User login
Brain imaging markers of breathlessness-expectation predict COPD rehabilitation success
In an experimental medicine study of D-cycloserine given during chronic obstructive pulmonary disease (COPD) rehabilitation, only models including brain imaging markers of breathlessness-expectation successfully predicted Dyspnea-12 score improvement. D-cycloserine was independently associated with breathlessness improvement, according to original research published in Thorax.
Chronic breathlessness persisting despite maximal medical therapy is a key feature of COPD. While pulmonary rehabilitation is the best treatment for chronic breathlessness in COPD, responses to treatment are variable, with 30% deriving no clinical benefit, Sarah L. Finnegan, PhD, with the Nuffield Department of Clinical Neurosciences, University of Oxford (England), and colleagues wrote.
While recent research has shown fear and anxiety to be key components of the expectation that plays an important role in the mechanisms and maintenance of breathlessness, expectation-related effects have not previously been considered in prediction studies of pulmonary rehabilitation outcomes. The authors’ prior research showed a clear correlation between improvements in breathlessness through pulmonary rehabilitation and expectation-related brain activity in areas that include the anterior insula, anterior cingulate cortex, and prefrontal cortex. That research methodology, however, did not attempt to predict individual responses.
The current study focused on brain activity changes within preselected regions associated with breathlessness-expectation and body and symptom perception. Its purpose was to predict improvements in breathlessness during pulmonary rehabilitation by analyzing baseline data from a longitudinal experimental medicine study of D-cycloserine on breathlessness during pulmonary rehabilitation. D-cycloserine, a partial agonist of brain N-methyl-D-aspartate receptors, was chosen because of its effects on neural plasticity and influence on brain expectation mechanisms associated with cognitive behavioral therapies. The authors hypothesized that baseline brain activity in response to breathlessness-related expectation would predict improvement in breathlessness through pulmonary rehabilitation, with D-cycloserine emerging as a significant factor in the prediction model.
The researchers recruited 71 participants (18 women, median age 71 years [46-85 years]) with mild to moderate COPD immediately prior to enrollment in a National Health Service–prescribed course of pulmonary rehabilitation. They were randomized double-blind to receive either 250 mg oral D-cycloserine or a matched placebo. Participants received a single dose on four occasions 30 minutes prior to the onset of the first four pulmonary rehabilitation sessions.
Baseline variables, including brain-activity, self-report questionnaires responses, clinical measures of respiratory function, and drug allocation were used to train three machine-learning models to predict the outcome, a minimally clinically relevant change in the Dyspnea-12 score.
Improvements in Dyspnea-12 score occurred only in the two models including brain imaging markers of breathlessness-expectation (sensitivity 0.88, specificity 0.77). The model that combined brain and behavior metrics produced the best classification performance (accuracy, 0.83 [95% confidence interval, 0.75-0.90]; sensitivity, 0.88; specificity, 0.77; P < 0.001). While the brain-only model was able to correctly categorize participants with statistically significant likelihood (accuracy, 0.70 [95% CI, 0.58-0.81]), it demonstrated poor goodness of fit, a measure of how well sample data fit a distribution from a population with a normal distribution. “By enriching the brain-only models with questionnaires and physiology measures improved performance considerably,” the researchers stated.
“Our findings demonstrate the first predictive model of change in breathlessness across pulmonary rehabilitation and, for the first time, the clinical relevance of expectation-related brain activity as a therapeutic target in the treatment of breathlessness. ... This was achieved using sensitive brain imaging techniques in order to capture personalized responses to breathlessness-expectation which has, until recently remained relatively unexplored.”
“This study raises interesting questions about breathlessness-expectations,” commented assistant professor of medicine Mary Jo S. Farmer, MD, PhD, director pulmonary hypertension service, University of Massachusetts, Worcester, in an interview. “There is much more to be understood about expectations pathways as to how these pathways are built upon prior experience and pave the way for reaction to future experiences. There is need for a similar study with larger sample size and clarification of the role of the effect of the agent D-cycloserine on breathlessness-expectation.”
The researchers noted their study’s limitations, pointing out that the small sample size precluded holding out a proportion of the original data to create an external validation dataset.
Dr. Finnegan and Dr. Farmer declared no disclosures relevant to this study. This work was supported by the JABBS Foundation and Dunhill Medical Trust. This research was funded in whole, or in part, by the Wellcome Trust.
In an experimental medicine study of D-cycloserine given during chronic obstructive pulmonary disease (COPD) rehabilitation, only models including brain imaging markers of breathlessness-expectation successfully predicted Dyspnea-12 score improvement. D-cycloserine was independently associated with breathlessness improvement, according to original research published in Thorax.
Chronic breathlessness persisting despite maximal medical therapy is a key feature of COPD. While pulmonary rehabilitation is the best treatment for chronic breathlessness in COPD, responses to treatment are variable, with 30% deriving no clinical benefit, Sarah L. Finnegan, PhD, with the Nuffield Department of Clinical Neurosciences, University of Oxford (England), and colleagues wrote.
While recent research has shown fear and anxiety to be key components of the expectation that plays an important role in the mechanisms and maintenance of breathlessness, expectation-related effects have not previously been considered in prediction studies of pulmonary rehabilitation outcomes. The authors’ prior research showed a clear correlation between improvements in breathlessness through pulmonary rehabilitation and expectation-related brain activity in areas that include the anterior insula, anterior cingulate cortex, and prefrontal cortex. That research methodology, however, did not attempt to predict individual responses.
The current study focused on brain activity changes within preselected regions associated with breathlessness-expectation and body and symptom perception. Its purpose was to predict improvements in breathlessness during pulmonary rehabilitation by analyzing baseline data from a longitudinal experimental medicine study of D-cycloserine on breathlessness during pulmonary rehabilitation. D-cycloserine, a partial agonist of brain N-methyl-D-aspartate receptors, was chosen because of its effects on neural plasticity and influence on brain expectation mechanisms associated with cognitive behavioral therapies. The authors hypothesized that baseline brain activity in response to breathlessness-related expectation would predict improvement in breathlessness through pulmonary rehabilitation, with D-cycloserine emerging as a significant factor in the prediction model.
The researchers recruited 71 participants (18 women, median age 71 years [46-85 years]) with mild to moderate COPD immediately prior to enrollment in a National Health Service–prescribed course of pulmonary rehabilitation. They were randomized double-blind to receive either 250 mg oral D-cycloserine or a matched placebo. Participants received a single dose on four occasions 30 minutes prior to the onset of the first four pulmonary rehabilitation sessions.
Baseline variables, including brain-activity, self-report questionnaires responses, clinical measures of respiratory function, and drug allocation were used to train three machine-learning models to predict the outcome, a minimally clinically relevant change in the Dyspnea-12 score.
Improvements in Dyspnea-12 score occurred only in the two models including brain imaging markers of breathlessness-expectation (sensitivity 0.88, specificity 0.77). The model that combined brain and behavior metrics produced the best classification performance (accuracy, 0.83 [95% confidence interval, 0.75-0.90]; sensitivity, 0.88; specificity, 0.77; P < 0.001). While the brain-only model was able to correctly categorize participants with statistically significant likelihood (accuracy, 0.70 [95% CI, 0.58-0.81]), it demonstrated poor goodness of fit, a measure of how well sample data fit a distribution from a population with a normal distribution. “By enriching the brain-only models with questionnaires and physiology measures improved performance considerably,” the researchers stated.
“Our findings demonstrate the first predictive model of change in breathlessness across pulmonary rehabilitation and, for the first time, the clinical relevance of expectation-related brain activity as a therapeutic target in the treatment of breathlessness. ... This was achieved using sensitive brain imaging techniques in order to capture personalized responses to breathlessness-expectation which has, until recently remained relatively unexplored.”
“This study raises interesting questions about breathlessness-expectations,” commented assistant professor of medicine Mary Jo S. Farmer, MD, PhD, director pulmonary hypertension service, University of Massachusetts, Worcester, in an interview. “There is much more to be understood about expectations pathways as to how these pathways are built upon prior experience and pave the way for reaction to future experiences. There is need for a similar study with larger sample size and clarification of the role of the effect of the agent D-cycloserine on breathlessness-expectation.”
The researchers noted their study’s limitations, pointing out that the small sample size precluded holding out a proportion of the original data to create an external validation dataset.
Dr. Finnegan and Dr. Farmer declared no disclosures relevant to this study. This work was supported by the JABBS Foundation and Dunhill Medical Trust. This research was funded in whole, or in part, by the Wellcome Trust.
In an experimental medicine study of D-cycloserine given during chronic obstructive pulmonary disease (COPD) rehabilitation, only models including brain imaging markers of breathlessness-expectation successfully predicted Dyspnea-12 score improvement. D-cycloserine was independently associated with breathlessness improvement, according to original research published in Thorax.
Chronic breathlessness persisting despite maximal medical therapy is a key feature of COPD. While pulmonary rehabilitation is the best treatment for chronic breathlessness in COPD, responses to treatment are variable, with 30% deriving no clinical benefit, Sarah L. Finnegan, PhD, with the Nuffield Department of Clinical Neurosciences, University of Oxford (England), and colleagues wrote.
While recent research has shown fear and anxiety to be key components of the expectation that plays an important role in the mechanisms and maintenance of breathlessness, expectation-related effects have not previously been considered in prediction studies of pulmonary rehabilitation outcomes. The authors’ prior research showed a clear correlation between improvements in breathlessness through pulmonary rehabilitation and expectation-related brain activity in areas that include the anterior insula, anterior cingulate cortex, and prefrontal cortex. That research methodology, however, did not attempt to predict individual responses.
The current study focused on brain activity changes within preselected regions associated with breathlessness-expectation and body and symptom perception. Its purpose was to predict improvements in breathlessness during pulmonary rehabilitation by analyzing baseline data from a longitudinal experimental medicine study of D-cycloserine on breathlessness during pulmonary rehabilitation. D-cycloserine, a partial agonist of brain N-methyl-D-aspartate receptors, was chosen because of its effects on neural plasticity and influence on brain expectation mechanisms associated with cognitive behavioral therapies. The authors hypothesized that baseline brain activity in response to breathlessness-related expectation would predict improvement in breathlessness through pulmonary rehabilitation, with D-cycloserine emerging as a significant factor in the prediction model.
The researchers recruited 71 participants (18 women, median age 71 years [46-85 years]) with mild to moderate COPD immediately prior to enrollment in a National Health Service–prescribed course of pulmonary rehabilitation. They were randomized double-blind to receive either 250 mg oral D-cycloserine or a matched placebo. Participants received a single dose on four occasions 30 minutes prior to the onset of the first four pulmonary rehabilitation sessions.
Baseline variables, including brain-activity, self-report questionnaires responses, clinical measures of respiratory function, and drug allocation were used to train three machine-learning models to predict the outcome, a minimally clinically relevant change in the Dyspnea-12 score.
Improvements in Dyspnea-12 score occurred only in the two models including brain imaging markers of breathlessness-expectation (sensitivity 0.88, specificity 0.77). The model that combined brain and behavior metrics produced the best classification performance (accuracy, 0.83 [95% confidence interval, 0.75-0.90]; sensitivity, 0.88; specificity, 0.77; P < 0.001). While the brain-only model was able to correctly categorize participants with statistically significant likelihood (accuracy, 0.70 [95% CI, 0.58-0.81]), it demonstrated poor goodness of fit, a measure of how well sample data fit a distribution from a population with a normal distribution. “By enriching the brain-only models with questionnaires and physiology measures improved performance considerably,” the researchers stated.
“Our findings demonstrate the first predictive model of change in breathlessness across pulmonary rehabilitation and, for the first time, the clinical relevance of expectation-related brain activity as a therapeutic target in the treatment of breathlessness. ... This was achieved using sensitive brain imaging techniques in order to capture personalized responses to breathlessness-expectation which has, until recently remained relatively unexplored.”
“This study raises interesting questions about breathlessness-expectations,” commented assistant professor of medicine Mary Jo S. Farmer, MD, PhD, director pulmonary hypertension service, University of Massachusetts, Worcester, in an interview. “There is much more to be understood about expectations pathways as to how these pathways are built upon prior experience and pave the way for reaction to future experiences. There is need for a similar study with larger sample size and clarification of the role of the effect of the agent D-cycloserine on breathlessness-expectation.”
