User login
Study reveals an association between atopic dermatitis and e-cigarette use among US adults
Key clinical point: Use of e-cigarettes is significantly associated with the development of atopic dermatitis (AD) in the US adult population.
Major finding: E-cigarette use was significantly associated with AD (adjusted odds ratio 1.35; P < .001). The association was significant in women (P < .001) but not in men (P = .5).
Study details: This population-based study analyzed the data of 28,563 adults from the US National Health Interview Survey 2021.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants, speakers, investigators, or advisors for or receiving speaking fees from various organizations.
Source: Smith B et al. Association between electronic cigarette use and atopic dermatitis among United States adults. J Am Acad Dermatol. 2023 (Feb 24). Doi: 10.1016/j.jaad.2023.02.027.
Key clinical point: Use of e-cigarettes is significantly associated with the development of atopic dermatitis (AD) in the US adult population.
Major finding: E-cigarette use was significantly associated with AD (adjusted odds ratio 1.35; P < .001). The association was significant in women (P < .001) but not in men (P = .5).
Study details: This population-based study analyzed the data of 28,563 adults from the US National Health Interview Survey 2021.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants, speakers, investigators, or advisors for or receiving speaking fees from various organizations.
Source: Smith B et al. Association between electronic cigarette use and atopic dermatitis among United States adults. J Am Acad Dermatol. 2023 (Feb 24). Doi: 10.1016/j.jaad.2023.02.027.
Key clinical point: Use of e-cigarettes is significantly associated with the development of atopic dermatitis (AD) in the US adult population.
Major finding: E-cigarette use was significantly associated with AD (adjusted odds ratio 1.35; P < .001). The association was significant in women (P < .001) but not in men (P = .5).
Study details: This population-based study analyzed the data of 28,563 adults from the US National Health Interview Survey 2021.
Disclosures: This study did not receive any funding. Some authors declared serving as consultants, speakers, investigators, or advisors for or receiving speaking fees from various organizations.
Source: Smith B et al. Association between electronic cigarette use and atopic dermatitis among United States adults. J Am Acad Dermatol. 2023 (Feb 24). Doi: 10.1016/j.jaad.2023.02.027.
Increased prevalence of allergic contact dermatitis in patients with atopic dermatitis
Key clinical point: After patch testing, the frequency of allergic contact dermatitis (ACD) diagnosis was higher among patients with atopic dermatitis (AD) than among individuals without AD.
Major finding: Among patients with AD vs individuals without AD, the diagnosis rate of ACD (54.8% vs 47.3%; P < .0001), particularly ACD to cosmetics (7.0% vs 5.7%; P = .0007), medicaments (2.3% vs 1.7%; P = .02), dyes (1.9% vs 1.4%; P = .036), and foods contacting the skin (0.4% vs 0.1%; P = .003), was significantly higher.
Study details: This retrospective study included 15,737 individuals who underwent patch testing, of which 5641 were diagnosed with AD.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Qian MF et al. Prevalence of allergic contact dermatitis following patch testing in patients with atopic dermatitis: A retrospective United States claims-based study. J Am Acad Dermatol. 2023 (Feb 10). Doi: 10.1016/j.jaad.2022.12.051
Key clinical point: After patch testing, the frequency of allergic contact dermatitis (ACD) diagnosis was higher among patients with atopic dermatitis (AD) than among individuals without AD.
Major finding: Among patients with AD vs individuals without AD, the diagnosis rate of ACD (54.8% vs 47.3%; P < .0001), particularly ACD to cosmetics (7.0% vs 5.7%; P = .0007), medicaments (2.3% vs 1.7%; P = .02), dyes (1.9% vs 1.4%; P = .036), and foods contacting the skin (0.4% vs 0.1%; P = .003), was significantly higher.
Study details: This retrospective study included 15,737 individuals who underwent patch testing, of which 5641 were diagnosed with AD.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Qian MF et al. Prevalence of allergic contact dermatitis following patch testing in patients with atopic dermatitis: A retrospective United States claims-based study. J Am Acad Dermatol. 2023 (Feb 10). Doi: 10.1016/j.jaad.2022.12.051
Key clinical point: After patch testing, the frequency of allergic contact dermatitis (ACD) diagnosis was higher among patients with atopic dermatitis (AD) than among individuals without AD.
Major finding: Among patients with AD vs individuals without AD, the diagnosis rate of ACD (54.8% vs 47.3%; P < .0001), particularly ACD to cosmetics (7.0% vs 5.7%; P = .0007), medicaments (2.3% vs 1.7%; P = .02), dyes (1.9% vs 1.4%; P = .036), and foods contacting the skin (0.4% vs 0.1%; P = .003), was significantly higher.
Study details: This retrospective study included 15,737 individuals who underwent patch testing, of which 5641 were diagnosed with AD.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Qian MF et al. Prevalence of allergic contact dermatitis following patch testing in patients with atopic dermatitis: A retrospective United States claims-based study. J Am Acad Dermatol. 2023 (Feb 10). Doi: 10.1016/j.jaad.2022.12.051
Upadacitinib effective for moderate-to-severe atopic dermatitis in daily practice
Key clinical point: In real-life settings, upadacitinib was effective and safe in patients with moderate-to-severe atopic dermatitis (AD), including those with prior inadequate response to dupilumab or baricitinib.
