 µg/dL, that’s a pretty robust cortisol and the patient probably doesn’t have adrenal insufficiency. But if the level is between 5 µg/dL and 15 µg/dL, you’re in a gray zone, and that’s where you might think about doing a stimulation (stim) test.
Dr. Kargi gave us a quick and dirty version of the stim test. Paul, have you had a chance to try this yet?
Dr. Williams: I have not. Have you? I’m sure you’ve been just waiting for the chance.
Dr. Watto: I would love to do this. I don›t know whether I›m set up to do it in the office right now. But this is an aspirational goal for my practice, and I›m sure some physicians are set up in their office already to do it. You can give either intramuscular or subcutaneous cosyntropin 250 µg. You don›t even have to get a baseline cortisol level right before the injection. Let›s say the patient›s previous cortisol level was between 5 µg/dL and 15 µg/dL, so you weren›t sure about the diagnosis. You bring them back to the office one day, give them a shot of cosyntropin, and then 30-60 minutes later, have a random cortisol drawn. If it›s > 19 µg/dL, you›ve ruled out adrenal insufficiency. If it›s anything else, send them to an endocrinologist to sort it out. You might be able to make the diagnosis yourself doing that.
Any treatment pearls to leave the audience with?
Dr. Williams: I hope endocrinologists don›t take issue with this. I say this with respect and admiration, but it feels kind of vibe-based to me. Without a lab value to guide treatment, you are dependent on the patient telling you how they feel much of the time. You have to let their symptoms guide you. It is probably worth noting that because hydrocortisone has a relatively short half-life, within hours, in fact, you typically have to do twice-daily dosing, sometimes even three times daily dosing to get patients to where they feel okay. It sounds like there›s a fair amount of trial and error and some adjustments that you have to make depending on what›s going on with the patient at any given time. You land somewhere between a dose of 15-30 mg per day, but there will be some variability, even within an individual patient, depending on what›s going on with them from a physiologic standpoint.
Dr. Watto: They are going to take one dose in the morning and then a second dose in the afternoon, but they don’t want them to take it too late in the evening because it could cause insomnia, and you want to try to mimic physiologic levels as much as you can. Two thirds of the daily dose is given early in the morning and then another third of the daily dose later in the day if you are prescribing two times daily dosing.
And Dr. Kargi had a low threshold for doubling the dose. If the patient has a cold, double the dose for 2 or 3 days. With a high fever, triple the dose for a few days. If they are going for surgery, they are probably going to be getting some intravenous hydrocortisone while they’re in the hospital.
We really turned over like every stone we could possibly think of on this podcast. There were so many great pearls that we don’t have time to go through them all here. But we talked about steroid tapers and a lot more. You can check it out here.
Dr. Watto has disclosed no relevant financial relationships.
Dr. Williams has disclosed the following relevant financial relationships:Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: The CurbsidersReceived income in an amount equal to or greater than $250 from: The Curbsiders.
A version of this article appeared on Medscape.com.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Watto, here with America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about some adrenal insufficiency? We had a great conversation with Dr. Atil Kargi, and I’d like you to start us off.
Paul N. Williams, MD: How about thinking about it? It’s a good place to start.
That’s one of the ways this episode changed my approach a little bit. I never really thought about the fact that . It’s such a protean sort of nonspecific presentation. But if you have someone with chronic malaise and poor appetite and maybe unexplained weight loss, and your GI workup is not really leading you anywhere, it’s probably worth thinking about adrenal insufficiency. Even though primary adrenal insufficiency is pretty rare — we’re talking cases per millions — secondary adrenal insufficiency is actually fairly common. It’s probably worth thinking about and testing for more often than I have in the past. So for me, it’s having a lower threshold to start looking for it.
Dr. Watto: When it’s adrenal crisis, you probably think about it, but then it’s too late. Ideally, you would think about it before that happens. But the symptoms can be quite vague. The mineralocorticoid symptoms, like salt cravings, dizziness, near syncope, muscle cramps, might make me think of it because they sound more like something endocrine is going on. But if it’s just a little weight loss, a little fatigue, or a little nausea, that’s everybody.
Dr. Williams: Right. If a patient came to me saying, “I’m craving salt,” that might hasten the workup a little bit, but that’s not the typical presentation.
Dr. Watto: If you are going to check a cortisol level, you should really check it in the morning, between 7 AM and 9 AM. If you check it too early, it might not have peaked yet, so you might get a level that looks low. But if you had checked an hour or 2 later, it might have been above a threshold, and then you would know you could rule out the diagnosis. The cutoffs depend on your source: < 3-5 µg/dL that early in the morning is pretty much diagnostic of adrenal insufficiency. If it’s > 15 µg/dL, that’s a pretty robust cortisol and the patient probably doesn’t have adrenal insufficiency. But if the level is between 5 µg/dL and 15 µg/dL, you’re in a gray zone, and that’s where you might think about doing a stimulation (stim) test.
Dr. Kargi gave us a quick and dirty version of the stim test. Paul, have you had a chance to try this yet?
Dr. Williams: I have not. Have you? I’m sure you’ve been just waiting for the chance.
Dr. Watto: I would love to do this. I don›t know whether I›m set up to do it in the office right now. But this is an aspirational goal for my practice, and I›m sure some physicians are set up in their office already to do it. You can give either intramuscular or subcutaneous cosyntropin 250 µg. You don›t even have to get a baseline cortisol level right before the injection. Let›s say the patient›s previous cortisol level was between 5 µg/dL and 15 µg/dL, so you weren›t sure about the diagnosis. You bring them back to the office one day, give them a shot of cosyntropin, and then 30-60 minutes later, have a random cortisol drawn. If it›s > 19 µg/dL, you›ve ruled out adrenal insufficiency. If it›s anything else, send them to an endocrinologist to sort it out. You might be able to make the diagnosis yourself doing that.
