User login
Lymphoma Debate: CAR T Not a Clear Winner
Acknowledging that hers was the weakest position, even the specialist who defended novel targeted therapies mounted a staunch defense of real-world patients being treated outside of tertiary centers.
“I was told by many of my colleagues that I got the short end of the stick in this debate, but I am actually here to convince everybody that targeted therapies continue to play an important role, despite the fact that they are the least sexy of these treatment options,” said Joanna Rhodes, MD, director of the Lymphoma Program at Rutgers Cancer Institute, Hoboken, New Jersey.
Targeted Therapies Still Relevant to Advanced FL
Although even the newest or coming targeted therapies, such as the EZH2 inhibitor tazemetostat or next-generation Bruton tyrosine kinase inhibitors, are not likely to achieve the deep responses and long-term progression-free survival possible with BsAbs or CAR T-cell therapy, the sustained disease control they offer for many patients with R/R FL is not trivial, according to Rhodes.
“The majority of these [advanced follicular lymphoma] patients are being managed in the community,” Rhodes argued at the 2024 Lymphoma, Leukemia, & Myeloma Congress. Access to tertiary centers where the most advanced therapies are available in some cases might not even be feasible.
Moreover, there are barriers to CAR T cells and BsAbs even at centers where these are available, Rhodes said. On a long list of barriers, lack of caregiver support is an example of one common disqualification at her own institution.
The experience with CAR T cells in R/R FL has been relatively short, so Rhodes used data on CAR T cells for B-cell lymphoma to make her point. It is not just that the proportion of eligible patients is limited.
“The majority of B-cell lymphoma patients who are eligible for CAR T cells are not getting them,” she said. “It will be the same for FL.”
In other words, Rhodes indicated that it is premature to count out targeted oral agents or lenalidomide despite the excitement surrounding BsAbs and CAR T cells. The targeted agents and immunomodulatory drugs remain appropriate choices for patients unable or unwilling to travel to tertiary centers for treatment, for frail patients, and for well-informed patients who understand their options and still consider better tolerated therapies to be more consistent with their perception of an adequate risk-benefit ratio.
BsAbs Vie With CAR T Cells in Advanced FL
Hers might be a valid summary, but it did not derail arguments about whether CAR T-cell therapy should be prioritized over BsAbs or the other way around for patients who are candidates for both.
There are two BsAbs currently approved for R/R FL: glofitamab and mosunetuzumab. More are coming, according to Nina Wagner-Johnston, MD, director of hematologic malignancies at Sidney Kimmel Cancer Center, Johns Hopkins University, Baltimore, Maryland. She provided several reasons why BsAbs might be considered before CAR T-cell therapies in at least some individuals.
“The biggest advantage is that these therapies…are off the shelf,” she said. This avoids the delay of T-cell manufacturing, the potential need for bridging therapies, and the need for conditioning regimens. With more experience, BsAbs offer the potential for treatment even in a community-practice setting, particularly for maintenance dosing.
“I do think this is a safe treatment in patients who are elderly or unfit,” Wagner-Johnston said, suggesting she tends to lean toward prioritizing BsAbs over CAR T cells when the ability to tolerate an aggressive strategy is a concern. She specified that these drugs are associated with a low relative incidence of grade 3 or higher cytokine release syndrome or immune effector cell–associated neurotoxicity syndrome, and faster B-cell aplasia recovery.
The third participant in the debate, who described the efficacy and safety of the three currently approved CAR T-cell therapies for R/R FL, did not agree with this characterization. Daniel J. Landsburg, MD, associate professor of clinical medicine at the University of Pennsylvania, Philadelphia, acknowledged that BsAb agents have an important role to play in the advanced FL setting, but he thinks that CAR T-cell therapies should be prioritized in at least some patients.
In particular, he would not rule out CAR T-cell therapy in patients with comorbidities or other characteristics that raise questions about fitness for aggressive treatment.
“In fact, you might want to treat a frail patient just one time with CAR T-cell therapy rather than dose after dose with a bispecific drug,” he said.
No Data to Compare BsAbs and CAR T-Cells Directly
Both agreed that there have been no trials directly comparing a BsAb therapy vs CAR T cells, so there is no definitive answer, and Landsburg was reluctant to take a hard line on reserving BsAbs until after CAR T-cell therapy has been tried.
“Because BsAbs and CAR Ts are approved in the third-line setting, you might consider debulking a patient getting ready for a CAR T with a bispecific,” Landsburg said. However, he acknowledged that the next step becomes complex if patients achieved a complete response after just a few BsAb doses.
“Do you stop what is already working?” Landsburg asked rhetorically, suggesting that the best way forward is not always clear.
For R/R FL, currently there are three approved products: axicabtagene ciloleucel (Yescarta), tisagenlecleucel (Kymriah), and lisocabtagene maraleucel (Breyanzi). The entry criteria and design of the three pivotal trials differed, so their specific indications vary. Looking across the trials, Landsburg suggested that there might be differences in activity as defined by objective response rates or risk for cytokine release syndrome, but these remain theoretical without head-to-head comparisons.
“My suspicion is we are going to see very similar — quote, unquote — long-term survival curves for patients treated with any of these therapies,” he said, noting that progression-free survival at 3 years has been in the vicinity of 50% for the trials that have had long enough follow-up to judge.
Rather than trying to pick the best agent, he suggested that it makes more sense now to concentrate on strategies to improve response irrespective of CAR T-cell product; these include paying attention to total metabolic tumor volume at the time of infusion, optimizing bridging therapies, and thinking about T-cell fitness, which might be impaired in some patients by recent exposure to bendamustine.
Overall, with multiple ongoing studies with both CAR T-cell therapies and BsAbs in R/R FL — as well with targeted small-molecule agents and immunomodulatory drugs — all of the debate participants acknowledged that choices in R/R FL will evolve.
“I actually think that combinations will be the future,” Wagner-Johnston said. Singling out tazemetostat and a BsAb and one approach that seems promising, she also predicted that some of the therapies in advanced disease are likely to be moved forward to earlier stages of FL therapy.
Rhodes reported ties with AbbVie, AstraZeneca, ADC Therapeutics, BeiGene, Bristol Myers Squibb, Epizyme, Genentech, Genmab, Janssen, Loxo Oncology, MorphoSys, Pharmacyclics, and Pfizer. Wagner-Johnston disclosed relationships with Cuno Science, Dava Oncology, Epizyme, Grünenthal, Karyopharm, and Seagen. Landsburg reported ties with ADC Therapeutics, Calithera, Curis, Epizyme, Karyopharm, MorphoSys, and Novartis.
A version of this article appeared on Medscape.com.
Acknowledging that hers was the weakest position, even the specialist who defended novel targeted therapies mounted a staunch defense of real-world patients being treated outside of tertiary centers.
“I was told by many of my colleagues that I got the short end of the stick in this debate, but I am actually here to convince everybody that targeted therapies continue to play an important role, despite the fact that they are the least sexy of these treatment options,” said Joanna Rhodes, MD, director of the Lymphoma Program at Rutgers Cancer Institute, Hoboken, New Jersey.
Targeted Therapies Still Relevant to Advanced FL
Although even the newest or coming targeted therapies, such as the EZH2 inhibitor tazemetostat or next-generation Bruton tyrosine kinase inhibitors, are not likely to achieve the deep responses and long-term progression-free survival possible with BsAbs or CAR T-cell therapy, the sustained disease control they offer for many patients with R/R FL is not trivial, according to Rhodes.
“The majority of these [advanced follicular lymphoma] patients are being managed in the community,” Rhodes argued at the 2024 Lymphoma, Leukemia, & Myeloma Congress. Access to tertiary centers where the most advanced therapies are available in some cases might not even be feasible.
Moreover, there are barriers to CAR T cells and BsAbs even at centers where these are available, Rhodes said. On a long list of barriers, lack of caregiver support is an example of one common disqualification at her own institution.
The experience with CAR T cells in R/R FL has been relatively short, so Rhodes used data on CAR T cells for B-cell lymphoma to make her point. It is not just that the proportion of eligible patients is limited.
“The majority of B-cell lymphoma patients who are eligible for CAR T cells are not getting them,” she said. “It will be the same for FL.”
In other words, Rhodes indicated that it is premature to count out targeted oral agents or lenalidomide despite the excitement surrounding BsAbs and CAR T cells. The targeted agents and immunomodulatory drugs remain appropriate choices for patients unable or unwilling to travel to tertiary centers for treatment, for frail patients, and for well-informed patients who understand their options and still consider better tolerated therapies to be more consistent with their perception of an adequate risk-benefit ratio.
BsAbs Vie With CAR T Cells in Advanced FL
Hers might be a valid summary, but it did not derail arguments about whether CAR T-cell therapy should be prioritized over BsAbs or the other way around for patients who are candidates for both.
There are two BsAbs currently approved for R/R FL: glofitamab and mosunetuzumab. More are coming, according to Nina Wagner-Johnston, MD, director of hematologic malignancies at Sidney Kimmel Cancer Center, Johns Hopkins University, Baltimore, Maryland. She provided several reasons why BsAbs might be considered before CAR T-cell therapies in at least some individuals.
“The biggest advantage is that these therapies…are off the shelf,” she said. This avoids the delay of T-cell manufacturing, the potential need for bridging therapies, and the need for conditioning regimens. With more experience, BsAbs offer the potential for treatment even in a community-practice setting, particularly for maintenance dosing.
“I do think this is a safe treatment in patients who are elderly or unfit,” Wagner-Johnston said, suggesting she tends to lean toward prioritizing BsAbs over CAR T cells when the ability to tolerate an aggressive strategy is a concern. She specified that these drugs are associated with a low relative incidence of grade 3 or higher cytokine release syndrome or immune effector cell–associated neurotoxicity syndrome, and faster B-cell aplasia recovery.
The third participant in the debate, who described the efficacy and safety of the three currently approved CAR T-cell therapies for R/R FL, did not agree with this characterization. Daniel J. Landsburg, MD, associate professor of clinical medicine at the University of Pennsylvania, Philadelphia, acknowledged that BsAb agents have an important role to play in the advanced FL setting, but he thinks that CAR T-cell therapies should be prioritized in at least some patients.
In particular, he would not rule out CAR T-cell therapy in patients with comorbidities or other characteristics that raise questions about fitness for aggressive treatment.
“In fact, you might want to treat a frail patient just one time with CAR T-cell therapy rather than dose after dose with a bispecific drug,” he said.
No Data to Compare BsAbs and CAR T-Cells Directly
Both agreed that there have been no trials directly comparing a BsAb therapy vs CAR T cells, so there is no definitive answer, and Landsburg was reluctant to take a hard line on reserving BsAbs until after CAR T-cell therapy has been tried.
“Because BsAbs and CAR Ts are approved in the third-line setting, you might consider debulking a patient getting ready for a CAR T with a bispecific,” Landsburg said. However, he acknowledged that the next step becomes complex if patients achieved a complete response after just a few BsAb doses.
“Do you stop what is already working?” Landsburg asked rhetorically, suggesting that the best way forward is not always clear.
For R/R FL, currently there are three approved products: axicabtagene ciloleucel (Yescarta), tisagenlecleucel (Kymriah), and lisocabtagene maraleucel (Breyanzi). The entry criteria and design of the three pivotal trials differed, so their specific indications vary. Looking across the trials, Landsburg suggested that there might be differences in activity as defined by objective response rates or risk for cytokine release syndrome, but these remain theoretical without head-to-head comparisons.
“My suspicion is we are going to see very similar — quote, unquote — long-term survival curves for patients treated with any of these therapies,” he said, noting that progression-free survival at 3 years has been in the vicinity of 50% for the trials that have had long enough follow-up to judge.
Rather than trying to pick the best agent, he suggested that it makes more sense now to concentrate on strategies to improve response irrespective of CAR T-cell product; these include paying attention to total metabolic tumor volume at the time of infusion, optimizing bridging therapies, and thinking about T-cell fitness, which might be impaired in some patients by recent exposure to bendamustine.
Overall, with multiple ongoing studies with both CAR T-cell therapies and BsAbs in R/R FL — as well with targeted small-molecule agents and immunomodulatory drugs — all of the debate participants acknowledged that choices in R/R FL will evolve.
“I actually think that combinations will be the future,” Wagner-Johnston said. Singling out tazemetostat and a BsAb and one approach that seems promising, she also predicted that some of the therapies in advanced disease are likely to be moved forward to earlier stages of FL therapy.
Rhodes reported ties with AbbVie, AstraZeneca, ADC Therapeutics, BeiGene, Bristol Myers Squibb, Epizyme, Genentech, Genmab, Janssen, Loxo Oncology, MorphoSys, Pharmacyclics, and Pfizer. Wagner-Johnston disclosed relationships with Cuno Science, Dava Oncology, Epizyme, Grünenthal, Karyopharm, and Seagen. Landsburg reported ties with ADC Therapeutics, Calithera, Curis, Epizyme, Karyopharm, MorphoSys, and Novartis.
A version of this article appeared on Medscape.com.
Acknowledging that hers was the weakest position, even the specialist who defended novel targeted therapies mounted a staunch defense of real-world patients being treated outside of tertiary centers.
