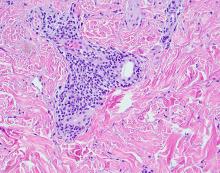
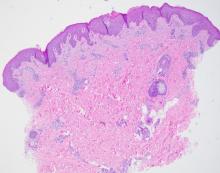
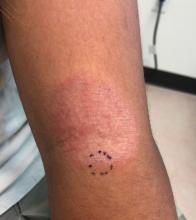
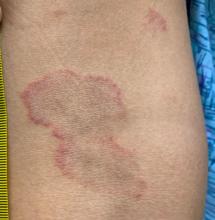
User login
Ob.gyns. on the day that Roe v. Wade was overturned
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
“I’m happy to contribute, but can you keep it anonymous? It’s a safety concern for me.”
On the day that the Supreme Court of the United States voted to strike down Roe v. Wade, I reached out to ob.gyn.s across the country, wanting to hear their reactions. My own response, like that of many doctors and women, was a visceral mix of anger, fear, and grief. I could only begin to imagine what the real experts on reproductive health care were going through.
When the first ob.gyn. responded to my request by expressing concerns around anonymity and personal safety, I was shocked – but I shouldn’t have been. For starters, there is already a storied history in this country of deadly attacks on abortion providers. David Gunn, MD; Barnett Slepian, MD; and George Tiller, MD, were all tragically murdered by antiabortion extremists. Then, there’s the existence of websites that keep logs of abortion providers and sometimes include photos, office contact information, or even home addresses.
The idea that any reproductive health care provider should have to think twice before offering their uniquely qualified opinion is profoundly disturbing, nearly as disturbing as the Supreme Court’s decision itself. But it’s more critical than ever for ob.gyn. voices to be amplified. This is the time for the healthcare community to rally around women’s health providers, to learn from them, to support them.
I asked ob.gyns. around the country to tell me what they were thinking and feeling on the day that Roe v. Wade was overturned. We agreed to keep the responses anonymous, given that several people expressed very understandable safety concerns.
Here’s what they had to say.
Tennessee ob.gyn.
“Today is an emotionally charged day for many people in this country, yet as I type this, with my ob.gyn. practice continuing around me, with my own almost 10-week pregnancy growing inside me, I feel quite blunted. I feel powerless to answer questions that are variations on ‘what next?’ or ‘how do we fight back?’ All I can think of is, I am so glad I do not have anyone on my schedule right now who does not want to be pregnant. But what will happen when that eventually changes? What about my colleagues who do have these patients on their schedules today? On a personal level, what if my prenatal genetic testing comes back abnormal? How can we so blatantly disregard a separation of church and state in this country? What ways will our government interfere with my practice next? My head is spinning, but I have to go see my next patient. She is a 25-year-old who is here to have an IUD placed, and that seems like the most important thing I can do today.”
South Carolina ob.gyn.
“I’m really scared. For my patients and for myself. I don’t know how to be a good ob.gyn. if my ability to offer safe and accessible abortion care is being threatened.”
Massachusetts ob.gyn.
“Livid and devastated and sad and terrified.”
California family planning specialist
“The fact is that about one in four people with uteruses have had an abortion. I can’t tell you how many abortions I’ve provided for people who say that they don’t ‘believe’ in them or that they thought they’d never be in this situation. ... The fact is that pregnancy is a life-threatening condition in and of itself. I am an ob.gyn., a medical doctor, and an abortion provider. I will not stop providing abortions or helping people access them. I will dedicate my life to ensuring this right to bodily autonomy. Today I am devastated by the Supreme Court’s decision to force parenthood that will result in increased maternal mortality. I am broken, but I have never been more proud to be an abortion provider.”
New York ob.gyn.
“Grateful to live in a state and work for a hospital where I can provide abortions but feel terrible for so many people less fortunate and underserved.”
Illinois maternal-fetal medicine specialist
“As a maternal-fetal medicine specialist, I fear for my patients who are at the highest risk of pregnancy complications having their freedom taken away. For the tragic ultrasound findings that make a pregnant person carry a baby who will never live. For the patients who cannot use most forms of contraception because of their medical comorbidities. For the patients who are victims of intimate partner violence or under the influence of their culture, to continue having children regardless of their desires or their health. ... The freedom to prevent or end a pregnancy has enabled women to become independent and productive members of society on their own terms, with or without children. My heart breaks for the children and adolescents and adults who are being told they are second-class citizens, not worthy of making their own decisions. Politicians and Supreme Court justices are not in the clinic room, ultrasound suite, operating room, or delivery room when we have these intense conversations and pregnancy outcomes. They have no idea that of which they speak, and it’s unconscionable that they can determine what healthcare decisions my patients can make for their own lives. Nobody knows a body better than the patient themselves.”