Major finding: At week 16, the mean Eczema Area and Severity Index and Numerical Rating Scale pruritus scores decreased significantly from 16.6 to 5.7 and 7.0 to 3.7, respectively (both P < .001), with rapid improvement being observed in the first 4 weeks. Adverse events were mostly mild in severity.
Study details: This prospective multicenter observational study included 47 adult patients with moderate-to-severe AD from the Dutch BioDay registry who received upadacitinib (15 or 30 mg once daily), of which 23 and 14 had not or inadequately responded to previous dupilumab and baricitinib therapies, respectively.
Disclosures: The BioDay registry is sponsored by Eli Lilly and others. Some authors reported ties with various sources, including the BioDay registry sponsors.
Source: Boesjes CM et al. Effectiveness of upadacitinib in patients with atopic dermatitis including those with inadequate response to dupilumab and/or baricitinib: Results from the BioDay Registry. Acta Derm Venereol. 2023;103:adv00872 (Feb 16). Doi: 10.2340/actadv.v103.5243
Key clinical point: In real-life settings, upadacitinib was effective and safe in patients with moderate-to-severe atopic dermatitis (AD), including those with prior inadequate response to dupilumab or baricitinib.
Major finding: At week 16, the mean Eczema Area and Severity Index and Numerical Rating Scale pruritus scores decreased significantly from 16.6 to 5.7 and 7.0 to 3.7, respectively (both P < .001), with rapid improvement being observed in the first 4 weeks. Adverse events were mostly mild in severity.
Study details: This prospective multicenter observational study included 47 adult patients with moderate-to-severe AD from the Dutch BioDay registry who received upadacitinib (15 or 30 mg once daily), of which 23 and 14 had not or inadequately responded to previous dupilumab and baricitinib therapies, respectively.
Disclosures: The BioDay registry is sponsored by Eli Lilly and others. Some authors reported ties with various sources, including the BioDay registry sponsors.
Source: Boesjes CM et al. Effectiveness of upadacitinib in patients with atopic dermatitis including those with inadequate response to dupilumab and/or baricitinib: Results from the BioDay Registry. Acta Derm Venereol. 2023;103:adv00872 (Feb 16). Doi: 10.2340/actadv.v103.5243
Key clinical point: In real-life settings, upadacitinib was effective and safe in patients with moderate-to-severe atopic dermatitis (AD), including those with prior inadequate response to dupilumab or baricitinib.
Major finding: At week 16, the mean Eczema Area and Severity Index and Numerical Rating Scale pruritus scores decreased significantly from 16.6 to 5.7 and 7.0 to 3.7, respectively (both P < .001), with rapid improvement being observed in the first 4 weeks. Adverse events were mostly mild in severity.
Study details: This prospective multicenter observational study included 47 adult patients with moderate-to-severe AD from the Dutch BioDay registry who received upadacitinib (15 or 30 mg once daily), of which 23 and 14 had not or inadequately responded to previous dupilumab and baricitinib therapies, respectively.
Disclosures: The BioDay registry is sponsored by Eli Lilly and others. Some authors reported ties with various sources, including the BioDay registry sponsors.
Source: Boesjes CM et al. Effectiveness of upadacitinib in patients with atopic dermatitis including those with inadequate response to dupilumab and/or baricitinib: Results from the BioDay Registry. Acta Derm Venereol. 2023;103:adv00872 (Feb 16). Doi: 10.2340/actadv.v103.5243
Dupilumab safe and effective in the elderly with moderate-to-severe atopic dermatitis
Key clinical point: Dupilumab is safe and improves atopic dermatitis (AD) signs and symptoms in patients aged ≥60 years with moderate-to-severe AD.
Major finding: At week 16, similar to the <60-year group, a significantly higher proportion of patients receiving dupilumab (every 2 weeks or every week) vs placebo in the ≥60-year group achieved an Investigator’s Global Assessment score of 0 or 1 (44.4% or 39.7% vs 7.1%, respectively; both P < .0001) and a 75% improvement in the Eczema Area and Severity Index (63.0% or 61.6% vs 14.3%, respectively; both P < .0001). Most treatment-emergent adverse events were of mild-to-moderate severity.
Study details: This post hoc pooled analysis of four phase 3 trials included 2444 patients (≥60 years, n = 183; <60 years, n = 2261) with moderate-to-severe AD who were randomly assigned to receive dupilumab or placebo.
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported various ties, including employment, with Sanofi, Regeneron, or others.
Source: Silverberg JI et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: Analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023 (Feb 20). Doi: 10.1007/s40257-022-00754-4
Key clinical point: Dupilumab is safe and improves atopic dermatitis (AD) signs and symptoms in patients aged ≥60 years with moderate-to-severe AD.
Major finding: At week 16, similar to the <60-year group, a significantly higher proportion of patients receiving dupilumab (every 2 weeks or every week) vs placebo in the ≥60-year group achieved an Investigator’s Global Assessment score of 0 or 1 (44.4% or 39.7% vs 7.1%, respectively; both P < .0001) and a 75% improvement in the Eczema Area and Severity Index (63.0% or 61.6% vs 14.3%, respectively; both P < .0001). Most treatment-emergent adverse events were of mild-to-moderate severity.
Study details: This post hoc pooled analysis of four phase 3 trials included 2444 patients (≥60 years, n = 183; <60 years, n = 2261) with moderate-to-severe AD who were randomly assigned to receive dupilumab or placebo.