Any treatment pearls to leave the audience with?
Dr. Williams: I hope endocrinologists don›t take issue with this. I say this with respect and admiration, but it feels kind of vibe-based to me. Without a lab value to guide treatment, you are dependent on the patient telling you how they feel much of the time. You have to let their symptoms guide you. It is probably worth noting that because hydrocortisone has a relatively short half-life, within hours, in fact, you typically have to do twice-daily dosing, sometimes even three times daily dosing to get patients to where they feel okay. It sounds like there›s a fair amount of trial and error and some adjustments that you have to make depending on what›s going on with the patient at any given time. You land somewhere between a dose of 15-30 mg per day, but there will be some variability, even within an individual patient, depending on what›s going on with them from a physiologic standpoint.
Dr. Watto: They are going to take one dose in the morning and then a second dose in the afternoon, but they don’t want them to take it too late in the evening because it could cause insomnia, and you want to try to mimic physiologic levels as much as you can. Two thirds of the daily dose is given early in the morning and then another third of the daily dose later in the day if you are prescribing two times daily dosing.
And Dr. Kargi had a low threshold for doubling the dose. If the patient has a cold, double the dose for 2 or 3 days. With a high fever, triple the dose for a few days. If they are going for surgery, they are probably going to be getting some intravenous hydrocortisone while they’re in the hospital.
We really turned over like every stone we could possibly think of on this podcast. There were so many great pearls that we don’t have time to go through them all here. But we talked about steroid tapers and a lot more. You can check it out here.
Dr. Watto has disclosed no relevant financial relationships.
Dr. Williams has disclosed the following relevant financial relationships:Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: The CurbsidersReceived income in an amount equal to or greater than $250 from: The Curbsiders.
A version of this article appeared on Medscape.com.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Watto, here with America’s primary care physician, Dr. Paul Nelson Williams. Paul, are you ready to talk about some adrenal insufficiency? We had a great conversation with Dr. Atil Kargi, and I’d like you to start us off.
Paul N. Williams, MD: How about thinking about it? It’s a good place to start.
That’s one of the ways this episode changed my approach a little bit. I never really thought about the fact that . It’s such a protean sort of nonspecific presentation. But if you have someone with chronic malaise and poor appetite and maybe unexplained weight loss, and your GI workup is not really leading you anywhere, it’s probably worth thinking about adrenal insufficiency. Even though primary adrenal insufficiency is pretty rare — we’re talking cases per millions — secondary adrenal insufficiency is actually fairly common. It’s probably worth thinking about and testing for more often than I have in the past. So for me, it’s having a lower threshold to start looking for it.
Dr. Watto: When it’s adrenal crisis, you probably think about it, but then it’s too late. Ideally, you would think about it before that happens. But the symptoms can be quite vague. The mineralocorticoid symptoms, like salt cravings, dizziness, near syncope, muscle cramps, might make me think of it because they sound more like something endocrine is going on. But if it’s just a little weight loss, a little fatigue, or a little nausea, that’s everybody.
Dr. Williams: Right. If a patient came to me saying, “I’m craving salt,” that might hasten the workup a little bit, but that’s not the typical presentation.
Dr. Watto: If you are going to check a cortisol level, you should really check it in the morning, between 7 AM and 9 AM. If you check it too early, it might not have peaked yet, so you might get a level that looks low. But if you had checked an hour or 2 later, it might have been above a threshold, and then you would know you could rule out the diagnosis. The cutoffs depend on your source: < 3-5 µg/dL that early in the morning is pretty much diagnostic of adrenal insufficiency. If it’s > 15 µg/dL, that’s a pretty robust cortisol and the patient probably doesn’t have adrenal insufficiency. But if the level is between 5 µg/dL and 15 µg/dL, you’re in a gray zone, and that’s where you might think about doing a stimulation (stim) test.
Dr. Kargi gave us a quick and dirty version of the stim test. Paul, have you had a chance to try this yet?
Dr. Williams: I have not. Have you? I’m sure you’ve been just waiting for the chance.
Dr. Watto: I would love to do this. I don›t know whether I›m set up to do it in the office right now. But this is an aspirational goal for my practice, and I›m sure some physicians are set up in their office already to do it. You can give either intramuscular or subcutaneous cosyntropin 250 µg. You don›t even have to get a baseline cortisol level right before the injection. Let›s say the patient›s previous cortisol level was between 5 µg/dL and 15 µg/dL, so you weren›t sure about the diagnosis. You bring them back to the office one day, give them a shot of cosyntropin, and then 30-60 minutes later, have a random cortisol drawn. If it›s > 19 µg/dL, you›ve ruled out adrenal insufficiency. If it›s anything else, send them to an endocrinologist to sort it out. You might be able to make the diagnosis yourself doing that.
Any treatment pearls to leave the audience with?
Dr. Williams: I hope endocrinologists don›t take issue with this. I say this with respect and admiration, but it feels kind of vibe-based to me. Without a lab value to guide treatment, you are dependent on the patient telling you how they feel much of the time. You have to let their symptoms guide you. It is probably worth noting that because hydrocortisone has a relatively short half-life, within hours, in fact, you typically have to do twice-daily dosing, sometimes even three times daily dosing to get patients to where they feel okay. It sounds like there›s a fair amount of trial and error and some adjustments that you have to make depending on what›s going on with the patient at any given time. You land somewhere between a dose of 15-30 mg per day, but there will be some variability, even within an individual patient, depending on what›s going on with them from a physiologic standpoint.
