User login
A New, Easily Identifiable Sign of Concussion?
Spontaneous Headshake After a Kinematic Event (SHAAKE) refers to the rapid, back-and-forth head movement athletes exhibit following a blow to the head. This voluntary motion typically occurs within seconds to minutes after impact and is a familiar response in athletes.
In a recent survey, 7 out of 10 adult athletes recalled making this movement after a collision, and three out of four times they attributed this back-and-forth head movement to a concussion. The association was strongest among football players, who reported that over 90% of SHAAKE episodes were associated with a concussion.
The results were published online in Diagnostics.
Call to Action
“Everyone” — including sports and medical organizations — “should be adding this to their list of potential concussion signs and their protocol immediately,” study investigator Chris Nowinski, PhD, CEO and co-founder of the Concussion Legacy Foundation, told this news organization.
Nowinski said it’s “fascinating” that this concussion sign hasn’t been formally studied or added to formal concussion screening metrics before now, given that it’s been depicted in movies, television, and cartoons for decades.
Coaches, medical professionals, and concussion spotters should be trained to recognize when a SHAAKE happens, he said.
“The interesting thing is, I don’t think coaches or parents need much training other than to officially tie this to suspicion of a concussion,” Nowinski added.
The Case of Miami Dolphins QB Tua Tagovailoa
Nowinski said he was tipped off to SHAAKE as a concussion sign after Miami Dolphins quarterback Tua Tagovailoa’s controversial undiagnosed concussion during a National Football League (NFL) game in 2022.
After Tagovailoa’s head hit the ground, he rapidly shook his head side to side, indicating displaying SHAAKE, before stumbling and collapsing. At the time, a sideline doctor attributed his collapse to a prior back injury.
If Tagovailoa had been diagnosed with a concussion, he likely would not have been playing in a game just 4 days later, where he lost consciousness after suffering a suspected second concussion and was removed from the field on a stretcher.
For the survey, Nowinski and colleagues showed 347 current and former athletes, including 109 football players, video examples of SHAAKE and them asked about their experiences with this potential indicator of concussion.
Nearly 69% of athletes reported exhibiting a SHAAKE during their career, and 93% of those reported a SHAAKE in association with concussion at least once. Athletes reported SHAAKE a median of five times in their lives.
Of the athletes who reported SHAAKE, 85% linked this head-shaking movement to concussion symptoms such as disorientation (71%) and dizziness (54%).
Across all sports, SHAAKE showed a sensitivity of 49.6% and a positive predictive value (PPV) of 72.4% for diagnosing concussions.
Among football players, sensitivity improved to 52.3%, with an estimated specificity of 99.9%, a PPV of 91.9%, and an estimated negative predictive value of 99.5%.
The main limitation of the survey was the potential for recall bias due to survey participants self-reporting prior concussions. The researchers called for future prospective studies to validate SHAAKE as a sign of concussion.
Instant Replay for Brain Injury?
Experts echoed the need for validation. SHAAKE represents a “promising advance” in objective TBI assessment, particularly for sideline evaluation, said Shaheen Lakhan, MD, PhD, neurologist, and researcher based in Miami, Florida, who wasn’t involved in the research.
The potential value of SHAAKE is “particularly notable given the well-documented tendency for athletes to minimize or conceal symptoms to maintain play eligibility, a limitation that has historically challenged our reliance on subjective reporting and observational assessments,” Lakhan said.
“Moving forward, validation through prospective studies incorporating real-time video analysis, helmet sensor data, and clinician-confirmed TBI diagnoses will be essential. With appropriate validation, SHAAKE could emerge as a valuable component of our sideline assessment arsenal, complementing rather than replacing existing diagnostic approaches,” Lakhan said.
“SHAAKE could be the ‘instant replay’ for brain injuries that sports medicine has been waiting for — but like any new technology, we need to make sure it works for every player, not just some,” Lakhan added.
Also weighing in, Richard Figler, MD, director of the Concussion Center, Cleveland Clinic Sports Medicine Center, Cleveland, cautioned that the survey participants were recruited from a concussion registry and self-reported an average of 23 concussions — more than one third of which happened 5-10 years prior — which begs the question, “How much are they actually remembering?”
“Our goal is to make sure that the athletes are safe and that we’re not missing concussions, and we don’t have great tools to start off with. This study opens up the door for some prospective studies [of SHAAKE] moving forward. I think we need more data before this should be listed as a definitive marker,” said Figler, who also wasn’t involved in the study.
In any case, he said, when it comes to suspected concussion in sports, “when in doubt, you sit them out,” Figler said.
This research received no external funding. Nowinski has received travel reimbursement from the NFL Players Association (NFLPA), NFL, World Rugby, WWE, and All Elite Wrestling; served as an expert witness in cases related to concussion and chronic traumatic encephalopathy; and is compensated for speaking appearances and serving on the NFL Concussion Settlement Player Advocacy Committee. Daniel H. Daneshvar served as an expert witness in legal cases involving brain injury and concussion and received funding from the Football Players Health Study at Harvard University, which is funded by the NFLPA and evaluates patients for the MGH Brain and Body TRUST Center, sponsored in part by the NFLPA. Lakhan and Figler had no relevant disclosures.
A version of this article appeared on Medscape.com.
Spontaneous Headshake After a Kinematic Event (SHAAKE) refers to the rapid, back-and-forth head movement athletes exhibit following a blow to the head. This voluntary motion typically occurs within seconds to minutes after impact and is a familiar response in athletes.
In a recent survey, 7 out of 10 adult athletes recalled making this movement after a collision, and three out of four times they attributed this back-and-forth head movement to a concussion. The association was strongest among football players, who reported that over 90% of SHAAKE episodes were associated with a concussion.
The results were published online in Diagnostics.
Call to Action
“Everyone” — including sports and medical organizations — “should be adding this to their list of potential concussion signs and their protocol immediately,” study investigator Chris Nowinski, PhD, CEO and co-founder of the Concussion Legacy Foundation, told this news organization.
Nowinski said it’s “fascinating” that this concussion sign hasn’t been formally studied or added to formal concussion screening metrics before now, given that it’s been depicted in movies, television, and cartoons for decades.
Coaches, medical professionals, and concussion spotters should be trained to recognize when a SHAAKE happens, he said.
“The interesting thing is, I don’t think coaches or parents need much training other than to officially tie this to suspicion of a concussion,” Nowinski added.
The Case of Miami Dolphins QB Tua Tagovailoa
Nowinski said he was tipped off to SHAAKE as a concussion sign after Miami Dolphins quarterback Tua Tagovailoa’s controversial undiagnosed concussion during a National Football League (NFL) game in 2022.
After Tagovailoa’s head hit the ground, he rapidly shook his head side to side, indicating displaying SHAAKE, before stumbling and collapsing. At the time, a sideline doctor attributed his collapse to a prior back injury.
If Tagovailoa had been diagnosed with a concussion, he likely would not have been playing in a game just 4 days later, where he lost consciousness after suffering a suspected second concussion and was removed from the field on a stretcher.
For the survey, Nowinski and colleagues showed 347 current and former athletes, including 109 football players, video examples of SHAAKE and them asked about their experiences with this potential indicator of concussion.
Nearly 69% of athletes reported exhibiting a SHAAKE during their career, and 93% of those reported a SHAAKE in association with concussion at least once. Athletes reported SHAAKE a median of five times in their lives.
Of the athletes who reported SHAAKE, 85% linked this head-shaking movement to concussion symptoms such as disorientation (71%) and dizziness (54%).
Across all sports, SHAAKE showed a sensitivity of 49.6% and a positive predictive value (PPV) of 72.4% for diagnosing concussions.
Among football players, sensitivity improved to 52.3%, with an estimated specificity of 99.9%, a PPV of 91.9%, and an estimated negative predictive value of 99.5%.
The main limitation of the survey was the potential for recall bias due to survey participants self-reporting prior concussions. The researchers called for future prospective studies to validate SHAAKE as a sign of concussion.
Instant Replay for Brain Injury?
Experts echoed the need for validation. SHAAKE represents a “promising advance” in objective TBI assessment, particularly for sideline evaluation, said Shaheen Lakhan, MD, PhD, neurologist, and researcher based in Miami, Florida, who wasn’t involved in the research.
The potential value of SHAAKE is “particularly notable given the well-documented tendency for athletes to minimize or conceal symptoms to maintain play eligibility, a limitation that has historically challenged our reliance on subjective reporting and observational assessments,” Lakhan said.
“Moving forward, validation through prospective studies incorporating real-time video analysis, helmet sensor data, and clinician-confirmed TBI diagnoses will be essential. With appropriate validation, SHAAKE could emerge as a valuable component of our sideline assessment arsenal, complementing rather than replacing existing diagnostic approaches,” Lakhan said.
“SHAAKE could be the ‘instant replay’ for brain injuries that sports medicine has been waiting for — but like any new technology, we need to make sure it works for every player, not just some,” Lakhan added.
Also weighing in, Richard Figler, MD, director of the Concussion Center, Cleveland Clinic Sports Medicine Center, Cleveland, cautioned that the survey participants were recruited from a concussion registry and self-reported an average of 23 concussions — more than one third of which happened 5-10 years prior — which begs the question, “How much are they actually remembering?”
“Our goal is to make sure that the athletes are safe and that we’re not missing concussions, and we don’t have great tools to start off with. This study opens up the door for some prospective studies [of SHAAKE] moving forward. I think we need more data before this should be listed as a definitive marker,” said Figler, who also wasn’t involved in the study.
In any case, he said, when it comes to suspected concussion in sports, “when in doubt, you sit them out,” Figler said.
This research received no external funding. Nowinski has received travel reimbursement from the NFL Players Association (NFLPA), NFL, World Rugby, WWE, and All Elite Wrestling; served as an expert witness in cases related to concussion and chronic traumatic encephalopathy; and is compensated for speaking appearances and serving on the NFL Concussion Settlement Player Advocacy Committee. Daniel H. Daneshvar served as an expert witness in legal cases involving brain injury and concussion and received funding from the Football Players Health Study at Harvard University, which is funded by the NFLPA and evaluates patients for the MGH Brain and Body TRUST Center, sponsored in part by the NFLPA. Lakhan and Figler had no relevant disclosures.
A version of this article appeared on Medscape.com.
Spontaneous Headshake After a Kinematic Event (SHAAKE) refers to the rapid, back-and-forth head movement athletes exhibit following a blow to the head. This voluntary motion typically occurs within seconds to minutes after impact and is a familiar response in athletes.
In a recent survey, 7 out of 10 adult athletes recalled making this movement after a collision, and three out of four times they attributed this back-and-forth head movement to a concussion. The association was strongest among football players, who reported that over 90% of SHAAKE episodes were associated with a concussion.
The results were published online in Diagnostics.
Call to Action
“Everyone” — including sports and medical organizations — “should be adding this to their list of potential concussion signs and their protocol immediately,” study investigator Chris Nowinski, PhD, CEO and co-founder of the Concussion Legacy Foundation, told this news organization.
Nowinski said it’s “fascinating” that this concussion sign hasn’t been formally studied or added to formal concussion screening metrics before now, given that it’s been depicted in movies, television, and cartoons for decades.
Coaches, medical professionals, and concussion spotters should be trained to recognize when a SHAAKE happens, he said.
“The interesting thing is, I don’t think coaches or parents need much training other than to officially tie this to suspicion of a concussion,” Nowinski added.
The Case of Miami Dolphins QB Tua Tagovailoa
Nowinski said he was tipped off to SHAAKE as a concussion sign after Miami Dolphins quarterback Tua Tagovailoa’s controversial undiagnosed concussion during a National Football League (NFL) game in 2022.
After Tagovailoa’s head hit the ground, he rapidly shook his head side to side, indicating displaying SHAAKE, before stumbling and collapsing. At the time, a sideline doctor attributed his collapse to a prior back injury.
If Tagovailoa had been diagnosed with a concussion, he likely would not have been playing in a game just 4 days later, where he lost consciousness after suffering a suspected second concussion and was removed from the field on a stretcher.
For the survey, Nowinski and colleagues showed 347 current and former athletes, including 109 football players, video examples of SHAAKE and them asked about their experiences with this potential indicator of concussion.
Nearly 69% of athletes reported exhibiting a SHAAKE during their career, and 93% of those reported a SHAAKE in association with concussion at least once. Athletes reported SHAAKE a median of five times in their lives.
Of the athletes who reported SHAAKE, 85% linked this head-shaking movement to concussion symptoms such as disorientation (71%) and dizziness (54%).
Across all sports, SHAAKE showed a sensitivity of 49.6% and a positive predictive value (PPV) of 72.4% for diagnosing concussions.
Among football players, sensitivity improved to 52.3%, with an estimated specificity of 99.9%, a PPV of 91.9%, and an estimated negative predictive value of 99.5%.
The main limitation of the survey was the potential for recall bias due to survey participants self-reporting prior concussions. The researchers called for future prospective studies to validate SHAAKE as a sign of concussion.
Instant Replay for Brain Injury?
Experts echoed the need for validation. SHAAKE represents a “promising advance” in objective TBI assessment, particularly for sideline evaluation, said Shaheen Lakhan, MD, PhD, neurologist, and researcher based in Miami, Florida, who wasn’t involved in the research.
The potential value of SHAAKE is “particularly notable given the well-documented tendency for athletes to minimize or conceal symptoms to maintain play eligibility, a limitation that has historically challenged our reliance on subjective reporting and observational assessments,” Lakhan said.
“Moving forward, validation through prospective studies incorporating real-time video analysis, helmet sensor data, and clinician-confirmed TBI diagnoses will be essential. With appropriate validation, SHAAKE could emerge as a valuable component of our sideline assessment arsenal, complementing rather than replacing existing diagnostic approaches,” Lakhan said.
“SHAAKE could be the ‘instant replay’ for brain injuries that sports medicine has been waiting for — but like any new technology, we need to make sure it works for every player, not just some,” Lakhan added.
Also weighing in, Richard Figler, MD, director of the Concussion Center, Cleveland Clinic Sports Medicine Center, Cleveland, cautioned that the survey participants were recruited from a concussion registry and self-reported an average of 23 concussions — more than one third of which happened 5-10 years prior — which begs the question, “How much are they actually remembering?”
“Our goal is to make sure that the athletes are safe and that we’re not missing concussions, and we don’t have great tools to start off with. This study opens up the door for some prospective studies [of SHAAKE] moving forward. I think we need more data before this should be listed as a definitive marker,” said Figler, who also wasn’t involved in the study.
In any case, he said, when it comes to suspected concussion in sports, “when in doubt, you sit them out,” Figler said.