Texas ob.gyn.
“In the area where I live and practice, it feels like guns and the people who use them have more legal rights than people with uteruses in desperate or life-threatening situations. I’m afraid for my personal safety as a women’s health practitioner in this political climate. I feel helpless, but I’m supposed to be able to help my patients.”
Missouri family planning specialist
“Abortion is an essential part of healthcare, and the only people that should get a say in it are the patient and their doctor. Period. The fact that some far-off court without any medical expertise can insert itself into individual medical decisions is oppressive and unethical.”
Georgia ob.gyn.
“I can’t even think straight right now. I feel sick. Honestly, I’ve been thinking about moving for a long time now. Somewhere where I would actually be able to offer good, comprehensive care.”
New York ob.gyn.
“I graduated from my ob.gyn. residency hours after the Roe v. Wade news broke. It was so emotional for me. I’ve dedicated my life to caring for people with uteruses and I will not let this heartbreaking news change that. I feel more committed than ever to women’s health. I fully plan to continue delivering babies, providing contraception, and performing abortions. I will be there to help women with desired pregnancies who received unspeakably bad news about fetal anomalies. I will be there to help women with life-threatening pregnancy complications before fetal viability. I will be there to help women with ectopic pregnancies. I will be there to help women who were raped or otherwise forced into pregnancy. I will always be there to help women.”
Dr. Croll is a neurovascular fellow at New York University Langone Health. She disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
My picks for best of ASCO 2022
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
AT ASCO 2022
Adjuvant vs. neoadjuvant? What has ASCO 2022 taught us regarding resectable NSCLC?
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
for non–small cell lung cancer (NSCLC). While there has been some notable progress in this area, we need phase 3 trials that compare the two therapeutic approaches.
Investigators reporting at the 2022 annual meeting of American Society of Clinical Oncology focused primarily on neoadjuvant treatment, which I’ll address here.
In the randomized, phase 2 NADIM II clinical trial reported at the meeting, researchers expanded on the results of NADIM published in 2020 in the Lancet Oncology and in May 2022 in the Journal of Clinical Oncology along with CheckMate 816 results published in the New England Journal of Medicine.
In each of these three studies, researchers compared nivolumab plus chemotherapy versus chemotherapy alone (abstract 8501) as a neoadjuvant treatment for resectable stage IIIA NSCLC. In the study reported at ASCO 2022, patients with resectable clinical stage IIIA-B (per American Joint Committee on Cancer 8th edition) NSCLC and no known EGFR/ALK alterations, were randomized to receive preoperative nivolumab plus chemotherapy (paclitaxel and carboplatin; n = 57) or chemotherapy (n = 29) alone followed by surgery.
The primary endpoint was pathological complete response (pCR); secondary endpoints included major pathological response, safety and tolerability, impact on surgical issues such as delayed or canceled surgeries or length of hospital stay, overall survival and progression free survival. The pCR rate was 36.8% in the neoadjuvant nivolumab plus chemotherapy arm and 6.9% in the chemotherapy alone arm. (P = .0068). 25% of patients on the nivolumab plus chemo arm had grade 3-4 adverse events, compared with 10.3% in the control arm. 93% of patients on the nivolumab plus chemo arm underwent definitive surgery whereas 69.0% of the patients on the chemo alone arm had definitive surgery. (P = .008)
What else did we learn about neoadjuvant treatment at the meeting?
Investigators looking at the optimal number of neoadjuvant cycles (abstract 8500) found that three cycles of sintilimab (an investigational PD-1 inhibitor) produced a numerically higher major pathological response rate, compared with two cycles (when given in concert with platinum-doublet chemotherapy). And, neoadjuvant chemoradiotherapy does not result in significant survival benefits when compared with neoadjuvant chemotherapy alone (abstract 8503).