“I was told by many of my colleagues that I got the short end of the stick in this debate, but I am actually here to convince everybody that targeted therapies continue to play an important role, despite the fact that they are the least sexy of these treatment options,” said Joanna Rhodes, MD, director of the Lymphoma Program at Rutgers Cancer Institute, Hoboken, New Jersey.
Targeted Therapies Still Relevant to Advanced FL
Although even the newest or coming targeted therapies, such as the EZH2 inhibitor tazemetostat or next-generation Bruton tyrosine kinase inhibitors, are not likely to achieve the deep responses and long-term progression-free survival possible with BsAbs or CAR T-cell therapy, the sustained disease control they offer for many patients with R/R FL is not trivial, according to Rhodes.
“The majority of these [advanced follicular lymphoma] patients are being managed in the community,” Rhodes argued at the 2024 Lymphoma, Leukemia, & Myeloma Congress. Access to tertiary centers where the most advanced therapies are available in some cases might not even be feasible.
Moreover, there are barriers to CAR T cells and BsAbs even at centers where these are available, Rhodes said. On a long list of barriers, lack of caregiver support is an example of one common disqualification at her own institution.
The experience with CAR T cells in R/R FL has been relatively short, so Rhodes used data on CAR T cells for B-cell lymphoma to make her point. It is not just that the proportion of eligible patients is limited.
“The majority of B-cell lymphoma patients who are eligible for CAR T cells are not getting them,” she said. “It will be the same for FL.”
In other words, Rhodes indicated that it is premature to count out targeted oral agents or lenalidomide despite the excitement surrounding BsAbs and CAR T cells. The targeted agents and immunomodulatory drugs remain appropriate choices for patients unable or unwilling to travel to tertiary centers for treatment, for frail patients, and for well-informed patients who understand their options and still consider better tolerated therapies to be more consistent with their perception of an adequate risk-benefit ratio.
BsAbs Vie With CAR T Cells in Advanced FL
Hers might be a valid summary, but it did not derail arguments about whether CAR T-cell therapy should be prioritized over BsAbs or the other way around for patients who are candidates for both.
There are two BsAbs currently approved for R/R FL: glofitamab and mosunetuzumab. More are coming, according to Nina Wagner-Johnston, MD, director of hematologic malignancies at Sidney Kimmel Cancer Center, Johns Hopkins University, Baltimore, Maryland. She provided several reasons why BsAbs might be considered before CAR T-cell therapies in at least some individuals.
“The biggest advantage is that these therapies…are off the shelf,” she said. This avoids the delay of T-cell manufacturing, the potential need for bridging therapies, and the need for conditioning regimens. With more experience, BsAbs offer the potential for treatment even in a community-practice setting, particularly for maintenance dosing.
“I do think this is a safe treatment in patients who are elderly or unfit,” Wagner-Johnston said, suggesting she tends to lean toward prioritizing BsAbs over CAR T cells when the ability to tolerate an aggressive strategy is a concern. She specified that these drugs are associated with a low relative incidence of grade 3 or higher cytokine release syndrome or immune effector cell–associated neurotoxicity syndrome, and faster B-cell aplasia recovery.
The third participant in the debate, who described the efficacy and safety of the three currently approved CAR T-cell therapies for R/R FL, did not agree with this characterization. Daniel J. Landsburg, MD, associate professor of clinical medicine at the University of Pennsylvania, Philadelphia, acknowledged that BsAb agents have an important role to play in the advanced FL setting, but he thinks that CAR T-cell therapies should be prioritized in at least some patients.
In particular, he would not rule out CAR T-cell therapy in patients with comorbidities or other characteristics that raise questions about fitness for aggressive treatment.
“In fact, you might want to treat a frail patient just one time with CAR T-cell therapy rather than dose after dose with a bispecific drug,” he said.
No Data to Compare BsAbs and CAR T-Cells Directly
Both agreed that there have been no trials directly comparing a BsAb therapy vs CAR T cells, so there is no definitive answer, and Landsburg was reluctant to take a hard line on reserving BsAbs until after CAR T-cell therapy has been tried.
“Because BsAbs and CAR Ts are approved in the third-line setting, you might consider debulking a patient getting ready for a CAR T with a bispecific,” Landsburg said. However, he acknowledged that the next step becomes complex if patients achieved a complete response after just a few BsAb doses.
“Do you stop what is already working?” Landsburg asked rhetorically, suggesting that the best way forward is not always clear.
For R/R FL, currently there are three approved products: axicabtagene ciloleucel (Yescarta), tisagenlecleucel (Kymriah), and lisocabtagene maraleucel (Breyanzi). The entry criteria and design of the three pivotal trials differed, so their specific indications vary. Looking across the trials, Landsburg suggested that there might be differences in activity as defined by objective response rates or risk for cytokine release syndrome, but these remain theoretical without head-to-head comparisons.
“My suspicion is we are going to see very similar — quote, unquote — long-term survival curves for patients treated with any of these therapies,” he said, noting that progression-free survival at 3 years has been in the vicinity of 50% for the trials that have had long enough follow-up to judge.
Rather than trying to pick the best agent, he suggested that it makes more sense now to concentrate on strategies to improve response irrespective of CAR T-cell product; these include paying attention to total metabolic tumor volume at the time of infusion, optimizing bridging therapies, and thinking about T-cell fitness, which might be impaired in some patients by recent exposure to bendamustine.
Overall, with multiple ongoing studies with both CAR T-cell therapies and BsAbs in R/R FL — as well with targeted small-molecule agents and immunomodulatory drugs — all of the debate participants acknowledged that choices in R/R FL will evolve.
“I actually think that combinations will be the future,” Wagner-Johnston said. Singling out tazemetostat and a BsAb and one approach that seems promising, she also predicted that some of the therapies in advanced disease are likely to be moved forward to earlier stages of FL therapy.
Rhodes reported ties with AbbVie, AstraZeneca, ADC Therapeutics, BeiGene, Bristol Myers Squibb, Epizyme, Genentech, Genmab, Janssen, Loxo Oncology, MorphoSys, Pharmacyclics, and Pfizer. Wagner-Johnston disclosed relationships with Cuno Science, Dava Oncology, Epizyme, Grünenthal, Karyopharm, and Seagen. Landsburg reported ties with ADC Therapeutics, Calithera, Curis, Epizyme, Karyopharm, MorphoSys, and Novartis.
A version of this article appeared on Medscape.com.
Expert Updates Therapy for Waldenström Macroglobulinemia
Most importantly, determining the mutational status of patients with WM has become a first or early step in guiding first- and second-line therapies, according to Edward A. Stadtmauer, MD, professor of medicine, University of Pennsylvania, Philadelphia.
Presenting at the 2024 Lymphoma, Leukemia & Myeloma Congress in New York City, Stadtmauer discussed how MYD88 and CXCR4 gene mutations influence his therapeutic choices.
While delivering the Bruce Waterfall Memorial Lecture, funded by the International WM Foundation, he explained that the vast majority of patients with WM have a MYD88 mutation that is highly sensitive to Bruton tyrosine kinase (BTK) inhibitors.
Due to greater specificity on the BTK target, which has implications for safety and efficacy, the first-generation BTK inhibitor ibrutinib has been largely supplanted by next generation drugs such as zanubrutinib.
Deep Responses in WM Remain Elusive
The support for next-generation BTK inhibitors over ibrutinib; bendamustine plus rituximab (BR); or cyclophosphamide, bortezomib, and dexamethasone (CyBorD) is, in his opinion, “a superior toxicity profile, high response rates, and prolonged response.” However, he conceded that the weaknesses of this approach include a low chance of a deep remission and the need for continuous therapy.
On account of these limitations, he typically favors the alkylating agent bendamustine plus the anti-CD20 rituximab over BTK inhibitors in the absence of MYD88 mutations. This once standard approach has become less commonly used in the era of BTK inhibitors, but it is also highly effective, is generally administered in a time-limited regimen, and may be more likely to push patients into a deep remission.
A similar rationale might be considered for CyBorD, but Stadtmauer believes that BR provides a higher rate of PFS with a lower risk for neuropathy, although he admitted this opinion is based on cross-study comparisons, not comparative trials.
While efforts to develop therapies capable of producing a deep response “should not be abandoned,” particularly with the T-cell engager therapies on the horizon, he is not convinced that the benefit-to-risk ratio of aggressive therapies is yet warranted in a disease the often progresses slowly.
“I must admit I am still under the philosophy that Waldenström’s is a chronic disease even if we are seeing a growing list of options for relapsed or poorly responding disease, so I am still not pushing patients too aggressively to knock them into a complete remission,” he said.
MYD88 mutations are not unique to WM, an uncommon, slow-growing form of non-Hodgkin lymphoma. They are found in a small proportion of patients with other hematologic disorders, such as marginal zone lymphomas, but Stadtmauer estimated they occur in 90% of patients with WM. They are common enough that they can help with diagnosis.
CXCR4 Mutations Predict Worse Outcomes
The CXCR4 mutation occurs in an estimated 40% of patients with WM. When present, they are associated with worse outcomes, including a faster time to progression and a reduced overall survival, according to Stadtmauer.
The prognostic impact of less common mutations, such as TP53 and TERT or deletions in LYN, are less well characterized, but Stadtmauer said that most mutations associated with WM result in constitutive or continuous activation in BTK, which, in turn drives WM cell proliferation and survival.
The importance of BTK in WM progression is the reason targeted inhibitors have assumed such a key role in first-line treatment, but Stadtmauer cautioned that these drugs, like other therapies, should not be initiated in asymptomatic patients. This has been stated in past and current guidelines.
More accurately, therapy should be held until just prior to symptomatic manifestations of disease, Stadtmauer specified.
For an optimal response, “you want to start therapy about 3 or 4 months before the symptoms begin,” said Stadtmauer characterizing efforts to do so as “the art of medicine.” Starting therapy just prior to symptoms is advantageous, but it involves following patients closely. Any single biomarker might not be enough.
“In an asymptomatic patient, the level of monoclonal IgM is not an indication to start therapy,” he said, citing studies showing no effect on subsequent disease control from treating this biomarker alone.
However, he listed the development of moderate peripheral neuropathy (PN) as an exception. Essentially, anything greater than mild PN is “still bad” in Stadtmauer’s opinion, so treatment is warranted.
The growing number of second-line options relieves some of the concern when patients progress. Stadtmauer said he is now using BR more often in the second-line drug now that he is using BTK inhibitor more in the first line.
The Bcl-2 inhibitor venetoclax is highly effective and is another first- or second-line option even if this agent, like BTK inhibitors, also appears to require continuous dosing, said Stadtmauer, citing a study that showed patients relapsed relatively rapidly when the drug was stopped.
He now thinks of regimens with proteasome inhibitors as third line.
In selected patients who do not tolerate the non-covalent second-generation BTK inhibitors in the first or second line, he said, “I move quickly to the covalent BTKi pirtobrutinib,” based on data suggesting responses that are at least as good but with a better tolerability profile.
T-Cell Engager Data Are Limited
Without spending much time on the T-cell engagers, such as CAR T-cells or bispecific antibodies, Stadtmauer said that the advances he sees on the horizon “are tremendous,” and the “future is bright.” Such approaches could yield deep responses that could extend control or even provide cure, but these are speculations until more patients have been treated and followed long term.
Morton Coleman, MD, director of the Center for Lymphoma and Myeloma at Weill Cornell Medicine and the chairperson of the LLM Congress, called the talk a valuable and practical summary from a knowledgeable source. BTK inhibitors have represented a major evolution in WM management, but Coleman appreciated the underlying concept that treatment still has to be individualized.
“I think one of the most important take home messages is that the characterization of the mutational profile in patients with Waldenström should be considered a standard of care,” Coleman said. Helpful now, the mutational profile is likely to have a more valuable role as treatment is increasingly individualized.
Stadtmauer reported financial relationships with AbbVie, Bristol Myers Squibb, Celgene, Janssen, and Sorrento. Coleman disclosed ties with AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead, Loxo Oncology, Janssen, and Pharmacyclics.
A version of this article appeared on Medscape.com.
Most importantly, determining the mutational status of patients with WM has become a first or early step in guiding first- and second-line therapies, according to Edward A. Stadtmauer, MD, professor of medicine, University of Pennsylvania, Philadelphia.
Presenting at the 2024 Lymphoma, Leukemia & Myeloma Congress in New York City, Stadtmauer discussed how MYD88 and CXCR4 gene mutations influence his therapeutic choices.
While delivering the Bruce Waterfall Memorial Lecture, funded by the International WM Foundation, he explained that the vast majority of patients with WM have a MYD88 mutation that is highly sensitive to Bruton tyrosine kinase (BTK) inhibitors.
Due to greater specificity on the BTK target, which has implications for safety and efficacy, the first-generation BTK inhibitor ibrutinib has been largely supplanted by next generation drugs such as zanubrutinib.
Deep Responses in WM Remain Elusive
The support for next-generation BTK inhibitors over ibrutinib; bendamustine plus rituximab (BR); or cyclophosphamide, bortezomib, and dexamethasone (CyBorD) is, in his opinion, “a superior toxicity profile, high response rates, and prolonged response.” However, he conceded that the weaknesses of this approach include a low chance of a deep remission and the need for continuous therapy.