This research received no external funding. Nowinski has received travel reimbursement from the NFL Players Association (NFLPA), NFL, World Rugby, WWE, and All Elite Wrestling; served as an expert witness in cases related to concussion and chronic traumatic encephalopathy; and is compensated for speaking appearances and serving on the NFL Concussion Settlement Player Advocacy Committee. Daniel H. Daneshvar served as an expert witness in legal cases involving brain injury and concussion and received funding from the Football Players Health Study at Harvard University, which is funded by the NFLPA and evaluates patients for the MGH Brain and Body TRUST Center, sponsored in part by the NFLPA. Lakhan and Figler had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM DIAGNOSTICS
For Radiation ‘Downwinders,’ Cancer Compensation Is On Hold
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
Increase in Troublesome Fungal Infections Requires All-Out Approach
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
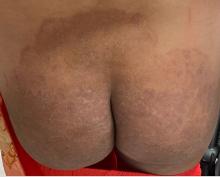
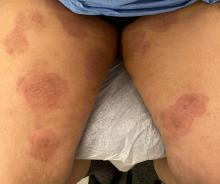
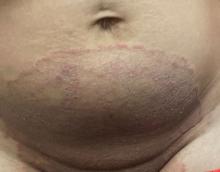
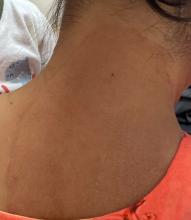
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
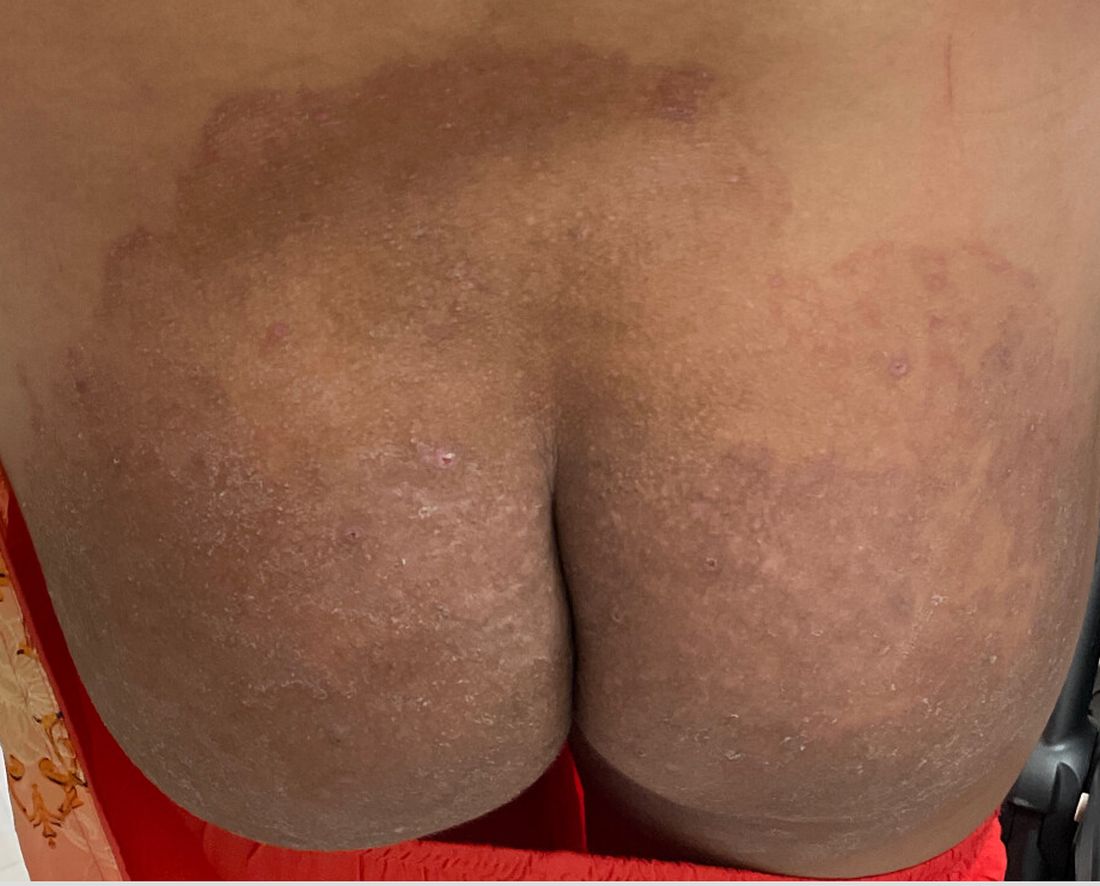
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
How Old Are You? Stand on One Leg and I’ll Tell You
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.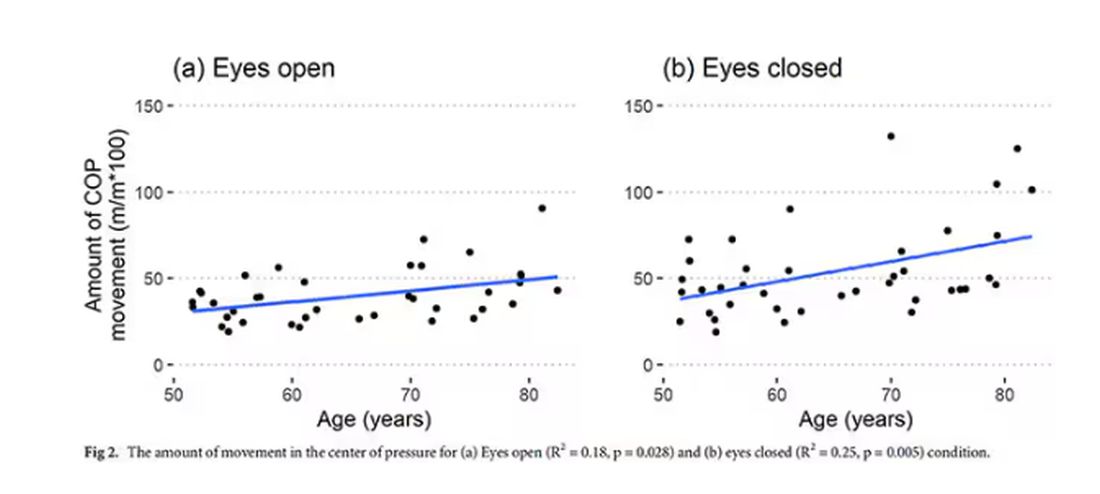
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Dermatologist’s Tips for Supporting LGBTQ Youth
HUNTINGTON BEACH, CALIFORNIA —
“Sometimes in dermatology we might say, ‘This gender care stuff, that’s really for pediatricians and primary care doctors,’ ” Boos, a pediatric dermatologist at Seattle Children’s Hospital, Seattle, said at the annual meeting of the Pacific Dermatologic Association. However, he added, “gender-affirming care happens not only with medications but with communication, curiosity, and respect.” For instance, an LGBTQ patient who is being treated with isotretinoin for acne is seen once a month by a dermatologist, which is probably more frequent than seeing their primary care physician, he said. “Every time you see that child, you can make them feel seen. You can respect them. You can let them know that you care about them. Hopefully then they understand what it feels like to get good care from a provider and then will not settle for poor care from someone else.”
According to Gallup polling, the proportion of people in the United States who identify as non-cisgender or nonheterosexual increased from 3.5% in 2012 to 7% in 2021. “The estimation is that 2.5%-3.5% of all teenagers identify as gay or bisexual, and another 1% identify as transgender, though some studies estimate the percentage of gender diverse youth to be as high as 9.2%,” said Boos.
He discussed several barriers to dermatologic care for LGBTQ youth, including availability. “There are only about 400 practicing pediatric dermatologists in the US, so there’s not a lot of pediatric dermatology care to go around for any child,” Boos said. “My plea to general dermatologists who see adolescents and teenagers: You can care for LGBTQ adolescents; they need your help.”
Accessibility is also an issue. For example, his clinic is in a wealthy and somewhat isolated area of Seattle, “which makes it hard for some patients to access our services because they may have to drive from far away or take multiple modes of public transportation to see us,” explained Boos, who came out as gay about 10 years ago after beginning his practice in Seattle. “Time matters, too. Children are in school. They don’t necessarily want to take time off to go to the doctor’s office. We want to make sure we have services at different times of day, including evenings or weekends if possible.”
Another potential barrier to care for this patient population is acceptability. “I can say that I welcome any patient to my practice, but if I’m not humble and informed about their concerns, especially queer or trans kids, if they feel that I’m not respecting them, that’s going to be a huge problem,” Boos said. “They won’t view that care as acceptable, and they’re not going to come back if they feel like I’m not looking out for their best interests.”
In a large cross-sectional study of patients with chronic inflammatory skin diseases published in 2023, sexual and gender minority (SGM) individuals were significantly more likely than non-SGM individuals to delay specialist care including dermatologic care (adjusted odds ratio [AOR], 1.23), mental health care (AOR, 1.62), and filing a prescription (AOR, 1.30) because of cost. The barriers for SGM patients were transportation issues, not having a healthcare practitioner (HCP) from the same racial or ethnic background, “and they were more likely to report not always being treated with respect by HCPs,” said Boos, who was not involved with the study. “SGM patients of minoritized racial identities such as Black, Hispanic, and Latino were also more likely to experience barriers to care.”
Boos offered several tips for improving the dermatologic care of LGBTQ youth:
Use inclusive language and follow your patient’s lead. “There are many ways that people identify, both with respect to their sexual orientation and their gender identity,” he said. “We often think that a person is either gay or straight, or cisgender or transgender. There are many folks who reject these binaries and may view their gender identity or sexual orientation outside of these descriptors. You can be bisexual. You can be asexual.” He also emphasized that sexual orientation is different from sexual behavior.
Be deliberate about your phrasing. Boos said he strives to make new patients feel comfortable by asking them such questions as what pronouns they use, how he should address them, and whether they have a partner or are in a relationship. “Then, in general, just follow your patient’s lead,” Boos said. “If they’re referring to their partner in a certain way or to themselves with certain pronouns, go along with it. When in doubt, just ask. And if you make a mistake like using the wrong pronouns or name of a patient, the best thing to do is immediately apologize and try your best not to repeat that error.”
When asking about sexual practices, don’t make assumptions. Boos recommends a 2019 article on dermatologic care of LGBT persons, published in the Journal of the American Academy of Dermatology, which includes specific examples of how to elicit a sexual history from adults and teens. One of the recommendations is “to be very direct, say, ‘This may feel uncomfortable, but I have to ask you these direct questions about what you’re doing sexually because I need to understand if you’re at risk for things like sexually transmitted infections,’ ” Boos said. “It’s also important to use terminology that our patients know. If I ask someone if they’ve had sex before, they usually understand that as penile-vaginal intercourse, but it’s also important to understand if they have oral or anal sex. But if you ask, ‘Have you had insertive anal sex?’ they may not know what that means as opposed to receptive anal sex. Instead, you might ask, ‘Are you a top or a bottom?’ which are more commonly used and understood terms in the queer community. It may feel really uncomfortable to use that kind of language, but we want to make sure patients understand what we’re asking them so we can take the best possible care of them.”
Pay attention to the details. One way to demonstrate inclusivity in your practice includes collecting pronoun and sexual orientation information for the electronic medical record so your entire staff can use proper pronouns for the patient. “Also, acknowledge that for queer folks, family can mean more than just biological family,” Boos added. “I do not buy into the stereotype that all queer kids are ostracized from their families and not loved by their families, but it is true that they are at risk for those experiences. So, sometimes a member of the patient’s ‘chosen family’ accompanies them on their visit.”
Privacy is also key. “You never know who else is in the room when you’re on a telehealth call, so you need to address that before you ask about personal things,” Boos said. “One sticking point that can also come up is that parents often fill out their child’s patient demographic form, which may not tell the real story. I typically start to have confidential time without parents and may take a sexual history as early as 12 or 13 years of age if it’s a patient that I’m seeing for an extended period or if I’m worried about a skin finding that might suggest an STI.”
He highlighted the unique opportunity dermatologists have to transform the healthcare landscape for LGBTQ children and adolescents. “It’s about extending yourself to nurture the growth of another person,” Boos said. “This can feel challenging, but you want to see each person for who they are and help get them to where they want to go. That’s what we went into medicine for, right? We want to care about people.”
Boos had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA —
“Sometimes in dermatology we might say, ‘This gender care stuff, that’s really for pediatricians and primary care doctors,’ ” Boos, a pediatric dermatologist at Seattle Children’s Hospital, Seattle, said at the annual meeting of the Pacific Dermatologic Association. However, he added, “gender-affirming care happens not only with medications but with communication, curiosity, and respect.” For instance, an LGBTQ patient who is being treated with isotretinoin for acne is seen once a month by a dermatologist, which is probably more frequent than seeing their primary care physician, he said. “Every time you see that child, you can make them feel seen. You can respect them. You can let them know that you care about them. Hopefully then they understand what it feels like to get good care from a provider and then will not settle for poor care from someone else.”
According to Gallup polling, the proportion of people in the United States who identify as non-cisgender or nonheterosexual increased from 3.5% in 2012 to 7% in 2021. “The estimation is that 2.5%-3.5% of all teenagers identify as gay or bisexual, and another 1% identify as transgender, though some studies estimate the percentage of gender diverse youth to be as high as 9.2%,” said Boos.
He discussed several barriers to dermatologic care for LGBTQ youth, including availability. “There are only about 400 practicing pediatric dermatologists in the US, so there’s not a lot of pediatric dermatology care to go around for any child,” Boos said. “My plea to general dermatologists who see adolescents and teenagers: You can care for LGBTQ adolescents; they need your help.”
Accessibility is also an issue. For example, his clinic is in a wealthy and somewhat isolated area of Seattle, “which makes it hard for some patients to access our services because they may have to drive from far away or take multiple modes of public transportation to see us,” explained Boos, who came out as gay about 10 years ago after beginning his practice in Seattle. “Time matters, too. Children are in school. They don’t necessarily want to take time off to go to the doctor’s office. We want to make sure we have services at different times of day, including evenings or weekends if possible.”
Another potential barrier to care for this patient population is acceptability. “I can say that I welcome any patient to my practice, but if I’m not humble and informed about their concerns, especially queer or trans kids, if they feel that I’m not respecting them, that’s going to be a huge problem,” Boos said. “They won’t view that care as acceptable, and they’re not going to come back if they feel like I’m not looking out for their best interests.”
In a large cross-sectional study of patients with chronic inflammatory skin diseases published in 2023, sexual and gender minority (SGM) individuals were significantly more likely than non-SGM individuals to delay specialist care including dermatologic care (adjusted odds ratio [AOR], 1.23), mental health care (AOR, 1.62), and filing a prescription (AOR, 1.30) because of cost. The barriers for SGM patients were transportation issues, not having a healthcare practitioner (HCP) from the same racial or ethnic background, “and they were more likely to report not always being treated with respect by HCPs,” said Boos, who was not involved with the study. “SGM patients of minoritized racial identities such as Black, Hispanic, and Latino were also more likely to experience barriers to care.”
Boos offered several tips for improving the dermatologic care of LGBTQ youth:
Use inclusive language and follow your patient’s lead. “There are many ways that people identify, both with respect to their sexual orientation and their gender identity,” he said. “We often think that a person is either gay or straight, or cisgender or transgender. There are many folks who reject these binaries and may view their gender identity or sexual orientation outside of these descriptors. You can be bisexual. You can be asexual.” He also emphasized that sexual orientation is different from sexual behavior.