Of course, when it comes to resectable NSCLC, the goal of treatment is to increase the cure rate and improve survival. No randomized studies have reported yet on overall survival, probably because they are too immature. Instead, disease-free survival (DFS) or event-free survival (EFS) are often used as surrogate endpoints. Since none of the studies reported at ASCO reported on DFS or EFS, we need to look elsewhere. CheckMate 816 was a phase 3 study which randomized patients with stages IB-IIIA NSCLC to receive neoadjuvant nivolumab plus platinum-based chemotherapy or neoadjuvant platinum-based chemotherapy alone, followed by resection. The median EFS was 31.6 months with nivolumab plus chemotherapy and 20.8 months with chemotherapy alone (P = .005). The percentage of patients with a pCR was 24.0% and 2.2%, respectively (P < .001).
We all know one has to be careful when doing cross-trial comparisons as these studies differ by the percentage of patients with various stages of disease, the type of immunotherapy and chemotherapy used, etc. However, I think we can agree that neoadjuvant chemoimmunotherapy results in better outcomes than chemotherapy alone.
Of course, resectable NSCLC is, by definition, resectable. And traditionally, resection is followed by adjuvant chemotherapy to eradicate micrometastases. Unfortunately, the current standard of care for completely resected early-stage NSCLC (stage I [tumor ≥ 4 cm] to IIIA) involves adjuvant platinum-based combination chemotherapy which results in only a modest 4%-5% improvement in survival versus observation.
Given these modest results, as in the neoadjuvant space, investigators have looked at the benefit of adding immunotherapy to adjuvant chemotherapy. One such study has been reported. IMpower 010 randomly assigned patients with completely resected stage IB (tumors ≥ 4 cm) to IIIA NSCLC, whose tumor cells expressed at least 1% PD-L1, to receive adjuvant atezolizumab or best supportive care after adjuvant platinum-based chemotherapy. In the stage II-IIIA population whose tumors expressed PD-L1 on 1% or more of tumor cells, 3-year DFS rates were 60% and 48% in the atezolizumab and best supportive care arms, respectively (hazard ratio, 0·66 P =·.0039). In all patients in the stage II-IIIA population, the 3-year DFS rates were 56% in the atezolizumab group and 49% in the best supportive care group, (HR, 0.79; P = .020).
KEYNOTE-091, reported at the 2021 annual meeting of the European Society for Medical Oncology, randomized early-stage NSCLC patients following complete resection and adjuvant chemotherapy to pembrolizumab or placebo. Median DFS for the overall population was 53.6 months for patients in the pembro arm versus 42 months in the placebo arm (HR, 0.76; P = .0014). Interestingly, the benefit was not seen in patients with PD-L1 with at least 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm (HR, 0.82; P = .14). Although the contradictory results of PD-L1 as a biomarker is puzzling, I think we can agree that the addition of immunotherapy following adjuvant chemotherapy improves outcomes compared to adjuvant chemotherapy alone.
What to do when a patient presents with resectable disease?
Cross-trial comparisons are fraught with danger. Until we have a phase 3 study comparing concurrent neoadjuvant chemo/immunotherapy with concurrent adjuvant chemo/immunotherapy, I do not think we can answer the question “which is better?” However, there are some caveats to keep in mind when deciding on which approach to recommend to our patients: First, neoadjuvant treatment requires biomarker testing to ensure the patient does not have EGFR or ALK mutations. This will necessitate a delay in the operation. Will patients be willing to wait? Will the surgeon? Or, would patients prefer to proceed with surgery while the results are pending? Yes, neoadjuvant therapy gives you information regarding the pCR rate, but does that help you in subsequent management of the patient? We do not know.
Secondly, the two adjuvant studies used adjuvant chemotherapy followed by adjuvant immunotherapy, as contrasted to the neoadjuvant study which used concurrent chemo/immunotherapy. Given the longer duration of treatment in postoperative sequential adjuvant studies, there tends to be more drop off because of patients being unwilling or unfit postoperatively to receive long courses of therapy. In IMpower 010, 1,269 patients completed adjuvant chemotherapy; 1,005 were randomized, and of the 507 assigned to the atezolizumab/chemo group, only 323 completed treatment.
Finally, we must beware of using neoadjuvant chemo/immunotherapy to “down-stage” a patient. KEYNOTE-091 included patients with IIIA disease and no benefit to adjuvant chemotherapy followed by immunotherapy was found in this subgroup of patients, which leads me to wonder if these patients were appropriately selected as surgical candidates. In the NADIM II trials, 9 of 29 patients on the neoadjuvant chemotherapy were not resected.