On account of these limitations, he typically favors the alkylating agent bendamustine plus the anti-CD20 rituximab over BTK inhibitors in the absence of MYD88 mutations. This once standard approach has become less commonly used in the era of BTK inhibitors, but it is also highly effective, is generally administered in a time-limited regimen, and may be more likely to push patients into a deep remission.
A similar rationale might be considered for CyBorD, but Stadtmauer believes that BR provides a higher rate of PFS with a lower risk for neuropathy, although he admitted this opinion is based on cross-study comparisons, not comparative trials.
While efforts to develop therapies capable of producing a deep response “should not be abandoned,” particularly with the T-cell engager therapies on the horizon, he is not convinced that the benefit-to-risk ratio of aggressive therapies is yet warranted in a disease the often progresses slowly.
“I must admit I am still under the philosophy that Waldenström’s is a chronic disease even if we are seeing a growing list of options for relapsed or poorly responding disease, so I am still not pushing patients too aggressively to knock them into a complete remission,” he said.
MYD88 mutations are not unique to WM, an uncommon, slow-growing form of non-Hodgkin lymphoma. They are found in a small proportion of patients with other hematologic disorders, such as marginal zone lymphomas, but Stadtmauer estimated they occur in 90% of patients with WM. They are common enough that they can help with diagnosis.
CXCR4 Mutations Predict Worse Outcomes
The CXCR4 mutation occurs in an estimated 40% of patients with WM. When present, they are associated with worse outcomes, including a faster time to progression and a reduced overall survival, according to Stadtmauer.
The prognostic impact of less common mutations, such as TP53 and TERT or deletions in LYN, are less well characterized, but Stadtmauer said that most mutations associated with WM result in constitutive or continuous activation in BTK, which, in turn drives WM cell proliferation and survival.
The importance of BTK in WM progression is the reason targeted inhibitors have assumed such a key role in first-line treatment, but Stadtmauer cautioned that these drugs, like other therapies, should not be initiated in asymptomatic patients. This has been stated in past and current guidelines.
More accurately, therapy should be held until just prior to symptomatic manifestations of disease, Stadtmauer specified.
For an optimal response, “you want to start therapy about 3 or 4 months before the symptoms begin,” said Stadtmauer characterizing efforts to do so as “the art of medicine.” Starting therapy just prior to symptoms is advantageous, but it involves following patients closely. Any single biomarker might not be enough.
“In an asymptomatic patient, the level of monoclonal IgM is not an indication to start therapy,” he said, citing studies showing no effect on subsequent disease control from treating this biomarker alone.
However, he listed the development of moderate peripheral neuropathy (PN) as an exception. Essentially, anything greater than mild PN is “still bad” in Stadtmauer’s opinion, so treatment is warranted.
The growing number of second-line options relieves some of the concern when patients progress. Stadtmauer said he is now using BR more often in the second-line drug now that he is using BTK inhibitor more in the first line.
The Bcl-2 inhibitor venetoclax is highly effective and is another first- or second-line option even if this agent, like BTK inhibitors, also appears to require continuous dosing, said Stadtmauer, citing a study that showed patients relapsed relatively rapidly when the drug was stopped.
He now thinks of regimens with proteasome inhibitors as third line.
In selected patients who do not tolerate the non-covalent second-generation BTK inhibitors in the first or second line, he said, “I move quickly to the covalent BTKi pirtobrutinib,” based on data suggesting responses that are at least as good but with a better tolerability profile.
T-Cell Engager Data Are Limited
Without spending much time on the T-cell engagers, such as CAR T-cells or bispecific antibodies, Stadtmauer said that the advances he sees on the horizon “are tremendous,” and the “future is bright.” Such approaches could yield deep responses that could extend control or even provide cure, but these are speculations until more patients have been treated and followed long term.
Morton Coleman, MD, director of the Center for Lymphoma and Myeloma at Weill Cornell Medicine and the chairperson of the LLM Congress, called the talk a valuable and practical summary from a knowledgeable source. BTK inhibitors have represented a major evolution in WM management, but Coleman appreciated the underlying concept that treatment still has to be individualized.
“I think one of the most important take home messages is that the characterization of the mutational profile in patients with Waldenström should be considered a standard of care,” Coleman said. Helpful now, the mutational profile is likely to have a more valuable role as treatment is increasingly individualized.
Stadtmauer reported financial relationships with AbbVie, Bristol Myers Squibb, Celgene, Janssen, and Sorrento. Coleman disclosed ties with AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead, Loxo Oncology, Janssen, and Pharmacyclics.
A version of this article appeared on Medscape.com.
Most importantly, determining the mutational status of patients with WM has become a first or early step in guiding first- and second-line therapies, according to Edward A. Stadtmauer, MD, professor of medicine, University of Pennsylvania, Philadelphia.
Presenting at the 2024 Lymphoma, Leukemia & Myeloma Congress in New York City, Stadtmauer discussed how MYD88 and CXCR4 gene mutations influence his therapeutic choices.
While delivering the Bruce Waterfall Memorial Lecture, funded by the International WM Foundation, he explained that the vast majority of patients with WM have a MYD88 mutation that is highly sensitive to Bruton tyrosine kinase (BTK) inhibitors.
Due to greater specificity on the BTK target, which has implications for safety and efficacy, the first-generation BTK inhibitor ibrutinib has been largely supplanted by next generation drugs such as zanubrutinib.
Deep Responses in WM Remain Elusive
The support for next-generation BTK inhibitors over ibrutinib; bendamustine plus rituximab (BR); or cyclophosphamide, bortezomib, and dexamethasone (CyBorD) is, in his opinion, “a superior toxicity profile, high response rates, and prolonged response.” However, he conceded that the weaknesses of this approach include a low chance of a deep remission and the need for continuous therapy.
On account of these limitations, he typically favors the alkylating agent bendamustine plus the anti-CD20 rituximab over BTK inhibitors in the absence of MYD88 mutations. This once standard approach has become less commonly used in the era of BTK inhibitors, but it is also highly effective, is generally administered in a time-limited regimen, and may be more likely to push patients into a deep remission.
A similar rationale might be considered for CyBorD, but Stadtmauer believes that BR provides a higher rate of PFS with a lower risk for neuropathy, although he admitted this opinion is based on cross-study comparisons, not comparative trials.
While efforts to develop therapies capable of producing a deep response “should not be abandoned,” particularly with the T-cell engager therapies on the horizon, he is not convinced that the benefit-to-risk ratio of aggressive therapies is yet warranted in a disease the often progresses slowly.
“I must admit I am still under the philosophy that Waldenström’s is a chronic disease even if we are seeing a growing list of options for relapsed or poorly responding disease, so I am still not pushing patients too aggressively to knock them into a complete remission,” he said.
MYD88 mutations are not unique to WM, an uncommon, slow-growing form of non-Hodgkin lymphoma. They are found in a small proportion of patients with other hematologic disorders, such as marginal zone lymphomas, but Stadtmauer estimated they occur in 90% of patients with WM. They are common enough that they can help with diagnosis.
CXCR4 Mutations Predict Worse Outcomes
The CXCR4 mutation occurs in an estimated 40% of patients with WM. When present, they are associated with worse outcomes, including a faster time to progression and a reduced overall survival, according to Stadtmauer.
The prognostic impact of less common mutations, such as TP53 and TERT or deletions in LYN, are less well characterized, but Stadtmauer said that most mutations associated with WM result in constitutive or continuous activation in BTK, which, in turn drives WM cell proliferation and survival.
The importance of BTK in WM progression is the reason targeted inhibitors have assumed such a key role in first-line treatment, but Stadtmauer cautioned that these drugs, like other therapies, should not be initiated in asymptomatic patients. This has been stated in past and current guidelines.
More accurately, therapy should be held until just prior to symptomatic manifestations of disease, Stadtmauer specified.
For an optimal response, “you want to start therapy about 3 or 4 months before the symptoms begin,” said Stadtmauer characterizing efforts to do so as “the art of medicine.” Starting therapy just prior to symptoms is advantageous, but it involves following patients closely. Any single biomarker might not be enough.
“In an asymptomatic patient, the level of monoclonal IgM is not an indication to start therapy,” he said, citing studies showing no effect on subsequent disease control from treating this biomarker alone.
However, he listed the development of moderate peripheral neuropathy (PN) as an exception. Essentially, anything greater than mild PN is “still bad” in Stadtmauer’s opinion, so treatment is warranted.
The growing number of second-line options relieves some of the concern when patients progress. Stadtmauer said he is now using BR more often in the second-line drug now that he is using BTK inhibitor more in the first line.
The Bcl-2 inhibitor venetoclax is highly effective and is another first- or second-line option even if this agent, like BTK inhibitors, also appears to require continuous dosing, said Stadtmauer, citing a study that showed patients relapsed relatively rapidly when the drug was stopped.
He now thinks of regimens with proteasome inhibitors as third line.
In selected patients who do not tolerate the non-covalent second-generation BTK inhibitors in the first or second line, he said, “I move quickly to the covalent BTKi pirtobrutinib,” based on data suggesting responses that are at least as good but with a better tolerability profile.
T-Cell Engager Data Are Limited
Without spending much time on the T-cell engagers, such as CAR T-cells or bispecific antibodies, Stadtmauer said that the advances he sees on the horizon “are tremendous,” and the “future is bright.” Such approaches could yield deep responses that could extend control or even provide cure, but these are speculations until more patients have been treated and followed long term.
Morton Coleman, MD, director of the Center for Lymphoma and Myeloma at Weill Cornell Medicine and the chairperson of the LLM Congress, called the talk a valuable and practical summary from a knowledgeable source. BTK inhibitors have represented a major evolution in WM management, but Coleman appreciated the underlying concept that treatment still has to be individualized.
“I think one of the most important take home messages is that the characterization of the mutational profile in patients with Waldenström should be considered a standard of care,” Coleman said. Helpful now, the mutational profile is likely to have a more valuable role as treatment is increasingly individualized.
Stadtmauer reported financial relationships with AbbVie, Bristol Myers Squibb, Celgene, Janssen, and Sorrento. Coleman disclosed ties with AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb, Gilead, Loxo Oncology, Janssen, and Pharmacyclics.
A version of this article appeared on Medscape.com.