Dr. Watto: They are going to take one dose in the morning and then a second dose in the afternoon, but they don’t want them to take it too late in the evening because it could cause insomnia, and you want to try to mimic physiologic levels as much as you can. Two thirds of the daily dose is given early in the morning and then another third of the daily dose later in the day if you are prescribing two times daily dosing.
And Dr. Kargi had a low threshold for doubling the dose. If the patient has a cold, double the dose for 2 or 3 days. With a high fever, triple the dose for a few days. If they are going for surgery, they are probably going to be getting some intravenous hydrocortisone while they’re in the hospital.
We really turned over like every stone we could possibly think of on this podcast. There were so many great pearls that we don’t have time to go through them all here. But we talked about steroid tapers and a lot more. You can check it out here.
Dr. Watto has disclosed no relevant financial relationships.
Dr. Williams has disclosed the following relevant financial relationships:Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: The CurbsidersReceived income in an amount equal to or greater than $250 from: The Curbsiders.
A version of this article appeared on Medscape.com.
Artificial Sweeteners Alter the Duodenal Microbiome
TOPLINE:
and composition and levels of circulating inflammatory markers.
METHODOLOGY:
- Researchers analyzed samples from the REIMAGINE (Revealing the Entire Intestinal Microbiota and its Associations with the Genetic, Immunologic, and Neuroendocrine Ecosystem) study to assess the potential effects of NSS consumption on the duodenal luminal microbiome.
- They analyzed subjects consuming non-aspartame nonsugar sweeteners (NANS; n = 35) and aspartame only (ASP; n = 9), who were compared with 55 control participants matched for age, sex, and body mass index.
- A subset of 40 participants provided stool samples for additional analysis.
TAKEAWAY:
- Duodenal alpha diversity was lower in NANS consumers vs controls.
- Duodenal relative abundance (RA) of Escherichia, Klebsiella, and Salmonella was lower in both NANS and ASP vs controls, whereas stool RA of these phylum Proteobacteria was increased in both NANS and ASP.
- Compared with controls, NANS and ASP differed in how they altered predicted duodenal microbial metabolic pathways, with NANS impacting polysaccharides biosynthesis and D-galactose degradation and ASP significantly enriching biosynthesis of cylindrospermopsin, a potential cancer-causing agent known to adversely impact the liver and nervous system.
- Circulating levels of interleukin (IL)-1b, a pro-inflammatory cytokine that plays a key role in the immune response, were significantly decreased in NANS vs controls, whereas IL-6 and IL-10, two cytokines with protective properties, were decreased in the ASP group vs controls.
IN PRACTICE:
“Given the crucial role played by small intestinal microbes in digestion, nutrient absorption, immune regulation, and endocrine functions, coupled with the substantial prevalence of NSS consumption among US adults (estimated at 41.4%), our findings have potential implications for metabolic and gastrointestinal health in a considerable proportion of the American adult population.”
SOURCE:
The study, conducted by Ava Hosseini, MPH, and colleagues at Cedars-Sinai, Los Angeles, was published online on November 22, 2023, in iScience.
LIMITATIONS:
The study population may not be representative of healthy individuals as they underwent upper endoscopy for various reasons (eg, evaluation of intestinal complaints). After exclusions, the duodenal sample size for the aspartame group was small. Samples were collected at a single timepoint, limiting the ability to establish causal relationships.
DISCLOSURES:
This research was supported by Frank Lee, the Monica Lester Charitable Trust, and the Elias, Genevieve, and Georgianna Atol Charitable Trust through their support of the Medically Associated Science and Technology Program, Cedars-Sinai, Los Angeles. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
and composition and levels of circulating inflammatory markers.
METHODOLOGY:
- Researchers analyzed samples from the REIMAGINE (Revealing the Entire Intestinal Microbiota and its Associations with the Genetic, Immunologic, and Neuroendocrine Ecosystem) study to assess the potential effects of NSS consumption on the duodenal luminal microbiome.
- They analyzed subjects consuming non-aspartame nonsugar sweeteners (NANS; n = 35) and aspartame only (ASP; n = 9), who were compared with 55 control participants matched for age, sex, and body mass index.
- A subset of 40 participants provided stool samples for additional analysis.
TAKEAWAY:
- Duodenal alpha diversity was lower in NANS consumers vs controls.
- Duodenal relative abundance (RA) of Escherichia, Klebsiella, and Salmonella was lower in both NANS and ASP vs controls, whereas stool RA of these phylum Proteobacteria was increased in both NANS and ASP.
- Compared with controls, NANS and ASP differed in how they altered predicted duodenal microbial metabolic pathways, with NANS impacting polysaccharides biosynthesis and D-galactose degradation and ASP significantly enriching biosynthesis of cylindrospermopsin, a potential cancer-causing agent known to adversely impact the liver and nervous system.
- Circulating levels of interleukin (IL)-1b, a pro-inflammatory cytokine that plays a key role in the immune response, were significantly decreased in NANS vs controls, whereas IL-6 and IL-10, two cytokines with protective properties, were decreased in the ASP group vs controls.
IN PRACTICE:
“Given the crucial role played by small intestinal microbes in digestion, nutrient absorption, immune regulation, and endocrine functions, coupled with the substantial prevalence of NSS consumption among US adults (estimated at 41.4%), our findings have potential implications for metabolic and gastrointestinal health in a considerable proportion of the American adult population.”
SOURCE:
The study, conducted by Ava Hosseini, MPH, and colleagues at Cedars-Sinai, Los Angeles, was published online on November 22, 2023, in iScience.