The researchers noted their study’s limitations, pointing out that the small sample size precluded holding out a proportion of the original data to create an external validation dataset.
Dr. Finnegan and Dr. Farmer declared no disclosures relevant to this study. This work was supported by the JABBS Foundation and Dunhill Medical Trust. This research was funded in whole, or in part, by the Wellcome Trust.
FROM THORAX
Treatment of craniofacial hyperhidrosis
Hyperhidrosis has a significant impact on a person’s physical, psychological, and social aspects of life. The lack of treatment options and associated stigma limits access to care and treatment options.
Primary hyperhidrosis does not have an underlying cause; is symmetrical; can worsen with anxiety, fear, or stress; and may have a familial component. Palmar and axillary hyperhidrosis are the most common types of hyperhidrosis. The incidence of craniofacial hyperhidrosis has not been clearly defined but it is most commonly reported on the forehead, where the concentration of eccrine sweat glands is highest.
Treatment options for craniofacial hyperhidrosis include topical aluminum chloride, which blocks the eccrine sweat duct or causes eccrine cell atrophy. Although this option is a common treatment for palmar and axillary hyperhidrosis, use on the face has not been thoroughly studied, and may also cause skin irritation.
Topical and oral glycopyrrolate can be effective for all types of hyperhidrosis, but must be used daily and can have systemic side effects, with variable efficacy and longevity. Oral oxybutynin, beta-blockers, clonidine, and benzodiazepines have also been used with some limited studies available in patients with generalized hyperhidrosis.
Surgical treatments such as videothoracoscopy sympathectomy can be used in severe or recalcitrant cases of hyperhidrosis with good efficacy. However, surgical complications and inherent surgical risks limit these treatment options unless other modalities are exhausted.
OnabotulinumtoxinA is Food and Drug Administration approved for treating severe primary axillary hyperhidrosis, but is used off label for palmar, plantar, and craniofacial hyperhidrosis with great results and few side effects. Clinical pearls and guidelines for the use of botulinum toxin A in craniofacial hyperhidrosis were outlined by Wolosker and colleagues in a review article. As with any injection of neurotoxin, knowledge of the facial anatomy is critical to avoiding muscle paralysis.
double diluted. Treatment effects usually last 3 months, similar to cosmetic uses. Wolosker uses a dilution of 100 U botulinum toxin in 1.0 mL saline, which I find slightly more difficult to control and more likely to have loss of toxin.
In my experience, I have found the following dosing to be most effective with the least side effects for the following (dosages vary and can be titrated up to response):
- Upper lip: 6-10 U.
- Chin: 6-10 U.
- Forehead: 15-30 U. (Avoid 1 cm above the brow unless risks of brow drop are reviewed and acceptable to the patient. In my experience patients would rather have a lower brow than obstructive sweating in their brow that can irritate the eyes, blur vision, and smudge skincare and makeup.)
- Nose: 10 U
- Cheeks: 10 U per side (staying very superficial with injections).
- Scalp: 30-50 U (using serial injections 1-2 cm apart in the area affected by hyperhidrosis).
Side effects include temporary erythema, bruising, and edema, as well as muscle paralysis and asymmetry if proper injection technique is not used, the dose is not diluted properly, or the injection is too deep.
There are scattered case studies of symbiotic techniques to help the penetration of botulinum toxin when treating craniofacial hyperhidrosis, including microneedling, radiofrequency, long-pulsed diode laser, and ultrasound. But the safety and efficacy of these procedures have not been properly evaluated.
In all of my patients with craniofacial hyperhidrosis treated with botulinum toxin, quality of life is significantly improved with almost no complications. Botulinum toxin is a safe, relatively quick in-office procedure to treat craniofacial hyperhidrosis that can be used to help patients – particularly those who experience anxiety or have social and occupational impairment related to their disease.
This procedure is cosmetic in nature, and therefore, not covered by insurance.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Parashar K et al. Am J Clin Dermatol. 2023 Mar;24(2):187-98.
Doolittle J et al. Arch Dermatol Res. 2016 Dec;308(10):743-9.
Wolosker N et al. J Vasc Bras. 2020 Nov 16;19:e20190152.
Garcia-Souto F et al. Dermatol Ther. 2021 Jan;34(1):e14658.
Ebrahim H et al. J Clin Aesthet Dermatol. 2022 Sep;15(9):40-4.
Campanati A et al. Toxins (Basel). 2022 May 27;14(6):3727.
Hyperhidrosis has a significant impact on a person’s physical, psychological, and social aspects of life. The lack of treatment options and associated stigma limits access to care and treatment options.
Primary hyperhidrosis does not have an underlying cause; is symmetrical; can worsen with anxiety, fear, or stress; and may have a familial component. Palmar and axillary hyperhidrosis are the most common types of hyperhidrosis. The incidence of craniofacial hyperhidrosis has not been clearly defined but it is most commonly reported on the forehead, where the concentration of eccrine sweat glands is highest.
Treatment options for craniofacial hyperhidrosis include topical aluminum chloride, which blocks the eccrine sweat duct or causes eccrine cell atrophy. Although this option is a common treatment for palmar and axillary hyperhidrosis, use on the face has not been thoroughly studied, and may also cause skin irritation.
Topical and oral glycopyrrolate can be effective for all types of hyperhidrosis, but must be used daily and can have systemic side effects, with variable efficacy and longevity. Oral oxybutynin, beta-blockers, clonidine, and benzodiazepines have also been used with some limited studies available in patients with generalized hyperhidrosis.
Surgical treatments such as videothoracoscopy sympathectomy can be used in severe or recalcitrant cases of hyperhidrosis with good efficacy. However, surgical complications and inherent surgical risks limit these treatment options unless other modalities are exhausted.
OnabotulinumtoxinA is Food and Drug Administration approved for treating severe primary axillary hyperhidrosis, but is used off label for palmar, plantar, and craniofacial hyperhidrosis with great results and few side effects. Clinical pearls and guidelines for the use of botulinum toxin A in craniofacial hyperhidrosis were outlined by Wolosker and colleagues in a review article. As with any injection of neurotoxin, knowledge of the facial anatomy is critical to avoiding muscle paralysis.
double diluted. Treatment effects usually last 3 months, similar to cosmetic uses. Wolosker uses a dilution of 100 U botulinum toxin in 1.0 mL saline, which I find slightly more difficult to control and more likely to have loss of toxin.
In my experience, I have found the following dosing to be most effective with the least side effects for the following (dosages vary and can be titrated up to response):
- Upper lip: 6-10 U.
- Chin: 6-10 U.
- Forehead: 15-30 U. (Avoid 1 cm above the brow unless risks of brow drop are reviewed and acceptable to the patient. In my experience patients would rather have a lower brow than obstructive sweating in their brow that can irritate the eyes, blur vision, and smudge skincare and makeup.)
- Nose: 10 U
- Cheeks: 10 U per side (staying very superficial with injections).
- Scalp: 30-50 U (using serial injections 1-2 cm apart in the area affected by hyperhidrosis).
Side effects include temporary erythema, bruising, and edema, as well as muscle paralysis and asymmetry if proper injection technique is not used, the dose is not diluted properly, or the injection is too deep.
There are scattered case studies of symbiotic techniques to help the penetration of botulinum toxin when treating craniofacial hyperhidrosis, including microneedling, radiofrequency, long-pulsed diode laser, and ultrasound. But the safety and efficacy of these procedures have not been properly evaluated.
In all of my patients with craniofacial hyperhidrosis treated with botulinum toxin, quality of life is significantly improved with almost no complications. Botulinum toxin is a safe, relatively quick in-office procedure to treat craniofacial hyperhidrosis that can be used to help patients – particularly those who experience anxiety or have social and occupational impairment related to their disease.
This procedure is cosmetic in nature, and therefore, not covered by insurance.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Parashar K et al. Am J Clin Dermatol. 2023 Mar;24(2):187-98.
Doolittle J et al. Arch Dermatol Res. 2016 Dec;308(10):743-9.
Wolosker N et al. J Vasc Bras. 2020 Nov 16;19:e20190152.
Garcia-Souto F et al. Dermatol Ther. 2021 Jan;34(1):e14658.
Ebrahim H et al. J Clin Aesthet Dermatol. 2022 Sep;15(9):40-4.
Campanati A et al. Toxins (Basel). 2022 May 27;14(6):3727.
Hyperhidrosis has a significant impact on a person’s physical, psychological, and social aspects of life. The lack of treatment options and associated stigma limits access to care and treatment options.
Primary hyperhidrosis does not have an underlying cause; is symmetrical; can worsen with anxiety, fear, or stress; and may have a familial component. Palmar and axillary hyperhidrosis are the most common types of hyperhidrosis. The incidence of craniofacial hyperhidrosis has not been clearly defined but it is most commonly reported on the forehead, where the concentration of eccrine sweat glands is highest.
Treatment options for craniofacial hyperhidrosis include topical aluminum chloride, which blocks the eccrine sweat duct or causes eccrine cell atrophy. Although this option is a common treatment for palmar and axillary hyperhidrosis, use on the face has not been thoroughly studied, and may also cause skin irritation.
Topical and oral glycopyrrolate can be effective for all types of hyperhidrosis, but must be used daily and can have systemic side effects, with variable efficacy and longevity. Oral oxybutynin, beta-blockers, clonidine, and benzodiazepines have also been used with some limited studies available in patients with generalized hyperhidrosis.
Surgical treatments such as videothoracoscopy sympathectomy can be used in severe or recalcitrant cases of hyperhidrosis with good efficacy. However, surgical complications and inherent surgical risks limit these treatment options unless other modalities are exhausted.
OnabotulinumtoxinA is Food and Drug Administration approved for treating severe primary axillary hyperhidrosis, but is used off label for palmar, plantar, and craniofacial hyperhidrosis with great results and few side effects. Clinical pearls and guidelines for the use of botulinum toxin A in craniofacial hyperhidrosis were outlined by Wolosker and colleagues in a review article. As with any injection of neurotoxin, knowledge of the facial anatomy is critical to avoiding muscle paralysis.
double diluted. Treatment effects usually last 3 months, similar to cosmetic uses. Wolosker uses a dilution of 100 U botulinum toxin in 1.0 mL saline, which I find slightly more difficult to control and more likely to have loss of toxin.
In my experience, I have found the following dosing to be most effective with the least side effects for the following (dosages vary and can be titrated up to response):
- Upper lip: 6-10 U.
- Chin: 6-10 U.
- Forehead: 15-30 U. (Avoid 1 cm above the brow unless risks of brow drop are reviewed and acceptable to the patient. In my experience patients would rather have a lower brow than obstructive sweating in their brow that can irritate the eyes, blur vision, and smudge skincare and makeup.)
- Nose: 10 U
- Cheeks: 10 U per side (staying very superficial with injections).
- Scalp: 30-50 U (using serial injections 1-2 cm apart in the area affected by hyperhidrosis).
Side effects include temporary erythema, bruising, and edema, as well as muscle paralysis and asymmetry if proper injection technique is not used, the dose is not diluted properly, or the injection is too deep.
There are scattered case studies of symbiotic techniques to help the penetration of botulinum toxin when treating craniofacial hyperhidrosis, including microneedling, radiofrequency, long-pulsed diode laser, and ultrasound. But the safety and efficacy of these procedures have not been properly evaluated.
In all of my patients with craniofacial hyperhidrosis treated with botulinum toxin, quality of life is significantly improved with almost no complications. Botulinum toxin is a safe, relatively quick in-office procedure to treat craniofacial hyperhidrosis that can be used to help patients – particularly those who experience anxiety or have social and occupational impairment related to their disease.
This procedure is cosmetic in nature, and therefore, not covered by insurance.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Parashar K et al. Am J Clin Dermatol. 2023 Mar;24(2):187-98.
Doolittle J et al. Arch Dermatol Res. 2016 Dec;308(10):743-9.
Wolosker N et al. J Vasc Bras. 2020 Nov 16;19:e20190152.
Garcia-Souto F et al. Dermatol Ther. 2021 Jan;34(1):e14658.
Ebrahim H et al. J Clin Aesthet Dermatol. 2022 Sep;15(9):40-4.
Campanati A et al. Toxins (Basel). 2022 May 27;14(6):3727.