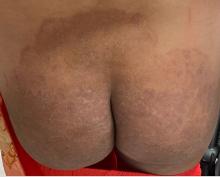
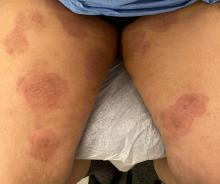
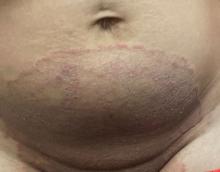
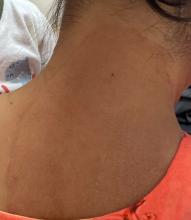
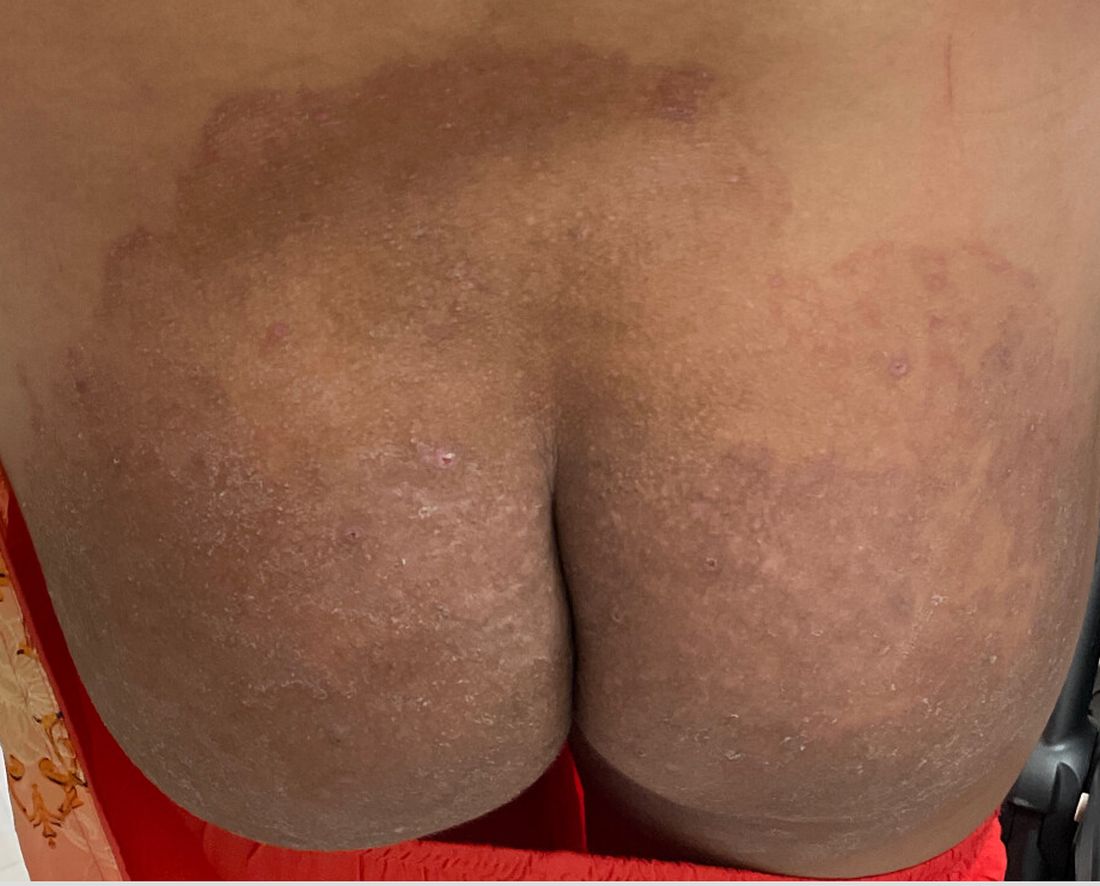
A 51-year-old woman presented for a routine full body skin exam after vacationing in Hawaii.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
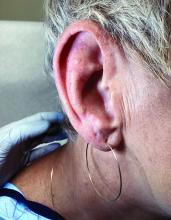
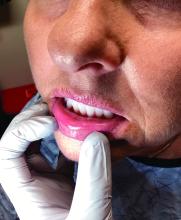
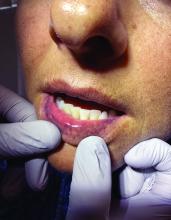
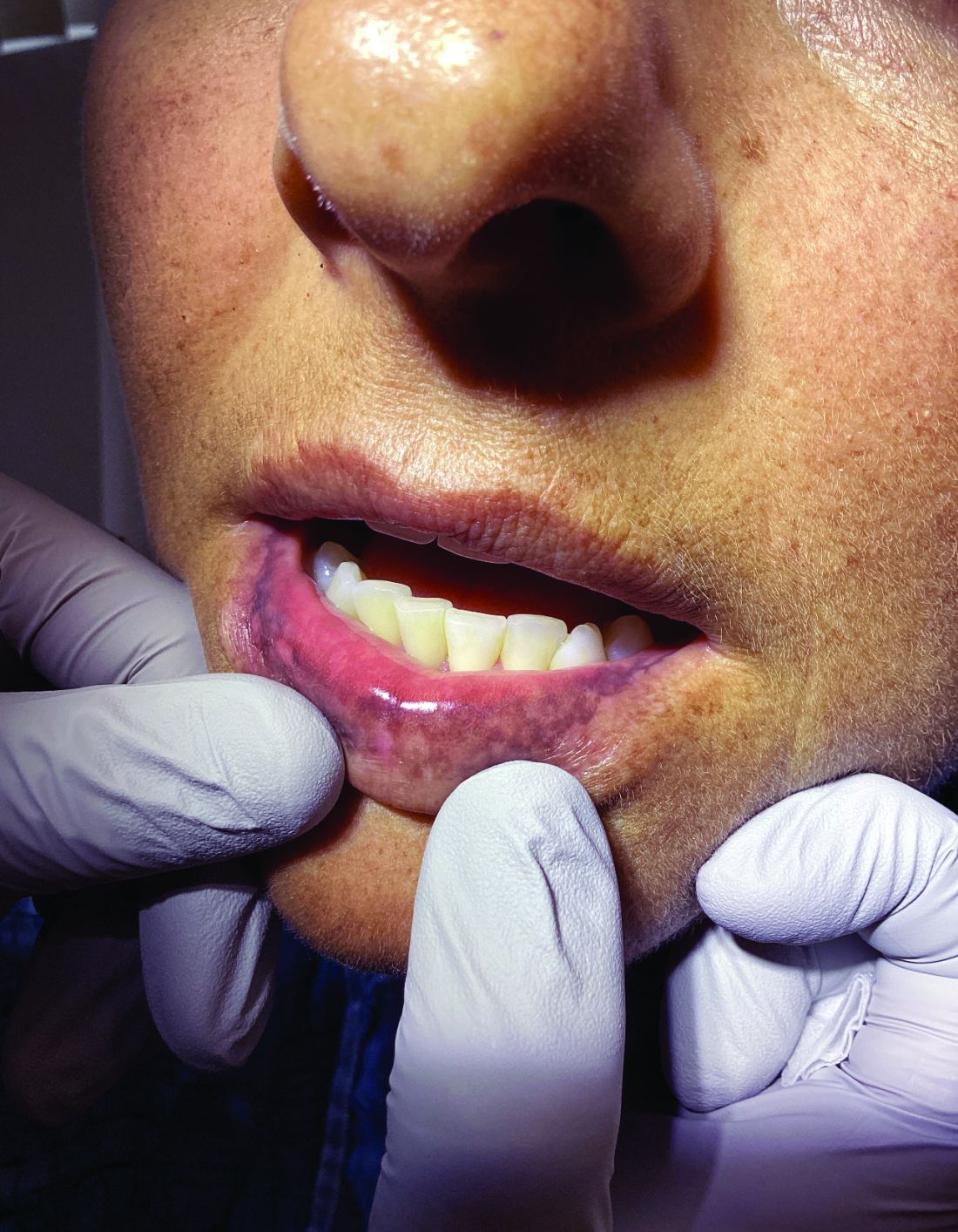
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
More Evidence Ties Semaglutide to Reduced Alzheimer’s Risk
Adults with type 2 diabetes who were prescribed the GLP-1 RA semaglutide had a significantly lower risk for Alzheimer’s disease compared with their peers who were prescribed any of seven other antidiabetic medications, including other types of GLP-1 receptor–targeting medications.
“These findings support further clinical trials to assess semaglutide’s potential in delaying or preventing Alzheimer’s disease,” wrote the investigators, led by Rong Xu, PhD, with Case Western Reserve School of Medicine, Cleveland, Ohio.
The study was published online on October 24 in Alzheimer’s & Dementia.
Real-World Data
Semaglutide has shown neuroprotective effects in animal models of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In animal models of Alzheimer’s disease, the drug reduced beta-amyloid deposition and improved spatial learning and memory, as well as glucose metabolism in the brain.
In a real-world analysis, Xu and colleagues used electronic health record data to identify 17,104 new users of semaglutide and 1,077,657 new users of seven other antidiabetic medications, including other GLP-1 RAs, insulin, metformin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, sulfonylurea, and thiazolidinedione.
Over 3 years, treatment with semaglutide was associated with significantly reduced risk of developing Alzheimer’s disease, most strongly compared with insulin (hazard ratio [HR], 0.33) and most weakly compared with other GLP-1 RAs (HR, 0.59).
Compared with the other medications, semaglutide was associated with a 40%-70% reduced risk for first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes, with similar reductions seen across obesity status and gender and age groups, the authors reported.
The findings align with recent evidence suggesting GLP-1 RAs may protect cognitive function.
For example, as previously reported, in the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 RA liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months compared with placebo.
Promising, but Preliminary
Reached for comment, Courtney Kloske, PhD, Alzheimer’s Association director of scientific engagement, noted that diabetes is a known risk factor for AD and managing diabetes with drugs such as semaglutide “could benefit brain health simply by managing diabetes.”
“However, we still need large clinical trials in representative populations to determine if semaglutide specifically lowers the risk of Alzheimer’s, so it is too early to recommend it for prevention,” Kloske said.
She noted that some research suggests that GLP-1 RAs “may help reduce inflammation and positively impact brain energy use. However, more research is needed to fully understand how these processes might contribute to preventing cognitive decline or Alzheimer’s,” Kloske cautioned.
The Alzheimer’s Association’s “Part the Cloud” initiative has invested more than $68 million to advance 65 clinical trials targeting a variety of compounds, including repurposed drugs that may address known and potential new aspects of the disease, Kloske said.
The study was supported by grants from the National Institute on Aging and the National Center for Advancing Translational Sciences. Xu and Kloske have no relevant conflicts.
A version of this article appeared on Medscape.com.
Adults with type 2 diabetes who were prescribed the GLP-1 RA semaglutide had a significantly lower risk for Alzheimer’s disease compared with their peers who were prescribed any of seven other antidiabetic medications, including other types of GLP-1 receptor–targeting medications.
“These findings support further clinical trials to assess semaglutide’s potential in delaying or preventing Alzheimer’s disease,” wrote the investigators, led by Rong Xu, PhD, with Case Western Reserve School of Medicine, Cleveland, Ohio.
The study was published online on October 24 in Alzheimer’s & Dementia.
Real-World Data
Semaglutide has shown neuroprotective effects in animal models of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In animal models of Alzheimer’s disease, the drug reduced beta-amyloid deposition and improved spatial learning and memory, as well as glucose metabolism in the brain.
In a real-world analysis, Xu and colleagues used electronic health record data to identify 17,104 new users of semaglutide and 1,077,657 new users of seven other antidiabetic medications, including other GLP-1 RAs, insulin, metformin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, sulfonylurea, and thiazolidinedione.
Over 3 years, treatment with semaglutide was associated with significantly reduced risk of developing Alzheimer’s disease, most strongly compared with insulin (hazard ratio [HR], 0.33) and most weakly compared with other GLP-1 RAs (HR, 0.59).
Compared with the other medications, semaglutide was associated with a 40%-70% reduced risk for first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes, with similar reductions seen across obesity status and gender and age groups, the authors reported.
The findings align with recent evidence suggesting GLP-1 RAs may protect cognitive function.
For example, as previously reported, in the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 RA liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months compared with placebo.
Promising, but Preliminary
Reached for comment, Courtney Kloske, PhD, Alzheimer’s Association director of scientific engagement, noted that diabetes is a known risk factor for AD and managing diabetes with drugs such as semaglutide “could benefit brain health simply by managing diabetes.”
“However, we still need large clinical trials in representative populations to determine if semaglutide specifically lowers the risk of Alzheimer’s, so it is too early to recommend it for prevention,” Kloske said.
She noted that some research suggests that GLP-1 RAs “may help reduce inflammation and positively impact brain energy use. However, more research is needed to fully understand how these processes might contribute to preventing cognitive decline or Alzheimer’s,” Kloske cautioned.
The Alzheimer’s Association’s “Part the Cloud” initiative has invested more than $68 million to advance 65 clinical trials targeting a variety of compounds, including repurposed drugs that may address known and potential new aspects of the disease, Kloske said.
The study was supported by grants from the National Institute on Aging and the National Center for Advancing Translational Sciences. Xu and Kloske have no relevant conflicts.
A version of this article appeared on Medscape.com.
Adults with type 2 diabetes who were prescribed the GLP-1 RA semaglutide had a significantly lower risk for Alzheimer’s disease compared with their peers who were prescribed any of seven other antidiabetic medications, including other types of GLP-1 receptor–targeting medications.
“These findings support further clinical trials to assess semaglutide’s potential in delaying or preventing Alzheimer’s disease,” wrote the investigators, led by Rong Xu, PhD, with Case Western Reserve School of Medicine, Cleveland, Ohio.
The study was published online on October 24 in Alzheimer’s & Dementia.
Real-World Data
Semaglutide has shown neuroprotective effects in animal models of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In animal models of Alzheimer’s disease, the drug reduced beta-amyloid deposition and improved spatial learning and memory, as well as glucose metabolism in the brain.
In a real-world analysis, Xu and colleagues used electronic health record data to identify 17,104 new users of semaglutide and 1,077,657 new users of seven other antidiabetic medications, including other GLP-1 RAs, insulin, metformin, dipeptidyl peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, sulfonylurea, and thiazolidinedione.
Over 3 years, treatment with semaglutide was associated with significantly reduced risk of developing Alzheimer’s disease, most strongly compared with insulin (hazard ratio [HR], 0.33) and most weakly compared with other GLP-1 RAs (HR, 0.59).
Compared with the other medications, semaglutide was associated with a 40%-70% reduced risk for first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes, with similar reductions seen across obesity status and gender and age groups, the authors reported.
The findings align with recent evidence suggesting GLP-1 RAs may protect cognitive function.
For example, as previously reported, in the phase 2b ELAD clinical trial, adults with early-stage Alzheimer’s disease taking the GLP-1 RA liraglutide exhibited slower decline in memory and thinking and experienced less brain atrophy over 12 months compared with placebo.
Promising, but Preliminary
Reached for comment, Courtney Kloske, PhD, Alzheimer’s Association director of scientific engagement, noted that diabetes is a known risk factor for AD and managing diabetes with drugs such as semaglutide “could benefit brain health simply by managing diabetes.”
“However, we still need large clinical trials in representative populations to determine if semaglutide specifically lowers the risk of Alzheimer’s, so it is too early to recommend it for prevention,” Kloske said.
She noted that some research suggests that GLP-1 RAs “may help reduce inflammation and positively impact brain energy use. However, more research is needed to fully understand how these processes might contribute to preventing cognitive decline or Alzheimer’s,” Kloske cautioned.
The Alzheimer’s Association’s “Part the Cloud” initiative has invested more than $68 million to advance 65 clinical trials targeting a variety of compounds, including repurposed drugs that may address known and potential new aspects of the disease, Kloske said.
The study was supported by grants from the National Institute on Aging and the National Center for Advancing Translational Sciences. Xu and Kloske have no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
A New, Easily Identifiable Sign of Concussion?
Spontaneous Headshake After a Kinematic Event (SHAAKE) refers to the rapid, back-and-forth head movement athletes exhibit following a blow to the head. This voluntary motion typically occurs within seconds to minutes after impact and is a familiar response in athletes.