LIMITATIONS:
The study population may not be representative of healthy individuals as they underwent upper endoscopy for various reasons (eg, evaluation of intestinal complaints). After exclusions, the duodenal sample size for the aspartame group was small. Samples were collected at a single timepoint, limiting the ability to establish causal relationships.
DISCLOSURES:
This research was supported by Frank Lee, the Monica Lester Charitable Trust, and the Elias, Genevieve, and Georgianna Atol Charitable Trust through their support of the Medically Associated Science and Technology Program, Cedars-Sinai, Los Angeles. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
and composition and levels of circulating inflammatory markers.
METHODOLOGY:
- Researchers analyzed samples from the REIMAGINE (Revealing the Entire Intestinal Microbiota and its Associations with the Genetic, Immunologic, and Neuroendocrine Ecosystem) study to assess the potential effects of NSS consumption on the duodenal luminal microbiome.
- They analyzed subjects consuming non-aspartame nonsugar sweeteners (NANS; n = 35) and aspartame only (ASP; n = 9), who were compared with 55 control participants matched for age, sex, and body mass index.
- A subset of 40 participants provided stool samples for additional analysis.
TAKEAWAY:
- Duodenal alpha diversity was lower in NANS consumers vs controls.
- Duodenal relative abundance (RA) of Escherichia, Klebsiella, and Salmonella was lower in both NANS and ASP vs controls, whereas stool RA of these phylum Proteobacteria was increased in both NANS and ASP.
- Compared with controls, NANS and ASP differed in how they altered predicted duodenal microbial metabolic pathways, with NANS impacting polysaccharides biosynthesis and D-galactose degradation and ASP significantly enriching biosynthesis of cylindrospermopsin, a potential cancer-causing agent known to adversely impact the liver and nervous system.
- Circulating levels of interleukin (IL)-1b, a pro-inflammatory cytokine that plays a key role in the immune response, were significantly decreased in NANS vs controls, whereas IL-6 and IL-10, two cytokines with protective properties, were decreased in the ASP group vs controls.
IN PRACTICE:
“Given the crucial role played by small intestinal microbes in digestion, nutrient absorption, immune regulation, and endocrine functions, coupled with the substantial prevalence of NSS consumption among US adults (estimated at 41.4%), our findings have potential implications for metabolic and gastrointestinal health in a considerable proportion of the American adult population.”
SOURCE:
The study, conducted by Ava Hosseini, MPH, and colleagues at Cedars-Sinai, Los Angeles, was published online on November 22, 2023, in iScience.
LIMITATIONS:
The study population may not be representative of healthy individuals as they underwent upper endoscopy for various reasons (eg, evaluation of intestinal complaints). After exclusions, the duodenal sample size for the aspartame group was small. Samples were collected at a single timepoint, limiting the ability to establish causal relationships.
DISCLOSURES:
This research was supported by Frank Lee, the Monica Lester Charitable Trust, and the Elias, Genevieve, and Georgianna Atol Charitable Trust through their support of the Medically Associated Science and Technology Program, Cedars-Sinai, Los Angeles. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Ascending Thoracic Aortic Aneurysms: A ‘Silver Lining’?
Often known as a “silent killer,” ascending thoracic aortic aneurysms (ATAAs) may grow asymptomatically until they rupture, at which point, mortality is over 90%.
But and by extension, for those who have one, a significantly reduced risk for coronary artery disease and myocardial infarction (MI).
“We noticed in the operating room that many patients we worked on who had an ATAA had pristine arteries, like a teenager’s,” said John Elefteriades, MD, William W.L. Glenn Professor of Cardiothoracic Surgery and former chief of cardiothoracic surgery at Yale University and Yale New Haven Hospital, New Haven, Connecticut. “The same was true of the femoral artery, which we use to hook up to the heart-lung machine.”
Elefteriades and colleagues have been investigating the implications of this association for more than two decades. Many of their studies are highlighted in a recent review of the evidence supporting the protective relationship between ATAAs and the development of atherosclerosis and the possible mechanisms driving the relationship.
“We see four different layers of protection,” said Sandip Mukherjee, MD, medical director of the Aortic Institute at Yale New Haven Hospital and a senior editor of the journal AORTA. Mukherjee collaborated with Elefteriades on many of the studies.
The first layer of protection is lower intima-media thickness, specifically, 0.131 mm lower than in individuals without an ATAA. “It may not seem like very much, but one point can actually translate into a 13%-15% decline in the rate of myocardial infarction or stroke,” Dr. Mukherjee said.
The second layer is lower levels of low-density lipoprotein (LDL) cholesterol. Lower LDL cholesterol levels (75 mg/dL) were associated with increased odds of ATAAs (odds ratio [OR], 1.21), whereas elevated levels (150 mg/dL and 200 mg/dL) were associated with decreased odds of ATAAs (OR, 0.62 and 0.29, respectively).
Lower calcification scores for the coronary arteries are the third layer of protection (6.73 vs 9.36 in one study).
The fourth protective layer is a significantly reduced prevalence of coronary artery disease. A study of individuals with ATAA compared to controls found 61 of those with ATAA had coronary artery disease vs 140 of controls, and 11 vs 83 had experienced an MI. Of note, patients with ATAAs were protected despite having higher body mass indices than controls.
Other MI risk factors such as age increased the risk even among those with an ATAA but, again, much less so than among controls; a multivariable binary logistic regression of data in the team’s review showed that patients with ATAAs were 298, 250, and 232 times less likely to have an MI than if they had a family history of MI, dyslipidemia, or hypertension, respectively.
Why the Protection?
The ligamentum arteriosum separates the ascending from the descending (thoracoabdominal) aorta. ATAAs, located above the ligamentum, tend to be pro-aneurysmal but anti-atherosclerotic. In the descending aorta, below the ligamentum, atherosclerotic aneurysms develop.