Be deliberate about your phrasing. Boos said he strives to make new patients feel comfortable by asking them such questions as what pronouns they use, how he should address them, and whether they have a partner or are in a relationship. “Then, in general, just follow your patient’s lead,” Boos said. “If they’re referring to their partner in a certain way or to themselves with certain pronouns, go along with it. When in doubt, just ask. And if you make a mistake like using the wrong pronouns or name of a patient, the best thing to do is immediately apologize and try your best not to repeat that error.”
When asking about sexual practices, don’t make assumptions. Boos recommends a 2019 article on dermatologic care of LGBT persons, published in the Journal of the American Academy of Dermatology, which includes specific examples of how to elicit a sexual history from adults and teens. One of the recommendations is “to be very direct, say, ‘This may feel uncomfortable, but I have to ask you these direct questions about what you’re doing sexually because I need to understand if you’re at risk for things like sexually transmitted infections,’ ” Boos said. “It’s also important to use terminology that our patients know. If I ask someone if they’ve had sex before, they usually understand that as penile-vaginal intercourse, but it’s also important to understand if they have oral or anal sex. But if you ask, ‘Have you had insertive anal sex?’ they may not know what that means as opposed to receptive anal sex. Instead, you might ask, ‘Are you a top or a bottom?’ which are more commonly used and understood terms in the queer community. It may feel really uncomfortable to use that kind of language, but we want to make sure patients understand what we’re asking them so we can take the best possible care of them.”
Pay attention to the details. One way to demonstrate inclusivity in your practice includes collecting pronoun and sexual orientation information for the electronic medical record so your entire staff can use proper pronouns for the patient. “Also, acknowledge that for queer folks, family can mean more than just biological family,” Boos added. “I do not buy into the stereotype that all queer kids are ostracized from their families and not loved by their families, but it is true that they are at risk for those experiences. So, sometimes a member of the patient’s ‘chosen family’ accompanies them on their visit.”
Privacy is also key. “You never know who else is in the room when you’re on a telehealth call, so you need to address that before you ask about personal things,” Boos said. “One sticking point that can also come up is that parents often fill out their child’s patient demographic form, which may not tell the real story. I typically start to have confidential time without parents and may take a sexual history as early as 12 or 13 years of age if it’s a patient that I’m seeing for an extended period or if I’m worried about a skin finding that might suggest an STI.”
He highlighted the unique opportunity dermatologists have to transform the healthcare landscape for LGBTQ children and adolescents. “It’s about extending yourself to nurture the growth of another person,” Boos said. “This can feel challenging, but you want to see each person for who they are and help get them to where they want to go. That’s what we went into medicine for, right? We want to care about people.”
Boos had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA —
“Sometimes in dermatology we might say, ‘This gender care stuff, that’s really for pediatricians and primary care doctors,’ ” Boos, a pediatric dermatologist at Seattle Children’s Hospital, Seattle, said at the annual meeting of the Pacific Dermatologic Association. However, he added, “gender-affirming care happens not only with medications but with communication, curiosity, and respect.” For instance, an LGBTQ patient who is being treated with isotretinoin for acne is seen once a month by a dermatologist, which is probably more frequent than seeing their primary care physician, he said. “Every time you see that child, you can make them feel seen. You can respect them. You can let them know that you care about them. Hopefully then they understand what it feels like to get good care from a provider and then will not settle for poor care from someone else.”
According to Gallup polling, the proportion of people in the United States who identify as non-cisgender or nonheterosexual increased from 3.5% in 2012 to 7% in 2021. “The estimation is that 2.5%-3.5% of all teenagers identify as gay or bisexual, and another 1% identify as transgender, though some studies estimate the percentage of gender diverse youth to be as high as 9.2%,” said Boos.
He discussed several barriers to dermatologic care for LGBTQ youth, including availability. “There are only about 400 practicing pediatric dermatologists in the US, so there’s not a lot of pediatric dermatology care to go around for any child,” Boos said. “My plea to general dermatologists who see adolescents and teenagers: You can care for LGBTQ adolescents; they need your help.”
Accessibility is also an issue. For example, his clinic is in a wealthy and somewhat isolated area of Seattle, “which makes it hard for some patients to access our services because they may have to drive from far away or take multiple modes of public transportation to see us,” explained Boos, who came out as gay about 10 years ago after beginning his practice in Seattle. “Time matters, too. Children are in school. They don’t necessarily want to take time off to go to the doctor’s office. We want to make sure we have services at different times of day, including evenings or weekends if possible.”
Another potential barrier to care for this patient population is acceptability. “I can say that I welcome any patient to my practice, but if I’m not humble and informed about their concerns, especially queer or trans kids, if they feel that I’m not respecting them, that’s going to be a huge problem,” Boos said. “They won’t view that care as acceptable, and they’re not going to come back if they feel like I’m not looking out for their best interests.”
In a large cross-sectional study of patients with chronic inflammatory skin diseases published in 2023, sexual and gender minority (SGM) individuals were significantly more likely than non-SGM individuals to delay specialist care including dermatologic care (adjusted odds ratio [AOR], 1.23), mental health care (AOR, 1.62), and filing a prescription (AOR, 1.30) because of cost. The barriers for SGM patients were transportation issues, not having a healthcare practitioner (HCP) from the same racial or ethnic background, “and they were more likely to report not always being treated with respect by HCPs,” said Boos, who was not involved with the study. “SGM patients of minoritized racial identities such as Black, Hispanic, and Latino were also more likely to experience barriers to care.”
Boos offered several tips for improving the dermatologic care of LGBTQ youth:
Use inclusive language and follow your patient’s lead. “There are many ways that people identify, both with respect to their sexual orientation and their gender identity,” he said. “We often think that a person is either gay or straight, or cisgender or transgender. There are many folks who reject these binaries and may view their gender identity or sexual orientation outside of these descriptors. You can be bisexual. You can be asexual.” He also emphasized that sexual orientation is different from sexual behavior.
Be deliberate about your phrasing. Boos said he strives to make new patients feel comfortable by asking them such questions as what pronouns they use, how he should address them, and whether they have a partner or are in a relationship. “Then, in general, just follow your patient’s lead,” Boos said. “If they’re referring to their partner in a certain way or to themselves with certain pronouns, go along with it. When in doubt, just ask. And if you make a mistake like using the wrong pronouns or name of a patient, the best thing to do is immediately apologize and try your best not to repeat that error.”
When asking about sexual practices, don’t make assumptions. Boos recommends a 2019 article on dermatologic care of LGBT persons, published in the Journal of the American Academy of Dermatology, which includes specific examples of how to elicit a sexual history from adults and teens. One of the recommendations is “to be very direct, say, ‘This may feel uncomfortable, but I have to ask you these direct questions about what you’re doing sexually because I need to understand if you’re at risk for things like sexually transmitted infections,’ ” Boos said. “It’s also important to use terminology that our patients know. If I ask someone if they’ve had sex before, they usually understand that as penile-vaginal intercourse, but it’s also important to understand if they have oral or anal sex. But if you ask, ‘Have you had insertive anal sex?’ they may not know what that means as opposed to receptive anal sex. Instead, you might ask, ‘Are you a top or a bottom?’ which are more commonly used and understood terms in the queer community. It may feel really uncomfortable to use that kind of language, but we want to make sure patients understand what we’re asking them so we can take the best possible care of them.”
Pay attention to the details. One way to demonstrate inclusivity in your practice includes collecting pronoun and sexual orientation information for the electronic medical record so your entire staff can use proper pronouns for the patient. “Also, acknowledge that for queer folks, family can mean more than just biological family,” Boos added. “I do not buy into the stereotype that all queer kids are ostracized from their families and not loved by their families, but it is true that they are at risk for those experiences. So, sometimes a member of the patient’s ‘chosen family’ accompanies them on their visit.”
Privacy is also key. “You never know who else is in the room when you’re on a telehealth call, so you need to address that before you ask about personal things,” Boos said. “One sticking point that can also come up is that parents often fill out their child’s patient demographic form, which may not tell the real story. I typically start to have confidential time without parents and may take a sexual history as early as 12 or 13 years of age if it’s a patient that I’m seeing for an extended period or if I’m worried about a skin finding that might suggest an STI.”
He highlighted the unique opportunity dermatologists have to transform the healthcare landscape for LGBTQ children and adolescents. “It’s about extending yourself to nurture the growth of another person,” Boos said. “This can feel challenging, but you want to see each person for who they are and help get them to where they want to go. That’s what we went into medicine for, right? We want to care about people.”
Boos had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM PDA 2024
H pylori: ACG Guideline Advises New Approaches to Treatment
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Should First-Line Dual Checkpoint Blockade Be Used for NSCLC With Specific Mutations?
according to the authors of a new paper.
These findings, drawn from a post hoc analysis of phase 3 data, are backed up by cell line and mouse data revealing clear mechanisms of efficacy, making the collective evidence compelling enough to reshape clinical practice, reported lead author Ferdinandos Skoulidis, MD, PhD, of The University of Texas MD Anderson Cancer Center, Houston.
“Although STK11 and KEAP1 mutations are associated with limited benefit from PD-1 or PD-L1 [PD-(L)1] inhibition, the association between these mutations and benefit from combinations of PD-(L)1 inhibitors with chemotherapy is not yet as well established,” the investigators wrote in Nature.
Skoulidis and colleagues conducted the subgroup analysis of POSEIDON trial data and characterized underlying biologic mechanisms using mouse models to address this knowledge gap.
What Were the Original Findings of POSEIDON?
The POSEIDON trial involved 1013 patients with metastatic NSCLC. Treatment arms included standard chemotherapy alone, chemotherapy plus programmed death ligand 1 (PD-L1) inhibitor durvalumab, and chemotherapy plus durvalumab and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab.
Adding durvalumab to chemotherapy significantly improved median progression-free survival (PFS) but not median overall survival (OS), while dual checkpoint blockade boosted both PFS and OS.
These findings provided support for the dual approach in the first-line setting, but not preferentially so. Experts called for more long-term data, questioned the survival benefit in terms of the increased toxicity, and noted the lack of biomarkers for patient selection.
What Did Post Hoc Analysis Highlight About POSEIDON?
The present analysis aimed to validate two actionable biomarkers.
“We and others have previously observed that alterations in STK11 and KEAP1 can promote an immunosuppressive tumor microenvironment and together might be responsible for half or more of the primary resistance to PD-(L)1 inhibition among patients with nsNSCLC when given as monotherapy,” Skoulidis and colleagues wrote.
From the original 1013 patients, 612 had non-squamous NSCLC and were evaluable for mutations. Among them, 87 had STK11 mutations and 37 had KEAP1 mutations.
As anticipated, patients in the STK11/KEAP1 subgroup saw little to no benefit from adding durvalumab to chemotherapy, but adding tremelimumab on top yielded notable improvement.
This was first observed in the objective response rate, which was 42.9% with dual checkpoint blockade plus chemotherapy vs 30.2% with single checkpoint blockade plus chemotherapy and 28% for chemotherapy alone. Durations of response improved in kind.
Survival outcomes also trended toward improvement in the dual checkpoint arm, which had a median OS of 15.8 months vs 7.3 months for durvalumab plus chemotherapy (hazard ratio [HR], 0.64; 95% CI, 0.40-1.04) and 10.5 months for chemotherapy alone (HR, 0.50; 95% CI, 0.29-0.87). PFS showed similar trends.
How Do Findings Relate to Previous NSCLC Subgroup Research?
Skoulidis and colleagues noted that their findings align with those of the CheckMate 9LA trial, which showed that patients with STK11 and/or KEAP1 mutations had better outcomes with dual checkpoint blockade plus chemotherapy than with chemotherapy alone.
“These data support the hypothesis that CTLA-4 inhibition can mitigate the resistance to chemotherapy plus PD-(L)1 inhibition observed in patients who have STK11 and/or KEAP1 mutations and suggest that this group of patients derives greater benefit from CTLA-4 inhibition than do patients who lack either alteration,” Skoulidis and colleagues wrote.
Grace Dy, MD, professor of oncology in the Department of Medicine at Roswell Park Comprehensive Cancer Center, Buffalo, New York, noted that in the present analysis, PD-L1 expression status did not predict outcomes; however, patients with STK11 and/or KEAP1 mutations typically have low or negative PD-L1 expression, which has been linked with better responses to CTLA-4 inhibition in multiple trials.
“In the CheckMate 227 and CheckMate 9LA studies, we have seen that patients with PD-L1–negative tumors appear to derive greater and more durable long-term overall survival benefit from dual immune checkpoint blockade compared to patients receiving anti-PD1-based therapy alone,” Dy said in a written comment. “While we take the necessary caveats on cross-trial comparisons, the same survival trend favoring CTLA-4-based immune checkpoint blockade is seen compared to the tail of the survival curves observed in PD-L1–negative patients enrolled in the KEYNOTE studies (KEYNOTE-189, KEYNOTE-407).”
Detecting improvements in survival within PD-L1 patients “may not be readily apparent until later when looking at the tail of the survival curves,” she added.
What Mechanisms of Action Explain Relative Benefits of Dual Checkpoint Blockade?
To elucidate underlying mechanisms of action, Skoulidis and colleagues conducted a series of experiments involving cell lines and mouse models of Stk11- and Keap1-deficient NSCLC.
“For us, it was critical to provide mechanistic support for the observed clinical benefit in POSEIDON, especially since this is based on a retrospective subgroup analysis,” Skoulidis said in an interview.
Their efforts revealed a strong link between the mutations and resistance to PD-(L)1 inhibition.
Inactivation of Stk11 and Keap1 promoted an immunosuppressive tumor microenvironment, marked by increased infiltration of suppressive myeloid cells and a reduction in CD8+ effector T cells. This immune imbalance contributed to evasion of immune destruction and limited the efficacy of programmed cell death protein 1 (PD-1) blockade.
Dual checkpoint blockade reprogrammed the immune microenvironment, leading to increased activation of CD4+ T helper (Th) cells, specifically the Th1 subtype, while inducing tumoricidal changes in myeloid cells. Consequently, antitumor responses improved, resulting in tumor regression and prolonged survival, compared with PD-1 monotherapy.
“Addition of CTLA-4 [inhibition] turns the two cardinal components of the suppressive microenvironment of these tumors on its head, and that’s why we believe we are observing this clinical benefit,” Skoulidis said. “This is not a mere association…but also based on very solid mechanistic data across a multitude of different models.”
Are Data Sufficient to Shift to First-Line Dual Checkpoint Blockade?
“Our work strengthens the available evidence that this regimen — and chemoimmunotherapy more broadly, with dual immune checkpoint blockade — constitutes a preferred approach for these patients,” Skoulidis said. “I personally, and I think physicians within MD Anderson, as well as a lot of physicians that I talk to, are already using — based on these data — the POSEIDON regimen, as well as, more broadly, chemoimmunotherapy with dual immune checkpoint for patients with these alterations.”
This view, however, remains contested by some oncologists.
Lei Deng, MD, assistant professor in the Division of Hematology and Oncology at the University of Washington, Fred Hutchinson Cancer Center, Seattle, called the new data “intriguing” and “hypothesis-generating,” but stopped short of supporting preferential first-line use.