In a recent survey, 7 out of 10 adult athletes recalled making this movement after a collision, and three out of four times they attributed this back-and-forth head movement to a concussion. The association was strongest among football players, who reported that over 90% of SHAAKE episodes were associated with a concussion.
The results were published online in Diagnostics.
Call to Action
“Everyone” — including sports and medical organizations — “should be adding this to their list of potential concussion signs and their protocol immediately,” study investigator Chris Nowinski, PhD, CEO and co-founder of the Concussion Legacy Foundation, told this news organization.
Nowinski said it’s “fascinating” that this concussion sign hasn’t been formally studied or added to formal concussion screening metrics before now, given that it’s been depicted in movies, television, and cartoons for decades.
Coaches, medical professionals, and concussion spotters should be trained to recognize when a SHAAKE happens, he said.
“The interesting thing is, I don’t think coaches or parents need much training other than to officially tie this to suspicion of a concussion,” Nowinski added.
The Case of Miami Dolphins QB Tua Tagovailoa
Nowinski said he was tipped off to SHAAKE as a concussion sign after Miami Dolphins quarterback Tua Tagovailoa’s controversial undiagnosed concussion during a National Football League (NFL) game in 2022.
After Tagovailoa’s head hit the ground, he rapidly shook his head side to side, indicating displaying SHAAKE, before stumbling and collapsing. At the time, a sideline doctor attributed his collapse to a prior back injury.
If Tagovailoa had been diagnosed with a concussion, he likely would not have been playing in a game just 4 days later, where he lost consciousness after suffering a suspected second concussion and was removed from the field on a stretcher.
For the survey, Nowinski and colleagues showed 347 current and former athletes, including 109 football players, video examples of SHAAKE and them asked about their experiences with this potential indicator of concussion.
Nearly 69% of athletes reported exhibiting a SHAAKE during their career, and 93% of those reported a SHAAKE in association with concussion at least once. Athletes reported SHAAKE a median of five times in their lives.
Of the athletes who reported SHAAKE, 85% linked this head-shaking movement to concussion symptoms such as disorientation (71%) and dizziness (54%).
Across all sports, SHAAKE showed a sensitivity of 49.6% and a positive predictive value (PPV) of 72.4% for diagnosing concussions.
Among football players, sensitivity improved to 52.3%, with an estimated specificity of 99.9%, a PPV of 91.9%, and an estimated negative predictive value of 99.5%.
The main limitation of the survey was the potential for recall bias due to survey participants self-reporting prior concussions. The researchers called for future prospective studies to validate SHAAKE as a sign of concussion.
Instant Replay for Brain Injury?
Experts echoed the need for validation. SHAAKE represents a “promising advance” in objective TBI assessment, particularly for sideline evaluation, said Shaheen Lakhan, MD, PhD, neurologist, and researcher based in Miami, Florida, who wasn’t involved in the research.
The potential value of SHAAKE is “particularly notable given the well-documented tendency for athletes to minimize or conceal symptoms to maintain play eligibility, a limitation that has historically challenged our reliance on subjective reporting and observational assessments,” Lakhan said.
“Moving forward, validation through prospective studies incorporating real-time video analysis, helmet sensor data, and clinician-confirmed TBI diagnoses will be essential. With appropriate validation, SHAAKE could emerge as a valuable component of our sideline assessment arsenal, complementing rather than replacing existing diagnostic approaches,” Lakhan said.
“SHAAKE could be the ‘instant replay’ for brain injuries that sports medicine has been waiting for — but like any new technology, we need to make sure it works for every player, not just some,” Lakhan added.
Also weighing in, Richard Figler, MD, director of the Concussion Center, Cleveland Clinic Sports Medicine Center, Cleveland, cautioned that the survey participants were recruited from a concussion registry and self-reported an average of 23 concussions — more than one third of which happened 5-10 years prior — which begs the question, “How much are they actually remembering?”
“Our goal is to make sure that the athletes are safe and that we’re not missing concussions, and we don’t have great tools to start off with. This study opens up the door for some prospective studies [of SHAAKE] moving forward. I think we need more data before this should be listed as a definitive marker,” said Figler, who also wasn’t involved in the study.
In any case, he said, when it comes to suspected concussion in sports, “when in doubt, you sit them out,” Figler said.
This research received no external funding. Nowinski has received travel reimbursement from the NFL Players Association (NFLPA), NFL, World Rugby, WWE, and All Elite Wrestling; served as an expert witness in cases related to concussion and chronic traumatic encephalopathy; and is compensated for speaking appearances and serving on the NFL Concussion Settlement Player Advocacy Committee. Daniel H. Daneshvar served as an expert witness in legal cases involving brain injury and concussion and received funding from the Football Players Health Study at Harvard University, which is funded by the NFLPA and evaluates patients for the MGH Brain and Body TRUST Center, sponsored in part by the NFLPA. Lakhan and Figler had no relevant disclosures.
A version of this article appeared on Medscape.com.
Spontaneous Headshake After a Kinematic Event (SHAAKE) refers to the rapid, back-and-forth head movement athletes exhibit following a blow to the head. This voluntary motion typically occurs within seconds to minutes after impact and is a familiar response in athletes.
In a recent survey, 7 out of 10 adult athletes recalled making this movement after a collision, and three out of four times they attributed this back-and-forth head movement to a concussion. The association was strongest among football players, who reported that over 90% of SHAAKE episodes were associated with a concussion.
The results were published online in Diagnostics.
Call to Action
“Everyone” — including sports and medical organizations — “should be adding this to their list of potential concussion signs and their protocol immediately,” study investigator Chris Nowinski, PhD, CEO and co-founder of the Concussion Legacy Foundation, told this news organization.
Nowinski said it’s “fascinating” that this concussion sign hasn’t been formally studied or added to formal concussion screening metrics before now, given that it’s been depicted in movies, television, and cartoons for decades.
Coaches, medical professionals, and concussion spotters should be trained to recognize when a SHAAKE happens, he said.
“The interesting thing is, I don’t think coaches or parents need much training other than to officially tie this to suspicion of a concussion,” Nowinski added.
The Case of Miami Dolphins QB Tua Tagovailoa
Nowinski said he was tipped off to SHAAKE as a concussion sign after Miami Dolphins quarterback Tua Tagovailoa’s controversial undiagnosed concussion during a National Football League (NFL) game in 2022.
After Tagovailoa’s head hit the ground, he rapidly shook his head side to side, indicating displaying SHAAKE, before stumbling and collapsing. At the time, a sideline doctor attributed his collapse to a prior back injury.
If Tagovailoa had been diagnosed with a concussion, he likely would not have been playing in a game just 4 days later, where he lost consciousness after suffering a suspected second concussion and was removed from the field on a stretcher.
For the survey, Nowinski and colleagues showed 347 current and former athletes, including 109 football players, video examples of SHAAKE and them asked about their experiences with this potential indicator of concussion.
Nearly 69% of athletes reported exhibiting a SHAAKE during their career, and 93% of those reported a SHAAKE in association with concussion at least once. Athletes reported SHAAKE a median of five times in their lives.
Of the athletes who reported SHAAKE, 85% linked this head-shaking movement to concussion symptoms such as disorientation (71%) and dizziness (54%).
Across all sports, SHAAKE showed a sensitivity of 49.6% and a positive predictive value (PPV) of 72.4% for diagnosing concussions.
Among football players, sensitivity improved to 52.3%, with an estimated specificity of 99.9%, a PPV of 91.9%, and an estimated negative predictive value of 99.5%.
The main limitation of the survey was the potential for recall bias due to survey participants self-reporting prior concussions. The researchers called for future prospective studies to validate SHAAKE as a sign of concussion.
Instant Replay for Brain Injury?
Experts echoed the need for validation. SHAAKE represents a “promising advance” in objective TBI assessment, particularly for sideline evaluation, said Shaheen Lakhan, MD, PhD, neurologist, and researcher based in Miami, Florida, who wasn’t involved in the research.
The potential value of SHAAKE is “particularly notable given the well-documented tendency for athletes to minimize or conceal symptoms to maintain play eligibility, a limitation that has historically challenged our reliance on subjective reporting and observational assessments,” Lakhan said.
“Moving forward, validation through prospective studies incorporating real-time video analysis, helmet sensor data, and clinician-confirmed TBI diagnoses will be essential. With appropriate validation, SHAAKE could emerge as a valuable component of our sideline assessment arsenal, complementing rather than replacing existing diagnostic approaches,” Lakhan said.
“SHAAKE could be the ‘instant replay’ for brain injuries that sports medicine has been waiting for — but like any new technology, we need to make sure it works for every player, not just some,” Lakhan added.
Also weighing in, Richard Figler, MD, director of the Concussion Center, Cleveland Clinic Sports Medicine Center, Cleveland, cautioned that the survey participants were recruited from a concussion registry and self-reported an average of 23 concussions — more than one third of which happened 5-10 years prior — which begs the question, “How much are they actually remembering?”
“Our goal is to make sure that the athletes are safe and that we’re not missing concussions, and we don’t have great tools to start off with. This study opens up the door for some prospective studies [of SHAAKE] moving forward. I think we need more data before this should be listed as a definitive marker,” said Figler, who also wasn’t involved in the study.
In any case, he said, when it comes to suspected concussion in sports, “when in doubt, you sit them out,” Figler said.
This research received no external funding. Nowinski has received travel reimbursement from the NFL Players Association (NFLPA), NFL, World Rugby, WWE, and All Elite Wrestling; served as an expert witness in cases related to concussion and chronic traumatic encephalopathy; and is compensated for speaking appearances and serving on the NFL Concussion Settlement Player Advocacy Committee. Daniel H. Daneshvar served as an expert witness in legal cases involving brain injury and concussion and received funding from the Football Players Health Study at Harvard University, which is funded by the NFLPA and evaluates patients for the MGH Brain and Body TRUST Center, sponsored in part by the NFLPA. Lakhan and Figler had no relevant disclosures.
A version of this article appeared on Medscape.com.
Spontaneous Headshake After a Kinematic Event (SHAAKE) refers to the rapid, back-and-forth head movement athletes exhibit following a blow to the head. This voluntary motion typically occurs within seconds to minutes after impact and is a familiar response in athletes.
In a recent survey, 7 out of 10 adult athletes recalled making this movement after a collision, and three out of four times they attributed this back-and-forth head movement to a concussion. The association was strongest among football players, who reported that over 90% of SHAAKE episodes were associated with a concussion.
The results were published online in Diagnostics.
Call to Action
“Everyone” — including sports and medical organizations — “should be adding this to their list of potential concussion signs and their protocol immediately,” study investigator Chris Nowinski, PhD, CEO and co-founder of the Concussion Legacy Foundation, told this news organization.
Nowinski said it’s “fascinating” that this concussion sign hasn’t been formally studied or added to formal concussion screening metrics before now, given that it’s been depicted in movies, television, and cartoons for decades.
Coaches, medical professionals, and concussion spotters should be trained to recognize when a SHAAKE happens, he said.
“The interesting thing is, I don’t think coaches or parents need much training other than to officially tie this to suspicion of a concussion,” Nowinski added.
The Case of Miami Dolphins QB Tua Tagovailoa
Nowinski said he was tipped off to SHAAKE as a concussion sign after Miami Dolphins quarterback Tua Tagovailoa’s controversial undiagnosed concussion during a National Football League (NFL) game in 2022.
After Tagovailoa’s head hit the ground, he rapidly shook his head side to side, indicating displaying SHAAKE, before stumbling and collapsing. At the time, a sideline doctor attributed his collapse to a prior back injury.
If Tagovailoa had been diagnosed with a concussion, he likely would not have been playing in a game just 4 days later, where he lost consciousness after suffering a suspected second concussion and was removed from the field on a stretcher.
For the survey, Nowinski and colleagues showed 347 current and former athletes, including 109 football players, video examples of SHAAKE and them asked about their experiences with this potential indicator of concussion.
Nearly 69% of athletes reported exhibiting a SHAAKE during their career, and 93% of those reported a SHAAKE in association with concussion at least once. Athletes reported SHAAKE a median of five times in their lives.
Of the athletes who reported SHAAKE, 85% linked this head-shaking movement to concussion symptoms such as disorientation (71%) and dizziness (54%).
Across all sports, SHAAKE showed a sensitivity of 49.6% and a positive predictive value (PPV) of 72.4% for diagnosing concussions.
Among football players, sensitivity improved to 52.3%, with an estimated specificity of 99.9%, a PPV of 91.9%, and an estimated negative predictive value of 99.5%.
The main limitation of the survey was the potential for recall bias due to survey participants self-reporting prior concussions. The researchers called for future prospective studies to validate SHAAKE as a sign of concussion.
Instant Replay for Brain Injury?
Experts echoed the need for validation. SHAAKE represents a “promising advance” in objective TBI assessment, particularly for sideline evaluation, said Shaheen Lakhan, MD, PhD, neurologist, and researcher based in Miami, Florida, who wasn’t involved in the research.
The potential value of SHAAKE is “particularly notable given the well-documented tendency for athletes to minimize or conceal symptoms to maintain play eligibility, a limitation that has historically challenged our reliance on subjective reporting and observational assessments,” Lakhan said.