The differences between the two sections of the aorta originate in the germ layer in the embryo, Dr. Elefteriades said. “The fundamental difference in tissue of origin translates into marked differences in the character of aneurysms in the different aortic segments.”
What specifically underlies the reduced cardiovascular risk? “We don’t really know, but we think that there may be two possible etiologies,” Dr. Mukherjee said. One hypothesis involves transforming growth factor–beta (TGF-beta), which is overexpressed in patients with ATAA and seems to increase their vulnerability to aneurysms while also conferring protection from coronary disease risk.
Some studies have shown differences in cellular responses to TGF-beta between the thoracic and abdominal aorta, including collagen production and contractility. Others have shown that some patients who have had an MI have polymorphisms that decrease their levels of TGF-beta.
Furthermore, TGF-beta plays a key role in the development of the intimal layer, which could underpin the lack of intimal thickening in patients with ATAA.
But overall, studies have been mixed and challenging to interpret, Dr. Elefteriades and Dr. Mukherjee agreed. TGF-beta has multiple remodeling roles in the body, and it is difficult at this point to isolate its exact role in aortic disease.
Another hypothesis involves matrix metalloproteinases (MMPs), which are dysregulated in patients with ATAA and may confer some protection, Mukherjee said. Several studies have shown higher plasma levels of certain MMPs in patients with ATAAs. MMPs also were found to be elevated in the thoracic aortic walls of patients with ATAA who had an aortic dissection, as well as in the aortic smooth muscle cells in the intima and media.
In addition, some studies have shown increased levels of MMP-2 in the aortas of patients with ATAAs compared with patients with coronary artery disease.
Adding to the mix of possibilities, “We recently found a gene that’s dysregulated in our aneurysm patients that is very intimately related to atherosclerosis,” Dr. Elefteriades said. “But the work is too preliminary to say anything more at this point.”
“It would be fabulous to prove what it is causing this protection,” Dr. Mukherjee added. “But the truth is we don’t know. These are hypotheses.”
“The most important message from our work is that most clinicians need to dissociate an ATAA from the concept of atherosclerosis,” Dr. Elefteriades said. “The ascending aorta is not an atherosclerotic phenomenon.”
How to Manage Patients With ATAA
What does the distinct character of ATAAs mean for patient management? “Finding a drug to treat ATAAs — to prevent growth, rupture, or dissection — has been like a search for the Holy Grail,” Dr. Elefteriades said. “Statins are not necessary, as this is a non-atherosclerotic process. Although sporadic studies have reported beneficial effects from beta-blockers or angiotensin II receptor blockers (ARBs), this has often been based on ‘soft’ evidence, requiring a combination of outcome measures to achieve significance.”
That said, he noted, “The mainstay, common sense treatment is to keep blood pressure controlled. This is usually achieved by a beta-blocker and an ARB, even if the benefit is not via a direct biologic effect on the aneurysmal degenerative process, but via simple hemodynamics — discouraging rupture by keeping pressure in the aorta low.”
Dr. Mukherjee suggested that these patients should be referred to a specialty aneurysm center where their genes will be evaluated, and then the aneurysm will be followed very closely.
“If the aneurysm is larger than 4.5 cm, we screen the patient every single year, and if they have chest pain, we treat them the same way as we treat other aneurysms,” he said. “As a rule of thumb, if the aneurysm reaches 5 cm, it should come out, although the size at which this should happen may differ between 4.5 cm and 5.5 cm, depending on the patient’s body size.”
As for lifestyle management, Dr. Elefteriades said, “Protection from atherosclerosis and MI won’t go away after the aneurysm is removed. We think it’s in the body’s chemistry. But even though it’s very hard for those patients to have a heart attack, we don’t recommend they eat roast beef every night — although I do think they’d be protected from such lifestyle aberrations.”
For now, he added, “Our team is on a hunt to find a drug to treat ascending disease directly and effectively. We have ongoing laboratory experiments with two drugs undergoing investigation at some level. We hope to embark soon on clinical trials.”
‘A Milestone’
James Hamilton Black III, MD, vice chair of the writing committee for the 2022 American College of Cardiology/American Heart Association Aortic Disease Guideline and chief of Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Medicine, Baltimore, commented on the review and the concept of ATAA’s atherosclerotic protection.
“The association of ascending aortic aneurysms with a lower risk for MI is an interesting one, but it’s probably influenced, at least in part, by the patient population.” That population is at least partially curated since people are coming to an academic center. In addition, Dr. Black noted, “the patients with ATAAs are younger, and so age may be a confounding factor in the analyses. We wouldn’t expect them to have the same burden of atherosclerosis” as older patients.
Nevertheless, he said, “the findings speak to an emerging body of literature suggesting that although the aorta is a single organ, there are certainly different areas, and these would respond quite differently to environmental or genetic or heritable stressors. This isn’t surprising, and there probably are a lot of factors involved.”
Overall, he said, the findings underscore “the precision medicine approaches we need to take with patients with aortic diseases.”
In a commentary on the team’s review article, published in 2022, John G.T. Augoustides, MD, professor of anesthesiology and critical care at the Perelman School of Medicine in Philadelphia, Pennsylvania, suggested that ATAA’s “silver lining” could advance the understanding of thoracic aortic aneurysm (TAA) management, be integrated with the expanding horizons in hereditary thoracic aortic disease, and might be explored in the context of bicuspid aortic valve disease.
Highlighting the “relative absence” of atherosclerosis in ascending aortic aneurysms and its importance is a “milestone in our understanding,” he concluded. “It is likely that future advances in TAAs will be significantly influenced by this observation.”
