User login
Is it OK to just be satisfied?
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Physician gender pay gap isn’t news; health inequity is rampant
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
Acid series: Azelaic acid
However, it has many positive qualities, including being gentle enough to use daily and is safe to use in pregnancy. It is antibacterial, comedolytic, keratolytic, and has antioxidant activity. Unfortunately, in the last decade the formulations of azelaic acid have not been changed considerably. The 20% cream, 15% gel, and 15% foam vehicles are often too irritating and drying to be used in the population it is intended for: those with rosacea, or with inflamed or sensitive skin.
Azelaic acid is a dicarboxylic acid produced by Pityrosporum ovale. It inhibits the synthesis of cellular proteins and is bactericidal against Propionibacterium acnes and Staphylococcus epidermidis. Azelaic acid is both keratolytic and comedolytic by decreasing keratohyalin granules and reducing filaggrin in the epidermis. It not only scavenges free oxygen radicals, thereby reducing inflammation, but is also a tyrosinase inhibitor – making it a safe, non–hydroquinone-based alternative to skin lightening.
Azelaic acid has little toxicity, it is ingested regularly as it is found in wheat, barley, and rye. Topical side effects are usually mild and can subside with increased use. The most common side effects include erythema, local stinging, pruritus, scaling, and a burning sensation. It is considered safe in pregnancy and a great alternative to medications for acne in pregnant or nursing patients.
The largest constraint with azelaic acid preparations on the market – and most likely the reason it has not been more widely used for acne, rosacea, antiaging, and hyperpigmentation – is the formulation. The foam and gel preparations are irritating and difficult to use on dry or sensitive skin. The 20% cream preparations are slightly better tolerated; however, in vitro skin-penetration studies have shown that cutaneous penetration of azelaic acid is greater after application of a 15% gel (aqueous-based vehicle) and 15% foam (hydrophilic oil-in-water emulsion) as compared with the 20% cream formulations.
In my clinical experience, azelaic acid can only be used in rosacea patients with oily or nonsensitive skin. The majority of my rosacea patients cannot tolerate the burning sensation, albeit transient and mild. Acne patients who do not have dry skin and pregnant patients with mild acne are a great population for integrating azelaic acid into an acne regimen. I also use azelaic acid as an alternative for mild melasma and lentigines in patients who are tapering off hydroquinone or cannot use hydroquinone. In the future, we need better, creamier, nonirritating formulations to be developed and more studies of higher concentrations of this acid for both prescription/patient at-home use, as well as more elegant in-office localized peel systems using azelaic acid.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Fitton A and Goa KL. Drugs. 1991 May;41(5):780-98.
Del Rosso JQ. J Clin Aesthet Dermatol. 2017 Mar;10(3):37-40.
Breathnach AC et al. Clin Dermatol. Apr-Jun 1989;7(2):106-19.
However, it has many positive qualities, including being gentle enough to use daily and is safe to use in pregnancy. It is antibacterial, comedolytic, keratolytic, and has antioxidant activity. Unfortunately, in the last decade the formulations of azelaic acid have not been changed considerably. The 20% cream, 15% gel, and 15% foam vehicles are often too irritating and drying to be used in the population it is intended for: those with rosacea, or with inflamed or sensitive skin.
Azelaic acid is a dicarboxylic acid produced by Pityrosporum ovale. It inhibits the synthesis of cellular proteins and is bactericidal against Propionibacterium acnes and Staphylococcus epidermidis. Azelaic acid is both keratolytic and comedolytic by decreasing keratohyalin granules and reducing filaggrin in the epidermis. It not only scavenges free oxygen radicals, thereby reducing inflammation, but is also a tyrosinase inhibitor – making it a safe, non–hydroquinone-based alternative to skin lightening.
Azelaic acid has little toxicity, it is ingested regularly as it is found in wheat, barley, and rye. Topical side effects are usually mild and can subside with increased use. The most common side effects include erythema, local stinging, pruritus, scaling, and a burning sensation. It is considered safe in pregnancy and a great alternative to medications for acne in pregnant or nursing patients.
The largest constraint with azelaic acid preparations on the market – and most likely the reason it has not been more widely used for acne, rosacea, antiaging, and hyperpigmentation – is the formulation. The foam and gel preparations are irritating and difficult to use on dry or sensitive skin. The 20% cream preparations are slightly better tolerated; however, in vitro skin-penetration studies have shown that cutaneous penetration of azelaic acid is greater after application of a 15% gel (aqueous-based vehicle) and 15% foam (hydrophilic oil-in-water emulsion) as compared with the 20% cream formulations.