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported various ties, including employment, with Sanofi, Regeneron, or others.
Source: Silverberg JI et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: Analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023 (Feb 20). Doi: 10.1007/s40257-022-00754-4
Key clinical point: Dupilumab is safe and improves atopic dermatitis (AD) signs and symptoms in patients aged ≥60 years with moderate-to-severe AD.
Major finding: At week 16, similar to the <60-year group, a significantly higher proportion of patients receiving dupilumab (every 2 weeks or every week) vs placebo in the ≥60-year group achieved an Investigator’s Global Assessment score of 0 or 1 (44.4% or 39.7% vs 7.1%, respectively; both P < .0001) and a 75% improvement in the Eczema Area and Severity Index (63.0% or 61.6% vs 14.3%, respectively; both P < .0001). Most treatment-emergent adverse events were of mild-to-moderate severity.
Study details: This post hoc pooled analysis of four phase 3 trials included 2444 patients (≥60 years, n = 183; <60 years, n = 2261) with moderate-to-severe AD who were randomly assigned to receive dupilumab or placebo.
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported various ties, including employment, with Sanofi, Regeneron, or others.
Source: Silverberg JI et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: Analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023 (Feb 20). Doi: 10.1007/s40257-022-00754-4
Psoriatic arthritis treatment for women falls short, study suggests
Women with psoriatic arthritis (PsA) presented with more severe disease at baseline and were less likely to achieve favorable outcomes after 12 months of treatment with either ustekinumab (Stelara) or a tumor necrosis factor (TNF) inhibitor, compared with men, according to a post hoc analysis of data from nearly 1,000 individuals.
Although data suggest that the overall prevalence of PsA is similar across genders, recent studies have identified differences in various aspects of PsA between men and women, wrote Arno W.R. Van Kuijk, MD, of Amsterdam Rheumatology and Immunology Center, and colleagues wrote in a study published in Rheumatology.
“Accumulating evidence in multiple rheumatic diseases indicates that gender may influence the likelihood of achieving the desired outcome with treatment,” but studies of differences in treatment response according to gender are limited, they said.
The researchers conducted a post hoc analysis of women and men with PsA who were part of PsABio, a noninterventional European study of patients with PsA. All participants were starting first-, second-, or third-line treatment with ustekinumab or a TNF inhibitor. The primary outcome was response to treatment at 12 months. Disease activity was assessed using the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and total score on the 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) questionnaire.
Baseline available data for 512 women and 417 men showed the mean duration of disease was similar between genders (6.7 years for females and 6.9 years for males); body mass index was similar, as was the proportion of male and female patients receiving concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Females scored significantly worse than males on disease activity assessments at baseline with mean cDAPSA scores of 32.3 and 26.8, respectively.
The final analysis of 895 patients with baseline data and a postbaseline assessment included 439 who started ustekinumab (247 females, 192 males), and 456 who started a TNF inhibitor (248 females, 208 males).
At 12 months, females showed smaller degrees of improvement than males; 57.8% and 80.3%, respectively, achieved low disease activity based on cDAPSA scores, while 33.7% and 55.5% of females and males, respectively, achieved minimal disease activity. Measures of disability were higher in females than males, with HAQ-DI scores of 0.85 versus 0.50. PsAID-12 scores also were higher for females, compared with males (3.5 vs. 2.4).
A total of 81.7% of patients were on their initial biologic DMARD after 12 months, but more females than males who were taking ustekinumab or a TNF inhibitor changed or discontinued treatment.
Treatment persistence was significantly lower in females than males (P = .01), and lack of effectiveness was the main reason for discontinuation regardless of gender.
“The analysis of gender subgroup results of the PsABio study has expanded previously published observations that men and women with PsA have different experiences with the disease activity, clinical manifestations, impact on health-related quality of life, response to [biologic] DMARDs, and drug persistence,” the researchers wrote.
The lack of a medication protocol in the PsABio study limited the conclusions that could be drawn from the post hoc analysis, but the results were strengthened by the relatively large and diverse sample size and the inclusion of responses to more than one medication, the researchers noted.
The study was supported by Janssen. Dr. Van Kuijk disclosed serving as a consultant and receiving grant support from Janssen and other companies; several coauthors also disclosed relationships with Janssen.
Women with psoriatic arthritis (PsA) presented with more severe disease at baseline and were less likely to achieve favorable outcomes after 12 months of treatment with either ustekinumab (Stelara) or a tumor necrosis factor (TNF) inhibitor, compared with men, according to a post hoc analysis of data from nearly 1,000 individuals.
Although data suggest that the overall prevalence of PsA is similar across genders, recent studies have identified differences in various aspects of PsA between men and women, wrote Arno W.R. Van Kuijk, MD, of Amsterdam Rheumatology and Immunology Center, and colleagues wrote in a study published in Rheumatology.
“Accumulating evidence in multiple rheumatic diseases indicates that gender may influence the likelihood of achieving the desired outcome with treatment,” but studies of differences in treatment response according to gender are limited, they said.
The researchers conducted a post hoc analysis of women and men with PsA who were part of PsABio, a noninterventional European study of patients with PsA. All participants were starting first-, second-, or third-line treatment with ustekinumab or a TNF inhibitor. The primary outcome was response to treatment at 12 months. Disease activity was assessed using the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and total score on the 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) questionnaire.