Dr. Elefteriades, Dr. Mukherjee, and Dr. Black have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Often known as a “silent killer,” ascending thoracic aortic aneurysms (ATAAs) may grow asymptomatically until they rupture, at which point, mortality is over 90%.
But and by extension, for those who have one, a significantly reduced risk for coronary artery disease and myocardial infarction (MI).
“We noticed in the operating room that many patients we worked on who had an ATAA had pristine arteries, like a teenager’s,” said John Elefteriades, MD, William W.L. Glenn Professor of Cardiothoracic Surgery and former chief of cardiothoracic surgery at Yale University and Yale New Haven Hospital, New Haven, Connecticut. “The same was true of the femoral artery, which we use to hook up to the heart-lung machine.”
Elefteriades and colleagues have been investigating the implications of this association for more than two decades. Many of their studies are highlighted in a recent review of the evidence supporting the protective relationship between ATAAs and the development of atherosclerosis and the possible mechanisms driving the relationship.
“We see four different layers of protection,” said Sandip Mukherjee, MD, medical director of the Aortic Institute at Yale New Haven Hospital and a senior editor of the journal AORTA. Mukherjee collaborated with Elefteriades on many of the studies.
The first layer of protection is lower intima-media thickness, specifically, 0.131 mm lower than in individuals without an ATAA. “It may not seem like very much, but one point can actually translate into a 13%-15% decline in the rate of myocardial infarction or stroke,” Dr. Mukherjee said.
The second layer is lower levels of low-density lipoprotein (LDL) cholesterol. Lower LDL cholesterol levels (75 mg/dL) were associated with increased odds of ATAAs (odds ratio [OR], 1.21), whereas elevated levels (150 mg/dL and 200 mg/dL) were associated with decreased odds of ATAAs (OR, 0.62 and 0.29, respectively).
Lower calcification scores for the coronary arteries are the third layer of protection (6.73 vs 9.36 in one study).
The fourth protective layer is a significantly reduced prevalence of coronary artery disease. A study of individuals with ATAA compared to controls found 61 of those with ATAA had coronary artery disease vs 140 of controls, and 11 vs 83 had experienced an MI. Of note, patients with ATAAs were protected despite having higher body mass indices than controls.
Other MI risk factors such as age increased the risk even among those with an ATAA but, again, much less so than among controls; a multivariable binary logistic regression of data in the team’s review showed that patients with ATAAs were 298, 250, and 232 times less likely to have an MI than if they had a family history of MI, dyslipidemia, or hypertension, respectively.
Why the Protection?
The ligamentum arteriosum separates the ascending from the descending (thoracoabdominal) aorta. ATAAs, located above the ligamentum, tend to be pro-aneurysmal but anti-atherosclerotic. In the descending aorta, below the ligamentum, atherosclerotic aneurysms develop.
The differences between the two sections of the aorta originate in the germ layer in the embryo, Dr. Elefteriades said. “The fundamental difference in tissue of origin translates into marked differences in the character of aneurysms in the different aortic segments.”
What specifically underlies the reduced cardiovascular risk? “We don’t really know, but we think that there may be two possible etiologies,” Dr. Mukherjee said. One hypothesis involves transforming growth factor–beta (TGF-beta), which is overexpressed in patients with ATAA and seems to increase their vulnerability to aneurysms while also conferring protection from coronary disease risk.
Some studies have shown differences in cellular responses to TGF-beta between the thoracic and abdominal aorta, including collagen production and contractility. Others have shown that some patients who have had an MI have polymorphisms that decrease their levels of TGF-beta.
Furthermore, TGF-beta plays a key role in the development of the intimal layer, which could underpin the lack of intimal thickening in patients with ATAA.
But overall, studies have been mixed and challenging to interpret, Dr. Elefteriades and Dr. Mukherjee agreed. TGF-beta has multiple remodeling roles in the body, and it is difficult at this point to isolate its exact role in aortic disease.
Another hypothesis involves matrix metalloproteinases (MMPs), which are dysregulated in patients with ATAA and may confer some protection, Mukherjee said. Several studies have shown higher plasma levels of certain MMPs in patients with ATAAs. MMPs also were found to be elevated in the thoracic aortic walls of patients with ATAA who had an aortic dissection, as well as in the aortic smooth muscle cells in the intima and media.
In addition, some studies have shown increased levels of MMP-2 in the aortas of patients with ATAAs compared with patients with coronary artery disease.
Adding to the mix of possibilities, “We recently found a gene that’s dysregulated in our aneurysm patients that is very intimately related to atherosclerosis,” Dr. Elefteriades said. “But the work is too preliminary to say anything more at this point.”
“It would be fabulous to prove what it is causing this protection,” Dr. Mukherjee added. “But the truth is we don’t know. These are hypotheses.”
“The most important message from our work is that most clinicians need to dissociate an ATAA from the concept of atherosclerosis,” Dr. Elefteriades said. “The ascending aorta is not an atherosclerotic phenomenon.”
How to Manage Patients With ATAA
What does the distinct character of ATAAs mean for patient management? “Finding a drug to treat ATAAs — to prevent growth, rupture, or dissection — has been like a search for the Holy Grail,” Dr. Elefteriades said. “Statins are not necessary, as this is a non-atherosclerotic process. Although sporadic studies have reported beneficial effects from beta-blockers or angiotensin II receptor blockers (ARBs), this has often been based on ‘soft’ evidence, requiring a combination of outcome measures to achieve significance.”
That said, he noted, “The mainstay, common sense treatment is to keep blood pressure controlled. This is usually achieved by a beta-blocker and an ARB, even if the benefit is not via a direct biologic effect on the aneurysmal degenerative process, but via simple hemodynamics — discouraging rupture by keeping pressure in the aorta low.”