In my clinical experience, azelaic acid can only be used in rosacea patients with oily or nonsensitive skin. The majority of my rosacea patients cannot tolerate the burning sensation, albeit transient and mild. Acne patients who do not have dry skin and pregnant patients with mild acne are a great population for integrating azelaic acid into an acne regimen. I also use azelaic acid as an alternative for mild melasma and lentigines in patients who are tapering off hydroquinone or cannot use hydroquinone. In the future, we need better, creamier, nonirritating formulations to be developed and more studies of higher concentrations of this acid for both prescription/patient at-home use, as well as more elegant in-office localized peel systems using azelaic acid.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Fitton A and Goa KL. Drugs. 1991 May;41(5):780-98.
Del Rosso JQ. J Clin Aesthet Dermatol. 2017 Mar;10(3):37-40.
Breathnach AC et al. Clin Dermatol. Apr-Jun 1989;7(2):106-19.
However, it has many positive qualities, including being gentle enough to use daily and is safe to use in pregnancy. It is antibacterial, comedolytic, keratolytic, and has antioxidant activity. Unfortunately, in the last decade the formulations of azelaic acid have not been changed considerably. The 20% cream, 15% gel, and 15% foam vehicles are often too irritating and drying to be used in the population it is intended for: those with rosacea, or with inflamed or sensitive skin.
Azelaic acid is a dicarboxylic acid produced by Pityrosporum ovale. It inhibits the synthesis of cellular proteins and is bactericidal against Propionibacterium acnes and Staphylococcus epidermidis. Azelaic acid is both keratolytic and comedolytic by decreasing keratohyalin granules and reducing filaggrin in the epidermis. It not only scavenges free oxygen radicals, thereby reducing inflammation, but is also a tyrosinase inhibitor – making it a safe, non–hydroquinone-based alternative to skin lightening.
Azelaic acid has little toxicity, it is ingested regularly as it is found in wheat, barley, and rye. Topical side effects are usually mild and can subside with increased use. The most common side effects include erythema, local stinging, pruritus, scaling, and a burning sensation. It is considered safe in pregnancy and a great alternative to medications for acne in pregnant or nursing patients.
The largest constraint with azelaic acid preparations on the market – and most likely the reason it has not been more widely used for acne, rosacea, antiaging, and hyperpigmentation – is the formulation. The foam and gel preparations are irritating and difficult to use on dry or sensitive skin. The 20% cream preparations are slightly better tolerated; however, in vitro skin-penetration studies have shown that cutaneous penetration of azelaic acid is greater after application of a 15% gel (aqueous-based vehicle) and 15% foam (hydrophilic oil-in-water emulsion) as compared with the 20% cream formulations.
In my clinical experience, azelaic acid can only be used in rosacea patients with oily or nonsensitive skin. The majority of my rosacea patients cannot tolerate the burning sensation, albeit transient and mild. Acne patients who do not have dry skin and pregnant patients with mild acne are a great population for integrating azelaic acid into an acne regimen. I also use azelaic acid as an alternative for mild melasma and lentigines in patients who are tapering off hydroquinone or cannot use hydroquinone. In the future, we need better, creamier, nonirritating formulations to be developed and more studies of higher concentrations of this acid for both prescription/patient at-home use, as well as more elegant in-office localized peel systems using azelaic acid.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Fitton A and Goa KL. Drugs. 1991 May;41(5):780-98.
Del Rosso JQ. J Clin Aesthet Dermatol. 2017 Mar;10(3):37-40.
Breathnach AC et al. Clin Dermatol. Apr-Jun 1989;7(2):106-19.
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
Moisturizers and skin barrier repair
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.