Baseline available data for 512 women and 417 men showed the mean duration of disease was similar between genders (6.7 years for females and 6.9 years for males); body mass index was similar, as was the proportion of male and female patients receiving concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Females scored significantly worse than males on disease activity assessments at baseline with mean cDAPSA scores of 32.3 and 26.8, respectively.
The final analysis of 895 patients with baseline data and a postbaseline assessment included 439 who started ustekinumab (247 females, 192 males), and 456 who started a TNF inhibitor (248 females, 208 males).
At 12 months, females showed smaller degrees of improvement than males; 57.8% and 80.3%, respectively, achieved low disease activity based on cDAPSA scores, while 33.7% and 55.5% of females and males, respectively, achieved minimal disease activity. Measures of disability were higher in females than males, with HAQ-DI scores of 0.85 versus 0.50. PsAID-12 scores also were higher for females, compared with males (3.5 vs. 2.4).
A total of 81.7% of patients were on their initial biologic DMARD after 12 months, but more females than males who were taking ustekinumab or a TNF inhibitor changed or discontinued treatment.
Treatment persistence was significantly lower in females than males (P = .01), and lack of effectiveness was the main reason for discontinuation regardless of gender.
“The analysis of gender subgroup results of the PsABio study has expanded previously published observations that men and women with PsA have different experiences with the disease activity, clinical manifestations, impact on health-related quality of life, response to [biologic] DMARDs, and drug persistence,” the researchers wrote.
The lack of a medication protocol in the PsABio study limited the conclusions that could be drawn from the post hoc analysis, but the results were strengthened by the relatively large and diverse sample size and the inclusion of responses to more than one medication, the researchers noted.
The study was supported by Janssen. Dr. Van Kuijk disclosed serving as a consultant and receiving grant support from Janssen and other companies; several coauthors also disclosed relationships with Janssen.
Women with psoriatic arthritis (PsA) presented with more severe disease at baseline and were less likely to achieve favorable outcomes after 12 months of treatment with either ustekinumab (Stelara) or a tumor necrosis factor (TNF) inhibitor, compared with men, according to a post hoc analysis of data from nearly 1,000 individuals.
Although data suggest that the overall prevalence of PsA is similar across genders, recent studies have identified differences in various aspects of PsA between men and women, wrote Arno W.R. Van Kuijk, MD, of Amsterdam Rheumatology and Immunology Center, and colleagues wrote in a study published in Rheumatology.
“Accumulating evidence in multiple rheumatic diseases indicates that gender may influence the likelihood of achieving the desired outcome with treatment,” but studies of differences in treatment response according to gender are limited, they said.
The researchers conducted a post hoc analysis of women and men with PsA who were part of PsABio, a noninterventional European study of patients with PsA. All participants were starting first-, second-, or third-line treatment with ustekinumab or a TNF inhibitor. The primary outcome was response to treatment at 12 months. Disease activity was assessed using the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and total score on the 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) questionnaire.
Baseline available data for 512 women and 417 men showed the mean duration of disease was similar between genders (6.7 years for females and 6.9 years for males); body mass index was similar, as was the proportion of male and female patients receiving concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Females scored significantly worse than males on disease activity assessments at baseline with mean cDAPSA scores of 32.3 and 26.8, respectively.
The final analysis of 895 patients with baseline data and a postbaseline assessment included 439 who started ustekinumab (247 females, 192 males), and 456 who started a TNF inhibitor (248 females, 208 males).
At 12 months, females showed smaller degrees of improvement than males; 57.8% and 80.3%, respectively, achieved low disease activity based on cDAPSA scores, while 33.7% and 55.5% of females and males, respectively, achieved minimal disease activity. Measures of disability were higher in females than males, with HAQ-DI scores of 0.85 versus 0.50. PsAID-12 scores also were higher for females, compared with males (3.5 vs. 2.4).
A total of 81.7% of patients were on their initial biologic DMARD after 12 months, but more females than males who were taking ustekinumab or a TNF inhibitor changed or discontinued treatment.
Treatment persistence was significantly lower in females than males (P = .01), and lack of effectiveness was the main reason for discontinuation regardless of gender.
“The analysis of gender subgroup results of the PsABio study has expanded previously published observations that men and women with PsA have different experiences with the disease activity, clinical manifestations, impact on health-related quality of life, response to [biologic] DMARDs, and drug persistence,” the researchers wrote.
The lack of a medication protocol in the PsABio study limited the conclusions that could be drawn from the post hoc analysis, but the results were strengthened by the relatively large and diverse sample size and the inclusion of responses to more than one medication, the researchers noted.
The study was supported by Janssen. Dr. Van Kuijk disclosed serving as a consultant and receiving grant support from Janssen and other companies; several coauthors also disclosed relationships with Janssen.
FROM RHEUMATOLOGY
Antibody-Drug Conjugates: Targeted Treatments Providing Hope for Patients With Breast Cancer
The restrictive therapeutic index of chemotherapy has led to the emergence of antibody-drug conjugates (ADCs), medicines that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy to deliver cytotoxic payloads to cancer cells. This targeted approach can reduce the side effects of chemotherapy and improve the effectiveness of treatment. Several ADCs, including ado-trastuzumab emtansine (Kadcyla), sacituzumab govitecan-hziy (Trodelvy), and fam-trastuzumab deruxtecan-nxki (Enhertu), are currently approved for treating some types of breast cancer (BC).