“This study is a post hoc analysis, so you don’t have a lot of patients,” Deng said. “It is still not strong enough or definitive enough to make it standard of care to use dual checkpoint blockade for [patients with STK11 and/or KEAP1 mutations].”
The cell line and mouse data help explain biologic mechanisms of efficacy, he said, but these findings do not obviate toxicity concerns.
“You are adding one more agent, and this agent is more toxic than single checkpoint blockade,” Deng said. “So, if you weigh the risk, it is known, [but] the benefit is suggestive. I am not sure if the risk-benefit ratio would argue for routine implementation of this regimen yet.”
On the other hand, he noted, the combination is the US Food and Drug Administration–approved in this setting, so “it is not wrong to use it.”
Jyoti Malhotra, MD, director of thoracic medical oncology at City of Hope Orange County in Irvine, California, had a similar take.
“The clinical data presented so far is exploratory and limited by the small sample size,” Malhotra said in a written comment. “Data from an ongoing phase 3 trial (TRITON) is awaited before dual checkpoint blockade becomes the standard of care in this setting.”
Hossein Borghaei, DO, chief of the Division of Thoracic Medical Oncology at Fox Chase Cancer Center, Philadelphia, was also unequivocal when asked if dual checkpoint blockade with chemotherapy should be considered the preferred first-line treatment option in patients with STK11 and/or KEAP1 mutations.
“No,” he said in a written comment. “The data and the hypothesis are very strong, but it is all based on retrospective clinical data, cell line data, and mouse models. We need a randomized study to test the hypothesis.”
Incidentally, Borghaei is on the steering committee for the TRITON trial. He shared this potential conflict of interest before praising Skoulidis and colleagues for their efforts, noting that the present findings underscore the broader importance of widespread tumor profiling and access to resultant data.
“This is a beautiful story that has developed over the last few years based on the research by the group from MD Anderson who has reported the current Nature article,” he said. “This highlights the possible utility of collecting sequencing data on [all] patients’ tumors. These sorts of understandings and new ideas could arise only if there is access to this information.”
The study was supported by AstraZeneca, the National Cancer Institute, the Gunnigar Fund, and others. The investigators disclosed additional relationships with Novartis, Merck, Amgen, and others. Deng disclosed relationships with Merck, BridgeBio, MJH Life Sciences, and others. Dy disclosed relationships with Eli Lilly and Company, Janssen Pharmaceuticals, Meru, and others. Malhotra has previously served as a consultant for AstraZeneca. Borghaei has served as a consultant for AstraZeneca and is on the steering committee for the TRITON trial.
A version of this article appeared on Medscape.com.
according to the authors of a new paper.
These findings, drawn from a post hoc analysis of phase 3 data, are backed up by cell line and mouse data revealing clear mechanisms of efficacy, making the collective evidence compelling enough to reshape clinical practice, reported lead author Ferdinandos Skoulidis, MD, PhD, of The University of Texas MD Anderson Cancer Center, Houston.
“Although STK11 and KEAP1 mutations are associated with limited benefit from PD-1 or PD-L1 [PD-(L)1] inhibition, the association between these mutations and benefit from combinations of PD-(L)1 inhibitors with chemotherapy is not yet as well established,” the investigators wrote in Nature.
Skoulidis and colleagues conducted the subgroup analysis of POSEIDON trial data and characterized underlying biologic mechanisms using mouse models to address this knowledge gap.
What Were the Original Findings of POSEIDON?
The POSEIDON trial involved 1013 patients with metastatic NSCLC. Treatment arms included standard chemotherapy alone, chemotherapy plus programmed death ligand 1 (PD-L1) inhibitor durvalumab, and chemotherapy plus durvalumab and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab.
Adding durvalumab to chemotherapy significantly improved median progression-free survival (PFS) but not median overall survival (OS), while dual checkpoint blockade boosted both PFS and OS.
These findings provided support for the dual approach in the first-line setting, but not preferentially so. Experts called for more long-term data, questioned the survival benefit in terms of the increased toxicity, and noted the lack of biomarkers for patient selection.
What Did Post Hoc Analysis Highlight About POSEIDON?
The present analysis aimed to validate two actionable biomarkers.
“We and others have previously observed that alterations in STK11 and KEAP1 can promote an immunosuppressive tumor microenvironment and together might be responsible for half or more of the primary resistance to PD-(L)1 inhibition among patients with nsNSCLC when given as monotherapy,” Skoulidis and colleagues wrote.
From the original 1013 patients, 612 had non-squamous NSCLC and were evaluable for mutations. Among them, 87 had STK11 mutations and 37 had KEAP1 mutations.
As anticipated, patients in the STK11/KEAP1 subgroup saw little to no benefit from adding durvalumab to chemotherapy, but adding tremelimumab on top yielded notable improvement.
This was first observed in the objective response rate, which was 42.9% with dual checkpoint blockade plus chemotherapy vs 30.2% with single checkpoint blockade plus chemotherapy and 28% for chemotherapy alone. Durations of response improved in kind.
Survival outcomes also trended toward improvement in the dual checkpoint arm, which had a median OS of 15.8 months vs 7.3 months for durvalumab plus chemotherapy (hazard ratio [HR], 0.64; 95% CI, 0.40-1.04) and 10.5 months for chemotherapy alone (HR, 0.50; 95% CI, 0.29-0.87). PFS showed similar trends.
How Do Findings Relate to Previous NSCLC Subgroup Research?
Skoulidis and colleagues noted that their findings align with those of the CheckMate 9LA trial, which showed that patients with STK11 and/or KEAP1 mutations had better outcomes with dual checkpoint blockade plus chemotherapy than with chemotherapy alone.
“These data support the hypothesis that CTLA-4 inhibition can mitigate the resistance to chemotherapy plus PD-(L)1 inhibition observed in patients who have STK11 and/or KEAP1 mutations and suggest that this group of patients derives greater benefit from CTLA-4 inhibition than do patients who lack either alteration,” Skoulidis and colleagues wrote.
Grace Dy, MD, professor of oncology in the Department of Medicine at Roswell Park Comprehensive Cancer Center, Buffalo, New York, noted that in the present analysis, PD-L1 expression status did not predict outcomes; however, patients with STK11 and/or KEAP1 mutations typically have low or negative PD-L1 expression, which has been linked with better responses to CTLA-4 inhibition in multiple trials.
“In the CheckMate 227 and CheckMate 9LA studies, we have seen that patients with PD-L1–negative tumors appear to derive greater and more durable long-term overall survival benefit from dual immune checkpoint blockade compared to patients receiving anti-PD1-based therapy alone,” Dy said in a written comment. “While we take the necessary caveats on cross-trial comparisons, the same survival trend favoring CTLA-4-based immune checkpoint blockade is seen compared to the tail of the survival curves observed in PD-L1–negative patients enrolled in the KEYNOTE studies (KEYNOTE-189, KEYNOTE-407).”
Detecting improvements in survival within PD-L1 patients “may not be readily apparent until later when looking at the tail of the survival curves,” she added.
What Mechanisms of Action Explain Relative Benefits of Dual Checkpoint Blockade?
To elucidate underlying mechanisms of action, Skoulidis and colleagues conducted a series of experiments involving cell lines and mouse models of Stk11- and Keap1-deficient NSCLC.
“For us, it was critical to provide mechanistic support for the observed clinical benefit in POSEIDON, especially since this is based on a retrospective subgroup analysis,” Skoulidis said in an interview.
Their efforts revealed a strong link between the mutations and resistance to PD-(L)1 inhibition.
Inactivation of Stk11 and Keap1 promoted an immunosuppressive tumor microenvironment, marked by increased infiltration of suppressive myeloid cells and a reduction in CD8+ effector T cells. This immune imbalance contributed to evasion of immune destruction and limited the efficacy of programmed cell death protein 1 (PD-1) blockade.
Dual checkpoint blockade reprogrammed the immune microenvironment, leading to increased activation of CD4+ T helper (Th) cells, specifically the Th1 subtype, while inducing tumoricidal changes in myeloid cells. Consequently, antitumor responses improved, resulting in tumor regression and prolonged survival, compared with PD-1 monotherapy.
“Addition of CTLA-4 [inhibition] turns the two cardinal components of the suppressive microenvironment of these tumors on its head, and that’s why we believe we are observing this clinical benefit,” Skoulidis said. “This is not a mere association…but also based on very solid mechanistic data across a multitude of different models.”
Are Data Sufficient to Shift to First-Line Dual Checkpoint Blockade?
“Our work strengthens the available evidence that this regimen — and chemoimmunotherapy more broadly, with dual immune checkpoint blockade — constitutes a preferred approach for these patients,” Skoulidis said. “I personally, and I think physicians within MD Anderson, as well as a lot of physicians that I talk to, are already using — based on these data — the POSEIDON regimen, as well as, more broadly, chemoimmunotherapy with dual immune checkpoint for patients with these alterations.”
This view, however, remains contested by some oncologists.
Lei Deng, MD, assistant professor in the Division of Hematology and Oncology at the University of Washington, Fred Hutchinson Cancer Center, Seattle, called the new data “intriguing” and “hypothesis-generating,” but stopped short of supporting preferential first-line use.
“This study is a post hoc analysis, so you don’t have a lot of patients,” Deng said. “It is still not strong enough or definitive enough to make it standard of care to use dual checkpoint blockade for [patients with STK11 and/or KEAP1 mutations].”
The cell line and mouse data help explain biologic mechanisms of efficacy, he said, but these findings do not obviate toxicity concerns.
“You are adding one more agent, and this agent is more toxic than single checkpoint blockade,” Deng said. “So, if you weigh the risk, it is known, [but] the benefit is suggestive. I am not sure if the risk-benefit ratio would argue for routine implementation of this regimen yet.”
On the other hand, he noted, the combination is the US Food and Drug Administration–approved in this setting, so “it is not wrong to use it.”
Jyoti Malhotra, MD, director of thoracic medical oncology at City of Hope Orange County in Irvine, California, had a similar take.
“The clinical data presented so far is exploratory and limited by the small sample size,” Malhotra said in a written comment. “Data from an ongoing phase 3 trial (TRITON) is awaited before dual checkpoint blockade becomes the standard of care in this setting.”
Hossein Borghaei, DO, chief of the Division of Thoracic Medical Oncology at Fox Chase Cancer Center, Philadelphia, was also unequivocal when asked if dual checkpoint blockade with chemotherapy should be considered the preferred first-line treatment option in patients with STK11 and/or KEAP1 mutations.
“No,” he said in a written comment. “The data and the hypothesis are very strong, but it is all based on retrospective clinical data, cell line data, and mouse models. We need a randomized study to test the hypothesis.”
Incidentally, Borghaei is on the steering committee for the TRITON trial. He shared this potential conflict of interest before praising Skoulidis and colleagues for their efforts, noting that the present findings underscore the broader importance of widespread tumor profiling and access to resultant data.
“This is a beautiful story that has developed over the last few years based on the research by the group from MD Anderson who has reported the current Nature article,” he said. “This highlights the possible utility of collecting sequencing data on [all] patients’ tumors. These sorts of understandings and new ideas could arise only if there is access to this information.”
The study was supported by AstraZeneca, the National Cancer Institute, the Gunnigar Fund, and others. The investigators disclosed additional relationships with Novartis, Merck, Amgen, and others. Deng disclosed relationships with Merck, BridgeBio, MJH Life Sciences, and others. Dy disclosed relationships with Eli Lilly and Company, Janssen Pharmaceuticals, Meru, and others. Malhotra has previously served as a consultant for AstraZeneca. Borghaei has served as a consultant for AstraZeneca and is on the steering committee for the TRITON trial.
A version of this article appeared on Medscape.com.
according to the authors of a new paper.
These findings, drawn from a post hoc analysis of phase 3 data, are backed up by cell line and mouse data revealing clear mechanisms of efficacy, making the collective evidence compelling enough to reshape clinical practice, reported lead author Ferdinandos Skoulidis, MD, PhD, of The University of Texas MD Anderson Cancer Center, Houston.
“Although STK11 and KEAP1 mutations are associated with limited benefit from PD-1 or PD-L1 [PD-(L)1] inhibition, the association between these mutations and benefit from combinations of PD-(L)1 inhibitors with chemotherapy is not yet as well established,” the investigators wrote in Nature.
Skoulidis and colleagues conducted the subgroup analysis of POSEIDON trial data and characterized underlying biologic mechanisms using mouse models to address this knowledge gap.
What Were the Original Findings of POSEIDON?
The POSEIDON trial involved 1013 patients with metastatic NSCLC. Treatment arms included standard chemotherapy alone, chemotherapy plus programmed death ligand 1 (PD-L1) inhibitor durvalumab, and chemotherapy plus durvalumab and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor tremelimumab.
Adding durvalumab to chemotherapy significantly improved median progression-free survival (PFS) but not median overall survival (OS), while dual checkpoint blockade boosted both PFS and OS.
These findings provided support for the dual approach in the first-line setting, but not preferentially so. Experts called for more long-term data, questioned the survival benefit in terms of the increased toxicity, and noted the lack of biomarkers for patient selection.
What Did Post Hoc Analysis Highlight About POSEIDON?
The present analysis aimed to validate two actionable biomarkers.
“We and others have previously observed that alterations in STK11 and KEAP1 can promote an immunosuppressive tumor microenvironment and together might be responsible for half or more of the primary resistance to PD-(L)1 inhibition among patients with nsNSCLC when given as monotherapy,” Skoulidis and colleagues wrote.
From the original 1013 patients, 612 had non-squamous NSCLC and were evaluable for mutations. Among them, 87 had STK11 mutations and 37 had KEAP1 mutations.
As anticipated, patients in the STK11/KEAP1 subgroup saw little to no benefit from adding durvalumab to chemotherapy, but adding tremelimumab on top yielded notable improvement.
This was first observed in the objective response rate, which was 42.9% with dual checkpoint blockade plus chemotherapy vs 30.2% with single checkpoint blockade plus chemotherapy and 28% for chemotherapy alone. Durations of response improved in kind.
Survival outcomes also trended toward improvement in the dual checkpoint arm, which had a median OS of 15.8 months vs 7.3 months for durvalumab plus chemotherapy (hazard ratio [HR], 0.64; 95% CI, 0.40-1.04) and 10.5 months for chemotherapy alone (HR, 0.50; 95% CI, 0.29-0.87). PFS showed similar trends.
How Do Findings Relate to Previous NSCLC Subgroup Research?
Skoulidis and colleagues noted that their findings align with those of the CheckMate 9LA trial, which showed that patients with STK11 and/or KEAP1 mutations had better outcomes with dual checkpoint blockade plus chemotherapy than with chemotherapy alone.
“These data support the hypothesis that CTLA-4 inhibition can mitigate the resistance to chemotherapy plus PD-(L)1 inhibition observed in patients who have STK11 and/or KEAP1 mutations and suggest that this group of patients derives greater benefit from CTLA-4 inhibition than do patients who lack either alteration,” Skoulidis and colleagues wrote.
Grace Dy, MD, professor of oncology in the Department of Medicine at Roswell Park Comprehensive Cancer Center, Buffalo, New York, noted that in the present analysis, PD-L1 expression status did not predict outcomes; however, patients with STK11 and/or KEAP1 mutations typically have low or negative PD-L1 expression, which has been linked with better responses to CTLA-4 inhibition in multiple trials.