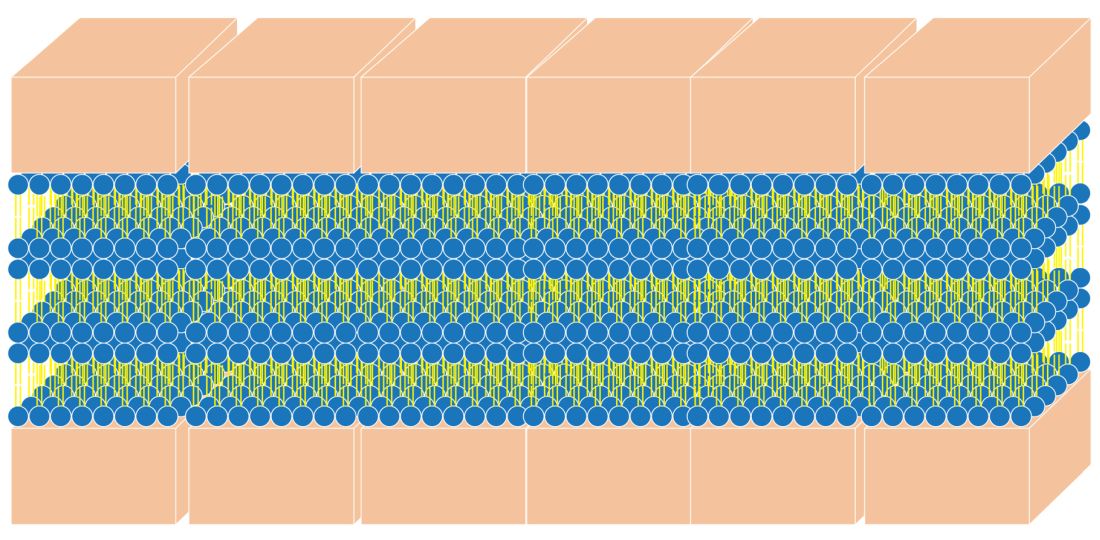
The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.

The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.

The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
A 22-year-old presented with erythematous papules on her fingers and toes
than men. Clinically, distal extremities such as toes, fingertips and heels, as well as the rims of the ears or nose develop erythematous to purple plaques. Lesions may be painful or pruritic. Over time, lesions may develop atrophy and resemble those of discoid lupus. While the pathogenesis is unknown, exposure to cold or wet environments can precipitate lesions.
Histopathology reveals a deep and superficial lymphocytic infiltrate with perieccrine involvement and fibrin deposition in vessels. Dermal edema is often present. Direct immunofluorescence shows an interface dermatitis positive for IgM, IgA, and C3.
The Mayo Clinic developed diagnostic criteria for diagnosing chilblains lupus. Two major criteria are acral skin lesions induced by cold exposure and evidence of lupus erythematosus in skin lesions (histopathologically or by direct immunofluorescence). Three minor criteria are the coexistence of systemic lupus erythematosus or discoid lupus erythematosus, response to antilupus treatment, and negative cryoglobulin and cold agglutinin studies.
Chilblains, or perniosis, has a similar clinical presentation to chilblain lupus erythematosus. However, serologic evidence of lupus, such as a positive antinuclear antibody (ANA), will be absent. Lupus pernio (Besnier-Tenneson syndrome) is a form of sarcoidosis that tends to favor the nose. These lesions are not precipitated by cold. It can be differentiated on histology. “COVID toes” is an entity described during the coronavirus pandemic, during which dermatologists noted pernio-like lesions in patients testing positive for coronavirus.
The patient’s labs revealed a positive ANA at 1:320 in a nucleolar speckled pattern, elevated double-stranded DNA, low C3 and C4 levels, elevated cardiolipin IgM Ab, and elevated sedimentation rate. COVID-19 antigen testing and COVID-19 antibodies were negative. A serum protein electrophoresis was negative. Cryoglobulins were negative.
Treatment includes protection from cold. Smoking cessation should be discussed. Topical steroids and topical calcineurin inhibitors are first-line treatments for mild disease. Antimalarials, such as hydroxychloroquine can be helpful. Systemic calcium channel blockers, systemic steroids, mycophenolate mofetil, and tacrolimus have all been reported as treatments. This patient responded well to hydroxychloroquine and topical steroids with full resolution of lesions.
This case was submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Su WP et al. Cutis. 1994 Dec;54(6):395-9.
Werth V and Newman S. Chilblain lupus (SLE pernio). Dermatology Advisor. 2017.
than men. Clinically, distal extremities such as toes, fingertips and heels, as well as the rims of the ears or nose develop erythematous to purple plaques. Lesions may be painful or pruritic. Over time, lesions may develop atrophy and resemble those of discoid lupus. While the pathogenesis is unknown, exposure to cold or wet environments can precipitate lesions.