The ADC trastuzumab emtansine was approved specifically for treating human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer (mBC) in patients who have previously been treated with trastuzumab and a taxane (paclitaxel or docetaxel) and who have already been treated for mBC or have developed tumor recurrence within 6 months of receiving adjuvant therapy. The US Food and Drug Administration (FDA) approval was based on the results of the EMILIA study, a phase 3 clinical trial that compared treatment with trastuzumab emtansine vs capecitabine + lapatinib in participants with HER2+, locally advanced, or metastatic BC. This trial emerged from the need for well-tolerated, HER2-directed therapies for patients with this type of cancer. Trastuzumab emtansine consists of trastuzumab, a mAb that targets HER2 (which is overexpressed in about 20% of BCs), linked to emtansine, a cytotoxic payload that inhibits cell division. The trastuzumab emtansine group had a median overall survival (OS) of 29.9 months vs 25.9 months in the capecitabine + lapatinib group, for a hazard ratio (HR) of 0.75 (95% CI: 0.64, 0.88).
Another ADC, sacituzumab govitecan, targets the Trop-2 protein, which is overexpressed in BC. This ADC includes a mAb that is linked to SN-38, a cytotoxic payload that inhibits DNA replication. Triple-negative breast cancer (TNBC) is a subtype of BC that does not have receptors for estrogen, progesterone, or HER2—making it more difficult to treat than other forms of BC. Sacituzumab govitecan is used to treat patients with metastatic TNBC who have received at least 2 prior therapies for metastatic disease. Sacituzumab govitecan is also approved for the treatment of patients with unresectable locally advanced or metastatic hormone-receptor–positive (HR+), and HER2-negative (HER2−) BC who have received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. Sacituzumab govitecan was the first Trop-2–directed ADC to demonstrate OS benefit in patients with HR+/HER2− mBC who had received prior endocrine-based therapy and at least 2 chemotherapies. It is now also recommended as a Category 1 preferred treatment for metastatic HR+/HER2− BC by the National Comprehensive Cancer Network.
The results of the TROPiCS-02 study, which led to the FDA approval of sacituzumab govitecan, demonstrated a median OS of 14.4 months with sacituzumab govitecan vs 11.2 months with treatment of physician’s choice (HR, 0.79; 95% CI: 0.65, 0.96; P = 0.02). This represents a 3.2-month improvement in survival and a 21% reduction in the risk for patient death. Before this medicine was approved, there were limited options to offer patients with BC after endocrine-based therapy and chemotherapy.
A third ADC, trastuzumab deruxtecan, targets the HER2 protein, like trastuzumab emtansine, but with a different cytotoxic payload. It consists of trastuzumab linked to deruxtecan, whose cytotoxicity inhibits DNA replication. It is approved for the treatment of HER2+ mBC. Its FDA approval was based on the results of the DESTINY-Breast04 phase 3 clinical trial, which demonstrated that treatment with trastuzumab deruxtecan, when compared with standard-of-care chemotherapy, doubles progression-free survival among patients with mBC that expresses low levels of HER2. The median OS for patients in the HR+ group who received trastuzumab deruxtecan was 23.9 months vs 17.5 months for those who received chemotherapy. In the hormone receptor-negative (HR−) group, the median OS for those who took trastuzumab deruxtecan was 16.6 months vs 10.3 months for those treated with chemotherapy.
The emergence of ADCs have demonstrated promising advancements in the treatment of BC, particularly in patients with HER2+ or triple-negative disease. ADCs have given new hope to and prolonged life for patients living with pretreated HR+/HER2− mBC. ADCs also have the potential to provide a more effective and less toxic treatment option for patients with BC. However, further research is needed to fully understand their long-term effects and to develop new ADCs that target other types of BC.
The restrictive therapeutic index of chemotherapy has led to the emergence of antibody-drug conjugates (ADCs), medicines that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy to deliver cytotoxic payloads to cancer cells. This targeted approach can reduce the side effects of chemotherapy and improve the effectiveness of treatment. Several ADCs, including ado-trastuzumab emtansine (Kadcyla), sacituzumab govitecan-hziy (Trodelvy), and fam-trastuzumab deruxtecan-nxki (Enhertu), are currently approved for treating some types of breast cancer (BC).
The ADC trastuzumab emtansine was approved specifically for treating human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer (mBC) in patients who have previously been treated with trastuzumab and a taxane (paclitaxel or docetaxel) and who have already been treated for mBC or have developed tumor recurrence within 6 months of receiving adjuvant therapy. The US Food and Drug Administration (FDA) approval was based on the results of the EMILIA study, a phase 3 clinical trial that compared treatment with trastuzumab emtansine vs capecitabine + lapatinib in participants with HER2+, locally advanced, or metastatic BC. This trial emerged from the need for well-tolerated, HER2-directed therapies for patients with this type of cancer. Trastuzumab emtansine consists of trastuzumab, a mAb that targets HER2 (which is overexpressed in about 20% of BCs), linked to emtansine, a cytotoxic payload that inhibits cell division. The trastuzumab emtansine group had a median overall survival (OS) of 29.9 months vs 25.9 months in the capecitabine + lapatinib group, for a hazard ratio (HR) of 0.75 (95% CI: 0.64, 0.88).