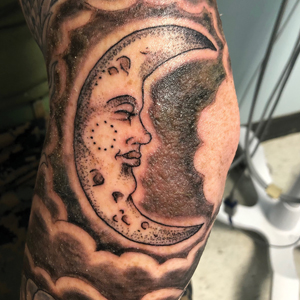
Papular Rash in a New Tattoo
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
A healthy 21-year-old woman presented with a pruritic papulovesicular rash on the left arm of 2 days’ duration. The day before rash onset, she received a black ink tattoo on the left arm to complete the second half of a monochromatic sleevestyle design. She previously underwent initial tattooing of the left arm by the same artist 2 weeks prior and experienced a similar but less extensive rash that self-resolved after 1 week. She had 8 older tattoos on various other body parts and denied any reactions. Physical examination showed numerous scattered papules and papulovesicles confined to areas of newly tattooed skin throughout the left arm. In the larger swaths of the tattoo, the papules coalesced into well-defined plaques. There was a discrete rim of faint erythema bordering the newly tattooed skin. No erosions, ulcerations, or purulent areas were observed, and there was no tenderness or excess warmth of the affected skin. Adjacent previously tattooed areas of the left arm were unaffected.

California picks generic drug company Civica to produce low-cost insulin
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Mediterranean diet linked to 24% reduction in CVD risk in women
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AI-assisted colonoscopy doesn’t always improve adenoma detection: Study
In a randomized clinical trial using EndoVigilant, there wasn’t a significant difference in adenomas per colonoscopy (APC) in procedures with the CADe tool versus those without it. In addition, the adenoma detection rate (ADR) and serrated polyp detection rate were similar in the CADe and non-CADe groups.
“Although we were disappointed that AI [artificial intelligence] did not improve detection of adenomas or serrated polyps in our study, we are still optimistic that this exciting technology will eventually impact endoscopy in a very positive way,” senior author Shai Friedland, MD, a professor of medicine at Stanford (Calif.) University and gastroenterologist with the Veterans Affairs Palo Alto Health Care System, said in an interview.
“The ultimate goal should be to improve the ability of colonoscopy to prevent morbidity and mortality from colon cancer, especially for endoscopists who may not be performing as well as they could be,” he said. “AI can potentially help prevent missed lesions due to fatigue or distraction, much like a warning system that averts car accidents. It can also potentially help endoscopists recognize dangerous – but rare – subtle lesions such as small, flat, and depressed cancers.”
The study was published online in the American Journal of Gastroenterology.
Analyzing detection rates
Several studies have evaluated the use of different CADe devices to reduce adenoma miss rates during colonoscopy, and some have found that the technology contributed to significantly higher ADR and APC, the study authors write. However, most of these studies have been performed in academic settings.
Dr. Friedland and colleagues conducted a randomized controlled trial, called AI-SEE, to evaluate the use of CADe during colonoscopy in four community-based endoscopy centers located in California, Connecticut, Maryland, and New Jersey between September 2020 and September 2021. The trial included seven board-certified clinicians, who had ADR of 25%-37% before the study. The participants were randomly assigned to colonoscopies with or without CADe in blocks of 16 patients to ensure masking. Both groups had similar patient demographics.
The research team enrolled patients aged 45 years or older who presented for screening or low-risk surveillance colonoscopy, which was defined as a patient qualifying for a surveillance interval of 3 years or greater based on the U.S. Multi-Society Task Force 2020 Guidelines. Patients were excluded if they had a history of inflammatory bowel disease, known or suspected polyposis or hereditary colon cancer syndrome, history of colon resection, or a referral for a diagnostic colonoscopy.
Among 769 enrolled patients, 387 were randomly assigned to undergo colonoscopy with EndoVigilant, an AI-enabled CADe software for colonoscopy. It augments existing white-light colonoscopy in real time by highlighting colon polyps and displaying a graphic box around the lesion on the monitor. It can be deployed as a single- or dual-monitor device. Although the study was originally designed to use two monitors, three investigators expressed strong preference for the single-monitor mode, so the protocol allowed endoscopists to choose.
Primary outcomes included APC and adenoma per extraction (APE), which is the percentage of polyps removed that are adenomas. Secondary endpoints included procedural time, ADR, serrated polyp detection rate, serrated polyps per colonoscopy, and nonadenomatous, nonserrated polyps per colonoscopy.
Overall, the use of CADe didn’t show a significant difference in APC, at 0.73, compared with 0.67 for non-CADe.
Although the use of CADe didn’t lead to increased identification of serrated polyps per colonoscopy – both at 0.08 – CADe led to increased identification of nonadenomatous, nonserrated polyps per colonoscopy, at 0.90 versus 0.51.
There also wasn’t a significant difference in distribution regarding adenomatous polyp location, size, or morphology. However, there was a trend toward greater identification of 6-9 mm APC using CADe, at 0.13 versus 0.08.
Mean withdrawal time was longer in the CADe group, at 11.7 minutes versus 10.7 minutes. However, when no polyps were identified, the withdrawal times were similar, at 9.1 minutes versus 8.8 minutes.
In addition, there was no difference in ADR for screening colonoscopies between the non-CADe and CADe groups, at 34.6% versus 34.3%, or for surveillance procedures, at 43.9% versus 40%. CADe also didn’t improve serrated polyp detection rates for screening or surveillance.
CADe was also associated with decreased APE in all colonoscopies (44.8 vs. 56.8) as well as in screening colonoscopies (43 vs. 57.8).
A comparison of single-monitor CADe with dual-monitor CADe found no significant difference in the average number of adenomas or serrated polyps identified per colonoscopy. However, dual-monitor CADe identified significantly more non-adenomatous, nonserrated polyps per colonoscopy (1.18 vs. 0.42), more adenomas sized at least 10 mm (0.19 vs. 0.05), and more flat polyps (0.18 vs. 0).
The study was terminated early after the interim analysis point, marked by 769 valid subjects. At this point, the comparison of APC between the two groups resulted in a new sample size estimate required for final analysis of 6,557 per group. This revised large study size estimate made it impractical to continue, the study authors wrote. No adverse events were observed during the study.
“What our study shows is that current systems – and the one we used in this study performs very well when tested on a database of images or videos – don’t make a major impact on very crude outcome measures, such as the total number of adenomas detected by a group of endoscopists at typical private endoscopy centers,” Dr. Friedland said. “I’m not convinced that we have a good answer yet for where to go from here, but we need to keep working with our AI colleagues to figure out how to use this exciting technology to improve outcomes in colon cancer.”
Additional considerations
In a separate evaluation of EndoVigilant, the frame level sensitivity was 0.9 and the frame level specificity was 0.97. These calculations were conducted on a dataset not used in training or validation of this model, the authors noted.
In this study, it’s possible that experienced community-based endoscopists are proficient at detecting the adenomas highlighted by the CADe system, so the technology may not detect a significant number of additional adenomas, the authors wrote. It’s also possible that some endoscopists ignore lesions highlighted by CADe, including small lesions that might be difficult to identify as adenomas or are seen as clinically unimportant, which could reduce the potential benefit of CADe.
“It’s important to remember that these tools are meant to be endoscopist assistance devices, not endoscopist replacements. They provide added benefit by pointing out polyps while we do the best exam we can,” Aasma Shaukat, MD, a professor of medicine and gastroenterologist at NYU Langone Health, New York, said in an interview.
Dr. Shaukat, who wasn’t involved with this study, has researched CADe for screening and surveillance colonoscopies. She and colleagues found that CADe use improved APC without an increase in resection of nonneoplastic lesions.
“Different trials have reported different results, and at the end of the day, it’s an endoscopist assistance tool, like spellcheck in a document,” she said. “It’s nice if spellcheck points to an incorrect spelling, but you don’t have to use it. Similarly, we often don’t know in these studies what an endoscopist felt or believed about the tool when using it.”
The benefits of CADe could vary based on its software, setting, number of patients, patient characteristics, number of clinicians, provider experience and training, dual- versus single-monitor setup, and even time of day, she noted. Future studies could clarify these factors, as well as improve the technology.
“This is just the beginning of AI in this field, and while bounding boxes to indicate potential polyps is a good start, it’s not the be-all, end-all,” Dr. Shaukat said.
“We want AI software to be able to tell us more about the size of the polyp, histology, prep quality, landmarks in the colon, adequacy of resection, and more. There’s some work being geared toward developing the algorithms to do these additional aspects,” she added.
The study was sponsored by EndoVigilant. Some of the authors reported consultant roles with Neptune Medical, AgilTx, Intuitive Surgical, Capsovision, and EndoVigilant. Dr. Shaukat reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial using EndoVigilant, there wasn’t a significant difference in adenomas per colonoscopy (APC) in procedures with the CADe tool versus those without it. In addition, the adenoma detection rate (ADR) and serrated polyp detection rate were similar in the CADe and non-CADe groups.
“Although we were disappointed that AI [artificial intelligence] did not improve detection of adenomas or serrated polyps in our study, we are still optimistic that this exciting technology will eventually impact endoscopy in a very positive way,” senior author Shai Friedland, MD, a professor of medicine at Stanford (Calif.) University and gastroenterologist with the Veterans Affairs Palo Alto Health Care System, said in an interview.
“The ultimate goal should be to improve the ability of colonoscopy to prevent morbidity and mortality from colon cancer, especially for endoscopists who may not be performing as well as they could be,” he said. “AI can potentially help prevent missed lesions due to fatigue or distraction, much like a warning system that averts car accidents. It can also potentially help endoscopists recognize dangerous – but rare – subtle lesions such as small, flat, and depressed cancers.”
The study was published online in the American Journal of Gastroenterology.
Analyzing detection rates
Several studies have evaluated the use of different CADe devices to reduce adenoma miss rates during colonoscopy, and some have found that the technology contributed to significantly higher ADR and APC, the study authors write. However, most of these studies have been performed in academic settings.
Dr. Friedland and colleagues conducted a randomized controlled trial, called AI-SEE, to evaluate the use of CADe during colonoscopy in four community-based endoscopy centers located in California, Connecticut, Maryland, and New Jersey between September 2020 and September 2021. The trial included seven board-certified clinicians, who had ADR of 25%-37% before the study. The participants were randomly assigned to colonoscopies with or without CADe in blocks of 16 patients to ensure masking. Both groups had similar patient demographics.
The research team enrolled patients aged 45 years or older who presented for screening or low-risk surveillance colonoscopy, which was defined as a patient qualifying for a surveillance interval of 3 years or greater based on the U.S. Multi-Society Task Force 2020 Guidelines. Patients were excluded if they had a history of inflammatory bowel disease, known or suspected polyposis or hereditary colon cancer syndrome, history of colon resection, or a referral for a diagnostic colonoscopy.
Among 769 enrolled patients, 387 were randomly assigned to undergo colonoscopy with EndoVigilant, an AI-enabled CADe software for colonoscopy. It augments existing white-light colonoscopy in real time by highlighting colon polyps and displaying a graphic box around the lesion on the monitor. It can be deployed as a single- or dual-monitor device. Although the study was originally designed to use two monitors, three investigators expressed strong preference for the single-monitor mode, so the protocol allowed endoscopists to choose.
Primary outcomes included APC and adenoma per extraction (APE), which is the percentage of polyps removed that are adenomas. Secondary endpoints included procedural time, ADR, serrated polyp detection rate, serrated polyps per colonoscopy, and nonadenomatous, nonserrated polyps per colonoscopy.
Overall, the use of CADe didn’t show a significant difference in APC, at 0.73, compared with 0.67 for non-CADe.
Although the use of CADe didn’t lead to increased identification of serrated polyps per colonoscopy – both at 0.08 – CADe led to increased identification of nonadenomatous, nonserrated polyps per colonoscopy, at 0.90 versus 0.51.
There also wasn’t a significant difference in distribution regarding adenomatous polyp location, size, or morphology. However, there was a trend toward greater identification of 6-9 mm APC using CADe, at 0.13 versus 0.08.
Mean withdrawal time was longer in the CADe group, at 11.7 minutes versus 10.7 minutes. However, when no polyps were identified, the withdrawal times were similar, at 9.1 minutes versus 8.8 minutes.
In addition, there was no difference in ADR for screening colonoscopies between the non-CADe and CADe groups, at 34.6% versus 34.3%, or for surveillance procedures, at 43.9% versus 40%. CADe also didn’t improve serrated polyp detection rates for screening or surveillance.
CADe was also associated with decreased APE in all colonoscopies (44.8 vs. 56.8) as well as in screening colonoscopies (43 vs. 57.8).