So, many questions remain. In addition to the ones we’ve raised, there is a clear and immediate need for predictive and prognostic biomarkers. In the NADIM II trial, PD-L1 expression was a predictive biomarker of response. The pCR rate for patients with a PD-L1 tumor expression of less than 1%, 1%-49%, and 50% or higher was 15%, 41.7%, and 61.1%, respectively. However, in KEYNOTE-091, the benefit was not seen in patients with PD-L1 of at least than 50%, where the 18-month DFS rate was 71.7% in the pembro arm and 70.2% in the placebo arm.
Another possible biomarker: circulating tumor DNA. In the first NADIM study, three low pretreatment levels of ctDNA were significantly associated with improved progression-free survival and overall survival (HR, 0.20 and HR, 0.07, respectively). Although clinical response did not predict survival outcomes, undetectable ctDNA levels after neoadjuvant treatment were significantly associated with progression-free survival and overall survival (HR, 0.26 and HR0.04, respectively). Similarly, in CheckMate 816, clearance of ctDNA was associated with longer EFS in patients with ctDNA clearance than in those without ctDNA clearance in both the nivolumab/chemotherapy group (HR, 0.60) and the chemotherapy-alone group (HR, 0.63).
Hopefully, ASCO 2023 will provide more answers.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Racial disparities in endometrial cancer
Endometrial cancer (EC) is the most common gynecologic malignancy and is the fourth most common cancer seen in U.S. women. It is the only major cancer that has continued to see a rise in incidence and mortality for the past 2 decades, and it is anticipated that nearly 66,000 new cases of EC will be diagnosed this year with 12,550 deaths.1 Given that the well-established risk factors for developing EC including obesity, diabetes, and insulin resistance, the obesity epidemic is indisputably playing a significant role in the increasing incidence.
Historically, White women were thought to have the highest incidence of EC; however, this incidence rate did not account for hysterectomy prevalence, which can vary widely by numerous factors including age, race, ethnicity, and geographic region. When correcting EC incidence rates for prevalence of hysterectomy, Black women have had the highest incidence of EC since 2007, and rates continue to climb.2 In fact, the average annual percent change (APC) in EC incidence from 2000 to 2015 was stable for White women at 0.2% while Black women had a near order of magnitude greater APC at 2.1%.2
Differing incidence rates of EC can also be seen by histologic subtype. Endometrioid EC is the more common and less lethal histology of EC that often coincides with the type I classification of EC. These tumors are estrogen driven; therefore, they are associated with conditions resulting in excess estrogen (for example, anovulation, obesity, and hyperlipidemia). Nonendometrioid histologies, primarily composed of serous tumors, are more rare, are typically more aggressive, are not estrogen driven, and are commonly classified as type II tumors. Racial differences between type I and type II tumors are seen with White women more commonly being diagnosed with type I tumors while Black women more typically have type II tumors. White women have the greatest incidence rate of endometrioid EC with an APC that remained relatively unchanged from 2000 to 2015. Black women’s APC in incidence rate of endometrioid EC has increased during this same period at 1.3%. For nonendometrioid tumors, an increasing incidence is seen in all races and ethnicities; however, Black women have a much higher incidence of these tumors, with a rate that continues to increase at an APC of 3.2%.2
EC incidence is increasing with a particularly concerning rise in those who report Black race, but are these same disparities being seen in EC mortality? Unfortunately, drastic disparities are seen in survival data for Black women afflicted with EC. Black patients are more likely to be diagnosed with advanced or metastatic EC and less likely to be diagnosed with localized tumors. While being diagnosed with a more advanced stage of disease does affect survival in EC, Black patients have worse survival regardless of stage of disease at the time of diagnosis.1 As discussed earlier, the more aggressive type II tumors are composed of nonendometrioid histologies and are more common in Black women. This could lead to the false assumption that these higher-risk tumors are why Black women are disproportionately dying from EC; however, when examining survival by histologic subtype, Black women are more frequently dying from the lower-risk endometrioid EC regardless of stage of disease. The same disparate survival outcomes are also seen in nonendometrioid histologies.2 Thus, Black patients have the lowest survival rates irrespective of stage at diagnosis or histologic subtype.