Another ADC, sacituzumab govitecan, targets the Trop-2 protein, which is overexpressed in BC. This ADC includes a mAb that is linked to SN-38, a cytotoxic payload that inhibits DNA replication. Triple-negative breast cancer (TNBC) is a subtype of BC that does not have receptors for estrogen, progesterone, or HER2—making it more difficult to treat than other forms of BC. Sacituzumab govitecan is used to treat patients with metastatic TNBC who have received at least 2 prior therapies for metastatic disease. Sacituzumab govitecan is also approved for the treatment of patients with unresectable locally advanced or metastatic hormone-receptor–positive (HR+), and HER2-negative (HER2−) BC who have received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. Sacituzumab govitecan was the first Trop-2–directed ADC to demonstrate OS benefit in patients with HR+/HER2− mBC who had received prior endocrine-based therapy and at least 2 chemotherapies. It is now also recommended as a Category 1 preferred treatment for metastatic HR+/HER2− BC by the National Comprehensive Cancer Network.
The results of the TROPiCS-02 study, which led to the FDA approval of sacituzumab govitecan, demonstrated a median OS of 14.4 months with sacituzumab govitecan vs 11.2 months with treatment of physician’s choice (HR, 0.79; 95% CI: 0.65, 0.96; P = 0.02). This represents a 3.2-month improvement in survival and a 21% reduction in the risk for patient death. Before this medicine was approved, there were limited options to offer patients with BC after endocrine-based therapy and chemotherapy.
A third ADC, trastuzumab deruxtecan, targets the HER2 protein, like trastuzumab emtansine, but with a different cytotoxic payload. It consists of trastuzumab linked to deruxtecan, whose cytotoxicity inhibits DNA replication. It is approved for the treatment of HER2+ mBC. Its FDA approval was based on the results of the DESTINY-Breast04 phase 3 clinical trial, which demonstrated that treatment with trastuzumab deruxtecan, when compared with standard-of-care chemotherapy, doubles progression-free survival among patients with mBC that expresses low levels of HER2. The median OS for patients in the HR+ group who received trastuzumab deruxtecan was 23.9 months vs 17.5 months for those who received chemotherapy. In the hormone receptor-negative (HR−) group, the median OS for those who took trastuzumab deruxtecan was 16.6 months vs 10.3 months for those treated with chemotherapy.
The emergence of ADCs have demonstrated promising advancements in the treatment of BC, particularly in patients with HER2+ or triple-negative disease. ADCs have given new hope to and prolonged life for patients living with pretreated HR+/HER2− mBC. ADCs also have the potential to provide a more effective and less toxic treatment option for patients with BC. However, further research is needed to fully understand their long-term effects and to develop new ADCs that target other types of BC.
The restrictive therapeutic index of chemotherapy has led to the emergence of antibody-drug conjugates (ADCs), medicines that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy to deliver cytotoxic payloads to cancer cells. This targeted approach can reduce the side effects of chemotherapy and improve the effectiveness of treatment. Several ADCs, including ado-trastuzumab emtansine (Kadcyla), sacituzumab govitecan-hziy (Trodelvy), and fam-trastuzumab deruxtecan-nxki (Enhertu), are currently approved for treating some types of breast cancer (BC).
The ADC trastuzumab emtansine was approved specifically for treating human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer (mBC) in patients who have previously been treated with trastuzumab and a taxane (paclitaxel or docetaxel) and who have already been treated for mBC or have developed tumor recurrence within 6 months of receiving adjuvant therapy. The US Food and Drug Administration (FDA) approval was based on the results of the EMILIA study, a phase 3 clinical trial that compared treatment with trastuzumab emtansine vs capecitabine + lapatinib in participants with HER2+, locally advanced, or metastatic BC. This trial emerged from the need for well-tolerated, HER2-directed therapies for patients with this type of cancer. Trastuzumab emtansine consists of trastuzumab, a mAb that targets HER2 (which is overexpressed in about 20% of BCs), linked to emtansine, a cytotoxic payload that inhibits cell division. The trastuzumab emtansine group had a median overall survival (OS) of 29.9 months vs 25.9 months in the capecitabine + lapatinib group, for a hazard ratio (HR) of 0.75 (95% CI: 0.64, 0.88).
Another ADC, sacituzumab govitecan, targets the Trop-2 protein, which is overexpressed in BC. This ADC includes a mAb that is linked to SN-38, a cytotoxic payload that inhibits DNA replication. Triple-negative breast cancer (TNBC) is a subtype of BC that does not have receptors for estrogen, progesterone, or HER2—making it more difficult to treat than other forms of BC. Sacituzumab govitecan is used to treat patients with metastatic TNBC who have received at least 2 prior therapies for metastatic disease. Sacituzumab govitecan is also approved for the treatment of patients with unresectable locally advanced or metastatic hormone-receptor–positive (HR+), and HER2-negative (HER2−) BC who have received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. Sacituzumab govitecan was the first Trop-2–directed ADC to demonstrate OS benefit in patients with HR+/HER2− mBC who had received prior endocrine-based therapy and at least 2 chemotherapies. It is now also recommended as a Category 1 preferred treatment for metastatic HR+/HER2− BC by the National Comprehensive Cancer Network.
The results of the TROPiCS-02 study, which led to the FDA approval of sacituzumab govitecan, demonstrated a median OS of 14.4 months with sacituzumab govitecan vs 11.2 months with treatment of physician’s choice (HR, 0.79; 95% CI: 0.65, 0.96; P = 0.02). This represents a 3.2-month improvement in survival and a 21% reduction in the risk for patient death. Before this medicine was approved, there were limited options to offer patients with BC after endocrine-based therapy and chemotherapy.