A comparison of single-monitor CADe with dual-monitor CADe found no significant difference in the average number of adenomas or serrated polyps identified per colonoscopy. However, dual-monitor CADe identified significantly more non-adenomatous, nonserrated polyps per colonoscopy (1.18 vs. 0.42), more adenomas sized at least 10 mm (0.19 vs. 0.05), and more flat polyps (0.18 vs. 0).
The study was terminated early after the interim analysis point, marked by 769 valid subjects. At this point, the comparison of APC between the two groups resulted in a new sample size estimate required for final analysis of 6,557 per group. This revised large study size estimate made it impractical to continue, the study authors wrote. No adverse events were observed during the study.
“What our study shows is that current systems – and the one we used in this study performs very well when tested on a database of images or videos – don’t make a major impact on very crude outcome measures, such as the total number of adenomas detected by a group of endoscopists at typical private endoscopy centers,” Dr. Friedland said. “I’m not convinced that we have a good answer yet for where to go from here, but we need to keep working with our AI colleagues to figure out how to use this exciting technology to improve outcomes in colon cancer.”
Additional considerations
In a separate evaluation of EndoVigilant, the frame level sensitivity was 0.9 and the frame level specificity was 0.97. These calculations were conducted on a dataset not used in training or validation of this model, the authors noted.
In this study, it’s possible that experienced community-based endoscopists are proficient at detecting the adenomas highlighted by the CADe system, so the technology may not detect a significant number of additional adenomas, the authors wrote. It’s also possible that some endoscopists ignore lesions highlighted by CADe, including small lesions that might be difficult to identify as adenomas or are seen as clinically unimportant, which could reduce the potential benefit of CADe.
“It’s important to remember that these tools are meant to be endoscopist assistance devices, not endoscopist replacements. They provide added benefit by pointing out polyps while we do the best exam we can,” Aasma Shaukat, MD, a professor of medicine and gastroenterologist at NYU Langone Health, New York, said in an interview.
Dr. Shaukat, who wasn’t involved with this study, has researched CADe for screening and surveillance colonoscopies. She and colleagues found that CADe use improved APC without an increase in resection of nonneoplastic lesions.
“Different trials have reported different results, and at the end of the day, it’s an endoscopist assistance tool, like spellcheck in a document,” she said. “It’s nice if spellcheck points to an incorrect spelling, but you don’t have to use it. Similarly, we often don’t know in these studies what an endoscopist felt or believed about the tool when using it.”
The benefits of CADe could vary based on its software, setting, number of patients, patient characteristics, number of clinicians, provider experience and training, dual- versus single-monitor setup, and even time of day, she noted. Future studies could clarify these factors, as well as improve the technology.
“This is just the beginning of AI in this field, and while bounding boxes to indicate potential polyps is a good start, it’s not the be-all, end-all,” Dr. Shaukat said.
“We want AI software to be able to tell us more about the size of the polyp, histology, prep quality, landmarks in the colon, adequacy of resection, and more. There’s some work being geared toward developing the algorithms to do these additional aspects,” she added.
The study was sponsored by EndoVigilant. Some of the authors reported consultant roles with Neptune Medical, AgilTx, Intuitive Surgical, Capsovision, and EndoVigilant. Dr. Shaukat reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial using EndoVigilant, there wasn’t a significant difference in adenomas per colonoscopy (APC) in procedures with the CADe tool versus those without it. In addition, the adenoma detection rate (ADR) and serrated polyp detection rate were similar in the CADe and non-CADe groups.
“Although we were disappointed that AI [artificial intelligence] did not improve detection of adenomas or serrated polyps in our study, we are still optimistic that this exciting technology will eventually impact endoscopy in a very positive way,” senior author Shai Friedland, MD, a professor of medicine at Stanford (Calif.) University and gastroenterologist with the Veterans Affairs Palo Alto Health Care System, said in an interview.
“The ultimate goal should be to improve the ability of colonoscopy to prevent morbidity and mortality from colon cancer, especially for endoscopists who may not be performing as well as they could be,” he said. “AI can potentially help prevent missed lesions due to fatigue or distraction, much like a warning system that averts car accidents. It can also potentially help endoscopists recognize dangerous – but rare – subtle lesions such as small, flat, and depressed cancers.”
The study was published online in the American Journal of Gastroenterology.
Analyzing detection rates
Several studies have evaluated the use of different CADe devices to reduce adenoma miss rates during colonoscopy, and some have found that the technology contributed to significantly higher ADR and APC, the study authors write. However, most of these studies have been performed in academic settings.
Dr. Friedland and colleagues conducted a randomized controlled trial, called AI-SEE, to evaluate the use of CADe during colonoscopy in four community-based endoscopy centers located in California, Connecticut, Maryland, and New Jersey between September 2020 and September 2021. The trial included seven board-certified clinicians, who had ADR of 25%-37% before the study. The participants were randomly assigned to colonoscopies with or without CADe in blocks of 16 patients to ensure masking. Both groups had similar patient demographics.
The research team enrolled patients aged 45 years or older who presented for screening or low-risk surveillance colonoscopy, which was defined as a patient qualifying for a surveillance interval of 3 years or greater based on the U.S. Multi-Society Task Force 2020 Guidelines. Patients were excluded if they had a history of inflammatory bowel disease, known or suspected polyposis or hereditary colon cancer syndrome, history of colon resection, or a referral for a diagnostic colonoscopy.
Among 769 enrolled patients, 387 were randomly assigned to undergo colonoscopy with EndoVigilant, an AI-enabled CADe software for colonoscopy. It augments existing white-light colonoscopy in real time by highlighting colon polyps and displaying a graphic box around the lesion on the monitor. It can be deployed as a single- or dual-monitor device. Although the study was originally designed to use two monitors, three investigators expressed strong preference for the single-monitor mode, so the protocol allowed endoscopists to choose.
Primary outcomes included APC and adenoma per extraction (APE), which is the percentage of polyps removed that are adenomas. Secondary endpoints included procedural time, ADR, serrated polyp detection rate, serrated polyps per colonoscopy, and nonadenomatous, nonserrated polyps per colonoscopy.
Overall, the use of CADe didn’t show a significant difference in APC, at 0.73, compared with 0.67 for non-CADe.
Although the use of CADe didn’t lead to increased identification of serrated polyps per colonoscopy – both at 0.08 – CADe led to increased identification of nonadenomatous, nonserrated polyps per colonoscopy, at 0.90 versus 0.51.
There also wasn’t a significant difference in distribution regarding adenomatous polyp location, size, or morphology. However, there was a trend toward greater identification of 6-9 mm APC using CADe, at 0.13 versus 0.08.
Mean withdrawal time was longer in the CADe group, at 11.7 minutes versus 10.7 minutes. However, when no polyps were identified, the withdrawal times were similar, at 9.1 minutes versus 8.8 minutes.
In addition, there was no difference in ADR for screening colonoscopies between the non-CADe and CADe groups, at 34.6% versus 34.3%, or for surveillance procedures, at 43.9% versus 40%. CADe also didn’t improve serrated polyp detection rates for screening or surveillance.
CADe was also associated with decreased APE in all colonoscopies (44.8 vs. 56.8) as well as in screening colonoscopies (43 vs. 57.8).
A comparison of single-monitor CADe with dual-monitor CADe found no significant difference in the average number of adenomas or serrated polyps identified per colonoscopy. However, dual-monitor CADe identified significantly more non-adenomatous, nonserrated polyps per colonoscopy (1.18 vs. 0.42), more adenomas sized at least 10 mm (0.19 vs. 0.05), and more flat polyps (0.18 vs. 0).
The study was terminated early after the interim analysis point, marked by 769 valid subjects. At this point, the comparison of APC between the two groups resulted in a new sample size estimate required for final analysis of 6,557 per group. This revised large study size estimate made it impractical to continue, the study authors wrote. No adverse events were observed during the study.
“What our study shows is that current systems – and the one we used in this study performs very well when tested on a database of images or videos – don’t make a major impact on very crude outcome measures, such as the total number of adenomas detected by a group of endoscopists at typical private endoscopy centers,” Dr. Friedland said. “I’m not convinced that we have a good answer yet for where to go from here, but we need to keep working with our AI colleagues to figure out how to use this exciting technology to improve outcomes in colon cancer.”
Additional considerations
In a separate evaluation of EndoVigilant, the frame level sensitivity was 0.9 and the frame level specificity was 0.97. These calculations were conducted on a dataset not used in training or validation of this model, the authors noted.
In this study, it’s possible that experienced community-based endoscopists are proficient at detecting the adenomas highlighted by the CADe system, so the technology may not detect a significant number of additional adenomas, the authors wrote. It’s also possible that some endoscopists ignore lesions highlighted by CADe, including small lesions that might be difficult to identify as adenomas or are seen as clinically unimportant, which could reduce the potential benefit of CADe.
“It’s important to remember that these tools are meant to be endoscopist assistance devices, not endoscopist replacements. They provide added benefit by pointing out polyps while we do the best exam we can,” Aasma Shaukat, MD, a professor of medicine and gastroenterologist at NYU Langone Health, New York, said in an interview.
Dr. Shaukat, who wasn’t involved with this study, has researched CADe for screening and surveillance colonoscopies. She and colleagues found that CADe use improved APC without an increase in resection of nonneoplastic lesions.
“Different trials have reported different results, and at the end of the day, it’s an endoscopist assistance tool, like spellcheck in a document,” she said. “It’s nice if spellcheck points to an incorrect spelling, but you don’t have to use it. Similarly, we often don’t know in these studies what an endoscopist felt or believed about the tool when using it.”
The benefits of CADe could vary based on its software, setting, number of patients, patient characteristics, number of clinicians, provider experience and training, dual- versus single-monitor setup, and even time of day, she noted. Future studies could clarify these factors, as well as improve the technology.
“This is just the beginning of AI in this field, and while bounding boxes to indicate potential polyps is a good start, it’s not the be-all, end-all,” Dr. Shaukat said.
“We want AI software to be able to tell us more about the size of the polyp, histology, prep quality, landmarks in the colon, adequacy of resection, and more. There’s some work being geared toward developing the algorithms to do these additional aspects,” she added.
The study was sponsored by EndoVigilant. Some of the authors reported consultant roles with Neptune Medical, AgilTx, Intuitive Surgical, Capsovision, and EndoVigilant. Dr. Shaukat reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Another FDA class I recall of Cardiosave Hybrid/Rescue IABPs
Datascope/Getinge is recalling certain Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic Balloon Pumps (IABPs) because the coiled cable connecting the display and base on some units may fail, causing an unexpected shutdown without warnings or alarms to alert the user.
The U.S. Food and Drug Administration has identified this as a class I recall, the most serious type of recall, because of the risk for serious injury or death.
The FDA warns that an unexpected pump shutdown and any interruption to therapy that occurs can lead to hemodynamic instability, organ damage, and/or death, especially in patients who are critically ill and most likely to receive therapy using these devices.
The devices are indicated for acute coronary syndrome, cardiac and noncardiac surgery, and complications of heart failure in adults.
From June 2019 to August 2022, Datascope/Getinge reported 44 complaints about damaged coiled cords resulting in unexpected shutdowns. There have been no reports of injuries or deaths related to this issue, according to the recall notice posted on the FDA’s website.
The recall includes a total of 2,300 CardioSave Hybrid or Rescue IABP units distributed prior to July 24, 2017, and/or coiled cord part number 0012-00-1801. Product model numbers for the recalled Cardiosave Hybrid and Cardiosave Rescue are available online.
The Cardiosave IABPs have previously been flagged by the FDA for subpar battery performance and fluid leaks.
To address the cable issue, Datascope/Getinge sent an urgent medical device correction letter to customers recommending that the coiled cable cord of the Cardiosave IABP be inspected for visible damage prior to use.
If an unexpected shutdown occurs, an attempt should be made to restart the Cardiosave IABP until an alternative pump is available. If the restart attempt is unsuccessful, an alternative IABP should be used. Any device that remains inoperable after a shutdown should be removed from patient care.
Customers should inspect their inventory to identify any Cardiosave Hybrid and/or Rescue IABPs that have the recalled coiled cord.
The company also asks customers to complete and sign the Medical Device Correction-Response form included with the letter and return it to Datascope/Getinge by emailing a scanned copy to cardiosave-sdhl23.act@getinge.com or by faxing the form to 1-877-660-5841.