Dr. Mukherjee suggested that these patients should be referred to a specialty aneurysm center where their genes will be evaluated, and then the aneurysm will be followed very closely.
“If the aneurysm is larger than 4.5 cm, we screen the patient every single year, and if they have chest pain, we treat them the same way as we treat other aneurysms,” he said. “As a rule of thumb, if the aneurysm reaches 5 cm, it should come out, although the size at which this should happen may differ between 4.5 cm and 5.5 cm, depending on the patient’s body size.”
As for lifestyle management, Dr. Elefteriades said, “Protection from atherosclerosis and MI won’t go away after the aneurysm is removed. We think it’s in the body’s chemistry. But even though it’s very hard for those patients to have a heart attack, we don’t recommend they eat roast beef every night — although I do think they’d be protected from such lifestyle aberrations.”
For now, he added, “Our team is on a hunt to find a drug to treat ascending disease directly and effectively. We have ongoing laboratory experiments with two drugs undergoing investigation at some level. We hope to embark soon on clinical trials.”
‘A Milestone’
James Hamilton Black III, MD, vice chair of the writing committee for the 2022 American College of Cardiology/American Heart Association Aortic Disease Guideline and chief of Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Medicine, Baltimore, commented on the review and the concept of ATAA’s atherosclerotic protection.
“The association of ascending aortic aneurysms with a lower risk for MI is an interesting one, but it’s probably influenced, at least in part, by the patient population.” That population is at least partially curated since people are coming to an academic center. In addition, Dr. Black noted, “the patients with ATAAs are younger, and so age may be a confounding factor in the analyses. We wouldn’t expect them to have the same burden of atherosclerosis” as older patients.
Nevertheless, he said, “the findings speak to an emerging body of literature suggesting that although the aorta is a single organ, there are certainly different areas, and these would respond quite differently to environmental or genetic or heritable stressors. This isn’t surprising, and there probably are a lot of factors involved.”
Overall, he said, the findings underscore “the precision medicine approaches we need to take with patients with aortic diseases.”
In a commentary on the team’s review article, published in 2022, John G.T. Augoustides, MD, professor of anesthesiology and critical care at the Perelman School of Medicine in Philadelphia, Pennsylvania, suggested that ATAA’s “silver lining” could advance the understanding of thoracic aortic aneurysm (TAA) management, be integrated with the expanding horizons in hereditary thoracic aortic disease, and might be explored in the context of bicuspid aortic valve disease.
Highlighting the “relative absence” of atherosclerosis in ascending aortic aneurysms and its importance is a “milestone in our understanding,” he concluded. “It is likely that future advances in TAAs will be significantly influenced by this observation.”
Dr. Elefteriades, Dr. Mukherjee, and Dr. Black have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Often known as a “silent killer,” ascending thoracic aortic aneurysms (ATAAs) may grow asymptomatically until they rupture, at which point, mortality is over 90%.
But and by extension, for those who have one, a significantly reduced risk for coronary artery disease and myocardial infarction (MI).
“We noticed in the operating room that many patients we worked on who had an ATAA had pristine arteries, like a teenager’s,” said John Elefteriades, MD, William W.L. Glenn Professor of Cardiothoracic Surgery and former chief of cardiothoracic surgery at Yale University and Yale New Haven Hospital, New Haven, Connecticut. “The same was true of the femoral artery, which we use to hook up to the heart-lung machine.”
Elefteriades and colleagues have been investigating the implications of this association for more than two decades. Many of their studies are highlighted in a recent review of the evidence supporting the protective relationship between ATAAs and the development of atherosclerosis and the possible mechanisms driving the relationship.
“We see four different layers of protection,” said Sandip Mukherjee, MD, medical director of the Aortic Institute at Yale New Haven Hospital and a senior editor of the journal AORTA. Mukherjee collaborated with Elefteriades on many of the studies.
The first layer of protection is lower intima-media thickness, specifically, 0.131 mm lower than in individuals without an ATAA. “It may not seem like very much, but one point can actually translate into a 13%-15% decline in the rate of myocardial infarction or stroke,” Dr. Mukherjee said.
The second layer is lower levels of low-density lipoprotein (LDL) cholesterol. Lower LDL cholesterol levels (75 mg/dL) were associated with increased odds of ATAAs (odds ratio [OR], 1.21), whereas elevated levels (150 mg/dL and 200 mg/dL) were associated with decreased odds of ATAAs (OR, 0.62 and 0.29, respectively).
Lower calcification scores for the coronary arteries are the third layer of protection (6.73 vs 9.36 in one study).
The fourth protective layer is a significantly reduced prevalence of coronary artery disease. A study of individuals with ATAA compared to controls found 61 of those with ATAA had coronary artery disease vs 140 of controls, and 11 vs 83 had experienced an MI. Of note, patients with ATAAs were protected despite having higher body mass indices than controls.
Other MI risk factors such as age increased the risk even among those with an ATAA but, again, much less so than among controls; a multivariable binary logistic regression of data in the team’s review showed that patients with ATAAs were 298, 250, and 232 times less likely to have an MI than if they had a family history of MI, dyslipidemia, or hypertension, respectively.
Why the Protection?
The ligamentum arteriosum separates the ascending from the descending (thoracoabdominal) aorta. ATAAs, located above the ligamentum, tend to be pro-aneurysmal but anti-atherosclerotic. In the descending aorta, below the ligamentum, atherosclerotic aneurysms develop.