“Moving forward, validation through prospective studies incorporating real-time video analysis, helmet sensor data, and clinician-confirmed TBI diagnoses will be essential. With appropriate validation, SHAAKE could emerge as a valuable component of our sideline assessment arsenal, complementing rather than replacing existing diagnostic approaches,” Lakhan said.
“SHAAKE could be the ‘instant replay’ for brain injuries that sports medicine has been waiting for — but like any new technology, we need to make sure it works for every player, not just some,” Lakhan added.
Also weighing in, Richard Figler, MD, director of the Concussion Center, Cleveland Clinic Sports Medicine Center, Cleveland, cautioned that the survey participants were recruited from a concussion registry and self-reported an average of 23 concussions — more than one third of which happened 5-10 years prior — which begs the question, “How much are they actually remembering?”
“Our goal is to make sure that the athletes are safe and that we’re not missing concussions, and we don’t have great tools to start off with. This study opens up the door for some prospective studies [of SHAAKE] moving forward. I think we need more data before this should be listed as a definitive marker,” said Figler, who also wasn’t involved in the study.
In any case, he said, when it comes to suspected concussion in sports, “when in doubt, you sit them out,” Figler said.
This research received no external funding. Nowinski has received travel reimbursement from the NFL Players Association (NFLPA), NFL, World Rugby, WWE, and All Elite Wrestling; served as an expert witness in cases related to concussion and chronic traumatic encephalopathy; and is compensated for speaking appearances and serving on the NFL Concussion Settlement Player Advocacy Committee. Daniel H. Daneshvar served as an expert witness in legal cases involving brain injury and concussion and received funding from the Football Players Health Study at Harvard University, which is funded by the NFLPA and evaluates patients for the MGH Brain and Body TRUST Center, sponsored in part by the NFLPA. Lakhan and Figler had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM DIAGNOSTICS
For Radiation ‘Downwinders,’ Cancer Compensation Is On Hold
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
As of 2022, more than 40,000 patients with cancer successfully applied for $2.6 billion in compensation. Recipients included “downwinders” who were eligible for $50,000 each if they lived in certain areas of Nevada, Utah, and Arizona during specified nuclear testing periods and developed a covered form of cancer.
In June 2024, however, the Radiation Exposure Compensation Program expired amid infighting among Republicans in Congress over whether to expand it. For now, no one can make a claim, even though many downwinders are still alive and continue to be diagnosed with covered cancers decades after they were exposed in the 1940s, 1950s, and 1960s.
There’s a glimmer of good news. The federal government continues to support free medical screenings for eligible people, including certain downwinders and uranium workers. Meanwhile, there are still important roles for clinicians across the country to play as politicians figure out what — if anything — to do next regarding those exposed to radiation.
“We are still here. We can still screen people,” Zachary Davis, program director for the Radiation Exposure Screening and Education Program, The University of New Mexico, in Albuquerque, New Mexico, said in an interview.
Still-Unfolding Legacy of Radiation Exposure
No one knew just how far radiation would spread when the first nuclear bomb was tested in New Mexico in July 1945. Would it cover the state? The entire Southwest? The whole nation?
It also wasn’t clear how radiation would affect people’s health. “There was an awareness that some cancers were caused by radiation, but there wasn’t a cohesive understanding of what the problem was,” Joseph Shonka, PhD, a health physicist who studies radiation exposure and has worked for decades in nuclear engineering, said in an interview.
Now, nearly eight decades later, scientists are still figuring out the full extent of radioactive fallout from nuclear testing. Just last year, a study suggested that radiation from 94 nuclear weapon tests in the Southwest from 1945 to 1962 reached 46 states along with Canada and Mexico.
Activists believe the tests triggered untold number of cancer cases in residents who were exposed in downwind areas:
“My brother died of stomach cancer; my mom died of bone cancer. One of my sisters is surviving brain tumors, and the other one is surviving thyroid cancer,” one New Mexico man recently told ABC-TV’s “Nightline.”
In Idaho, a downwinder advocate told Idaho Capital Sun that everyone who attended a reception for her newly married parents in 1952 — just weeks after a nuclear test — developed cancer or “weird medical complications.” That included her parents, who both had cancer. Her two older brothers, born in 1953 and 1955, also developed cancer, and she’s tracked many other cases in the small town of Emmett.
In Utah, another downwinder advocate told Utah News Dispatch that cancer was common in Salt Lake City neighborhood, where she grew up, which was exposed to fallout. She developed thyroid cancer, her younger sister developed stomach cancer, and an older sister died of lupus, which is connected to radiation exposure. But Salt Lake City isn’t in one of the regions of Utah covered by the federal compensation program, so the advocate can’t get a $50,000 payment.
Downwinders who lived in New Mexico, Idaho, and the Salt Lake City area of Utah are not covered by the federal compensation program. That means none of these people or their descendants are eligible for payments — yet.
Decades After Nuclear Testing, the Government Responds
In 1990, Congress passed the Radiation Exposure Compensation Act, which allowed compensation to people with cancer at several levels. It was later expanded. Downwinders — including those who’ve moved elsewhere over the years — were eligible for $50,000. Onsite participants in nuclear testing could get $75,000. Uranium miners, millers, and ore transporters in 11 states west of the Mississippi River could get $100,000.
Among downwinders, eligible cancers included blood cancers (leukemias with the exception of chronic lymphocytic leukemia, multiple myeloma, and non-Hodgkin’s lymphomas) and a long list of solid organ cancers such as thyroid, breast, stomach, brain, lung, colon, and liver cancers.
“When it comes to blood-related cancers, we do see leukemias, lymphomas, and multiple myeloma, but these cancers were more likely to occur sooner after fallout exposure,” said Laura Shaw, MD, principal investigator who oversees the radiation exposure screening program at the University of Nevada, Las Vegas. “At this point, we see more pancreatic, thyroid, lung, stomach, bladder, and breast cancer.”
The compensation program had major limitations, critics said. “It left out a lot of communities that were exposed,” said Lilly Adams, senior outreach coordinator with the Union of Concerned Scientists (UCS), which supports expanding the program. A national nonprofit organization, UCS was founded more than 50 years ago by scientists and students at the Massachusetts Institute of Technology.
“You have this pretty small amount of one-time compensation, and that’s it,” Adams said in an interview. “You can’t get reimbursed for medical costs or lost wages.” Still, “as flawed as the program is, it’s really valuable for the people who are eligible,” she noted.
Now Congress Is Divided on Next Steps
Some lawmakers have recognized the need to do more for those who developed cancer that’s potentially linked to radiation exposure. As the June 2024 expiration of the Radiation Exposure Compensation Act loomed, Democrats and Republicans in Congress worked together to extend and expand the program.
They introduced a bill for higher compensation — $100,000 per person — and the widening of covered downwinder areas to all of Arizona, Nevada, and Utah (which had only been partially covered), along with all of Colorado, Idaho, New Mexico, Montana, and Guam. Under the legislation, the program also would expand to cover some uranium workers who were on the job after 1971 and residents exposed to nuclear waste in Kentucky, Missouri, and Tennessee.
In March, the new legislation easily passed the US Senate by a vote of 69-30, with support from both political parties — but the Republican-led House hasn’t taken it up. As a result, the Radiation Exposure Compensation Act expired in June, and no one can submit new applications for compensation.
A spokesman for House Speaker Mike Johnson told Missouri Independent “unfortunately, the current Senate bill is estimated to cost $50-$60 billion in new mandatory spending with no offsets and was supported by only 20 of 49 Republicans in the Senate.”
Adams rejected these arguments. “The government spends literally trillions of dollars on our nuclear weapons. Whether or not you support that spending, the human cost of building those weapons should be factored in,” she said. She added that she hopes the House will act by the end of the year to pass the bill, but that’s uncertain.
As Compensation Is On Hold, Medical Screening Continues
A major benefit is still available for downwinders and uranium workers: Free medical screening and referrals for medical treatment. The Radiation Exposure Screening and Education Program’s funding has not been affected by the congressional impasse, so screenings are continuing for eligible people exposed to radiation.
Radiation exposure clinics offer screening in Arizona, Colorado, Nevada, New Mexico, and Utah, and health providers can get funding to offer screening in other affected states.
In Nevada, “we hold screening clinics throughout the state: Caliente, Ely, and Winnemucca. Also, in Reno and Las Vegas, which are not in designated downwind areas, but many downwinders have migrated there,” said Shaw in an interview. Among downwinders, “our youngest patients are in their 60s and range up to a few in their 90s,” she said.
Patients fill out questionnaires that ask about their medical problems, family history, and medications. “Ely patients in particular seem to have extensive family histories of cancer, and this may be due to their location directly downwind of the Nevada Test Site,” Shaw said. (Ely is a remote town in central eastern Nevada near the Utah border.)
The screenings cover both cancer and noncancer conditions. Shaw said clinicians often diagnose problems other than the covered cancers — new cases of atrial fibrillation, diabetes, and hypertension. “We see a ton of prostate and skin cancer” but don’t make patients eligible for the compensation program because they’re not covered, she said.
Even as compensation is on hold, doctors can get the word out that screenings are still available, Shaw said. “We continue to get contacted by individuals who in these communities who have never heard of this program, even though we’ve been holding clinics since 2005,” Shaw said. “Despite outreach activities and advertising through newspapers and radio, we find the most successful method of reaching these patients is through word of mouth — either from other patients or their doctors. That is why we feel it is so important to reach other physicians as well.”
Affected Patients Don’t Just Live in the West
On the outreach front, clinicians in states outside of the western US region can be helpful, too. Shaw urged oncologists nationwide to ask older patients where they lived in the 1950s and 1960s. “Did they live in Nevada, Arizona, Utah, and other Western states that are downwind? They may qualify for needed services and future compensation.”
With regard to compensation, she noted that applicants need to prove that they lived in affected areas many decades ago. And, of course, they must prove that they’ve had cancer. Locating residency records “has often been an enormous challenge.” Old utility bills, pay stubs, and high school annuals can be helpful, “but these records tend to disappear. People and their families throw stuff away.”
Even proving a cancer diagnosis can be a challenge because records can be missing. In Nevada, the law says clinicians only need to keep medical records for 5 years, Shaw said. “Imaging and pathology reports are destroyed. Patients that have been diagnosed with cancer can’t prove it.”
Shaw said she hopes oncologists will offer these messages to patients: “Be an advocate for your own health and keep copies of your own records. Discuss your diagnosis with your family and contact a cancer registry if you are diagnosed with cancer.”
A version of this article appeared on Medscape.com.
Increase in Troublesome Fungal Infections Requires All-Out Approach
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
How Old Are You? Stand on One Leg and I’ll Tell You
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.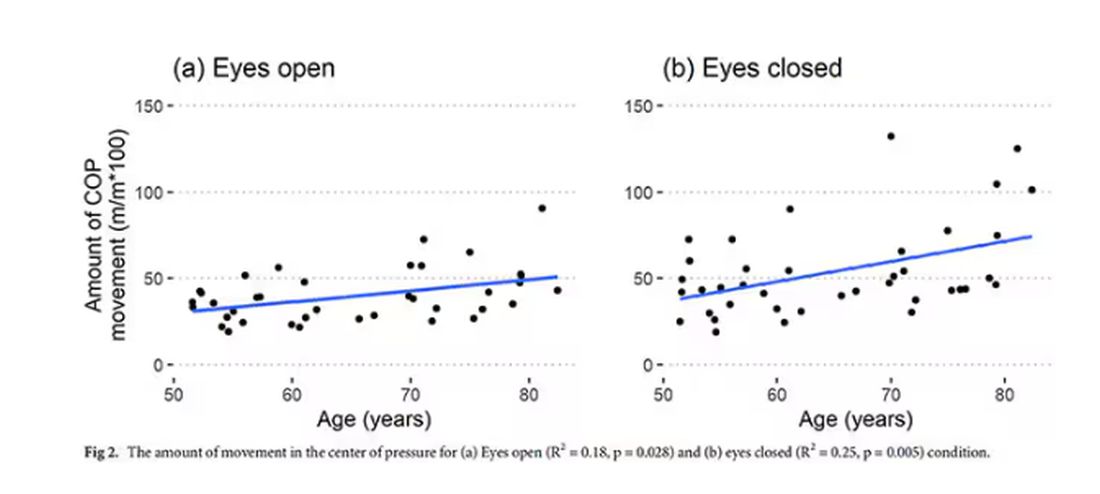
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
So I was lying in bed the other night, trying to read my phone, and started complaining to my wife about how my vision keeps getting worse, and then how stiff I feel when I wake up in the morning, and how a recent injury is taking too long to heal, and she said, “Well, yeah. You’re 44. That’s when things start to head downhill.”
And I was like, “Forty-four? That seems very specific. I thought 50 was what people complain about.” And she said, “No, it’s a thing — 44 years old and 60 years old. There’s a drop-off there.”
And you know what? She was right.
A study, “Nonlinear dynamics of multi-omics profiles during human aging,” published in Nature Aging in August 2024, analyzed a ton of proteins and metabolites in people of various ages and found, when you put it all together, that I should know better than to doubt my brilliant spouse.
But deep down, I believe the cliché that age is just a number. I don’t particularly care about being 44, or turning 50 or 60. I care about how my body and brain are aging. If I can be a happy, healthy, 80-year-old in full command of my faculties, I would consider that a major win no matter what the calendar says.