“In the CheckMate 227 and CheckMate 9LA studies, we have seen that patients with PD-L1–negative tumors appear to derive greater and more durable long-term overall survival benefit from dual immune checkpoint blockade compared to patients receiving anti-PD1-based therapy alone,” Dy said in a written comment. “While we take the necessary caveats on cross-trial comparisons, the same survival trend favoring CTLA-4-based immune checkpoint blockade is seen compared to the tail of the survival curves observed in PD-L1–negative patients enrolled in the KEYNOTE studies (KEYNOTE-189, KEYNOTE-407).”
Detecting improvements in survival within PD-L1 patients “may not be readily apparent until later when looking at the tail of the survival curves,” she added.
What Mechanisms of Action Explain Relative Benefits of Dual Checkpoint Blockade?
To elucidate underlying mechanisms of action, Skoulidis and colleagues conducted a series of experiments involving cell lines and mouse models of Stk11- and Keap1-deficient NSCLC.
“For us, it was critical to provide mechanistic support for the observed clinical benefit in POSEIDON, especially since this is based on a retrospective subgroup analysis,” Skoulidis said in an interview.
Their efforts revealed a strong link between the mutations and resistance to PD-(L)1 inhibition.
Inactivation of Stk11 and Keap1 promoted an immunosuppressive tumor microenvironment, marked by increased infiltration of suppressive myeloid cells and a reduction in CD8+ effector T cells. This immune imbalance contributed to evasion of immune destruction and limited the efficacy of programmed cell death protein 1 (PD-1) blockade.
Dual checkpoint blockade reprogrammed the immune microenvironment, leading to increased activation of CD4+ T helper (Th) cells, specifically the Th1 subtype, while inducing tumoricidal changes in myeloid cells. Consequently, antitumor responses improved, resulting in tumor regression and prolonged survival, compared with PD-1 monotherapy.
“Addition of CTLA-4 [inhibition] turns the two cardinal components of the suppressive microenvironment of these tumors on its head, and that’s why we believe we are observing this clinical benefit,” Skoulidis said. “This is not a mere association…but also based on very solid mechanistic data across a multitude of different models.”
Are Data Sufficient to Shift to First-Line Dual Checkpoint Blockade?
“Our work strengthens the available evidence that this regimen — and chemoimmunotherapy more broadly, with dual immune checkpoint blockade — constitutes a preferred approach for these patients,” Skoulidis said. “I personally, and I think physicians within MD Anderson, as well as a lot of physicians that I talk to, are already using — based on these data — the POSEIDON regimen, as well as, more broadly, chemoimmunotherapy with dual immune checkpoint for patients with these alterations.”
This view, however, remains contested by some oncologists.
Lei Deng, MD, assistant professor in the Division of Hematology and Oncology at the University of Washington, Fred Hutchinson Cancer Center, Seattle, called the new data “intriguing” and “hypothesis-generating,” but stopped short of supporting preferential first-line use.
“This study is a post hoc analysis, so you don’t have a lot of patients,” Deng said. “It is still not strong enough or definitive enough to make it standard of care to use dual checkpoint blockade for [patients with STK11 and/or KEAP1 mutations].”
The cell line and mouse data help explain biologic mechanisms of efficacy, he said, but these findings do not obviate toxicity concerns.
“You are adding one more agent, and this agent is more toxic than single checkpoint blockade,” Deng said. “So, if you weigh the risk, it is known, [but] the benefit is suggestive. I am not sure if the risk-benefit ratio would argue for routine implementation of this regimen yet.”
On the other hand, he noted, the combination is the US Food and Drug Administration–approved in this setting, so “it is not wrong to use it.”
Jyoti Malhotra, MD, director of thoracic medical oncology at City of Hope Orange County in Irvine, California, had a similar take.
“The clinical data presented so far is exploratory and limited by the small sample size,” Malhotra said in a written comment. “Data from an ongoing phase 3 trial (TRITON) is awaited before dual checkpoint blockade becomes the standard of care in this setting.”
Hossein Borghaei, DO, chief of the Division of Thoracic Medical Oncology at Fox Chase Cancer Center, Philadelphia, was also unequivocal when asked if dual checkpoint blockade with chemotherapy should be considered the preferred first-line treatment option in patients with STK11 and/or KEAP1 mutations.
“No,” he said in a written comment. “The data and the hypothesis are very strong, but it is all based on retrospective clinical data, cell line data, and mouse models. We need a randomized study to test the hypothesis.”
Incidentally, Borghaei is on the steering committee for the TRITON trial. He shared this potential conflict of interest before praising Skoulidis and colleagues for their efforts, noting that the present findings underscore the broader importance of widespread tumor profiling and access to resultant data.
“This is a beautiful story that has developed over the last few years based on the research by the group from MD Anderson who has reported the current Nature article,” he said. “This highlights the possible utility of collecting sequencing data on [all] patients’ tumors. These sorts of understandings and new ideas could arise only if there is access to this information.”
The study was supported by AstraZeneca, the National Cancer Institute, the Gunnigar Fund, and others. The investigators disclosed additional relationships with Novartis, Merck, Amgen, and others. Deng disclosed relationships with Merck, BridgeBio, MJH Life Sciences, and others. Dy disclosed relationships with Eli Lilly and Company, Janssen Pharmaceuticals, Meru, and others. Malhotra has previously served as a consultant for AstraZeneca. Borghaei has served as a consultant for AstraZeneca and is on the steering committee for the TRITON trial.
A version of this article appeared on Medscape.com.
Help Your Patients Reap the Benefits of Plant-Based Diets
Research pooled from nearly 100 studies has indicated that people who adhere to a vegan diet (ie, completely devoid of animal products) or a vegetarian diet (ie, devoid of meat, but may include dairy and eggs) are able to ward off some chronic diseases, such as cardiovascular disease, optimize glycemic control, and decrease their risk for cancer compared with those who consume omnivorous diets.
Vegan and vegetarian diets, or flexitarian diets — which are less reliant on animal protein than the standard US diet but do not completely exclude meat, fish, eggs, or dairy — may promote homeostasis and decrease inflammation by providing more fiber, antioxidants, and unsaturated fatty acids than the typical Western diet.
Inflammation and Obesity
Adipose tissue is a major producer of pro-inflammatory cytokines like interleukin (IL)-6, whose presence then triggers a rush of acute-phase reactants such as C-reactive protein (CRP) by the liver. This process develops into chronic low-grade inflammation that can increase a person’s chances of developing diabetes, cardiovascular disease, kidney disease, metabolic syndrome, and related complications.
Adopting a plant-based diet can improve markers of chronic low-grade inflammation that can lead to chronic disease and worsen existent chronic disease. A meta-analysis of 29 studies encompassing nearly 2700 participants found that initiation of a plant-based diet showed significant improvement in CRP, IL-6, and soluble intercellular adhesion molecule 1.
If we want to prevent these inflammatory disease states and their complications, the obvious response is to counsel patients to avoid excessive weight gain or to lose weight if obesity is their baseline. This can be tough for some patients, but it is nonetheless an important step in chronic disease prevention and management.
Plant-Based Diet for Type 2 Diabetes
According to a review of nine studies of patients living with type 2 diabetes who adhered to a plant-based diet, all but one found that this approach led to significantly lower A1c values than those seen in control groups. Six of the included studies reported that participants were able to decrease or discontinue medications for the management of diabetes. Researchers across all included studies also noted a decrease in total cholesterol, low-density lipoprotein cholesterol, and triglycerides, as well as increased weight loss in participants in each intervention group.
Such improvements are probably the result of the increase in fiber intake that occurs with a plant-based diet. A high-fiber diet is known to promote improved glucose and lipid metabolism as well as weight loss.
It is also worth noting that participants in the intervention groups also experienced improvements in depression and less chronic pain than did those in the control groups.
Plant-Based Diet for Chronic Kidney Disease (CKD)
Although the use of a plant-based diet in the prevention of CKD is well documented, adopting such diets for the treatment of CKD may intimidate both patients and practitioners owing to the high potassium and phosphorus content of many fruits and vegetables.
However, research indicates that the bioavailability of both potassium and phosphorus is lower in plant-based, whole foods than in preservatives and the highly processed food items that incorporate them. This makes a plant-based diet more viable than previously thought.
Diets rich in vegetables, whole grains, nuts, and legumes have been shown to decrease dietary acid load, both preventing and treating metabolic acidosis. Such diets have also been shown to decrease blood pressure and the risk for a decline in estimated glomerular filtration rate. This type of diet would also prioritize the unsaturated fatty acids and fiber-rich proteins such as avocados, beans, and nuts shown to improve dyslipidemia, which may occur alongside CKD.
Realistic Options for Patients on Medical Diets
There is one question that I always seem to get from when recommending a plant-based diet: “These patients already have so many restrictions. Why would you add more?” And my answer is also always the same: I don’t.
I rarely, if ever, recommend completely cutting out any food item or food group. Instead, I ask the patient to increase their intake of plant-based foods and only limit highly processed foods and fatty meats. By shifting a patient’s focus to beans; nuts; and low-carbohydrate, high-fiber fruits and vegetables, I am often opening up a whole new world of possibilities.
Instead of a sandwich with low-sodium turkey and cheese on white bread with a side of unsalted pretzels, I recommend a caprese salad with blueberries and almonds or a Southwest salad with black beans, corn, and avocado. I don’t encourage my patients to skip the foods that they love, but instead to only think about all the delicious plant-based options that will provide them with more than just calories.
Meat, dairy, seafood, and eggs can certainly be a part of a healthy diet, but what if our chronically ill patients, especially those with diabetes, had more options than just grilled chicken and green beans for every meal? What if we focus on decreasing dietary restrictions, incorporating a variety of nourishing foods, and educating our patients, instead of on portion control and moderation?
This is how I choose to incorporate plant-based diets into my practice to treat and prevent these chronic inflammatory conditions and promote sustainable, realistic change in my clients’ health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Research pooled from nearly 100 studies has indicated that people who adhere to a vegan diet (ie, completely devoid of animal products) or a vegetarian diet (ie, devoid of meat, but may include dairy and eggs) are able to ward off some chronic diseases, such as cardiovascular disease, optimize glycemic control, and decrease their risk for cancer compared with those who consume omnivorous diets.
Vegan and vegetarian diets, or flexitarian diets — which are less reliant on animal protein than the standard US diet but do not completely exclude meat, fish, eggs, or dairy — may promote homeostasis and decrease inflammation by providing more fiber, antioxidants, and unsaturated fatty acids than the typical Western diet.
Inflammation and Obesity
Adipose tissue is a major producer of pro-inflammatory cytokines like interleukin (IL)-6, whose presence then triggers a rush of acute-phase reactants such as C-reactive protein (CRP) by the liver. This process develops into chronic low-grade inflammation that can increase a person’s chances of developing diabetes, cardiovascular disease, kidney disease, metabolic syndrome, and related complications.
Adopting a plant-based diet can improve markers of chronic low-grade inflammation that can lead to chronic disease and worsen existent chronic disease. A meta-analysis of 29 studies encompassing nearly 2700 participants found that initiation of a plant-based diet showed significant improvement in CRP, IL-6, and soluble intercellular adhesion molecule 1.
If we want to prevent these inflammatory disease states and their complications, the obvious response is to counsel patients to avoid excessive weight gain or to lose weight if obesity is their baseline. This can be tough for some patients, but it is nonetheless an important step in chronic disease prevention and management.
Plant-Based Diet for Type 2 Diabetes
According to a review of nine studies of patients living with type 2 diabetes who adhered to a plant-based diet, all but one found that this approach led to significantly lower A1c values than those seen in control groups. Six of the included studies reported that participants were able to decrease or discontinue medications for the management of diabetes. Researchers across all included studies also noted a decrease in total cholesterol, low-density lipoprotein cholesterol, and triglycerides, as well as increased weight loss in participants in each intervention group.
Such improvements are probably the result of the increase in fiber intake that occurs with a plant-based diet. A high-fiber diet is known to promote improved glucose and lipid metabolism as well as weight loss.
It is also worth noting that participants in the intervention groups also experienced improvements in depression and less chronic pain than did those in the control groups.
Plant-Based Diet for Chronic Kidney Disease (CKD)
Although the use of a plant-based diet in the prevention of CKD is well documented, adopting such diets for the treatment of CKD may intimidate both patients and practitioners owing to the high potassium and phosphorus content of many fruits and vegetables.
However, research indicates that the bioavailability of both potassium and phosphorus is lower in plant-based, whole foods than in preservatives and the highly processed food items that incorporate them. This makes a plant-based diet more viable than previously thought.
Diets rich in vegetables, whole grains, nuts, and legumes have been shown to decrease dietary acid load, both preventing and treating metabolic acidosis. Such diets have also been shown to decrease blood pressure and the risk for a decline in estimated glomerular filtration rate. This type of diet would also prioritize the unsaturated fatty acids and fiber-rich proteins such as avocados, beans, and nuts shown to improve dyslipidemia, which may occur alongside CKD.
Realistic Options for Patients on Medical Diets
There is one question that I always seem to get from when recommending a plant-based diet: “These patients already have so many restrictions. Why would you add more?” And my answer is also always the same: I don’t.
I rarely, if ever, recommend completely cutting out any food item or food group. Instead, I ask the patient to increase their intake of plant-based foods and only limit highly processed foods and fatty meats. By shifting a patient’s focus to beans; nuts; and low-carbohydrate, high-fiber fruits and vegetables, I am often opening up a whole new world of possibilities.
Instead of a sandwich with low-sodium turkey and cheese on white bread with a side of unsalted pretzels, I recommend a caprese salad with blueberries and almonds or a Southwest salad with black beans, corn, and avocado. I don’t encourage my patients to skip the foods that they love, but instead to only think about all the delicious plant-based options that will provide them with more than just calories.
Meat, dairy, seafood, and eggs can certainly be a part of a healthy diet, but what if our chronically ill patients, especially those with diabetes, had more options than just grilled chicken and green beans for every meal? What if we focus on decreasing dietary restrictions, incorporating a variety of nourishing foods, and educating our patients, instead of on portion control and moderation?
This is how I choose to incorporate plant-based diets into my practice to treat and prevent these chronic inflammatory conditions and promote sustainable, realistic change in my clients’ health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Research pooled from nearly 100 studies has indicated that people who adhere to a vegan diet (ie, completely devoid of animal products) or a vegetarian diet (ie, devoid of meat, but may include dairy and eggs) are able to ward off some chronic diseases, such as cardiovascular disease, optimize glycemic control, and decrease their risk for cancer compared with those who consume omnivorous diets.
Vegan and vegetarian diets, or flexitarian diets — which are less reliant on animal protein than the standard US diet but do not completely exclude meat, fish, eggs, or dairy — may promote homeostasis and decrease inflammation by providing more fiber, antioxidants, and unsaturated fatty acids than the typical Western diet.