Histopathology reveals a deep and superficial lymphocytic infiltrate with perieccrine involvement and fibrin deposition in vessels. Dermal edema is often present. Direct immunofluorescence shows an interface dermatitis positive for IgM, IgA, and C3.
The Mayo Clinic developed diagnostic criteria for diagnosing chilblains lupus. Two major criteria are acral skin lesions induced by cold exposure and evidence of lupus erythematosus in skin lesions (histopathologically or by direct immunofluorescence). Three minor criteria are the coexistence of systemic lupus erythematosus or discoid lupus erythematosus, response to antilupus treatment, and negative cryoglobulin and cold agglutinin studies.
Chilblains, or perniosis, has a similar clinical presentation to chilblain lupus erythematosus. However, serologic evidence of lupus, such as a positive antinuclear antibody (ANA), will be absent. Lupus pernio (Besnier-Tenneson syndrome) is a form of sarcoidosis that tends to favor the nose. These lesions are not precipitated by cold. It can be differentiated on histology. “COVID toes” is an entity described during the coronavirus pandemic, during which dermatologists noted pernio-like lesions in patients testing positive for coronavirus.
The patient’s labs revealed a positive ANA at 1:320 in a nucleolar speckled pattern, elevated double-stranded DNA, low C3 and C4 levels, elevated cardiolipin IgM Ab, and elevated sedimentation rate. COVID-19 antigen testing and COVID-19 antibodies were negative. A serum protein electrophoresis was negative. Cryoglobulins were negative.
Treatment includes protection from cold. Smoking cessation should be discussed. Topical steroids and topical calcineurin inhibitors are first-line treatments for mild disease. Antimalarials, such as hydroxychloroquine can be helpful. Systemic calcium channel blockers, systemic steroids, mycophenolate mofetil, and tacrolimus have all been reported as treatments. This patient responded well to hydroxychloroquine and topical steroids with full resolution of lesions.
This case was submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Su WP et al. Cutis. 1994 Dec;54(6):395-9.
Werth V and Newman S. Chilblain lupus (SLE pernio). Dermatology Advisor. 2017.
than men. Clinically, distal extremities such as toes, fingertips and heels, as well as the rims of the ears or nose develop erythematous to purple plaques. Lesions may be painful or pruritic. Over time, lesions may develop atrophy and resemble those of discoid lupus. While the pathogenesis is unknown, exposure to cold or wet environments can precipitate lesions.
Histopathology reveals a deep and superficial lymphocytic infiltrate with perieccrine involvement and fibrin deposition in vessels. Dermal edema is often present. Direct immunofluorescence shows an interface dermatitis positive for IgM, IgA, and C3.
The Mayo Clinic developed diagnostic criteria for diagnosing chilblains lupus. Two major criteria are acral skin lesions induced by cold exposure and evidence of lupus erythematosus in skin lesions (histopathologically or by direct immunofluorescence). Three minor criteria are the coexistence of systemic lupus erythematosus or discoid lupus erythematosus, response to antilupus treatment, and negative cryoglobulin and cold agglutinin studies.
Chilblains, or perniosis, has a similar clinical presentation to chilblain lupus erythematosus. However, serologic evidence of lupus, such as a positive antinuclear antibody (ANA), will be absent. Lupus pernio (Besnier-Tenneson syndrome) is a form of sarcoidosis that tends to favor the nose. These lesions are not precipitated by cold. It can be differentiated on histology. “COVID toes” is an entity described during the coronavirus pandemic, during which dermatologists noted pernio-like lesions in patients testing positive for coronavirus.
The patient’s labs revealed a positive ANA at 1:320 in a nucleolar speckled pattern, elevated double-stranded DNA, low C3 and C4 levels, elevated cardiolipin IgM Ab, and elevated sedimentation rate. COVID-19 antigen testing and COVID-19 antibodies were negative. A serum protein electrophoresis was negative. Cryoglobulins were negative.
Treatment includes protection from cold. Smoking cessation should be discussed. Topical steroids and topical calcineurin inhibitors are first-line treatments for mild disease. Antimalarials, such as hydroxychloroquine can be helpful. Systemic calcium channel blockers, systemic steroids, mycophenolate mofetil, and tacrolimus have all been reported as treatments. This patient responded well to hydroxychloroquine and topical steroids with full resolution of lesions.