So I’m always interested in ways to quantify how my body is aging, independent of how many birthdays I have passed. And, according to a new study, there’s actually a really easy way to do this: Just stand on one leg.
The surprising results come from “Age-related changes in gait, balance, and strength parameters: A cross-sectional study,” appearing in PLOS One, which analyzed 40 individuals — half under age 65 and half over age 65 — across a variety of domains of strength, balance, and gait. The conceit of the study? We all know that things like strength and balance worsen over time, but what worsens fastest? What might be the best metric to tell us how our bodies are aging?
To that end, you have a variety of correlations between various metrics and calendar age.
As age increases, grip strength goes down. Men (inexplicably in pink) have higher grip strength overall, and women (confusingly in blue) lower. Somewhat less strong correlations were seen for knee strength.
What about balance?
To assess this, the researchers had the participants stand on a pressure plate. In one scenario, they did this with eyes open, and the next with eyes closed. They then measured how much the pressure varied around the center of the individual on the plate — basically, how much the person swayed while they were standing there.
Sway increased as age increased. Sway increased a bit more with eyes closed than with eyes open.
But the strongest correlation between any of these metrics and age was a simple one: How long can you stand on one leg?
Particularly for the nondominant leg, what you see here is a pretty dramatic drop-off in balance time around age 65, with younger people able to do 10 seconds with ease and some older people barely being able to make it to 2.
Of course, I had to try this for myself. And as I was standing around on one leg, it became clear to me exactly why this might be a good metric. It really integrates balance and strength in a way that the other tests don’t: balance, clearly, since you have to stay vertical over a relatively small base; but strength as well, because, well, one leg is holding up all the rest of you. You do feel it after a while.
So this metric passes the smell test to me, at least as a potential proxy for age-related physical decline.
But I should be careful to note that this was a cross-sectional study; the researchers looked at various people who were all different ages, not the same people over time to watch how these things change as they aged.
Also, the use of the correlation coefficient in graphs like this implies a certain linear relationship between age and standing-on-one-foot time. The raw data — the points on this graph — don’t appear that linear to me. As I mentioned above, it seems like there might be a bit of a sharp drop-off somewhere in the mid-60s. That means that we may not be able to use this as a sensitive test for aging that slowly changes as your body gets older. It might be that you’re able to essentially stand on one leg as long as you want until, one day, you can’t. That gives us less warning and less to act on.
And finally, we don’t know that changing this metric will change your health for the better. I’m sure a good physiatrist or physical therapist could design some exercises to increase any of our standing-on-one leg times. And no doubt, with practice, you could get your numbers way up. But that doesn’t necessarily mean you’re healthier. It’s like “teaching to the test”; you might score better on the standardized exam but you didn’t really learn the material.
So I am not adding one-leg standing to my daily exercise routine. But I won’t lie and tell you that, from time to time, and certainly on my 60th birthday, you may find me standing like a flamingo with a stopwatch in my hand.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
A Dermatologist’s Tips for Supporting LGBTQ Youth
HUNTINGTON BEACH, CALIFORNIA —
“Sometimes in dermatology we might say, ‘This gender care stuff, that’s really for pediatricians and primary care doctors,’ ” Boos, a pediatric dermatologist at Seattle Children’s Hospital, Seattle, said at the annual meeting of the Pacific Dermatologic Association. However, he added, “gender-affirming care happens not only with medications but with communication, curiosity, and respect.” For instance, an LGBTQ patient who is being treated with isotretinoin for acne is seen once a month by a dermatologist, which is probably more frequent than seeing their primary care physician, he said. “Every time you see that child, you can make them feel seen. You can respect them. You can let them know that you care about them. Hopefully then they understand what it feels like to get good care from a provider and then will not settle for poor care from someone else.”
According to Gallup polling, the proportion of people in the United States who identify as non-cisgender or nonheterosexual increased from 3.5% in 2012 to 7% in 2021. “The estimation is that 2.5%-3.5% of all teenagers identify as gay or bisexual, and another 1% identify as transgender, though some studies estimate the percentage of gender diverse youth to be as high as 9.2%,” said Boos.
He discussed several barriers to dermatologic care for LGBTQ youth, including availability. “There are only about 400 practicing pediatric dermatologists in the US, so there’s not a lot of pediatric dermatology care to go around for any child,” Boos said. “My plea to general dermatologists who see adolescents and teenagers: You can care for LGBTQ adolescents; they need your help.”
Accessibility is also an issue. For example, his clinic is in a wealthy and somewhat isolated area of Seattle, “which makes it hard for some patients to access our services because they may have to drive from far away or take multiple modes of public transportation to see us,” explained Boos, who came out as gay about 10 years ago after beginning his practice in Seattle. “Time matters, too. Children are in school. They don’t necessarily want to take time off to go to the doctor’s office. We want to make sure we have services at different times of day, including evenings or weekends if possible.”
Another potential barrier to care for this patient population is acceptability. “I can say that I welcome any patient to my practice, but if I’m not humble and informed about their concerns, especially queer or trans kids, if they feel that I’m not respecting them, that’s going to be a huge problem,” Boos said. “They won’t view that care as acceptable, and they’re not going to come back if they feel like I’m not looking out for their best interests.”
In a large cross-sectional study of patients with chronic inflammatory skin diseases published in 2023, sexual and gender minority (SGM) individuals were significantly more likely than non-SGM individuals to delay specialist care including dermatologic care (adjusted odds ratio [AOR], 1.23), mental health care (AOR, 1.62), and filing a prescription (AOR, 1.30) because of cost. The barriers for SGM patients were transportation issues, not having a healthcare practitioner (HCP) from the same racial or ethnic background, “and they were more likely to report not always being treated with respect by HCPs,” said Boos, who was not involved with the study. “SGM patients of minoritized racial identities such as Black, Hispanic, and Latino were also more likely to experience barriers to care.”
Boos offered several tips for improving the dermatologic care of LGBTQ youth:
Use inclusive language and follow your patient’s lead. “There are many ways that people identify, both with respect to their sexual orientation and their gender identity,” he said. “We often think that a person is either gay or straight, or cisgender or transgender. There are many folks who reject these binaries and may view their gender identity or sexual orientation outside of these descriptors. You can be bisexual. You can be asexual.” He also emphasized that sexual orientation is different from sexual behavior.
Be deliberate about your phrasing. Boos said he strives to make new patients feel comfortable by asking them such questions as what pronouns they use, how he should address them, and whether they have a partner or are in a relationship. “Then, in general, just follow your patient’s lead,” Boos said. “If they’re referring to their partner in a certain way or to themselves with certain pronouns, go along with it. When in doubt, just ask. And if you make a mistake like using the wrong pronouns or name of a patient, the best thing to do is immediately apologize and try your best not to repeat that error.”
When asking about sexual practices, don’t make assumptions. Boos recommends a 2019 article on dermatologic care of LGBT persons, published in the Journal of the American Academy of Dermatology, which includes specific examples of how to elicit a sexual history from adults and teens. One of the recommendations is “to be very direct, say, ‘This may feel uncomfortable, but I have to ask you these direct questions about what you’re doing sexually because I need to understand if you’re at risk for things like sexually transmitted infections,’ ” Boos said. “It’s also important to use terminology that our patients know. If I ask someone if they’ve had sex before, they usually understand that as penile-vaginal intercourse, but it’s also important to understand if they have oral or anal sex. But if you ask, ‘Have you had insertive anal sex?’ they may not know what that means as opposed to receptive anal sex. Instead, you might ask, ‘Are you a top or a bottom?’ which are more commonly used and understood terms in the queer community. It may feel really uncomfortable to use that kind of language, but we want to make sure patients understand what we’re asking them so we can take the best possible care of them.”
Pay attention to the details. One way to demonstrate inclusivity in your practice includes collecting pronoun and sexual orientation information for the electronic medical record so your entire staff can use proper pronouns for the patient. “Also, acknowledge that for queer folks, family can mean more than just biological family,” Boos added. “I do not buy into the stereotype that all queer kids are ostracized from their families and not loved by their families, but it is true that they are at risk for those experiences. So, sometimes a member of the patient’s ‘chosen family’ accompanies them on their visit.”
Privacy is also key. “You never know who else is in the room when you’re on a telehealth call, so you need to address that before you ask about personal things,” Boos said. “One sticking point that can also come up is that parents often fill out their child’s patient demographic form, which may not tell the real story. I typically start to have confidential time without parents and may take a sexual history as early as 12 or 13 years of age if it’s a patient that I’m seeing for an extended period or if I’m worried about a skin finding that might suggest an STI.”
He highlighted the unique opportunity dermatologists have to transform the healthcare landscape for LGBTQ children and adolescents. “It’s about extending yourself to nurture the growth of another person,” Boos said. “This can feel challenging, but you want to see each person for who they are and help get them to where they want to go. That’s what we went into medicine for, right? We want to care about people.”
Boos had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA —
“Sometimes in dermatology we might say, ‘This gender care stuff, that’s really for pediatricians and primary care doctors,’ ” Boos, a pediatric dermatologist at Seattle Children’s Hospital, Seattle, said at the annual meeting of the Pacific Dermatologic Association. However, he added, “gender-affirming care happens not only with medications but with communication, curiosity, and respect.” For instance, an LGBTQ patient who is being treated with isotretinoin for acne is seen once a month by a dermatologist, which is probably more frequent than seeing their primary care physician, he said. “Every time you see that child, you can make them feel seen. You can respect them. You can let them know that you care about them. Hopefully then they understand what it feels like to get good care from a provider and then will not settle for poor care from someone else.”
According to Gallup polling, the proportion of people in the United States who identify as non-cisgender or nonheterosexual increased from 3.5% in 2012 to 7% in 2021. “The estimation is that 2.5%-3.5% of all teenagers identify as gay or bisexual, and another 1% identify as transgender, though some studies estimate the percentage of gender diverse youth to be as high as 9.2%,” said Boos.
He discussed several barriers to dermatologic care for LGBTQ youth, including availability. “There are only about 400 practicing pediatric dermatologists in the US, so there’s not a lot of pediatric dermatology care to go around for any child,” Boos said. “My plea to general dermatologists who see adolescents and teenagers: You can care for LGBTQ adolescents; they need your help.”
Accessibility is also an issue. For example, his clinic is in a wealthy and somewhat isolated area of Seattle, “which makes it hard for some patients to access our services because they may have to drive from far away or take multiple modes of public transportation to see us,” explained Boos, who came out as gay about 10 years ago after beginning his practice in Seattle. “Time matters, too. Children are in school. They don’t necessarily want to take time off to go to the doctor’s office. We want to make sure we have services at different times of day, including evenings or weekends if possible.”
Another potential barrier to care for this patient population is acceptability. “I can say that I welcome any patient to my practice, but if I’m not humble and informed about their concerns, especially queer or trans kids, if they feel that I’m not respecting them, that’s going to be a huge problem,” Boos said. “They won’t view that care as acceptable, and they’re not going to come back if they feel like I’m not looking out for their best interests.”
In a large cross-sectional study of patients with chronic inflammatory skin diseases published in 2023, sexual and gender minority (SGM) individuals were significantly more likely than non-SGM individuals to delay specialist care including dermatologic care (adjusted odds ratio [AOR], 1.23), mental health care (AOR, 1.62), and filing a prescription (AOR, 1.30) because of cost. The barriers for SGM patients were transportation issues, not having a healthcare practitioner (HCP) from the same racial or ethnic background, “and they were more likely to report not always being treated with respect by HCPs,” said Boos, who was not involved with the study. “SGM patients of minoritized racial identities such as Black, Hispanic, and Latino were also more likely to experience barriers to care.”
Boos offered several tips for improving the dermatologic care of LGBTQ youth:
Use inclusive language and follow your patient’s lead. “There are many ways that people identify, both with respect to their sexual orientation and their gender identity,” he said. “We often think that a person is either gay or straight, or cisgender or transgender. There are many folks who reject these binaries and may view their gender identity or sexual orientation outside of these descriptors. You can be bisexual. You can be asexual.” He also emphasized that sexual orientation is different from sexual behavior.
Be deliberate about your phrasing. Boos said he strives to make new patients feel comfortable by asking them such questions as what pronouns they use, how he should address them, and whether they have a partner or are in a relationship. “Then, in general, just follow your patient’s lead,” Boos said. “If they’re referring to their partner in a certain way or to themselves with certain pronouns, go along with it. When in doubt, just ask. And if you make a mistake like using the wrong pronouns or name of a patient, the best thing to do is immediately apologize and try your best not to repeat that error.”
When asking about sexual practices, don’t make assumptions. Boos recommends a 2019 article on dermatologic care of LGBT persons, published in the Journal of the American Academy of Dermatology, which includes specific examples of how to elicit a sexual history from adults and teens. One of the recommendations is “to be very direct, say, ‘This may feel uncomfortable, but I have to ask you these direct questions about what you’re doing sexually because I need to understand if you’re at risk for things like sexually transmitted infections,’ ” Boos said. “It’s also important to use terminology that our patients know. If I ask someone if they’ve had sex before, they usually understand that as penile-vaginal intercourse, but it’s also important to understand if they have oral or anal sex. But if you ask, ‘Have you had insertive anal sex?’ they may not know what that means as opposed to receptive anal sex. Instead, you might ask, ‘Are you a top or a bottom?’ which are more commonly used and understood terms in the queer community. It may feel really uncomfortable to use that kind of language, but we want to make sure patients understand what we’re asking them so we can take the best possible care of them.”