A third ADC, trastuzumab deruxtecan, targets the HER2 protein, like trastuzumab emtansine, but with a different cytotoxic payload. It consists of trastuzumab linked to deruxtecan, whose cytotoxicity inhibits DNA replication. It is approved for the treatment of HER2+ mBC. Its FDA approval was based on the results of the DESTINY-Breast04 phase 3 clinical trial, which demonstrated that treatment with trastuzumab deruxtecan, when compared with standard-of-care chemotherapy, doubles progression-free survival among patients with mBC that expresses low levels of HER2. The median OS for patients in the HR+ group who received trastuzumab deruxtecan was 23.9 months vs 17.5 months for those who received chemotherapy. In the hormone receptor-negative (HR−) group, the median OS for those who took trastuzumab deruxtecan was 16.6 months vs 10.3 months for those treated with chemotherapy.
The emergence of ADCs have demonstrated promising advancements in the treatment of BC, particularly in patients with HER2+ or triple-negative disease. ADCs have given new hope to and prolonged life for patients living with pretreated HR+/HER2− mBC. ADCs also have the potential to provide a more effective and less toxic treatment option for patients with BC. However, further research is needed to fully understand their long-term effects and to develop new ADCs that target other types of BC.
The air up there: Oxygen could be a bit overrated
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Hair Repigmentation as a Melanoma Warning Sign
To the Editor:
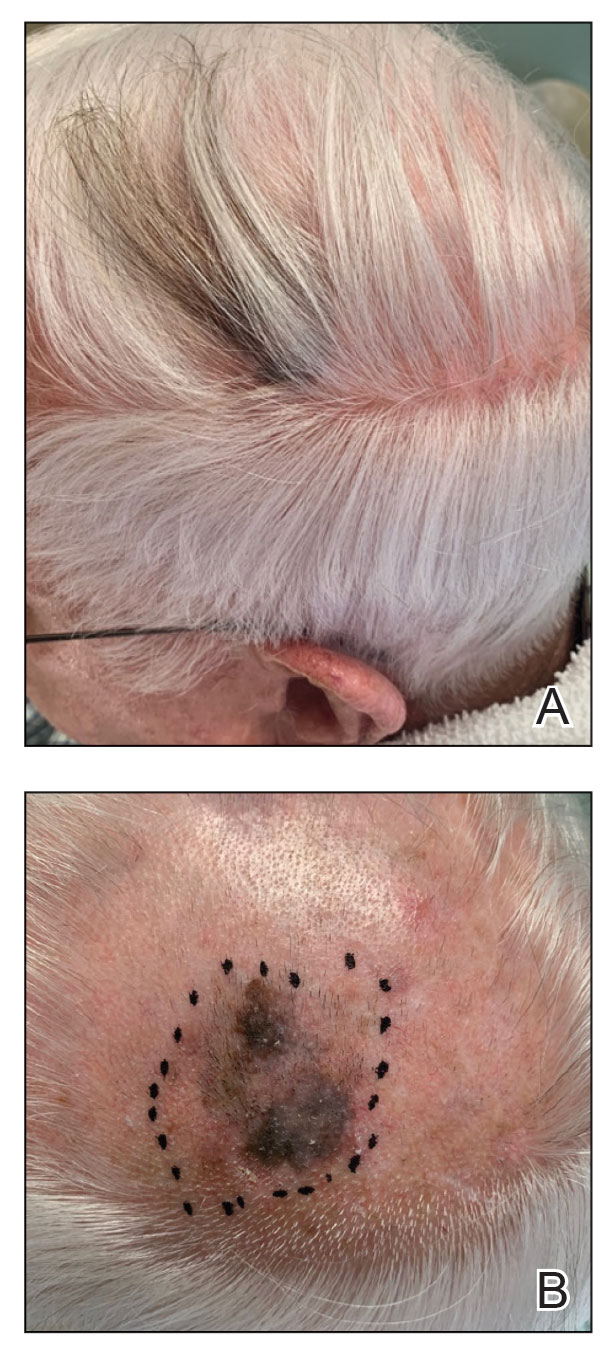
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.