Customers with questions about this recall should contact their Datascope/Getinge representative or call Datascope/Getinge technical support at 1-888-943-8872, Monday through Friday, between 8:00 AM and 6:00 PM ET.
The company has developed a hardware correction to address this issue and says a service representative will contact customers to schedule installation of the correction when the correction kit is available.
Any adverse events or suspected adverse events related to the recalled CardioSave Hybrid/Rescue IABPs should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article first appeared on Medscape.com.
Datascope/Getinge is recalling certain Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic Balloon Pumps (IABPs) because the coiled cable connecting the display and base on some units may fail, causing an unexpected shutdown without warnings or alarms to alert the user.
The U.S. Food and Drug Administration has identified this as a class I recall, the most serious type of recall, because of the risk for serious injury or death.
The FDA warns that an unexpected pump shutdown and any interruption to therapy that occurs can lead to hemodynamic instability, organ damage, and/or death, especially in patients who are critically ill and most likely to receive therapy using these devices.
The devices are indicated for acute coronary syndrome, cardiac and noncardiac surgery, and complications of heart failure in adults.
From June 2019 to August 2022, Datascope/Getinge reported 44 complaints about damaged coiled cords resulting in unexpected shutdowns. There have been no reports of injuries or deaths related to this issue, according to the recall notice posted on the FDA’s website.
The recall includes a total of 2,300 CardioSave Hybrid or Rescue IABP units distributed prior to July 24, 2017, and/or coiled cord part number 0012-00-1801. Product model numbers for the recalled Cardiosave Hybrid and Cardiosave Rescue are available online.
The Cardiosave IABPs have previously been flagged by the FDA for subpar battery performance and fluid leaks.
To address the cable issue, Datascope/Getinge sent an urgent medical device correction letter to customers recommending that the coiled cable cord of the Cardiosave IABP be inspected for visible damage prior to use.
If an unexpected shutdown occurs, an attempt should be made to restart the Cardiosave IABP until an alternative pump is available. If the restart attempt is unsuccessful, an alternative IABP should be used. Any device that remains inoperable after a shutdown should be removed from patient care.
Customers should inspect their inventory to identify any Cardiosave Hybrid and/or Rescue IABPs that have the recalled coiled cord.
The company also asks customers to complete and sign the Medical Device Correction-Response form included with the letter and return it to Datascope/Getinge by emailing a scanned copy to cardiosave-sdhl23.act@getinge.com or by faxing the form to 1-877-660-5841.
Customers with questions about this recall should contact their Datascope/Getinge representative or call Datascope/Getinge technical support at 1-888-943-8872, Monday through Friday, between 8:00 AM and 6:00 PM ET.
The company has developed a hardware correction to address this issue and says a service representative will contact customers to schedule installation of the correction when the correction kit is available.
Any adverse events or suspected adverse events related to the recalled CardioSave Hybrid/Rescue IABPs should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article first appeared on Medscape.com.
Datascope/Getinge is recalling certain Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic Balloon Pumps (IABPs) because the coiled cable connecting the display and base on some units may fail, causing an unexpected shutdown without warnings or alarms to alert the user.
The U.S. Food and Drug Administration has identified this as a class I recall, the most serious type of recall, because of the risk for serious injury or death.
The FDA warns that an unexpected pump shutdown and any interruption to therapy that occurs can lead to hemodynamic instability, organ damage, and/or death, especially in patients who are critically ill and most likely to receive therapy using these devices.
The devices are indicated for acute coronary syndrome, cardiac and noncardiac surgery, and complications of heart failure in adults.
From June 2019 to August 2022, Datascope/Getinge reported 44 complaints about damaged coiled cords resulting in unexpected shutdowns. There have been no reports of injuries or deaths related to this issue, according to the recall notice posted on the FDA’s website.
The recall includes a total of 2,300 CardioSave Hybrid or Rescue IABP units distributed prior to July 24, 2017, and/or coiled cord part number 0012-00-1801. Product model numbers for the recalled Cardiosave Hybrid and Cardiosave Rescue are available online.
The Cardiosave IABPs have previously been flagged by the FDA for subpar battery performance and fluid leaks.
To address the cable issue, Datascope/Getinge sent an urgent medical device correction letter to customers recommending that the coiled cable cord of the Cardiosave IABP be inspected for visible damage prior to use.
If an unexpected shutdown occurs, an attempt should be made to restart the Cardiosave IABP until an alternative pump is available. If the restart attempt is unsuccessful, an alternative IABP should be used. Any device that remains inoperable after a shutdown should be removed from patient care.
Customers should inspect their inventory to identify any Cardiosave Hybrid and/or Rescue IABPs that have the recalled coiled cord.
The company also asks customers to complete and sign the Medical Device Correction-Response form included with the letter and return it to Datascope/Getinge by emailing a scanned copy to cardiosave-sdhl23.act@getinge.com or by faxing the form to 1-877-660-5841.
Customers with questions about this recall should contact their Datascope/Getinge representative or call Datascope/Getinge technical support at 1-888-943-8872, Monday through Friday, between 8:00 AM and 6:00 PM ET.
The company has developed a hardware correction to address this issue and says a service representative will contact customers to schedule installation of the correction when the correction kit is available.
Any adverse events or suspected adverse events related to the recalled CardioSave Hybrid/Rescue IABPs should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article first appeared on Medscape.com.
Screen time and teenagers: Principles for parents
The Centers for Disease Control and Prevention recently released results of the most recent Youth Risk Behavior Survey, their once-a-decade survey of youth mental health and risk-taking behaviors. The headlines aren’t good: Self-reported rates of anxiety, depression, suicidal thoughts, and suicide attempts in adolescents have increased substantially from 2011 to 2021. This echoes epidemiologic data showing increasing rates of anxiety and depression over the last decade in 12- to 24-year-olds, but not in older age cohorts.
This trend started well before COVID, coinciding with the explosive growth in use of smartphones, apps, and social media platforms. Facebook launched in 2004, the iPhone in 2007, Instagram in 2010, and TikTok in 2016. A 2018 Pew Research survey of 13- to 17-year-olds found that 97% of them used at least one social media platform and 45% described themselves as online “almost constantly.” Social media does have great potential benefits for adolescents.
We all experienced how it supported relationships during COVID. It can provide supportive networks for teenagers isolated by exclusion, illness, or disability. It can support exploration of esoteric interests, expression of identity, entertainment, and relaxation. But certain children, as was true before social media, seem vulnerable to the bullying, loneliness, isolation, and disengagement that social media may exacerbate.
Several studies have shown an association between high daily screen time and adolescent anxiety and depression. These findings have not been consistently duplicated, and those that were could not establish causality. There appears to be a strong link between certain illnesses (ADHD, depression, anorexia nervosa) and excessive screen use, which can in turn worsen symptoms. But it is hard to know which came first or how they are related.
Now, a very large long-term observational study has suggested that there may be critical windows in adolescence (11-13 years in girls and 14-16 in boys and again at 19 years for both) during which time excessive screen time can put that child’s developing mental health at risk. This is nuanced and interesting progress, but you don’t have to wait another decade to offer the families in your practice some common sense guidance when they are asking how to balance their children’s needs to be independent and socially connected (and the fact that smartphones and social media are pervasive) with the risks of overuse. Equipped with these guiding principles, parents can set individualized, flexible ground rules, and adjust them as their children grow into young adults.
First: Know your child
Parents are, of course, the experts on their own child – their talents, interests, challenges, vulnerabilities, and developmental progress. Children with poor impulse control (including those with ADHD) are going to have greater difficulty turning away from highly addictive activities on their devices. Children who are anxious and shy may be prone to avoiding the stress of real-life situations, preferring virtual ones. Children with a history of depression may be vulnerable to relapse if their sleep and exercise routines are disrupted by excessive use. And children with eating disorders are especially vulnerable to the superficial social comparisons and “likes” that Instagram offers. Children with these vulnerabilities will benefit if their parents are aware of and can talk about these vulnerabilities, ideally with their child. They should be prepared to work with their teens to develop strategies that can help them learn how to manage their social media usage. These might include stopping screen use after a certain hour, leaving devices outside of bedrooms at night, and setting up apps that monitor and alert them about excessive use. They might use resources such as the AAP’s Family Media Plan (Media and Children [aap.org]), but simply taking the time to have regular, open, honest conversations about what is known and unknown about the potential risks of social media use is very protective.
Second: Use adolescent development as your guide
For those children who do not have a known vulnerability to overuse, consider the following areas that are essential to healthy development in adolescence as guideposts to help parents in setting reasonable ground rules: building independence, cultivating healthy social relationships, learning about their identity, managing their strong emotions, and developing the skills of self-care. If screen time supports these developmental areas, then it’s probably healthy. If it interferes with them, then not. And remember, parents should routinely discuss these principles with their children as well.
Independence
Key questions. Does their use of a device enable them to function more independently – that is, to arrange for rides, manage their schedules, homework, shifts, and so forth – on their own? Could it be done with a “dumb” device (text/call only)?
Social relationships
One-way viewing (Instagram, Facebook) with superficial acquaintances may promote isolation, anxiety, and depression, does not facilitate deepened relationships, and may be using up time that they could be investing in genuine social connections. But if they are using their devices to stay connected to good friends who live far away or just have different schedules, they can promote genuine, satisfying, bilateral social connections.
Key questions. Are they engaged in two-way communication with their devices? Are they staying connected to friends with whom they have a genuine, substantial relationship?
Investigating and experimenting with interests (identity)
Teenagers are supposed to be learning in deep and nuanced ways about their own interests and abilities during these years. This requires a lot of time invested in exploration and experimentation and a considerable amount of failure. Any activity that consumes a lot of their time without deepening meaningful knowledge of their interests and abilities (that is, activity that is only an escape or distraction) will interfere with their discovering their authentic identity.
Key questions. Is their use of devices facilitating this genuine exploration (setting up internships, practicing programming, or exploring interests that must be virtual)? Or is their device use just consuming precious time they could be using to genuinely explore potential interests?
Managing anxiety or distress
Exploring their identity and building social connections will involve a lot of stress, failure, disappointment, and even heartbreak. Learning to manage these uncomfortable feelings is an important part of adolescence. Distraction with a diverting entertainment can be one of several strategies for managing stress and distress. But if it becomes the only strategy, it can keep teens from getting “back in the game” and experiencing the fun, success, meaning, and joy that are also a big part of this exploration.
Key questions. Do they turn to their devices first when sad or stressed? Are they also able to use other strategies, such as talking with friends/family, exercising, or engaging in a meaningful pursuit to help them manage stress? Do they feel better after a little time spent on their device, or as if they will only feel good if they can stay on the device?
Self-care
Getting adequate, restful sleep (8-10 hours/night), finding regular time for exercise, cultivating healthy eating habits, and discovering what healthy strategies help them to unwind or relax is critical to a teenager’s healthiest development, and to healthy adult life. Some screens may help with motivating and tracking exercise, but screens in the bedroom interfere with going to bed, and with falling and staying asleep. Most teenagers are very busy and managing a lot of (normal) stress; the senseless fun or relaxation that are part of video games or surfing the Web are quick, practical, and effective ways to unwind. Don’t discourage your teenager from enjoying them. Instead, focus on also helping them to find other healthy ways to relax: hot baths, exercise, time with pets, crafts, reading, and listening to music are just a few examples. As they are building their identity, they should also be discovering how they best slow down and calm down.
Key questions. How many hours of sleep do they usually get on a school night? Is their phone (or other screen) in their bedroom during sleep? How do they relax? Do they have several strategies that do not require screens? Do they exercise regularly (3-5 times weekly)? Do they complain that they do not have enough time for exercise?
Third: Be mindful of what you model
Many of these principles can apply to our own use of smartphones, computers, and so on. Remind parents that their teenager will ultimately consider and follow their example much more than their commands. They should be prepared to talk about how they are thinking about the risks and benefits of social media use, how they are developing rules and expectations, and why they decided on them. These conversations model thoughtful and flexible decision-making.
It is critical that parents acknowledge that there are wonderful benefits to technology, including senseless fun. Then, it is easier to discuss how escaping into screen use can be hard to resist, and why it is important to practice resisting some temptations. Parents should find ways to follow the same rules they set for their teenager, or making them “family rules.” It’s important for our teenagers to learn about how to set these limits, as eventually they will be setting their own!