Pay attention to the details. One way to demonstrate inclusivity in your practice includes collecting pronoun and sexual orientation information for the electronic medical record so your entire staff can use proper pronouns for the patient. “Also, acknowledge that for queer folks, family can mean more than just biological family,” Boos added. “I do not buy into the stereotype that all queer kids are ostracized from their families and not loved by their families, but it is true that they are at risk for those experiences. So, sometimes a member of the patient’s ‘chosen family’ accompanies them on their visit.”
Privacy is also key. “You never know who else is in the room when you’re on a telehealth call, so you need to address that before you ask about personal things,” Boos said. “One sticking point that can also come up is that parents often fill out their child’s patient demographic form, which may not tell the real story. I typically start to have confidential time without parents and may take a sexual history as early as 12 or 13 years of age if it’s a patient that I’m seeing for an extended period or if I’m worried about a skin finding that might suggest an STI.”
He highlighted the unique opportunity dermatologists have to transform the healthcare landscape for LGBTQ children and adolescents. “It’s about extending yourself to nurture the growth of another person,” Boos said. “This can feel challenging, but you want to see each person for who they are and help get them to where they want to go. That’s what we went into medicine for, right? We want to care about people.”
Boos had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA —
“Sometimes in dermatology we might say, ‘This gender care stuff, that’s really for pediatricians and primary care doctors,’ ” Boos, a pediatric dermatologist at Seattle Children’s Hospital, Seattle, said at the annual meeting of the Pacific Dermatologic Association. However, he added, “gender-affirming care happens not only with medications but with communication, curiosity, and respect.” For instance, an LGBTQ patient who is being treated with isotretinoin for acne is seen once a month by a dermatologist, which is probably more frequent than seeing their primary care physician, he said. “Every time you see that child, you can make them feel seen. You can respect them. You can let them know that you care about them. Hopefully then they understand what it feels like to get good care from a provider and then will not settle for poor care from someone else.”
According to Gallup polling, the proportion of people in the United States who identify as non-cisgender or nonheterosexual increased from 3.5% in 2012 to 7% in 2021. “The estimation is that 2.5%-3.5% of all teenagers identify as gay or bisexual, and another 1% identify as transgender, though some studies estimate the percentage of gender diverse youth to be as high as 9.2%,” said Boos.
He discussed several barriers to dermatologic care for LGBTQ youth, including availability. “There are only about 400 practicing pediatric dermatologists in the US, so there’s not a lot of pediatric dermatology care to go around for any child,” Boos said. “My plea to general dermatologists who see adolescents and teenagers: You can care for LGBTQ adolescents; they need your help.”
Accessibility is also an issue. For example, his clinic is in a wealthy and somewhat isolated area of Seattle, “which makes it hard for some patients to access our services because they may have to drive from far away or take multiple modes of public transportation to see us,” explained Boos, who came out as gay about 10 years ago after beginning his practice in Seattle. “Time matters, too. Children are in school. They don’t necessarily want to take time off to go to the doctor’s office. We want to make sure we have services at different times of day, including evenings or weekends if possible.”
Another potential barrier to care for this patient population is acceptability. “I can say that I welcome any patient to my practice, but if I’m not humble and informed about their concerns, especially queer or trans kids, if they feel that I’m not respecting them, that’s going to be a huge problem,” Boos said. “They won’t view that care as acceptable, and they’re not going to come back if they feel like I’m not looking out for their best interests.”
In a large cross-sectional study of patients with chronic inflammatory skin diseases published in 2023, sexual and gender minority (SGM) individuals were significantly more likely than non-SGM individuals to delay specialist care including dermatologic care (adjusted odds ratio [AOR], 1.23), mental health care (AOR, 1.62), and filing a prescription (AOR, 1.30) because of cost. The barriers for SGM patients were transportation issues, not having a healthcare practitioner (HCP) from the same racial or ethnic background, “and they were more likely to report not always being treated with respect by HCPs,” said Boos, who was not involved with the study. “SGM patients of minoritized racial identities such as Black, Hispanic, and Latino were also more likely to experience barriers to care.”
Boos offered several tips for improving the dermatologic care of LGBTQ youth:
Use inclusive language and follow your patient’s lead. “There are many ways that people identify, both with respect to their sexual orientation and their gender identity,” he said. “We often think that a person is either gay or straight, or cisgender or transgender. There are many folks who reject these binaries and may view their gender identity or sexual orientation outside of these descriptors. You can be bisexual. You can be asexual.” He also emphasized that sexual orientation is different from sexual behavior.
Be deliberate about your phrasing. Boos said he strives to make new patients feel comfortable by asking them such questions as what pronouns they use, how he should address them, and whether they have a partner or are in a relationship. “Then, in general, just follow your patient’s lead,” Boos said. “If they’re referring to their partner in a certain way or to themselves with certain pronouns, go along with it. When in doubt, just ask. And if you make a mistake like using the wrong pronouns or name of a patient, the best thing to do is immediately apologize and try your best not to repeat that error.”
When asking about sexual practices, don’t make assumptions. Boos recommends a 2019 article on dermatologic care of LGBT persons, published in the Journal of the American Academy of Dermatology, which includes specific examples of how to elicit a sexual history from adults and teens. One of the recommendations is “to be very direct, say, ‘This may feel uncomfortable, but I have to ask you these direct questions about what you’re doing sexually because I need to understand if you’re at risk for things like sexually transmitted infections,’ ” Boos said. “It’s also important to use terminology that our patients know. If I ask someone if they’ve had sex before, they usually understand that as penile-vaginal intercourse, but it’s also important to understand if they have oral or anal sex. But if you ask, ‘Have you had insertive anal sex?’ they may not know what that means as opposed to receptive anal sex. Instead, you might ask, ‘Are you a top or a bottom?’ which are more commonly used and understood terms in the queer community. It may feel really uncomfortable to use that kind of language, but we want to make sure patients understand what we’re asking them so we can take the best possible care of them.”
Pay attention to the details. One way to demonstrate inclusivity in your practice includes collecting pronoun and sexual orientation information for the electronic medical record so your entire staff can use proper pronouns for the patient. “Also, acknowledge that for queer folks, family can mean more than just biological family,” Boos added. “I do not buy into the stereotype that all queer kids are ostracized from their families and not loved by their families, but it is true that they are at risk for those experiences. So, sometimes a member of the patient’s ‘chosen family’ accompanies them on their visit.”
Privacy is also key. “You never know who else is in the room when you’re on a telehealth call, so you need to address that before you ask about personal things,” Boos said. “One sticking point that can also come up is that parents often fill out their child’s patient demographic form, which may not tell the real story. I typically start to have confidential time without parents and may take a sexual history as early as 12 or 13 years of age if it’s a patient that I’m seeing for an extended period or if I’m worried about a skin finding that might suggest an STI.”
He highlighted the unique opportunity dermatologists have to transform the healthcare landscape for LGBTQ children and adolescents. “It’s about extending yourself to nurture the growth of another person,” Boos said. “This can feel challenging, but you want to see each person for who they are and help get them to where they want to go. That’s what we went into medicine for, right? We want to care about people.”
Boos had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM PDA 2024
H pylori: ACG Guideline Advises New Approaches to Treatment
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Helicobacter pylori is one of the most common human bacterial chronic infections globally. Its prevalence has actually decreased in North America in recent years, although its current range of approximately 30%-40% remains substantial given the potential clinical implications of infection.
Standards have changed considerably regarding the testing, treatment, and follow-up of H pylori. This is made clear by the just-published clinical practice guideline from the American College of Gastroenterology (ACG), which provides several new recommendations based on recent scientific evidence that should change your clinical approach to managing this common infection.
This discussion aims to synthesize and highlight key concepts from the ACG’s comprehensive publication.
Who Should Be Tested and Treated?
The cardinal diseases caused by H pylori have traditionally included peptic ulcer disease, marginal zone B-cell lymphoma, gastric adenocarcinoma, and dyspepsia.
Additional associations have been made with idiopathic thrombocytopenic purpura and otherwise unexplained iron deficiency.
New evidence suggests that patients taking long-term nonsteroidal anti-inflammatory drugs, including low-dose aspirin, are relatively more susceptible to infection.
The ACG’s guideline also recommends testing persons at an increased risk for gastric adenocarcinoma (eg, those with autoimmune gastritis, current or history of premalignant conditions, or first-degree relative with gastric cancer), as well as household members of patients with a positive nonserologic test for H pylori.
The authors note that those with an indication for testing should be offered treatment if determined to have an infection. These patients should also undergo a posttreatment test-of-cure, which should occur at least 4 weeks afterwards using a urea breath test, fecal antigen test, or gastric biopsy.
Caveats to Treatment
Patients with H pylori infections are advised to undergo treatment for a duration of 14 days. Some of the commercial prepackaged H pylori treatment options (eg, Pylera, which contains bismuth subcitrate/metronidazole/tetracycline) are dispensed in regimens lasting only 10 days and currently are viewed as inadequate.
In the United States, the patterns of antibiotic resistance for the previously used standard drugs in the treatment of H pylori have increased considerably. They range from 32% for clarithromycin, 38% for levofloxacin, and 42% for metronidazole, in contrast to 3% for amoxicillin, 1% for tetracycline, and 0% for rifabutin.
Clarithromycin- and levofloxacin-containing treatments should be avoided in treatment-naive patients unless specifically directed following the results of susceptibility tests with either a phenotypic method (culture-based) or a molecular method (polymerase chain reaction or next-generation sequencing). Notably, the mutations responsible for both clarithromycin and levofloxacin resistance may be detectable by stool-based testing.
Maintenance of intragastric acid suppression is key to H pylori eradication, as elevated intragastric pH promotes active replication of H pylori and makes it more susceptible to bactericidal antibiotics.
Therefore, the use of histamine-2 receptors is not recommended, as they are inadequate for achieving acid suppression. Instead, a dual-based therapy of either the potassium-competitive acid blocker (PCAB) vonoprazan (20 mg) or a high-dose proton pump inhibitor (PPI) and amoxicillin, administered twice daily, is effective, although this finding is based on limited evidence.
Treatment-Naive Patients
In treatment-naive patients without penicillin allergy and for whom antibiotic susceptibility testing has not been obtained, the guideline offers its strongest recommendation for bismuth quadruple therapy. This therapy typically consists of a PPI, bismuth subcitrate or subsalicylate, tetracycline, and metronidazole.
Among those with a penicillin allergy, bismuth quadruple therapy is also the primary treatment choice. The authors suggest that patients with a suspected allergy are referred to an allergist for possible penicillin desensitization, given that less than 1% of the population is thought to present with a “true” allergy.
The guideline also presented conditional recommendations, based on low- to moderate-quality evidence, for using a rifabutin-based triple regimen of omeprazole, amoxicillin, and rifabutin (Talicia); a PCAB-based dual regimen of vonoprazan and amoxicillin (Voquezna Dual Pak); and a PCAB-based triple regimen of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak). In patients with unknown clarithromycin susceptibility, the PCAB-based triple therapy is preferred over PPI-clarithromycin triple therapy.
Although probiotics have been suggested to possibly lead to increased effectiveness or tolerability for H pylori eradication, this was based on studies with significant heterogeneity in their designs. At present, no high-quality data support probiotic therapy.
Clinicians may substitute doxycycline for tetracycline due to availability or cost, and also may prescribe metronidazole at a lower dose than recommended (1.5-2 g/d) to limit side effects. Both modifications have been associated with lower rates of H pylori eradication and are not recommended.
Treatment-Experienced Patients
Quadruple bismuth therapy is the optimal approach among treatment-experienced patients with persistent H pylori infection who have not previously received this therapy. However, this recommendation was rated as conditional, given that it was based on a low quality of evidence.
The guideline offered other recommendations for treatment-experienced patients with persistent infection who had received bismuth quadruple therapy — also conditionally based on a low quality of evidence.
In such patients, it is recommended to consider the use of a rifabutin-based triple therapy (ie, a PPI standard to double dose, amoxicillin, and rifabutin) and a levofloxacin-based triple therapy (ie, a PPI standard dose, levofloxacin, and amoxicillin or metronidazole).
Although significant evidence gaps prevented the authors from providing formal recommendations, they included a PCAB-based triple therapy of vonoprazan, clarithromycin, and amoxicillin (Voquezna Triple Pak) and a high-dose dual therapy of either vonoprazan (20 mg) or PPI (double dose) and amoxicillin among their suggested salvage regimens for these patients.
A New Standard
We must recognize, however, that there are still substantial evidence gaps, particularly around the use of a PCAB-based regimen and its relative advantages over a standard or high-dose PPI-based regimen. This may be of particular importance based on the variable prevalence of cytochrome P450 2C19 (CYP2C19) polymorphisms in the specific patient populations, as PCABs are not metabolized by CYP2C19.
Reviewing the entirety of the ACG’s clinical guideline is encouraged for additional details about the management of H pylori beyond what is highlighted herein.
Dr. Johnson, Professor of Medicine, Chief of Gastroenterology, Eastern Virginia Medical School, Norfolk, Virginia, disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.