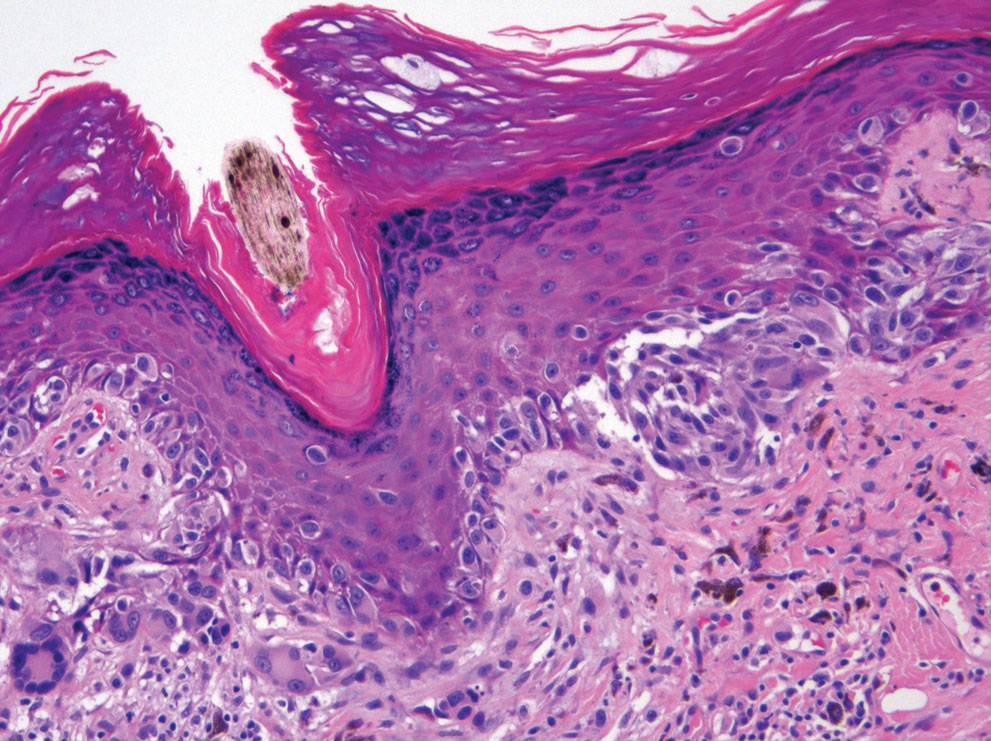
The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).
The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
To the Editor:
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.

The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).

The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
To the Editor:
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.

The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).

The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
Practice Points
- Localized repigmentation of the hair is a rare phenomenon that may indicate underlying melanoma.
- Careful clinicopathologic correlation is necessary to appropriately diagnose and manage this unusual presentation of melanoma.
Generalized Essential Telangiectasia Treated With Pulsed Dye Laser
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.
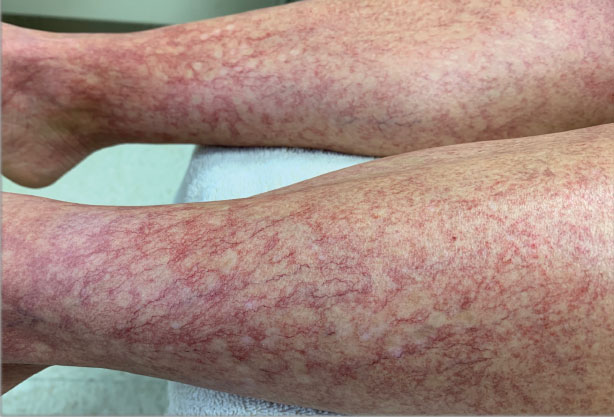
Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).
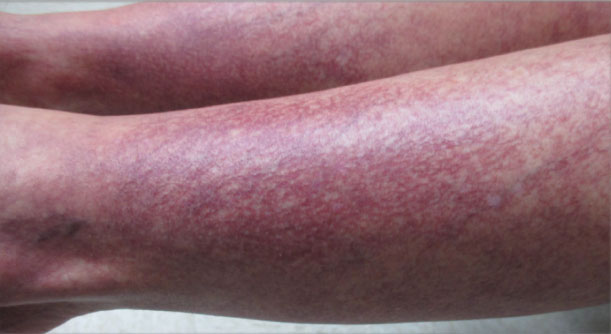
Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.

Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).

Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.

Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).

Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
Practice Points
- Generalized essential telangiectasia (GET) is a primary benign skin condition in which there is progressive development of telangiectases but a lack of systemic symptoms.
- Although patients should be assured that GET is a benign disease, its manifestation on the skin may cause negative psychologic impacts that should not be overlooked.
- Pulsed dye laser therapy does lead to improvement of the condition, but it does not prevent progression.
Rash and edema
Lab work was ordered and came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Patients also often have associated facial or acral edema (72%).1 Children with significant edema of the feet may find walking difficult, which should not be confused with arthritis or arthralgias.
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing. When performed, laboratory studies—including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis—are routinely normal. The differential diagnosis in this case included erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis.1,2,4
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved, and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
This case was adapted from: Cook JS, Angles A, Morley E. Urticaria and edema in a 2-year-old boy. J Fam Pract. 2021;70:353-355.
Photos courtesy of Jeffrey S. Cook, MD, FAAFP
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.

Lab work was ordered and came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Patients also often have associated facial or acral edema (72%).1 Children with significant edema of the feet may find walking difficult, which should not be confused with arthritis or arthralgias.
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing. When performed, laboratory studies—including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis—are routinely normal. The differential diagnosis in this case included erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis.1,2,4
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved, and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
This case was adapted from: Cook JS, Angles A, Morley E. Urticaria and edema in a 2-year-old boy. J Fam Pract. 2021;70:353-355.
Photos courtesy of Jeffrey S. Cook, MD, FAAFP

Lab work was ordered and came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Patients also often have associated facial or acral edema (72%).1 Children with significant edema of the feet may find walking difficult, which should not be confused with arthritis or arthralgias.
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing. When performed, laboratory studies—including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis—are routinely normal. The differential diagnosis in this case included erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis.1,2,4
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved, and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
This case was adapted from: Cook JS, Angles A, Morley E. Urticaria and edema in a 2-year-old boy. J Fam Pract. 2021;70:353-355.
Photos courtesy of Jeffrey S. Cook, MD, FAAFP
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.