This case was submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Su WP et al. Cutis. 1994 Dec;54(6):395-9.
Werth V and Newman S. Chilblain lupus (SLE pernio). Dermatology Advisor. 2017.
The Angel of Death in Clarksburg
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
Readers of this column may recall that since I have been the Editor-in-Chief of Federal Practitioner, my December editorial focuses on the best and worst of the year in federal medicine. In 2021, these evaluative terms fail to capture the sadness and global devastation that mark this grim epoch of the continuing pandemic, increasing climate disasters, rising political tensions, and racial violence. Thus, this year my editorial is framed in terms of the philosophical or theological categories of good and evil as the only concepts that can even begin to express the horrendous events that occurred in West Virginia.
On June 28, 2018, then US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) Executive-in-Charge, Carolyn Clancy, MD, contacted Inspector General Michael Missal to alert him that “there may be an ‘Angel of Death’ in Clarksburg [West Virginia].”1 Two years later Reta Mays, a 46-year-old VA nursing assistant, entered a guilty plea in federal court to the deaths of 7 vulnerable veterans. The legal charges were second-degree murder and 1 count of assault with intent to commit murder by injecting insulin. The victims were all patients on Ward 3 at the Louis A. Johnson VA, Medical Center in Clarksburg, where Mays worked the night shift from 2015 to 2018.2 Mays was sentenced in May of this year to 7 consecutive life terms for each of the veterans whose lives she cruelly ended and an additional 240 months for the eighth patient who survived her murder attempt.3
The term angel of death has religious roots in Judaism, although not strictly in the Hebrew scriptures. Neither the Jewish nor Christian Bible identifies a specific figure who is the angel of death. The idea first appears in Rabbinic literature and Jewish tradition.4 The angel God sends as a messenger of death is known as malakh ha-mavet in Hebrew. The revered Jewish physician and philosopher Moses Maimonides taught in his Guide for the Perplexed the angel of death is synonymous with the devil, and the evil inclination that dwells in the mind of all human beings.5In modern times, the concept of an angel of death has come to designate a serial killer who is a health care professional (HCP). A group of forensic scientists, HCPs, and attorneys, including former VA Under Secretary for Health Dr. Kenneth Kizer, published a study of HCPs who had been prosecuted or convicted of serial murder. Nurses constituted the largest group of offenders (60%) with nursing aides like Mays responsible for 18% of murders, and physicians 12%. The review found that though health care serial killers are rare, they operate in nations across the Western world, in many different states in this country, and in almost all health care settings, including previous VA angels of death.6Nursing aides who are not supposed to have access to medications—a major problem in Mays’ case—nor permitted to administer them more often resort to noncontrolled substances to kill their victims.1 Mays chose insulin as her murder weapon as did 13% of serial killers. Just as insulin may be difficult to detect in toxicology, so Mays and others like her committed their crimes on the night shift when they were less likely to be discovered.6
Many of us feel compelled to seek a rational motivation for why healers would mutate into killers: If we can find a reason for this heinous behavior it somehow helps us feel the world is more intelligible and controllable. Unfortunately, despite intensive forensic investigations of multiple angels of death, there is little definitive understanding of the motives of these murders.6 Mays disclosed more than most. As part of a plea bargain, she provided investigators with 2 rationales for her killing: She wanted to ease the patients’ suffering. Such claims of being an angel of mercy are common among HCP serial murders, which the patterns of the killings generally disprove. The patients Mays lethally injected, while mostly old and ill, were all expected to recover and leave the hospital. The Inspector General report uncovered a cautionary detail that has at least indirect bearing on the nursing assistant’s contention that she “wanted to let the patient’s die gently”: Contrary to VHA requirements, the facility had no functioning palliative care team. This finding in no way excuses or even explains Mays’ actions; it does, however, reinforce the essential value of palliative care expertise in an aging veteran population with many life-limiting conditions.7
Mays’ second motivation seems more plausible, based on her life narrative and the literature on HCP serial killers. Mays disclosed to investigators that she “had a lot of stress and chaos in [her] personal and professional life and these actions gave [her] a sense of control.”1 Her prior use of excessive force when employed at a prison as well as forensic science indicating that feelings of wielding power over life and death often drive health care murders, suggest this may have been a factor in Mays’ horrific conduct.8
It seems blasphemous to associate the word good in the same pages with this terrible evil. Nothing can compensate or justify the betrayal of the sacred oath of an HCP and the public trust of a VHA employee. Yet that very impossibility carries with it an obligation to ask, as did the author of an article about a recent Canadian nurse serial killer, “What can we learn from the [Mays] story?”9
Mays could never have taken the lives of 8 patients without clinical and administrative lapses and shortcuts at all levels of the health care system. Indeed, the 100-plus page Inspector General report makes 15 recommendations for the VHA, the Veterans Integrated Service Network, and the facility, encompassing areas of personnel hiring and performance evaluation, medication management and security, reporting and responding to unexplained events, quality and safety programs oversight, leaders’ responses, corrective actions, and even computer systems data analysis.