Inflammation and Obesity
Adipose tissue is a major producer of pro-inflammatory cytokines like interleukin (IL)-6, whose presence then triggers a rush of acute-phase reactants such as C-reactive protein (CRP) by the liver. This process develops into chronic low-grade inflammation that can increase a person’s chances of developing diabetes, cardiovascular disease, kidney disease, metabolic syndrome, and related complications.
Adopting a plant-based diet can improve markers of chronic low-grade inflammation that can lead to chronic disease and worsen existent chronic disease. A meta-analysis of 29 studies encompassing nearly 2700 participants found that initiation of a plant-based diet showed significant improvement in CRP, IL-6, and soluble intercellular adhesion molecule 1.
If we want to prevent these inflammatory disease states and their complications, the obvious response is to counsel patients to avoid excessive weight gain or to lose weight if obesity is their baseline. This can be tough for some patients, but it is nonetheless an important step in chronic disease prevention and management.
Plant-Based Diet for Type 2 Diabetes
According to a review of nine studies of patients living with type 2 diabetes who adhered to a plant-based diet, all but one found that this approach led to significantly lower A1c values than those seen in control groups. Six of the included studies reported that participants were able to decrease or discontinue medications for the management of diabetes. Researchers across all included studies also noted a decrease in total cholesterol, low-density lipoprotein cholesterol, and triglycerides, as well as increased weight loss in participants in each intervention group.
Such improvements are probably the result of the increase in fiber intake that occurs with a plant-based diet. A high-fiber diet is known to promote improved glucose and lipid metabolism as well as weight loss.
It is also worth noting that participants in the intervention groups also experienced improvements in depression and less chronic pain than did those in the control groups.
Plant-Based Diet for Chronic Kidney Disease (CKD)
Although the use of a plant-based diet in the prevention of CKD is well documented, adopting such diets for the treatment of CKD may intimidate both patients and practitioners owing to the high potassium and phosphorus content of many fruits and vegetables.
However, research indicates that the bioavailability of both potassium and phosphorus is lower in plant-based, whole foods than in preservatives and the highly processed food items that incorporate them. This makes a plant-based diet more viable than previously thought.
Diets rich in vegetables, whole grains, nuts, and legumes have been shown to decrease dietary acid load, both preventing and treating metabolic acidosis. Such diets have also been shown to decrease blood pressure and the risk for a decline in estimated glomerular filtration rate. This type of diet would also prioritize the unsaturated fatty acids and fiber-rich proteins such as avocados, beans, and nuts shown to improve dyslipidemia, which may occur alongside CKD.
Realistic Options for Patients on Medical Diets
There is one question that I always seem to get from when recommending a plant-based diet: “These patients already have so many restrictions. Why would you add more?” And my answer is also always the same: I don’t.
I rarely, if ever, recommend completely cutting out any food item or food group. Instead, I ask the patient to increase their intake of plant-based foods and only limit highly processed foods and fatty meats. By shifting a patient’s focus to beans; nuts; and low-carbohydrate, high-fiber fruits and vegetables, I am often opening up a whole new world of possibilities.
Instead of a sandwich with low-sodium turkey and cheese on white bread with a side of unsalted pretzels, I recommend a caprese salad with blueberries and almonds or a Southwest salad with black beans, corn, and avocado. I don’t encourage my patients to skip the foods that they love, but instead to only think about all the delicious plant-based options that will provide them with more than just calories.
Meat, dairy, seafood, and eggs can certainly be a part of a healthy diet, but what if our chronically ill patients, especially those with diabetes, had more options than just grilled chicken and green beans for every meal? What if we focus on decreasing dietary restrictions, incorporating a variety of nourishing foods, and educating our patients, instead of on portion control and moderation?
This is how I choose to incorporate plant-based diets into my practice to treat and prevent these chronic inflammatory conditions and promote sustainable, realistic change in my clients’ health.
Brandy Winfree Root, a renal dietitian in private practice in Mary Esther, Florida, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Why Scientists Are Linking More Diseases to Light at Night
This October, millions of Americans missed out on two of the most spectacular shows in the universe: the northern lights and a rare comet. Even if you were aware of them, light pollution made them difficult to see, unless you went to a dark area and let your eyes adjust.
It’s not getting any easier — the night sky over North America has been growing brighter by about 10% per year since 2011. More and more research is linking all that light pollution to a surprising range of health consequences: cancer, heart disease, diabetes, Alzheimer’s disease, and even low sperm quality, though the reasons for these troubling associations are not always clear.
“We’ve lost the contrast between light and dark, and we are confusing our physiology on a regular basis,” said John Hanifin, PhD, associate director of Thomas Jefferson University’s Light Research Program.
Our own galaxy is invisible to nearly 80% of people in North America. In 1994, an earthquake-triggered blackout in Los Angeles led to calls to the Griffith Observatory from people wondering about that hazy blob of light in the night sky. It was the Milky Way.
Glaring headlights, illuminated buildings, blazing billboards, and streetlights fill our urban skies with a glow that even affects rural residents. Inside, since the invention of the lightbulb, we’ve kept our homes bright at night. Now, we’ve also added blue light-emitting devices — smartphones, television screens, tablets — which have been linked to sleep problems.
But outdoor light may matter for our health, too. “Every photon counts,” Hanifin said.
Bright Lights, Big Problems
For one 2024 study researchers used satellite data to measure light pollution at residential addresses of over 13,000 people. They found that those who lived in places with the brightest skies at night had a 31% higher risk of high blood pressure. Another study out of Hong Kong showed a 29% higher risk of death from coronary heart disease. And yet another found a 17%higher risk of cerebrovascular disease, such as strokes or brain aneurysms.
Of course, urban areas also have air pollution, noise, and a lack of greenery. So, for some studies, scientists controlled for these factors, and the correlation remained strong (although air pollution with fine particulate matter appeared to be worse for heart health than outdoor light).
Research has found links between the nighttime glow outside and other diseases:
Breast cancer. “It’s a very strong correlation,” said Randy Nelson, PhD, a neuroscientist at West Virginia University. A study of over 100,000 teachers in California revealed that women living in areas with the most light pollution had a 12%higher risk. That effect is comparable to increasing your intake of ultra-processed foods by 10%.
Alzheimer’s disease. In a study published this fall, outdoor light at night was more strongly linked to the disease than even alcohol misuse or obesity.
Diabetes. In one recent study, people living in the most illuminated areas had a 28% higher risk of diabetes than those residing in much darker places. In a country like China, scientists concluded that 9 million cases of diabetes could be linked to light pollution.
What Happens in Your Body When You’re Exposed to Light at Night
, the “hormone of darkness.” “Darkness is very important,” Hanifin said. When he and his colleagues decades ago started studying the effects of light on human physiology, “people thought we were borderline crazy,” he said.
Nighttime illumination affects the health and behavior of species as diverse as Siberian hamsters, zebra finches, mice, crickets, and mosquitoes. Like most creatures on Earth, humans have internal clocks that are synced to the 24-hour cycle of day and night. The master clock is in your hypothalamus, a diamond-shaped part of the brain, but every cell in your body has its own clock, too. Many physiological processes run on circadian rhythms (a term derived from a Latin phrase meaning “about a day”), from sleep-wake cycle to hormone secretion, as well as processes involved in cancer progression, such as cell division.
“There are special photoreceptors in the eye that don’t deal with visual information. They just send light information,” Nelson said. “If you get light at the wrong time, you’re resetting the clocks.”
This internal clock “prepares the body for various recurrent challenges, such as eating,” said Christian Benedict, PhD, a sleep researcher at Uppsala University, Sweden. “Light exposure [at night] can mess up this very important system.” This could mean, for instance, that your insulin is released at the wrong time, Benedict said, causing “a jet lag-ish condition that will then impair the ability to handle blood sugar.” Animal studies confirm that exposure to light at night can reduce glucose tolerance and alter insulin secretion – potential pathways to diabetes.
The hormone melatonin, produced when it’s dark by the pineal gland in the brain, is a key player in this modern struggle. Melatonin helps you sleep, synchronizes the body’s circadian rhythms, protects neurons from damage, regulates the immune system, and fights inflammation. But even a sliver of light at night can suppress its secretion. Less than 30 lux of light, about the level of a pedestrian street at night, can slash melatonin by half.
When lab animals are exposed to nighttime light, they “show enormous neuroinflammation” — that is, inflammation of nervous tissue, Nelson said. In one experiment on humans, those who slept immersed in weak light had higher levels of C-reactive protein in their blood, a marker of inflammation.
Low melatonin has also been linked to cancer. It “allows the metabolic machinery of the cancer cells to be active,” Hanifin said. One of melatonin’s effects is stimulation of natural killer cells, which can recognize and destroy cancer cells. What’s more, when melatonin plunges, estrogen may go up, which could explain the link between light at night and breast cancer (estrogen fuels tumor growth in breast cancers).
Researchers concede that satellite data might be too coarse to estimate how much light people are actually exposed to while they sleep. Plus, many of us are staring at bright screens. “But the studies keep coming,” Nelson said, suggesting that outdoor light pollution does have an impact.
When researchers put wrist-worn light sensors on over 80,000 British people, they found that the more light the device registered between half-past midnight and 6 a.m., the more its wearer was at risk of having diabetes several years down the road — no matter how long they’ve actually slept. This, according to the study’s authors, supports the findings of satellite data.
A similar study that used actigraphy with built-in light sensors, measuring whether people had been sleeping in complete darkness for at least five hours, found that light pollution upped the risk of heart disease by 74%.
What Can You Do About This?
Not everyone’s melatonin is affected by nighttime light to the same degree. “Some people are very much sensitive to very dim light, whereas others are not as sensitive and need far, far more light stimulation [to impact melatonin],” Benedict said. In one study, some volunteers needed 350 lux to lower their melatonin by half. For such people, flipping on the light in the bathroom at night wouldn’t matter; for others, though, a mere 6 lux was already as harmful – which is darker than twilight.
You can protect yourself by keeping your bedroom lights off and your screens stashed away, but avoiding outdoor light pollution may be harder. You can invest in high-quality blackout curtains, of course, although some light may still seep inside. You can plant trees in front of your windows, reorient any motion-detector lights, and even petition your local government to reduce over-illumination of buildings and to choose better streetlights. You can support organizations, such as the International Dark-Sky Association, that work to preserve darkness.
Last but not least, you might want to change your habits. If you live in a particularly light-polluted area, such as the District of Columbia, America’s top place for urban blaze, you might reconsider late-night walks or drives around the neighborhood. Instead, Hanifin said, read a book in bed, while keeping the light “as dim as you can.” It’s “a much better idea versus being outside in midtown Manhattan,” he said. According to recent recommendations published by Hanifin and his colleagues, when you sleep, there should be no more than 1 lux of illumination at the level of your eyes — about as much as you’d get from having a lit candle 1 meter away.
And if we manage to preserve outdoor darkness, and the stars reappear (including the breathtaking Milky Way), we could reap more benefits — some research suggests that stargazing can elicit positive emotions, a sense of personal growth, and “a variety of transcendent thoughts and experiences.”
A version of this article appeared on WebMD.com.
This October, millions of Americans missed out on two of the most spectacular shows in the universe: the northern lights and a rare comet. Even if you were aware of them, light pollution made them difficult to see, unless you went to a dark area and let your eyes adjust.
It’s not getting any easier — the night sky over North America has been growing brighter by about 10% per year since 2011. More and more research is linking all that light pollution to a surprising range of health consequences: cancer, heart disease, diabetes, Alzheimer’s disease, and even low sperm quality, though the reasons for these troubling associations are not always clear.
“We’ve lost the contrast between light and dark, and we are confusing our physiology on a regular basis,” said John Hanifin, PhD, associate director of Thomas Jefferson University’s Light Research Program.
Our own galaxy is invisible to nearly 80% of people in North America. In 1994, an earthquake-triggered blackout in Los Angeles led to calls to the Griffith Observatory from people wondering about that hazy blob of light in the night sky. It was the Milky Way.
Glaring headlights, illuminated buildings, blazing billboards, and streetlights fill our urban skies with a glow that even affects rural residents. Inside, since the invention of the lightbulb, we’ve kept our homes bright at night. Now, we’ve also added blue light-emitting devices — smartphones, television screens, tablets — which have been linked to sleep problems.
But outdoor light may matter for our health, too. “Every photon counts,” Hanifin said.
Bright Lights, Big Problems
For one 2024 study researchers used satellite data to measure light pollution at residential addresses of over 13,000 people. They found that those who lived in places with the brightest skies at night had a 31% higher risk of high blood pressure. Another study out of Hong Kong showed a 29% higher risk of death from coronary heart disease. And yet another found a 17%higher risk of cerebrovascular disease, such as strokes or brain aneurysms.
Of course, urban areas also have air pollution, noise, and a lack of greenery. So, for some studies, scientists controlled for these factors, and the correlation remained strong (although air pollution with fine particulate matter appeared to be worse for heart health than outdoor light).
Research has found links between the nighttime glow outside and other diseases:
Breast cancer. “It’s a very strong correlation,” said Randy Nelson, PhD, a neuroscientist at West Virginia University. A study of over 100,000 teachers in California revealed that women living in areas with the most light pollution had a 12%higher risk. That effect is comparable to increasing your intake of ultra-processed foods by 10%.
Alzheimer’s disease. In a study published this fall, outdoor light at night was more strongly linked to the disease than even alcohol misuse or obesity.
Diabetes. In one recent study, people living in the most illuminated areas had a 28% higher risk of diabetes than those residing in much darker places. In a country like China, scientists concluded that 9 million cases of diabetes could be linked to light pollution.
What Happens in Your Body When You’re Exposed to Light at Night
, the “hormone of darkness.” “Darkness is very important,” Hanifin said. When he and his colleagues decades ago started studying the effects of light on human physiology, “people thought we were borderline crazy,” he said.
Nighttime illumination affects the health and behavior of species as diverse as Siberian hamsters, zebra finches, mice, crickets, and mosquitoes. Like most creatures on Earth, humans have internal clocks that are synced to the 24-hour cycle of day and night. The master clock is in your hypothalamus, a diamond-shaped part of the brain, but every cell in your body has its own clock, too. Many physiological processes run on circadian rhythms (a term derived from a Latin phrase meaning “about a day”), from sleep-wake cycle to hormone secretion, as well as processes involved in cancer progression, such as cell division.
“There are special photoreceptors in the eye that don’t deal with visual information. They just send light information,” Nelson said. “If you get light at the wrong time, you’re resetting the clocks.”
This internal clock “prepares the body for various recurrent challenges, such as eating,” said Christian Benedict, PhD, a sleep researcher at Uppsala University, Sweden. “Light exposure [at night] can mess up this very important system.” This could mean, for instance, that your insulin is released at the wrong time, Benedict said, causing “a jet lag-ish condition that will then impair the ability to handle blood sugar.” Animal studies confirm that exposure to light at night can reduce glucose tolerance and alter insulin secretion – potential pathways to diabetes.
The hormone melatonin, produced when it’s dark by the pineal gland in the brain, is a key player in this modern struggle. Melatonin helps you sleep, synchronizes the body’s circadian rhythms, protects neurons from damage, regulates the immune system, and fights inflammation. But even a sliver of light at night can suppress its secretion. Less than 30 lux of light, about the level of a pedestrian street at night, can slash melatonin by half.