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
The Centers for Disease Control and Prevention recently released results of the most recent Youth Risk Behavior Survey, their once-a-decade survey of youth mental health and risk-taking behaviors. The headlines aren’t good: Self-reported rates of anxiety, depression, suicidal thoughts, and suicide attempts in adolescents have increased substantially from 2011 to 2021. This echoes epidemiologic data showing increasing rates of anxiety and depression over the last decade in 12- to 24-year-olds, but not in older age cohorts.
This trend started well before COVID, coinciding with the explosive growth in use of smartphones, apps, and social media platforms. Facebook launched in 2004, the iPhone in 2007, Instagram in 2010, and TikTok in 2016. A 2018 Pew Research survey of 13- to 17-year-olds found that 97% of them used at least one social media platform and 45% described themselves as online “almost constantly.” Social media does have great potential benefits for adolescents.
We all experienced how it supported relationships during COVID. It can provide supportive networks for teenagers isolated by exclusion, illness, or disability. It can support exploration of esoteric interests, expression of identity, entertainment, and relaxation. But certain children, as was true before social media, seem vulnerable to the bullying, loneliness, isolation, and disengagement that social media may exacerbate.
Several studies have shown an association between high daily screen time and adolescent anxiety and depression. These findings have not been consistently duplicated, and those that were could not establish causality. There appears to be a strong link between certain illnesses (ADHD, depression, anorexia nervosa) and excessive screen use, which can in turn worsen symptoms. But it is hard to know which came first or how they are related.
Now, a very large long-term observational study has suggested that there may be critical windows in adolescence (11-13 years in girls and 14-16 in boys and again at 19 years for both) during which time excessive screen time can put that child’s developing mental health at risk. This is nuanced and interesting progress, but you don’t have to wait another decade to offer the families in your practice some common sense guidance when they are asking how to balance their children’s needs to be independent and socially connected (and the fact that smartphones and social media are pervasive) with the risks of overuse. Equipped with these guiding principles, parents can set individualized, flexible ground rules, and adjust them as their children grow into young adults.
First: Know your child
Parents are, of course, the experts on their own child – their talents, interests, challenges, vulnerabilities, and developmental progress. Children with poor impulse control (including those with ADHD) are going to have greater difficulty turning away from highly addictive activities on their devices. Children who are anxious and shy may be prone to avoiding the stress of real-life situations, preferring virtual ones. Children with a history of depression may be vulnerable to relapse if their sleep and exercise routines are disrupted by excessive use. And children with eating disorders are especially vulnerable to the superficial social comparisons and “likes” that Instagram offers. Children with these vulnerabilities will benefit if their parents are aware of and can talk about these vulnerabilities, ideally with their child. They should be prepared to work with their teens to develop strategies that can help them learn how to manage their social media usage. These might include stopping screen use after a certain hour, leaving devices outside of bedrooms at night, and setting up apps that monitor and alert them about excessive use. They might use resources such as the AAP’s Family Media Plan (Media and Children [aap.org]), but simply taking the time to have regular, open, honest conversations about what is known and unknown about the potential risks of social media use is very protective.
Second: Use adolescent development as your guide
For those children who do not have a known vulnerability to overuse, consider the following areas that are essential to healthy development in adolescence as guideposts to help parents in setting reasonable ground rules: building independence, cultivating healthy social relationships, learning about their identity, managing their strong emotions, and developing the skills of self-care. If screen time supports these developmental areas, then it’s probably healthy. If it interferes with them, then not. And remember, parents should routinely discuss these principles with their children as well.
Independence
Key questions. Does their use of a device enable them to function more independently – that is, to arrange for rides, manage their schedules, homework, shifts, and so forth – on their own? Could it be done with a “dumb” device (text/call only)?
Social relationships
One-way viewing (Instagram, Facebook) with superficial acquaintances may promote isolation, anxiety, and depression, does not facilitate deepened relationships, and may be using up time that they could be investing in genuine social connections. But if they are using their devices to stay connected to good friends who live far away or just have different schedules, they can promote genuine, satisfying, bilateral social connections.
Key questions. Are they engaged in two-way communication with their devices? Are they staying connected to friends with whom they have a genuine, substantial relationship?
Investigating and experimenting with interests (identity)
Teenagers are supposed to be learning in deep and nuanced ways about their own interests and abilities during these years. This requires a lot of time invested in exploration and experimentation and a considerable amount of failure. Any activity that consumes a lot of their time without deepening meaningful knowledge of their interests and abilities (that is, activity that is only an escape or distraction) will interfere with their discovering their authentic identity.
Key questions. Is their use of devices facilitating this genuine exploration (setting up internships, practicing programming, or exploring interests that must be virtual)? Or is their device use just consuming precious time they could be using to genuinely explore potential interests?
Managing anxiety or distress
Exploring their identity and building social connections will involve a lot of stress, failure, disappointment, and even heartbreak. Learning to manage these uncomfortable feelings is an important part of adolescence. Distraction with a diverting entertainment can be one of several strategies for managing stress and distress. But if it becomes the only strategy, it can keep teens from getting “back in the game” and experiencing the fun, success, meaning, and joy that are also a big part of this exploration.
Key questions. Do they turn to their devices first when sad or stressed? Are they also able to use other strategies, such as talking with friends/family, exercising, or engaging in a meaningful pursuit to help them manage stress? Do they feel better after a little time spent on their device, or as if they will only feel good if they can stay on the device?
Self-care
Getting adequate, restful sleep (8-10 hours/night), finding regular time for exercise, cultivating healthy eating habits, and discovering what healthy strategies help them to unwind or relax is critical to a teenager’s healthiest development, and to healthy adult life. Some screens may help with motivating and tracking exercise, but screens in the bedroom interfere with going to bed, and with falling and staying asleep. Most teenagers are very busy and managing a lot of (normal) stress; the senseless fun or relaxation that are part of video games or surfing the Web are quick, practical, and effective ways to unwind. Don’t discourage your teenager from enjoying them. Instead, focus on also helping them to find other healthy ways to relax: hot baths, exercise, time with pets, crafts, reading, and listening to music are just a few examples. As they are building their identity, they should also be discovering how they best slow down and calm down.
Key questions. How many hours of sleep do they usually get on a school night? Is their phone (or other screen) in their bedroom during sleep? How do they relax? Do they have several strategies that do not require screens? Do they exercise regularly (3-5 times weekly)? Do they complain that they do not have enough time for exercise?
Third: Be mindful of what you model
Many of these principles can apply to our own use of smartphones, computers, and so on. Remind parents that their teenager will ultimately consider and follow their example much more than their commands. They should be prepared to talk about how they are thinking about the risks and benefits of social media use, how they are developing rules and expectations, and why they decided on them. These conversations model thoughtful and flexible decision-making.
It is critical that parents acknowledge that there are wonderful benefits to technology, including senseless fun. Then, it is easier to discuss how escaping into screen use can be hard to resist, and why it is important to practice resisting some temptations. Parents should find ways to follow the same rules they set for their teenager, or making them “family rules.” It’s important for our teenagers to learn about how to set these limits, as eventually they will be setting their own!
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
The Centers for Disease Control and Prevention recently released results of the most recent Youth Risk Behavior Survey, their once-a-decade survey of youth mental health and risk-taking behaviors. The headlines aren’t good: Self-reported rates of anxiety, depression, suicidal thoughts, and suicide attempts in adolescents have increased substantially from 2011 to 2021. This echoes epidemiologic data showing increasing rates of anxiety and depression over the last decade in 12- to 24-year-olds, but not in older age cohorts.
This trend started well before COVID, coinciding with the explosive growth in use of smartphones, apps, and social media platforms. Facebook launched in 2004, the iPhone in 2007, Instagram in 2010, and TikTok in 2016. A 2018 Pew Research survey of 13- to 17-year-olds found that 97% of them used at least one social media platform and 45% described themselves as online “almost constantly.” Social media does have great potential benefits for adolescents.
We all experienced how it supported relationships during COVID. It can provide supportive networks for teenagers isolated by exclusion, illness, or disability. It can support exploration of esoteric interests, expression of identity, entertainment, and relaxation. But certain children, as was true before social media, seem vulnerable to the bullying, loneliness, isolation, and disengagement that social media may exacerbate.
Several studies have shown an association between high daily screen time and adolescent anxiety and depression. These findings have not been consistently duplicated, and those that were could not establish causality. There appears to be a strong link between certain illnesses (ADHD, depression, anorexia nervosa) and excessive screen use, which can in turn worsen symptoms. But it is hard to know which came first or how they are related.
Now, a very large long-term observational study has suggested that there may be critical windows in adolescence (11-13 years in girls and 14-16 in boys and again at 19 years for both) during which time excessive screen time can put that child’s developing mental health at risk. This is nuanced and interesting progress, but you don’t have to wait another decade to offer the families in your practice some common sense guidance when they are asking how to balance their children’s needs to be independent and socially connected (and the fact that smartphones and social media are pervasive) with the risks of overuse. Equipped with these guiding principles, parents can set individualized, flexible ground rules, and adjust them as their children grow into young adults.
First: Know your child
Parents are, of course, the experts on their own child – their talents, interests, challenges, vulnerabilities, and developmental progress. Children with poor impulse control (including those with ADHD) are going to have greater difficulty turning away from highly addictive activities on their devices. Children who are anxious and shy may be prone to avoiding the stress of real-life situations, preferring virtual ones. Children with a history of depression may be vulnerable to relapse if their sleep and exercise routines are disrupted by excessive use. And children with eating disorders are especially vulnerable to the superficial social comparisons and “likes” that Instagram offers. Children with these vulnerabilities will benefit if their parents are aware of and can talk about these vulnerabilities, ideally with their child. They should be prepared to work with their teens to develop strategies that can help them learn how to manage their social media usage. These might include stopping screen use after a certain hour, leaving devices outside of bedrooms at night, and setting up apps that monitor and alert them about excessive use. They might use resources such as the AAP’s Family Media Plan (Media and Children [aap.org]), but simply taking the time to have regular, open, honest conversations about what is known and unknown about the potential risks of social media use is very protective.
Second: Use adolescent development as your guide
For those children who do not have a known vulnerability to overuse, consider the following areas that are essential to healthy development in adolescence as guideposts to help parents in setting reasonable ground rules: building independence, cultivating healthy social relationships, learning about their identity, managing their strong emotions, and developing the skills of self-care. If screen time supports these developmental areas, then it’s probably healthy. If it interferes with them, then not. And remember, parents should routinely discuss these principles with their children as well.
Independence
Key questions. Does their use of a device enable them to function more independently – that is, to arrange for rides, manage their schedules, homework, shifts, and so forth – on their own? Could it be done with a “dumb” device (text/call only)?
Social relationships
One-way viewing (Instagram, Facebook) with superficial acquaintances may promote isolation, anxiety, and depression, does not facilitate deepened relationships, and may be using up time that they could be investing in genuine social connections. But if they are using their devices to stay connected to good friends who live far away or just have different schedules, they can promote genuine, satisfying, bilateral social connections.
Key questions. Are they engaged in two-way communication with their devices? Are they staying connected to friends with whom they have a genuine, substantial relationship?
Investigating and experimenting with interests (identity)
Teenagers are supposed to be learning in deep and nuanced ways about their own interests and abilities during these years. This requires a lot of time invested in exploration and experimentation and a considerable amount of failure. Any activity that consumes a lot of their time without deepening meaningful knowledge of their interests and abilities (that is, activity that is only an escape or distraction) will interfere with their discovering their authentic identity.
Key questions. Is their use of devices facilitating this genuine exploration (setting up internships, practicing programming, or exploring interests that must be virtual)? Or is their device use just consuming precious time they could be using to genuinely explore potential interests?
Managing anxiety or distress
Exploring their identity and building social connections will involve a lot of stress, failure, disappointment, and even heartbreak. Learning to manage these uncomfortable feelings is an important part of adolescence. Distraction with a diverting entertainment can be one of several strategies for managing stress and distress. But if it becomes the only strategy, it can keep teens from getting “back in the game” and experiencing the fun, success, meaning, and joy that are also a big part of this exploration.
Key questions. Do they turn to their devices first when sad or stressed? Are they also able to use other strategies, such as talking with friends/family, exercising, or engaging in a meaningful pursuit to help them manage stress? Do they feel better after a little time spent on their device, or as if they will only feel good if they can stay on the device?