I want to suggest 2 ethical additions to this list addressed to all of us as VHA staff and especially to those of us who are HCPs. From the perspective of virtue ethics, Reta Mays is a tragedy about complacency and compromise in everyday work that the pandemic has made even more frequent and challenging to avoid and resist. This is what the Roman Virgil means in the epigraph that the road down to hell is easy and the road back very difficult.
I propose the need for discernment in trying to listen to our moral intuitions that tell us something is amiss and diligence in adhering to best practices even when we are fearful, exhausted, demoralized, or apathetic. These 2 habits of commitment to veterans, one of compassion and the other of competence, can help us follow the good inclinations of our hearts and together with system changes can bar the doors of our hospitals to the visits of future angels of death. This dedication is the least we owe to the families of the patients at Clarksburg whose loved ones never came home and whose questions likely can never be fully answered.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
1. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration: care and oversight deficiencies related to multiple homicides at the Louis A. Johnson VA Medical Center in Clarksburg, West Virginia. Healthcare Inspection Report #20-035993-140. Published May 11 2021. Accessed November 22, 2021. https://www.va.gov/oig/pubs/VAOIG-20-03593-140.pdf
2. Kennedy M, Schwartz M. Former VA medical worker pleads guilty to murdering 7 patients in West Virginia. Published July 14, 2020. Accessed November 22, 2021. https://www.npr.org/2020/07/14/890776010/former-va-medical-worker-charged-with-7-murders-in-west-virginia
3. US Department of Justice, US Attorney’s Office Northern District of West Virginia. Former VA hospital nursing assistant sentenced to seven consecutive life sentences for murdering seven veterans and assault with intent to commit murder of an eighth [press release]. Published May 11, 2021. Accessed November 22, 2021. https://www.justice.gov/usao-ndwv/pr/former-va-hospital-nursing-assistant-sentenced-seven-consecutive-life-sentences.
4. Jacobs L. The Jewish Religion: A Companion. 1st ed. Oxford University Press;1995:116.
5. Maimonides. Guide for the Perplexed. Frielander M, trans. Routledge and Kegan Paul Ltd; 1904:pt 3, chap 22.
6. Yorker BC, Kizer KW, Lampe P, Forrest AR, Lannan JM, Russell DA. Serial murder by healthcare professionals. J Forensic Sci. 2006;51(6):1362-1371. doi:10.1111/j.1556-4029.2006.00273.x
7. VHA Directive 1139. Palliative care consult teams (PCCT) and VISN leads. Published June 14, 2017.
8. Rourke S, Ward T. Healthcare serial killers: patterns and policies. Published August 14, 2017. Accessed November 22, 2021. https://www.medscape.com/viewarticle/884136
9. Frank C. Health care serial murder: what can we learn from the Wettlaufer story? Can Fam Physician. 2020;66(10):719-722.
Mumps: Sometimes forgotten but not gone
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
The 7-year-old boy sat at the edge of a stretcher in the emergency department, looking miserable, as his mother recounted his symptoms to a senior resident physician on duty. Low-grade fever, fatigue, and myalgias prompted rapid SARS-CoV-2 testing at his school. That test, as well as a repeat test at the pediatrician’s office, were negative. A triage protocol in the emergency department prompted a third test, which was also negative.
“Everyone has told me that it’s likely just a different virus,” the mother said. “But then his cheek started to swell. Have you ever seen anything like this?”
The boy turned his head, revealing a diffuse swelling that extended down his right cheek to the angle of his jaw.
“Only in textbooks,” the resident physician responded.