When lab animals are exposed to nighttime light, they “show enormous neuroinflammation” — that is, inflammation of nervous tissue, Nelson said. In one experiment on humans, those who slept immersed in weak light had higher levels of C-reactive protein in their blood, a marker of inflammation.
Low melatonin has also been linked to cancer. It “allows the metabolic machinery of the cancer cells to be active,” Hanifin said. One of melatonin’s effects is stimulation of natural killer cells, which can recognize and destroy cancer cells. What’s more, when melatonin plunges, estrogen may go up, which could explain the link between light at night and breast cancer (estrogen fuels tumor growth in breast cancers).
Researchers concede that satellite data might be too coarse to estimate how much light people are actually exposed to while they sleep. Plus, many of us are staring at bright screens. “But the studies keep coming,” Nelson said, suggesting that outdoor light pollution does have an impact.
When researchers put wrist-worn light sensors on over 80,000 British people, they found that the more light the device registered between half-past midnight and 6 a.m., the more its wearer was at risk of having diabetes several years down the road — no matter how long they’ve actually slept. This, according to the study’s authors, supports the findings of satellite data.
A similar study that used actigraphy with built-in light sensors, measuring whether people had been sleeping in complete darkness for at least five hours, found that light pollution upped the risk of heart disease by 74%.
What Can You Do About This?
Not everyone’s melatonin is affected by nighttime light to the same degree. “Some people are very much sensitive to very dim light, whereas others are not as sensitive and need far, far more light stimulation [to impact melatonin],” Benedict said. In one study, some volunteers needed 350 lux to lower their melatonin by half. For such people, flipping on the light in the bathroom at night wouldn’t matter; for others, though, a mere 6 lux was already as harmful – which is darker than twilight.
You can protect yourself by keeping your bedroom lights off and your screens stashed away, but avoiding outdoor light pollution may be harder. You can invest in high-quality blackout curtains, of course, although some light may still seep inside. You can plant trees in front of your windows, reorient any motion-detector lights, and even petition your local government to reduce over-illumination of buildings and to choose better streetlights. You can support organizations, such as the International Dark-Sky Association, that work to preserve darkness.
Last but not least, you might want to change your habits. If you live in a particularly light-polluted area, such as the District of Columbia, America’s top place for urban blaze, you might reconsider late-night walks or drives around the neighborhood. Instead, Hanifin said, read a book in bed, while keeping the light “as dim as you can.” It’s “a much better idea versus being outside in midtown Manhattan,” he said. According to recent recommendations published by Hanifin and his colleagues, when you sleep, there should be no more than 1 lux of illumination at the level of your eyes — about as much as you’d get from having a lit candle 1 meter away.
And if we manage to preserve outdoor darkness, and the stars reappear (including the breathtaking Milky Way), we could reap more benefits — some research suggests that stargazing can elicit positive emotions, a sense of personal growth, and “a variety of transcendent thoughts and experiences.”
A version of this article appeared on WebMD.com.
This October, millions of Americans missed out on two of the most spectacular shows in the universe: the northern lights and a rare comet. Even if you were aware of them, light pollution made them difficult to see, unless you went to a dark area and let your eyes adjust.
It’s not getting any easier — the night sky over North America has been growing brighter by about 10% per year since 2011. More and more research is linking all that light pollution to a surprising range of health consequences: cancer, heart disease, diabetes, Alzheimer’s disease, and even low sperm quality, though the reasons for these troubling associations are not always clear.
“We’ve lost the contrast between light and dark, and we are confusing our physiology on a regular basis,” said John Hanifin, PhD, associate director of Thomas Jefferson University’s Light Research Program.
Our own galaxy is invisible to nearly 80% of people in North America. In 1994, an earthquake-triggered blackout in Los Angeles led to calls to the Griffith Observatory from people wondering about that hazy blob of light in the night sky. It was the Milky Way.
Glaring headlights, illuminated buildings, blazing billboards, and streetlights fill our urban skies with a glow that even affects rural residents. Inside, since the invention of the lightbulb, we’ve kept our homes bright at night. Now, we’ve also added blue light-emitting devices — smartphones, television screens, tablets — which have been linked to sleep problems.
But outdoor light may matter for our health, too. “Every photon counts,” Hanifin said.
Bright Lights, Big Problems
For one 2024 study researchers used satellite data to measure light pollution at residential addresses of over 13,000 people. They found that those who lived in places with the brightest skies at night had a 31% higher risk of high blood pressure. Another study out of Hong Kong showed a 29% higher risk of death from coronary heart disease. And yet another found a 17%higher risk of cerebrovascular disease, such as strokes or brain aneurysms.
Of course, urban areas also have air pollution, noise, and a lack of greenery. So, for some studies, scientists controlled for these factors, and the correlation remained strong (although air pollution with fine particulate matter appeared to be worse for heart health than outdoor light).
Research has found links between the nighttime glow outside and other diseases:
Breast cancer. “It’s a very strong correlation,” said Randy Nelson, PhD, a neuroscientist at West Virginia University. A study of over 100,000 teachers in California revealed that women living in areas with the most light pollution had a 12%higher risk. That effect is comparable to increasing your intake of ultra-processed foods by 10%.
Alzheimer’s disease. In a study published this fall, outdoor light at night was more strongly linked to the disease than even alcohol misuse or obesity.
Diabetes. In one recent study, people living in the most illuminated areas had a 28% higher risk of diabetes than those residing in much darker places. In a country like China, scientists concluded that 9 million cases of diabetes could be linked to light pollution.
What Happens in Your Body When You’re Exposed to Light at Night
, the “hormone of darkness.” “Darkness is very important,” Hanifin said. When he and his colleagues decades ago started studying the effects of light on human physiology, “people thought we were borderline crazy,” he said.
Nighttime illumination affects the health and behavior of species as diverse as Siberian hamsters, zebra finches, mice, crickets, and mosquitoes. Like most creatures on Earth, humans have internal clocks that are synced to the 24-hour cycle of day and night. The master clock is in your hypothalamus, a diamond-shaped part of the brain, but every cell in your body has its own clock, too. Many physiological processes run on circadian rhythms (a term derived from a Latin phrase meaning “about a day”), from sleep-wake cycle to hormone secretion, as well as processes involved in cancer progression, such as cell division.
“There are special photoreceptors in the eye that don’t deal with visual information. They just send light information,” Nelson said. “If you get light at the wrong time, you’re resetting the clocks.”
This internal clock “prepares the body for various recurrent challenges, such as eating,” said Christian Benedict, PhD, a sleep researcher at Uppsala University, Sweden. “Light exposure [at night] can mess up this very important system.” This could mean, for instance, that your insulin is released at the wrong time, Benedict said, causing “a jet lag-ish condition that will then impair the ability to handle blood sugar.” Animal studies confirm that exposure to light at night can reduce glucose tolerance and alter insulin secretion – potential pathways to diabetes.
The hormone melatonin, produced when it’s dark by the pineal gland in the brain, is a key player in this modern struggle. Melatonin helps you sleep, synchronizes the body’s circadian rhythms, protects neurons from damage, regulates the immune system, and fights inflammation. But even a sliver of light at night can suppress its secretion. Less than 30 lux of light, about the level of a pedestrian street at night, can slash melatonin by half.
When lab animals are exposed to nighttime light, they “show enormous neuroinflammation” — that is, inflammation of nervous tissue, Nelson said. In one experiment on humans, those who slept immersed in weak light had higher levels of C-reactive protein in their blood, a marker of inflammation.
Low melatonin has also been linked to cancer. It “allows the metabolic machinery of the cancer cells to be active,” Hanifin said. One of melatonin’s effects is stimulation of natural killer cells, which can recognize and destroy cancer cells. What’s more, when melatonin plunges, estrogen may go up, which could explain the link between light at night and breast cancer (estrogen fuels tumor growth in breast cancers).
Researchers concede that satellite data might be too coarse to estimate how much light people are actually exposed to while they sleep. Plus, many of us are staring at bright screens. “But the studies keep coming,” Nelson said, suggesting that outdoor light pollution does have an impact.
When researchers put wrist-worn light sensors on over 80,000 British people, they found that the more light the device registered between half-past midnight and 6 a.m., the more its wearer was at risk of having diabetes several years down the road — no matter how long they’ve actually slept. This, according to the study’s authors, supports the findings of satellite data.
A similar study that used actigraphy with built-in light sensors, measuring whether people had been sleeping in complete darkness for at least five hours, found that light pollution upped the risk of heart disease by 74%.
What Can You Do About This?
Not everyone’s melatonin is affected by nighttime light to the same degree. “Some people are very much sensitive to very dim light, whereas others are not as sensitive and need far, far more light stimulation [to impact melatonin],” Benedict said. In one study, some volunteers needed 350 lux to lower their melatonin by half. For such people, flipping on the light in the bathroom at night wouldn’t matter; for others, though, a mere 6 lux was already as harmful – which is darker than twilight.
You can protect yourself by keeping your bedroom lights off and your screens stashed away, but avoiding outdoor light pollution may be harder. You can invest in high-quality blackout curtains, of course, although some light may still seep inside. You can plant trees in front of your windows, reorient any motion-detector lights, and even petition your local government to reduce over-illumination of buildings and to choose better streetlights. You can support organizations, such as the International Dark-Sky Association, that work to preserve darkness.
Last but not least, you might want to change your habits. If you live in a particularly light-polluted area, such as the District of Columbia, America’s top place for urban blaze, you might reconsider late-night walks or drives around the neighborhood. Instead, Hanifin said, read a book in bed, while keeping the light “as dim as you can.” It’s “a much better idea versus being outside in midtown Manhattan,” he said. According to recent recommendations published by Hanifin and his colleagues, when you sleep, there should be no more than 1 lux of illumination at the level of your eyes — about as much as you’d get from having a lit candle 1 meter away.
And if we manage to preserve outdoor darkness, and the stars reappear (including the breathtaking Milky Way), we could reap more benefits — some research suggests that stargazing can elicit positive emotions, a sense of personal growth, and “a variety of transcendent thoughts and experiences.”
A version of this article appeared on WebMD.com.
Blood Tests for Alzheimer’s Are Here... Are Clinicians Ready?
With the approval of anti-amyloid monoclonal antibodies to treat early-stage Alzheimer’s disease, the need for accurate and early diagnosis is crucial.
Recently, an expert workgroup convened by the Global CEO Initiative on Alzheimer’s Disease published recommendations for the clinical implementation of Alzheimer’s disease blood-based biomarkers.
“Our hope was to provide some recommendations that clinicians could use to develop the best pathways for their clinical practice,” said workgroup co-chair Michelle M. Mielke, PhD, with Wake Forest University School of Medicine, Winston-Salem, North Carolina.
Triage and Confirmatory Pathways
The group recommends two implementation pathways for Alzheimer’s disease blood biomarkers — one for current use for triaging and another for future use to confirm amyloid pathology once blood biomarker tests have reached sufficient performance for this purpose.
In the triage pathway, a negative blood biomarker test would flag individuals unlikely to have detectable brain amyloid pathology. This outcome would prompt clinicians to focus on evaluating non–Alzheimer’s disease-related causes of cognitive impairment, which may streamline the diagnosis of other causes of cognitive impairment, the authors said.
A positive triage blood test would suggest a higher likelihood of amyloid pathology and prompt referral to secondary care for further assessment and consideration for a second, more accurate test, such as amyloid PET or CSF for amyloid confirmation.
In the confirmatory pathway, a positive blood biomarker test result would identify amyloid pathology without the need for a second test, providing a faster route to diagnosis, the authors noted.
Mielke emphasized that these recommendations represent a “first step” and will need to be updated as experiences with the Alzheimer’s disease blood biomarkers in clinical care increase and additional barriers and facilitators are identified.
“These updates will likely include community-informed approaches that incorporate feedback from patients as well as healthcare providers, alongside results from validation in diverse real-world settings,” said workgroup co-chair Chi Udeh-Momoh, PhD, MSc, with Wake Forest University School of Medicine and the Brain and Mind Institute, Aga Khan University, Nairobi, Kenya.
The Alzheimer’s Association published “appropriate use” recommendations for blood biomarkers in 2022.
“Currently, the Alzheimer’s Association is building an updated library of clinical guidance that distills the scientific evidence using de novo systematic reviews and translates them into clear and actionable recommendations for clinical practice,” said Rebecca M. Edelmayer, PhD, vice president of scientific engagement, Alzheimer’s Association.
“The first major effort with our new process will be the upcoming Evidence-based Clinical Practice Guideline on the Use of Blood-based Biomarkers (BBMs) in Specialty Care Settings. This guideline’s recommendations will be published in early 2025,” Edelmayer said.
Availability and Accuracy
Research has shown that amyloid beta and tau protein blood biomarkers — especially a high plasma phosphorylated (p)–tau217 levels — are highly accurate in identifying Alzheimer’s disease in patients with cognitive symptoms attending primary and secondary care clinics.
Several tests targeting plasma p-tau217 are now available for use. They include the PrecivityAD2 blood test from C2N Diagnostics and the Simoa p-Tau 217 Planar Kit and LucentAD p-Tau 217 — both from Quanterix.
In a recent head-to-head comparison of seven leading blood tests for AD pathology, measures of plasma p-tau217, either individually or in combination with other plasma biomarkers, had the strongest relationships with Alzheimer’s disease outcomes.
A recent Swedish study showed that the PrecivityAD2 test had an accuracy of 91% for correctly classifying clinical, biomarker-verified Alzheimer’s disease.
“We’ve been using these blood biomarkers in research for a long time and we’re now taking the jump to start using them in clinic to risk stratify patients,” said Fanny Elahi, MD, PhD, director of fluid biomarker research for the Barbara and Maurice Deane Center for Wellness and Cognitive Health at Icahn Mount Sinai in New York City.
New York’s Mount Sinai Health System is among the first in the northeast to offer blood tests across primary and specialty care settings for early diagnosis of AD and related dementias.
Edelmayer cautioned, “There is no single, stand-alone test to diagnose Alzheimer’s disease today. Blood testing is one piece of the diagnostic process.”
“Currently, physicians use well-established diagnostic tools combined with medical history and other information, including neurological exams, cognitive and functional assessments as well as brain imaging and spinal fluid analysis and blood to make an accurate diagnosis and to understand which patients are eligible for approved treatments,” she said.
There are also emerging biomarkers in the research pipeline, Edelmayer said.
“For example, some researchers think retinal imaging has the potential to detect biological signs of Alzheimer’s disease within certain areas of the eye,” she explained.
“Other emerging biomarkers include examining components in saliva and the skin for signals that may indicate early biological changes in the brain. These biomarkers are still very exploratory, and more research is needed before these tests or biomarkers can be used more routinely to study risk or aid in diagnosis,” Edelmayer said.
Ideal Candidates for Alzheimer’s Disease Blood Testing?
Experts agree that blood tests represent a convenient and scalable option to address the anticipated surge in demand for biomarker testing with the availability of disease-modifying treatments. For now, however, they are not for all older adults worried about their memory.