Self-care
Getting adequate, restful sleep (8-10 hours/night), finding regular time for exercise, cultivating healthy eating habits, and discovering what healthy strategies help them to unwind or relax is critical to a teenager’s healthiest development, and to healthy adult life. Some screens may help with motivating and tracking exercise, but screens in the bedroom interfere with going to bed, and with falling and staying asleep. Most teenagers are very busy and managing a lot of (normal) stress; the senseless fun or relaxation that are part of video games or surfing the Web are quick, practical, and effective ways to unwind. Don’t discourage your teenager from enjoying them. Instead, focus on also helping them to find other healthy ways to relax: hot baths, exercise, time with pets, crafts, reading, and listening to music are just a few examples. As they are building their identity, they should also be discovering how they best slow down and calm down.
Key questions. How many hours of sleep do they usually get on a school night? Is their phone (or other screen) in their bedroom during sleep? How do they relax? Do they have several strategies that do not require screens? Do they exercise regularly (3-5 times weekly)? Do they complain that they do not have enough time for exercise?
Third: Be mindful of what you model
Many of these principles can apply to our own use of smartphones, computers, and so on. Remind parents that their teenager will ultimately consider and follow their example much more than their commands. They should be prepared to talk about how they are thinking about the risks and benefits of social media use, how they are developing rules and expectations, and why they decided on them. These conversations model thoughtful and flexible decision-making.
It is critical that parents acknowledge that there are wonderful benefits to technology, including senseless fun. Then, it is easier to discuss how escaping into screen use can be hard to resist, and why it is important to practice resisting some temptations. Parents should find ways to follow the same rules they set for their teenager, or making them “family rules.” It’s important for our teenagers to learn about how to set these limits, as eventually they will be setting their own!
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
Children with ASD less likely to get vision screening
Children with autism spectrum disorder (ASD) are significantly less likely to have vision screening at well visits for 3- to 5-year-olds than are typically developing children, researchers have found.
The report, by Kimberly Hoover, MD, of Thomas Jefferson University in Philadelphia, and colleagues, was published online in Pediatrics.
While 59.9% of children without ASD got vision screening in these visits, only 36.5% of children with ASD got the screening. Both screening rates miss the mark set by American Academy of Pediatrics guidelines.
The AAP recommends “annual instrument-based vision screening, if available, at well visits for children starting at age 12 months to 3 years, and direct visual acuity testing beginning at 4 years of age. However, in children with developmental delays, the AAP recommends instrument-based screening, such as photoscreening, as a useful alternative at any age.”
Racial, age disparities as well
Racial disparities were evident in the data as well. Of the children who had ASD, Black children had the lowest rates of screening (27.6%), while the rate for White children was 39.7%. The rate for other/multiracial children with ASD was 39.8%.
The lowest rates of screening occurred in the youngest children, at the 3-year visit.
The researchers analyzed data from 63,829 well-child visits between January 2016 and December 2019, collected from the large primary care database PEDSnet.
Photoscreening vs. acuity screening
The authors pointed out that children with ASD are less likely to complete a vision test, which can be problematic in a busy primary care office.
“Children with ASD were significantly less likely to have at least one completed vision screening (43.2%) compared with children without ASD (72.1%; P <. 01),” the authors wrote, “with only 6.9% of children with ASD having had two or more vision screenings compared with 22.3% of children without ASD.”
The researchers saw higher vision test completion rates with photoscreening, using a sophisticated camera, compared with acuity screening, which uses a wall chart and requires responses.
Less patient participation is required for photoscreening and it can be done in less than 2 minutes.
If ability to complete the vision tests is a concern, the authors wrote, photoscreening may be a better solution.
Photoscreening takes 90 seconds
“Photoscreening has high sensitivity in detecting ocular conditions in children with ASD and has an average screening time of 90 seconds, and [it has] been validated in both children with ASD and developmental delays,” the authors wrote.
Andrew Adesman, MD, chief of developmental and behavioral pediatrics at Cohen Children’s Medical Center in New Hyde Park, N.Y., said the authors of this study quantify the gap between need and reality for vision tests for those with ASD.
“Other studies have shown that children on the autism spectrum have more than three times greater risk of having eye disease or vision problems,” he said in an interview. “You’ve got a high-risk population in need of assessment and the likelihood of them getting an assessment is much reduced.”
He said in addition to attention problems in taking the test, vision screening may get lost in the plethora of concerns parents want to talk about in well-child visits.
“If you’re the parent of a child with developmental delays, language delays, poor social engagement, there are a multitude of things the visit could be focused on and it may be that vision screening possibly gets compromised or not done,” Dr. Adesman said.
That, he said, may be a focus area for improving the screening numbers.
Neither parents nor providers should forget that vision screening is important, despite the myriad other issues to address, he said. “They don’t have to take a long time.”
When it comes to vision problems and children, “the earlier they’re identified the better,” Dr. Adesman says, particularly to identify the need for eye muscle surgery or corrective lenses, the two major interventions for strabismus or refractive error.
“If those problems are significant and go untreated, there’s a risk of loss of vision in the affected eye,” he said.
Reimbursement concerns for photoscreening
This study strongly supports the use of routine photoscreening to help eliminate the vision screening gap in children with ASD, the authors wrote.
They noted, however, that would require insurance reimbursement for primary care practices to effectively use that screening.
The researchers advised, “Providers treating patients with race, ethnicity, region, or age categories that reduce the adjusted odds of photoscreening can take steps in their practices to address these disparities, particularly in children with ASD.”
The study authors and Dr. Adesman reported no relevant financial relationships.
Children with autism spectrum disorder (ASD) are significantly less likely to have vision screening at well visits for 3- to 5-year-olds than are typically developing children, researchers have found.
The report, by Kimberly Hoover, MD, of Thomas Jefferson University in Philadelphia, and colleagues, was published online in Pediatrics.
While 59.9% of children without ASD got vision screening in these visits, only 36.5% of children with ASD got the screening. Both screening rates miss the mark set by American Academy of Pediatrics guidelines.
The AAP recommends “annual instrument-based vision screening, if available, at well visits for children starting at age 12 months to 3 years, and direct visual acuity testing beginning at 4 years of age. However, in children with developmental delays, the AAP recommends instrument-based screening, such as photoscreening, as a useful alternative at any age.”
Racial, age disparities as well
Racial disparities were evident in the data as well. Of the children who had ASD, Black children had the lowest rates of screening (27.6%), while the rate for White children was 39.7%. The rate for other/multiracial children with ASD was 39.8%.
The lowest rates of screening occurred in the youngest children, at the 3-year visit.
The researchers analyzed data from 63,829 well-child visits between January 2016 and December 2019, collected from the large primary care database PEDSnet.
Photoscreening vs. acuity screening
The authors pointed out that children with ASD are less likely to complete a vision test, which can be problematic in a busy primary care office.
“Children with ASD were significantly less likely to have at least one completed vision screening (43.2%) compared with children without ASD (72.1%; P <. 01),” the authors wrote, “with only 6.9% of children with ASD having had two or more vision screenings compared with 22.3% of children without ASD.”
The researchers saw higher vision test completion rates with photoscreening, using a sophisticated camera, compared with acuity screening, which uses a wall chart and requires responses.
Less patient participation is required for photoscreening and it can be done in less than 2 minutes.
If ability to complete the vision tests is a concern, the authors wrote, photoscreening may be a better solution.
Photoscreening takes 90 seconds
“Photoscreening has high sensitivity in detecting ocular conditions in children with ASD and has an average screening time of 90 seconds, and [it has] been validated in both children with ASD and developmental delays,” the authors wrote.
Andrew Adesman, MD, chief of developmental and behavioral pediatrics at Cohen Children’s Medical Center in New Hyde Park, N.Y., said the authors of this study quantify the gap between need and reality for vision tests for those with ASD.
“Other studies have shown that children on the autism spectrum have more than three times greater risk of having eye disease or vision problems,” he said in an interview. “You’ve got a high-risk population in need of assessment and the likelihood of them getting an assessment is much reduced.”
He said in addition to attention problems in taking the test, vision screening may get lost in the plethora of concerns parents want to talk about in well-child visits.
“If you’re the parent of a child with developmental delays, language delays, poor social engagement, there are a multitude of things the visit could be focused on and it may be that vision screening possibly gets compromised or not done,” Dr. Adesman said.
That, he said, may be a focus area for improving the screening numbers.
Neither parents nor providers should forget that vision screening is important, despite the myriad other issues to address, he said. “They don’t have to take a long time.”
When it comes to vision problems and children, “the earlier they’re identified the better,” Dr. Adesman says, particularly to identify the need for eye muscle surgery or corrective lenses, the two major interventions for strabismus or refractive error.
“If those problems are significant and go untreated, there’s a risk of loss of vision in the affected eye,” he said.
Reimbursement concerns for photoscreening
This study strongly supports the use of routine photoscreening to help eliminate the vision screening gap in children with ASD, the authors wrote.
They noted, however, that would require insurance reimbursement for primary care practices to effectively use that screening.
The researchers advised, “Providers treating patients with race, ethnicity, region, or age categories that reduce the adjusted odds of photoscreening can take steps in their practices to address these disparities, particularly in children with ASD.”
The study authors and Dr. Adesman reported no relevant financial relationships.
Children with autism spectrum disorder (ASD) are significantly less likely to have vision screening at well visits for 3- to 5-year-olds than are typically developing children, researchers have found.
The report, by Kimberly Hoover, MD, of Thomas Jefferson University in Philadelphia, and colleagues, was published online in Pediatrics.
While 59.9% of children without ASD got vision screening in these visits, only 36.5% of children with ASD got the screening. Both screening rates miss the mark set by American Academy of Pediatrics guidelines.
The AAP recommends “annual instrument-based vision screening, if available, at well visits for children starting at age 12 months to 3 years, and direct visual acuity testing beginning at 4 years of age. However, in children with developmental delays, the AAP recommends instrument-based screening, such as photoscreening, as a useful alternative at any age.”
Racial, age disparities as well
Racial disparities were evident in the data as well. Of the children who had ASD, Black children had the lowest rates of screening (27.6%), while the rate for White children was 39.7%. The rate for other/multiracial children with ASD was 39.8%.
The lowest rates of screening occurred in the youngest children, at the 3-year visit.
The researchers analyzed data from 63,829 well-child visits between January 2016 and December 2019, collected from the large primary care database PEDSnet.
Photoscreening vs. acuity screening
The authors pointed out that children with ASD are less likely to complete a vision test, which can be problematic in a busy primary care office.
“Children with ASD were significantly less likely to have at least one completed vision screening (43.2%) compared with children without ASD (72.1%; P <. 01),” the authors wrote, “with only 6.9% of children with ASD having had two or more vision screenings compared with 22.3% of children without ASD.”
The researchers saw higher vision test completion rates with photoscreening, using a sophisticated camera, compared with acuity screening, which uses a wall chart and requires responses.
Less patient participation is required for photoscreening and it can be done in less than 2 minutes.
If ability to complete the vision tests is a concern, the authors wrote, photoscreening may be a better solution.
Photoscreening takes 90 seconds
“Photoscreening has high sensitivity in detecting ocular conditions in children with ASD and has an average screening time of 90 seconds, and [it has] been validated in both children with ASD and developmental delays,” the authors wrote.
Andrew Adesman, MD, chief of developmental and behavioral pediatrics at Cohen Children’s Medical Center in New Hyde Park, N.Y., said the authors of this study quantify the gap between need and reality for vision tests for those with ASD.
“Other studies have shown that children on the autism spectrum have more than three times greater risk of having eye disease or vision problems,” he said in an interview. “You’ve got a high-risk population in need of assessment and the likelihood of them getting an assessment is much reduced.”
He said in addition to attention problems in taking the test, vision screening may get lost in the plethora of concerns parents want to talk about in well-child visits.
“If you’re the parent of a child with developmental delays, language delays, poor social engagement, there are a multitude of things the visit could be focused on and it may be that vision screening possibly gets compromised or not done,” Dr. Adesman said.
That, he said, may be a focus area for improving the screening numbers.
Neither parents nor providers should forget that vision screening is important, despite the myriad other issues to address, he said. “They don’t have to take a long time.”
When it comes to vision problems and children, “the earlier they’re identified the better,” Dr. Adesman says, particularly to identify the need for eye muscle surgery or corrective lenses, the two major interventions for strabismus or refractive error.