The differences between the two sections of the aorta originate in the germ layer in the embryo, Dr. Elefteriades said. “The fundamental difference in tissue of origin translates into marked differences in the character of aneurysms in the different aortic segments.”
What specifically underlies the reduced cardiovascular risk? “We don’t really know, but we think that there may be two possible etiologies,” Dr. Mukherjee said. One hypothesis involves transforming growth factor–beta (TGF-beta), which is overexpressed in patients with ATAA and seems to increase their vulnerability to aneurysms while also conferring protection from coronary disease risk.
Some studies have shown differences in cellular responses to TGF-beta between the thoracic and abdominal aorta, including collagen production and contractility. Others have shown that some patients who have had an MI have polymorphisms that decrease their levels of TGF-beta.
Furthermore, TGF-beta plays a key role in the development of the intimal layer, which could underpin the lack of intimal thickening in patients with ATAA.
But overall, studies have been mixed and challenging to interpret, Dr. Elefteriades and Dr. Mukherjee agreed. TGF-beta has multiple remodeling roles in the body, and it is difficult at this point to isolate its exact role in aortic disease.
Another hypothesis involves matrix metalloproteinases (MMPs), which are dysregulated in patients with ATAA and may confer some protection, Mukherjee said. Several studies have shown higher plasma levels of certain MMPs in patients with ATAAs. MMPs also were found to be elevated in the thoracic aortic walls of patients with ATAA who had an aortic dissection, as well as in the aortic smooth muscle cells in the intima and media.
In addition, some studies have shown increased levels of MMP-2 in the aortas of patients with ATAAs compared with patients with coronary artery disease.
Adding to the mix of possibilities, “We recently found a gene that’s dysregulated in our aneurysm patients that is very intimately related to atherosclerosis,” Dr. Elefteriades said. “But the work is too preliminary to say anything more at this point.”
“It would be fabulous to prove what it is causing this protection,” Dr. Mukherjee added. “But the truth is we don’t know. These are hypotheses.”
“The most important message from our work is that most clinicians need to dissociate an ATAA from the concept of atherosclerosis,” Dr. Elefteriades said. “The ascending aorta is not an atherosclerotic phenomenon.”
How to Manage Patients With ATAA
What does the distinct character of ATAAs mean for patient management? “Finding a drug to treat ATAAs — to prevent growth, rupture, or dissection — has been like a search for the Holy Grail,” Dr. Elefteriades said. “Statins are not necessary, as this is a non-atherosclerotic process. Although sporadic studies have reported beneficial effects from beta-blockers or angiotensin II receptor blockers (ARBs), this has often been based on ‘soft’ evidence, requiring a combination of outcome measures to achieve significance.”
That said, he noted, “The mainstay, common sense treatment is to keep blood pressure controlled. This is usually achieved by a beta-blocker and an ARB, even if the benefit is not via a direct biologic effect on the aneurysmal degenerative process, but via simple hemodynamics — discouraging rupture by keeping pressure in the aorta low.”
Dr. Mukherjee suggested that these patients should be referred to a specialty aneurysm center where their genes will be evaluated, and then the aneurysm will be followed very closely.
“If the aneurysm is larger than 4.5 cm, we screen the patient every single year, and if they have chest pain, we treat them the same way as we treat other aneurysms,” he said. “As a rule of thumb, if the aneurysm reaches 5 cm, it should come out, although the size at which this should happen may differ between 4.5 cm and 5.5 cm, depending on the patient’s body size.”
As for lifestyle management, Dr. Elefteriades said, “Protection from atherosclerosis and MI won’t go away after the aneurysm is removed. We think it’s in the body’s chemistry. But even though it’s very hard for those patients to have a heart attack, we don’t recommend they eat roast beef every night — although I do think they’d be protected from such lifestyle aberrations.”
For now, he added, “Our team is on a hunt to find a drug to treat ascending disease directly and effectively. We have ongoing laboratory experiments with two drugs undergoing investigation at some level. We hope to embark soon on clinical trials.”
‘A Milestone’
James Hamilton Black III, MD, vice chair of the writing committee for the 2022 American College of Cardiology/American Heart Association Aortic Disease Guideline and chief of Division of Vascular Surgery and Endovascular Therapy at Johns Hopkins Medicine, Baltimore, commented on the review and the concept of ATAA’s atherosclerotic protection.
“The association of ascending aortic aneurysms with a lower risk for MI is an interesting one, but it’s probably influenced, at least in part, by the patient population.” That population is at least partially curated since people are coming to an academic center. In addition, Dr. Black noted, “the patients with ATAAs are younger, and so age may be a confounding factor in the analyses. We wouldn’t expect them to have the same burden of atherosclerosis” as older patients.
Nevertheless, he said, “the findings speak to an emerging body of literature suggesting that although the aorta is a single organ, there are certainly different areas, and these would respond quite differently to environmental or genetic or heritable stressors. This isn’t surprising, and there probably are a lot of factors involved.”
Overall, he said, the findings underscore “the precision medicine approaches we need to take with patients with aortic diseases.”
In a commentary on the team’s review article, published in 2022, John G.T. Augoustides, MD, professor of anesthesiology and critical care at the Perelman School of Medicine in Philadelphia, Pennsylvania, suggested that ATAA’s “silver lining” could advance the understanding of thoracic aortic aneurysm (TAA) management, be integrated with the expanding horizons in hereditary thoracic aortic disease, and might be explored in the context of bicuspid aortic valve disease.
Highlighting the “relative absence” of atherosclerosis in ascending aortic aneurysms and its importance is a “milestone in our understanding,” he concluded. “It is likely that future advances in TAAs will be significantly influenced by this observation.”
Dr. Elefteriades, Dr. Mukherjee, and Dr. Black have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.