It is a credit to our national immunization program that most practicing clinicians have never actually seen a case of mumps. Before vaccination was introduced in 1967, infection in childhood was nearly universal. Unilateral or bilateral tender swelling of the parotid gland is the typical clinical finding. Low-grade fever, myalgias, decreased appetite, malaise, and headache may precede parotid swelling in some patients. Other patients infected with mumps may have only respiratory symptoms, and some may have no symptoms at all.
Two doses of measles-mumps-rubella vaccine have been recommended for children in the United States since 1989, with the first dose administered at 12-15 months of age. According to data collected through the National Immunization Survey, more than 92% of children in the United States receive at least one dose of measles-mumps-rubella vaccine by 24 months of age. The vaccine is immunogenic, with 94% of recipients developing measurable mumps antibody (range, 89%-97%). The vaccine has been a public health success: Overall, mumps cases declined more than 99% between 1967 and 2005.
But in the mid-2000s, mumps cases started to rise again, with more than 28,000 reported between 2007 and 2019. Annual cases ranged from 229 to 6,369 and while large, localized outbreaks have contributed to peak years, mumps has been reported from all 50 states and the District of Columbia. According to a recently published paper in Pediatrics, nearly a third of these cases occurred in children <18 years of age and most had been appropriately immunized for age.
Of the 9,172 cases reported in children, 5,461 or 60% occurred between 2015 and 2019. Of these, 55% were in boys. While cases occurred in children of all ages, 54% were in children 11-17 years of age, and 33% were in children 5-10 years of age. Non-Hispanic Asian and/or Pacific Islander children accounted for 38% of cases. Only 2% of cases were associated with international travel and were presumed to have been acquired outside the United States
The reason for the increase in mumps cases in recent years is not well understood. Outbreaks in fully immunized college students have prompted concern about poor B-cell memory after vaccination resulting in waning immunity over time. In the past, antibodies against mumps were boosted by exposure to wild-type mumps virus but such exposures have become fortunately rare for most of us. Cases in recently immunized children suggest there is more to the story. Notably, there is a mismatch between the genotype A mumps virus contained in the current MMR and MMRV vaccines and the genotype G virus currently circulating in the United States.
With the onset of the pandemic and implementation of mitigation measures to prevent the spread of COVID-19, circulation of some common respiratory viruses, including respiratory syncytial virus and influenza, was sharply curtailed. Mumps continued to circulate, albeit at reduced levels, with 616 cases reported in 2020. In 2021, 30 states and jurisdictions reported 139 cases through Dec. 1.
Clinicians should suspect mumps in all cases of parotitis, regardless of an individual’s age, vaccination status, or travel history. Laboratory testing is required to distinguish mumps from other infectious and noninfectious causes of parotitis. Infectious causes include gram-positive and gram-negative bacterial infection, as well as other viral infections, including Epstein-Barr virus, coxsackie viruses, parainfluenza, and rarely, influenza. Case reports also describe parotitis coincident with SARS-CoV-2 infection.
When parotitis has been present for 3 days or less, a buccal swab for RT-PCR should be obtained, massaging the parotid gland for 30 seconds before specimen collection. When parotitis has been present for >3 days, a mumps Immunoglobulin M serum antibody should be collected in addition to the buccal swab PCR. A negative IgM does not exclude the possibility of infection, especially in immunized individuals. Mumps is a nationally notifiable disease, and all confirmed and suspect cases should be reported to the state or local health department.
Back in the emergency department, the mother was counseled about the potential diagnosis of mumps and the need for her son to isolate at home for 5 days after the onset of the parotid swelling. She was also educated about potential complications of mumps, including orchitis, aseptic meningitis and encephalitis, and hearing loss. Fortunately, complications are less common in individuals who have been immunized, and orchitis rarely occurs in prepubertal boys.
The resident physician also confirmed that other members of the household had been appropriately immunized for age. While the MMR vaccine does not prevent illness in those already infected with mumps and is not indicated as postexposure prophylaxis, providing vaccine to those not already immunized can protect against future exposures. A third dose of MMR vaccine is only indicated in the setting of an outbreak and when specifically recommended by public health authorities for those deemed to be in a high-risk group. Additional information about mumps is available at www.cdc.gov/mumps/hcp.html#report.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Spam filter failure: Selling physician emails equals big $$
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to spam@uce.gov, then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to spam@uce.gov, then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to spam@uce.gov, then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Closing your practice
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.