“Current practice should focus on using these blood biomarkers in individuals with cognitive impairment rather than in those with normal cognition or subjective cognitive decline until further research demonstrates effective interventions for individuals considered cognitively normal with elevated levels of amyloid,” the authors of a recent JAMA editorial noted.
At Mount Sinai, “we’re not starting with stone-cold asymptomatic individuals. But ultimately, this is what the blood tests are intended for — screening,” Elahi noted.
She also noted that Mount Sinai has a “very diverse population” — some with young onset cognitive symptoms, so the entry criteria for testing are “very wide.”
“Anyone above age 40 with symptoms can qualify to get a blood test. We do ask at this stage that either the individual report symptoms or someone in their life or their clinician be worried about their cognition or their brain function,” Elahi said.
Ethical Considerations, Counseling
Elahi emphasized the importance of counseling patients who come to the clinic seeking an Alzheimer’s disease blood test. This should include how the diagnostic process will unfold and what the next steps are with a given result.
Elahi said patients need to be informed that Alzheimer’s disease blood biomarkers are still “relatively new,” and a test can help a patient “know the likelihood of having the disease, but it won’t be 100% definitive.”
To ensure the ethical principle of “do no harm,” counseling should ensure that patients are fully prepared for the implications of the test results and ensure that the decision to test aligns with the patient’s readiness and well-being, Elahi said.
Edelmayer said the forthcoming clinical practice guidelines will provide “evidence-based recommendations for physicians to help guide them through the decision-making process around who should be tested and when. In the meantime, the Alzheimer’s Association urges providers to refer to the 2022 appropriate use recommendations for blood tests in clinical practice and trial settings.”
Mielke has served on scientific advisory boards and/or having consulted for Acadia, Biogen, Eisai, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio. Edelmayer and Elahi had no relevant disclosures.
A version of this article appeared on Medscape.com.
With the approval of anti-amyloid monoclonal antibodies to treat early-stage Alzheimer’s disease, the need for accurate and early diagnosis is crucial.
Recently, an expert workgroup convened by the Global CEO Initiative on Alzheimer’s Disease published recommendations for the clinical implementation of Alzheimer’s disease blood-based biomarkers.
“Our hope was to provide some recommendations that clinicians could use to develop the best pathways for their clinical practice,” said workgroup co-chair Michelle M. Mielke, PhD, with Wake Forest University School of Medicine, Winston-Salem, North Carolina.
Triage and Confirmatory Pathways
The group recommends two implementation pathways for Alzheimer’s disease blood biomarkers — one for current use for triaging and another for future use to confirm amyloid pathology once blood biomarker tests have reached sufficient performance for this purpose.
In the triage pathway, a negative blood biomarker test would flag individuals unlikely to have detectable brain amyloid pathology. This outcome would prompt clinicians to focus on evaluating non–Alzheimer’s disease-related causes of cognitive impairment, which may streamline the diagnosis of other causes of cognitive impairment, the authors said.
A positive triage blood test would suggest a higher likelihood of amyloid pathology and prompt referral to secondary care for further assessment and consideration for a second, more accurate test, such as amyloid PET or CSF for amyloid confirmation.
In the confirmatory pathway, a positive blood biomarker test result would identify amyloid pathology without the need for a second test, providing a faster route to diagnosis, the authors noted.
Mielke emphasized that these recommendations represent a “first step” and will need to be updated as experiences with the Alzheimer’s disease blood biomarkers in clinical care increase and additional barriers and facilitators are identified.
“These updates will likely include community-informed approaches that incorporate feedback from patients as well as healthcare providers, alongside results from validation in diverse real-world settings,” said workgroup co-chair Chi Udeh-Momoh, PhD, MSc, with Wake Forest University School of Medicine and the Brain and Mind Institute, Aga Khan University, Nairobi, Kenya.
The Alzheimer’s Association published “appropriate use” recommendations for blood biomarkers in 2022.
“Currently, the Alzheimer’s Association is building an updated library of clinical guidance that distills the scientific evidence using de novo systematic reviews and translates them into clear and actionable recommendations for clinical practice,” said Rebecca M. Edelmayer, PhD, vice president of scientific engagement, Alzheimer’s Association.
“The first major effort with our new process will be the upcoming Evidence-based Clinical Practice Guideline on the Use of Blood-based Biomarkers (BBMs) in Specialty Care Settings. This guideline’s recommendations will be published in early 2025,” Edelmayer said.
Availability and Accuracy
Research has shown that amyloid beta and tau protein blood biomarkers — especially a high plasma phosphorylated (p)–tau217 levels — are highly accurate in identifying Alzheimer’s disease in patients with cognitive symptoms attending primary and secondary care clinics.
Several tests targeting plasma p-tau217 are now available for use. They include the PrecivityAD2 blood test from C2N Diagnostics and the Simoa p-Tau 217 Planar Kit and LucentAD p-Tau 217 — both from Quanterix.
In a recent head-to-head comparison of seven leading blood tests for AD pathology, measures of plasma p-tau217, either individually or in combination with other plasma biomarkers, had the strongest relationships with Alzheimer’s disease outcomes.
A recent Swedish study showed that the PrecivityAD2 test had an accuracy of 91% for correctly classifying clinical, biomarker-verified Alzheimer’s disease.
“We’ve been using these blood biomarkers in research for a long time and we’re now taking the jump to start using them in clinic to risk stratify patients,” said Fanny Elahi, MD, PhD, director of fluid biomarker research for the Barbara and Maurice Deane Center for Wellness and Cognitive Health at Icahn Mount Sinai in New York City.
New York’s Mount Sinai Health System is among the first in the northeast to offer blood tests across primary and specialty care settings for early diagnosis of AD and related dementias.
Edelmayer cautioned, “There is no single, stand-alone test to diagnose Alzheimer’s disease today. Blood testing is one piece of the diagnostic process.”
“Currently, physicians use well-established diagnostic tools combined with medical history and other information, including neurological exams, cognitive and functional assessments as well as brain imaging and spinal fluid analysis and blood to make an accurate diagnosis and to understand which patients are eligible for approved treatments,” she said.
There are also emerging biomarkers in the research pipeline, Edelmayer said.
“For example, some researchers think retinal imaging has the potential to detect biological signs of Alzheimer’s disease within certain areas of the eye,” she explained.
“Other emerging biomarkers include examining components in saliva and the skin for signals that may indicate early biological changes in the brain. These biomarkers are still very exploratory, and more research is needed before these tests or biomarkers can be used more routinely to study risk or aid in diagnosis,” Edelmayer said.
Ideal Candidates for Alzheimer’s Disease Blood Testing?
Experts agree that blood tests represent a convenient and scalable option to address the anticipated surge in demand for biomarker testing with the availability of disease-modifying treatments. For now, however, they are not for all older adults worried about their memory.
“Current practice should focus on using these blood biomarkers in individuals with cognitive impairment rather than in those with normal cognition or subjective cognitive decline until further research demonstrates effective interventions for individuals considered cognitively normal with elevated levels of amyloid,” the authors of a recent JAMA editorial noted.
At Mount Sinai, “we’re not starting with stone-cold asymptomatic individuals. But ultimately, this is what the blood tests are intended for — screening,” Elahi noted.
She also noted that Mount Sinai has a “very diverse population” — some with young onset cognitive symptoms, so the entry criteria for testing are “very wide.”
“Anyone above age 40 with symptoms can qualify to get a blood test. We do ask at this stage that either the individual report symptoms or someone in their life or their clinician be worried about their cognition or their brain function,” Elahi said.
Ethical Considerations, Counseling
Elahi emphasized the importance of counseling patients who come to the clinic seeking an Alzheimer’s disease blood test. This should include how the diagnostic process will unfold and what the next steps are with a given result.
Elahi said patients need to be informed that Alzheimer’s disease blood biomarkers are still “relatively new,” and a test can help a patient “know the likelihood of having the disease, but it won’t be 100% definitive.”
To ensure the ethical principle of “do no harm,” counseling should ensure that patients are fully prepared for the implications of the test results and ensure that the decision to test aligns with the patient’s readiness and well-being, Elahi said.
Edelmayer said the forthcoming clinical practice guidelines will provide “evidence-based recommendations for physicians to help guide them through the decision-making process around who should be tested and when. In the meantime, the Alzheimer’s Association urges providers to refer to the 2022 appropriate use recommendations for blood tests in clinical practice and trial settings.”
Mielke has served on scientific advisory boards and/or having consulted for Acadia, Biogen, Eisai, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio. Edelmayer and Elahi had no relevant disclosures.
A version of this article appeared on Medscape.com.
With the approval of anti-amyloid monoclonal antibodies to treat early-stage Alzheimer’s disease, the need for accurate and early diagnosis is crucial.
Recently, an expert workgroup convened by the Global CEO Initiative on Alzheimer’s Disease published recommendations for the clinical implementation of Alzheimer’s disease blood-based biomarkers.
“Our hope was to provide some recommendations that clinicians could use to develop the best pathways for their clinical practice,” said workgroup co-chair Michelle M. Mielke, PhD, with Wake Forest University School of Medicine, Winston-Salem, North Carolina.
Triage and Confirmatory Pathways
The group recommends two implementation pathways for Alzheimer’s disease blood biomarkers — one for current use for triaging and another for future use to confirm amyloid pathology once blood biomarker tests have reached sufficient performance for this purpose.
In the triage pathway, a negative blood biomarker test would flag individuals unlikely to have detectable brain amyloid pathology. This outcome would prompt clinicians to focus on evaluating non–Alzheimer’s disease-related causes of cognitive impairment, which may streamline the diagnosis of other causes of cognitive impairment, the authors said.
A positive triage blood test would suggest a higher likelihood of amyloid pathology and prompt referral to secondary care for further assessment and consideration for a second, more accurate test, such as amyloid PET or CSF for amyloid confirmation.
In the confirmatory pathway, a positive blood biomarker test result would identify amyloid pathology without the need for a second test, providing a faster route to diagnosis, the authors noted.
Mielke emphasized that these recommendations represent a “first step” and will need to be updated as experiences with the Alzheimer’s disease blood biomarkers in clinical care increase and additional barriers and facilitators are identified.
“These updates will likely include community-informed approaches that incorporate feedback from patients as well as healthcare providers, alongside results from validation in diverse real-world settings,” said workgroup co-chair Chi Udeh-Momoh, PhD, MSc, with Wake Forest University School of Medicine and the Brain and Mind Institute, Aga Khan University, Nairobi, Kenya.
The Alzheimer’s Association published “appropriate use” recommendations for blood biomarkers in 2022.
“Currently, the Alzheimer’s Association is building an updated library of clinical guidance that distills the scientific evidence using de novo systematic reviews and translates them into clear and actionable recommendations for clinical practice,” said Rebecca M. Edelmayer, PhD, vice president of scientific engagement, Alzheimer’s Association.
“The first major effort with our new process will be the upcoming Evidence-based Clinical Practice Guideline on the Use of Blood-based Biomarkers (BBMs) in Specialty Care Settings. This guideline’s recommendations will be published in early 2025,” Edelmayer said.
Availability and Accuracy
Research has shown that amyloid beta and tau protein blood biomarkers — especially a high plasma phosphorylated (p)–tau217 levels — are highly accurate in identifying Alzheimer’s disease in patients with cognitive symptoms attending primary and secondary care clinics.
Several tests targeting plasma p-tau217 are now available for use. They include the PrecivityAD2 blood test from C2N Diagnostics and the Simoa p-Tau 217 Planar Kit and LucentAD p-Tau 217 — both from Quanterix.
In a recent head-to-head comparison of seven leading blood tests for AD pathology, measures of plasma p-tau217, either individually or in combination with other plasma biomarkers, had the strongest relationships with Alzheimer’s disease outcomes.
A recent Swedish study showed that the PrecivityAD2 test had an accuracy of 91% for correctly classifying clinical, biomarker-verified Alzheimer’s disease.
“We’ve been using these blood biomarkers in research for a long time and we’re now taking the jump to start using them in clinic to risk stratify patients,” said Fanny Elahi, MD, PhD, director of fluid biomarker research for the Barbara and Maurice Deane Center for Wellness and Cognitive Health at Icahn Mount Sinai in New York City.
New York’s Mount Sinai Health System is among the first in the northeast to offer blood tests across primary and specialty care settings for early diagnosis of AD and related dementias.
Edelmayer cautioned, “There is no single, stand-alone test to diagnose Alzheimer’s disease today. Blood testing is one piece of the diagnostic process.”
“Currently, physicians use well-established diagnostic tools combined with medical history and other information, including neurological exams, cognitive and functional assessments as well as brain imaging and spinal fluid analysis and blood to make an accurate diagnosis and to understand which patients are eligible for approved treatments,” she said.
There are also emerging biomarkers in the research pipeline, Edelmayer said.
“For example, some researchers think retinal imaging has the potential to detect biological signs of Alzheimer’s disease within certain areas of the eye,” she explained.
“Other emerging biomarkers include examining components in saliva and the skin for signals that may indicate early biological changes in the brain. These biomarkers are still very exploratory, and more research is needed before these tests or biomarkers can be used more routinely to study risk or aid in diagnosis,” Edelmayer said.
Ideal Candidates for Alzheimer’s Disease Blood Testing?
Experts agree that blood tests represent a convenient and scalable option to address the anticipated surge in demand for biomarker testing with the availability of disease-modifying treatments. For now, however, they are not for all older adults worried about their memory.
“Current practice should focus on using these blood biomarkers in individuals with cognitive impairment rather than in those with normal cognition or subjective cognitive decline until further research demonstrates effective interventions for individuals considered cognitively normal with elevated levels of amyloid,” the authors of a recent JAMA editorial noted.
At Mount Sinai, “we’re not starting with stone-cold asymptomatic individuals. But ultimately, this is what the blood tests are intended for — screening,” Elahi noted.
She also noted that Mount Sinai has a “very diverse population” — some with young onset cognitive symptoms, so the entry criteria for testing are “very wide.”
“Anyone above age 40 with symptoms can qualify to get a blood test. We do ask at this stage that either the individual report symptoms or someone in their life or their clinician be worried about their cognition or their brain function,” Elahi said.
Ethical Considerations, Counseling
Elahi emphasized the importance of counseling patients who come to the clinic seeking an Alzheimer’s disease blood test. This should include how the diagnostic process will unfold and what the next steps are with a given result.
Elahi said patients need to be informed that Alzheimer’s disease blood biomarkers are still “relatively new,” and a test can help a patient “know the likelihood of having the disease, but it won’t be 100% definitive.”
To ensure the ethical principle of “do no harm,” counseling should ensure that patients are fully prepared for the implications of the test results and ensure that the decision to test aligns with the patient’s readiness and well-being, Elahi said.
Edelmayer said the forthcoming clinical practice guidelines will provide “evidence-based recommendations for physicians to help guide them through the decision-making process around who should be tested and when. In the meantime, the Alzheimer’s Association urges providers to refer to the 2022 appropriate use recommendations for blood tests in clinical practice and trial settings.”
Mielke has served on scientific advisory boards and/or having consulted for Acadia, Biogen, Eisai, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio. Edelmayer and Elahi had no relevant disclosures.
A version of this article appeared on Medscape.com.