“If those problems are significant and go untreated, there’s a risk of loss of vision in the affected eye,” he said.
Reimbursement concerns for photoscreening
This study strongly supports the use of routine photoscreening to help eliminate the vision screening gap in children with ASD, the authors wrote.
They noted, however, that would require insurance reimbursement for primary care practices to effectively use that screening.
The researchers advised, “Providers treating patients with race, ethnicity, region, or age categories that reduce the adjusted odds of photoscreening can take steps in their practices to address these disparities, particularly in children with ASD.”
The study authors and Dr. Adesman reported no relevant financial relationships.
FROM PEDIATRICS
CLL and surgery are more compatible than ever
In the past decade, as targeted therapies have permitted better management of CLL, a new realm of possibilities has opened up for patients with this blood cancer.
“Previously, patients may not have been candidates for elective surgeries, such as hip replacements,” said hematologist-oncologist Helen Ma, MD, of the University of Irvine (Calif.) and VA Long Beach Healthcare System. She is the lead author of the report, which appeared in the British Journal of Hematology.
“Now that targeted therapies are controlling CLL well, patients may elect to have procedures that they may not have considered if their blood counts were very low or they felt too unwell to go through such invasive surgeries,” said Dr. Ma in an interview. In fact, the study authors noted that, “with currently available treatments, many patients with CLL are living considerably longer than the 1-year life expectancy threshold that proceduralists require.”
But extra surgical risks persist. “Both CLL and its treatment can increase the risk of complications during and after procedures, though available data are not consistently stratified by stage and whether patients are undergoing treatment,” the report authors noted.
Research has linked CLL to higher rates of blood transfusions in cardiac surgeries: One study, conducted partially in the era of targeted therapy, found that 87% of these surgery patients with CLL needed blood products vs. 65% of those who didn’t have CLL (P = .01). Studies didn’t find any extra risk of infections in patients with CLL, however, and there are conflicting findings about whether hospital mortality is higher.
Another study, also conducted partially in the era of targeted therapy, found that patients with CLL who had percutaneous coronary intervention procedures “developed higher rates of in-hospital mortality, any complication, bleeding and postoperative stroke compared to those seen in patients without leukemia.”
The authors of the new report noted that “patients with more advanced stage are at increased risk of bleeding and thromboembolic events relevant to their disease and invasive procedures.” Patients at more than minimal risk should undergo electrocardiograms prior to cardiac procedures, they wrote. Stress tests, coronary angiography, and percutaneous coronary intervention may also be warranted.
“To optimize evaluation and perioperative management, we strongly recommend the prospective collaborative inclusion of a multidisciplinary team including hematologists/oncologists, cardiologists (ideally cardio-oncologists), surgeons and anesthetists, as well as their ongoing involvement during the postoperative period,” the authors wrote.
As for medications, the researchers said that “generally, antibody therapy has no impact on surgery.” They added, “There is no evidence to hold treatment with anti-CD20 monoclonal antibodies prior to procedures unless the patient has cytopenias that may be a contra-indication. If that is the case, we recommend holding until counts recover to the parameters required for the procedure.”
In regard to Bruton’s tyrosine kinase inhibitors such as ibrutinib, “patients undergoing major surgeries with high risk of bleeding should hold Bruton’s tyrosine kinase inhibitors for a week prior to surgery to ensure adequate platelet function recovery given the disruption between collagen and platelet aggregation. Medications can be resumed 3-7 days after achieving postoperative hemostasis, depending on the type of surgery and risk of bleeding.”
As for venetoclax, “prior to surgery, patients should receive granulocyte colony-stimulating factor for neutropenia, blood transfusions for anemia, and platelet transfusions for thrombocytopenia to maintain procedural parameters.”
In the big picture, study lead author Dr. Ma said, “patients with CLL are doing well on continuous targeted treatments, and if there are otherwise no contraindications, they should be considered for procedures to improve their quality of life.”
In an interview, Stanford (Calif.) University surgeon Joe Forrester MD, MSc, who’s familiar with the report findings, said its conclusions are valid. “The nice thing is that a lot of the [CLL] therapies don’t have a lot of surgical side effects. Most should not preclude a patient from going to surgery.”
He advised colleagues to make sure to be open with patients about the heightened surgical risks due to CLL, such when they need emergency procedures. And it’s important to be realistic about whether patients will live long enough to benefit from the rare surgeries – such as weight-loss procedures – that won’t show major benefits for 5-10 years, he said.
The Lymphoma Research Foundation supported the study. Dr. Ma, several coauthors, and Dr. Forrester report no disclosures. One coauthor reports multiple relationships with industry.
In the past decade, as targeted therapies have permitted better management of CLL, a new realm of possibilities has opened up for patients with this blood cancer.
“Previously, patients may not have been candidates for elective surgeries, such as hip replacements,” said hematologist-oncologist Helen Ma, MD, of the University of Irvine (Calif.) and VA Long Beach Healthcare System. She is the lead author of the report, which appeared in the British Journal of Hematology.
“Now that targeted therapies are controlling CLL well, patients may elect to have procedures that they may not have considered if their blood counts were very low or they felt too unwell to go through such invasive surgeries,” said Dr. Ma in an interview. In fact, the study authors noted that, “with currently available treatments, many patients with CLL are living considerably longer than the 1-year life expectancy threshold that proceduralists require.”
But extra surgical risks persist. “Both CLL and its treatment can increase the risk of complications during and after procedures, though available data are not consistently stratified by stage and whether patients are undergoing treatment,” the report authors noted.
Research has linked CLL to higher rates of blood transfusions in cardiac surgeries: One study, conducted partially in the era of targeted therapy, found that 87% of these surgery patients with CLL needed blood products vs. 65% of those who didn’t have CLL (P = .01). Studies didn’t find any extra risk of infections in patients with CLL, however, and there are conflicting findings about whether hospital mortality is higher.
Another study, also conducted partially in the era of targeted therapy, found that patients with CLL who had percutaneous coronary intervention procedures “developed higher rates of in-hospital mortality, any complication, bleeding and postoperative stroke compared to those seen in patients without leukemia.”
The authors of the new report noted that “patients with more advanced stage are at increased risk of bleeding and thromboembolic events relevant to their disease and invasive procedures.” Patients at more than minimal risk should undergo electrocardiograms prior to cardiac procedures, they wrote. Stress tests, coronary angiography, and percutaneous coronary intervention may also be warranted.
“To optimize evaluation and perioperative management, we strongly recommend the prospective collaborative inclusion of a multidisciplinary team including hematologists/oncologists, cardiologists (ideally cardio-oncologists), surgeons and anesthetists, as well as their ongoing involvement during the postoperative period,” the authors wrote.
As for medications, the researchers said that “generally, antibody therapy has no impact on surgery.” They added, “There is no evidence to hold treatment with anti-CD20 monoclonal antibodies prior to procedures unless the patient has cytopenias that may be a contra-indication. If that is the case, we recommend holding until counts recover to the parameters required for the procedure.”
In regard to Bruton’s tyrosine kinase inhibitors such as ibrutinib, “patients undergoing major surgeries with high risk of bleeding should hold Bruton’s tyrosine kinase inhibitors for a week prior to surgery to ensure adequate platelet function recovery given the disruption between collagen and platelet aggregation. Medications can be resumed 3-7 days after achieving postoperative hemostasis, depending on the type of surgery and risk of bleeding.”
As for venetoclax, “prior to surgery, patients should receive granulocyte colony-stimulating factor for neutropenia, blood transfusions for anemia, and platelet transfusions for thrombocytopenia to maintain procedural parameters.”
In the big picture, study lead author Dr. Ma said, “patients with CLL are doing well on continuous targeted treatments, and if there are otherwise no contraindications, they should be considered for procedures to improve their quality of life.”
In an interview, Stanford (Calif.) University surgeon Joe Forrester MD, MSc, who’s familiar with the report findings, said its conclusions are valid. “The nice thing is that a lot of the [CLL] therapies don’t have a lot of surgical side effects. Most should not preclude a patient from going to surgery.”
He advised colleagues to make sure to be open with patients about the heightened surgical risks due to CLL, such when they need emergency procedures. And it’s important to be realistic about whether patients will live long enough to benefit from the rare surgeries – such as weight-loss procedures – that won’t show major benefits for 5-10 years, he said.
The Lymphoma Research Foundation supported the study. Dr. Ma, several coauthors, and Dr. Forrester report no disclosures. One coauthor reports multiple relationships with industry.
In the past decade, as targeted therapies have permitted better management of CLL, a new realm of possibilities has opened up for patients with this blood cancer.
“Previously, patients may not have been candidates for elective surgeries, such as hip replacements,” said hematologist-oncologist Helen Ma, MD, of the University of Irvine (Calif.) and VA Long Beach Healthcare System. She is the lead author of the report, which appeared in the British Journal of Hematology.
“Now that targeted therapies are controlling CLL well, patients may elect to have procedures that they may not have considered if their blood counts were very low or they felt too unwell to go through such invasive surgeries,” said Dr. Ma in an interview. In fact, the study authors noted that, “with currently available treatments, many patients with CLL are living considerably longer than the 1-year life expectancy threshold that proceduralists require.”
But extra surgical risks persist. “Both CLL and its treatment can increase the risk of complications during and after procedures, though available data are not consistently stratified by stage and whether patients are undergoing treatment,” the report authors noted.
Research has linked CLL to higher rates of blood transfusions in cardiac surgeries: One study, conducted partially in the era of targeted therapy, found that 87% of these surgery patients with CLL needed blood products vs. 65% of those who didn’t have CLL (P = .01). Studies didn’t find any extra risk of infections in patients with CLL, however, and there are conflicting findings about whether hospital mortality is higher.
Another study, also conducted partially in the era of targeted therapy, found that patients with CLL who had percutaneous coronary intervention procedures “developed higher rates of in-hospital mortality, any complication, bleeding and postoperative stroke compared to those seen in patients without leukemia.”
The authors of the new report noted that “patients with more advanced stage are at increased risk of bleeding and thromboembolic events relevant to their disease and invasive procedures.” Patients at more than minimal risk should undergo electrocardiograms prior to cardiac procedures, they wrote. Stress tests, coronary angiography, and percutaneous coronary intervention may also be warranted.
“To optimize evaluation and perioperative management, we strongly recommend the prospective collaborative inclusion of a multidisciplinary team including hematologists/oncologists, cardiologists (ideally cardio-oncologists), surgeons and anesthetists, as well as their ongoing involvement during the postoperative period,” the authors wrote.
As for medications, the researchers said that “generally, antibody therapy has no impact on surgery.” They added, “There is no evidence to hold treatment with anti-CD20 monoclonal antibodies prior to procedures unless the patient has cytopenias that may be a contra-indication. If that is the case, we recommend holding until counts recover to the parameters required for the procedure.”
In regard to Bruton’s tyrosine kinase inhibitors such as ibrutinib, “patients undergoing major surgeries with high risk of bleeding should hold Bruton’s tyrosine kinase inhibitors for a week prior to surgery to ensure adequate platelet function recovery given the disruption between collagen and platelet aggregation. Medications can be resumed 3-7 days after achieving postoperative hemostasis, depending on the type of surgery and risk of bleeding.”
As for venetoclax, “prior to surgery, patients should receive granulocyte colony-stimulating factor for neutropenia, blood transfusions for anemia, and platelet transfusions for thrombocytopenia to maintain procedural parameters.”
In the big picture, study lead author Dr. Ma said, “patients with CLL are doing well on continuous targeted treatments, and if there are otherwise no contraindications, they should be considered for procedures to improve their quality of life.”
In an interview, Stanford (Calif.) University surgeon Joe Forrester MD, MSc, who’s familiar with the report findings, said its conclusions are valid. “The nice thing is that a lot of the [CLL] therapies don’t have a lot of surgical side effects. Most should not preclude a patient from going to surgery.”
He advised colleagues to make sure to be open with patients about the heightened surgical risks due to CLL, such when they need emergency procedures. And it’s important to be realistic about whether patients will live long enough to benefit from the rare surgeries – such as weight-loss procedures – that won’t show major benefits for 5-10 years, he said.
The Lymphoma Research Foundation supported the study. Dr. Ma, several coauthors, and Dr. Forrester report no disclosures. One coauthor reports multiple relationships with industry.
FROM THE BRITISH JOURNAL OF HEMATOLOGY