The disparities seen in EC mortality are not new. They can be seen in data for over 30 years and are only widening. While there has been an increase in mortality rates from EC across all races and ethnicities from 2015 to 2019 compared with 1990 to 1994, the mortality rate ratio for Black women compared with White women has increased from 1.83 in 1990-1994 to 1.98 in 2015-2019.3 In the early 1990s, the risk of death from ovarian cancer was twice that of EC. The mortality of EC is now similar to that of ovarian cancer. This threshold in mortality ratio of EC to ovarian cancer has already been seen in Black women, who have experienced greater mortality in EC compared with ovarian cancer since 2005. In fact, the EC mortality of Black women in 2019 was similar to the mortality of White women with ovarian cancer nearly 30 years ago.3
Decades of data have demonstrated the glaring racial disparities seen in EC, and yet, no significant progress has been made in addressing this inequity. Oncology research is now beginning to move beyond describing these differences to a strategy of achieving equitable cancer care. While the study frameworks and novel investigations aimed at addressing the disparities in EC is outside the scope of this article, disparities in clinical trial enrollment continue to exist.
A recent example can be seen in the practice-changing KEYNOTE-775 trial, which led to the Food and Drug Administration approval of lenvatinib plus pembrolizumab in EC treatment.4 A total of 827 patients with EC that progressed or recurred following treatment with platinum-based chemotherapy were enrolled in this multinational, multicenter trial. Thirty-one (3.7%) of the patients enrolled were Black. Of those who were enrolled in the United States, 14% were Black. The authors report that this proportion of Black patients in the United States is consistent with 2020 census data, which reported 13.4% of people identified as Black. However, using census data as a benchmark for equitable enrollment is inappropriate. Certain demographic groups are historically more difficult to count, and the COVID-19 pandemic exacerbated the challenge in obtaining an accurate count through job loss, government distrust, and access restrictions resulting in an estimated net undercount of 2.45% in those who report Black race.5 Composition of trial enrollment should mirror the population that will be affected by the study results. As advanced EC disproportionately affects Black patients, their enrollment must be higher in these pivotal trials. How else are we to know if these novel therapeutics will work in the population that is most afflicted by EC?
Future studies must account for socioeconomic factors while acknowledging the role of social determinants of health. It is imperative that we use the knowledge that race is a social construct created to control access to power and that there are biologic responses to environmental stresses, including that of racism, affecting health and disease. Changes at every level, from individual practitioners up to federal policies, will need to be enacted or else the unacceptable status quo will continue.
Dr. Burkett is a clinical fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina at Chapel Hill.
References
1. Siegel RL et al. CA Cancer J Clin. 2022;72:7-33.
2. Clarke MA et al. J Clin Oncol. 2019;37:1895-908.
3. Giaquinto AN et al. Obstet Gynecol. 2022;139:440-2.
4. Makker V et al. N Engl J Med. 2022;386:437-48.
5. Elliott D et al. Simulating the 2020 Census: Miscounts and the fairness of outcomes. Urban Institute; 2021.
Endometrial cancer (EC) is the most common gynecologic malignancy and is the fourth most common cancer seen in U.S. women. It is the only major cancer that has continued to see a rise in incidence and mortality for the past 2 decades, and it is anticipated that nearly 66,000 new cases of EC will be diagnosed this year with 12,550 deaths.1 Given that the well-established risk factors for developing EC including obesity, diabetes, and insulin resistance, the obesity epidemic is indisputably playing a significant role in the increasing incidence.
Historically, White women were thought to have the highest incidence of EC; however, this incidence rate did not account for hysterectomy prevalence, which can vary widely by numerous factors including age, race, ethnicity, and geographic region. When correcting EC incidence rates for prevalence of hysterectomy, Black women have had the highest incidence of EC since 2007, and rates continue to climb.2 In fact, the average annual percent change (APC) in EC incidence from 2000 to 2015 was stable for White women at 0.2% while Black women had a near order of magnitude greater APC at 2.1%.2
Differing incidence rates of EC can also be seen by histologic subtype. Endometrioid EC is the more common and less lethal histology of EC that often coincides with the type I classification of EC. These tumors are estrogen driven; therefore, they are associated with conditions resulting in excess estrogen (for example, anovulation, obesity, and hyperlipidemia). Nonendometrioid histologies, primarily composed of serous tumors, are more rare, are typically more aggressive, are not estrogen driven, and are commonly classified as type II tumors. Racial differences between type I and type II tumors are seen with White women more commonly being diagnosed with type I tumors while Black women more typically have type II tumors. White women have the greatest incidence rate of endometrioid EC with an APC that remained relatively unchanged from 2000 to 2015. Black women’s APC in incidence rate of endometrioid EC has increased during this same period at 1.3%. For nonendometrioid tumors, an increasing incidence is seen in all races and ethnicities; however, Black women have a much higher incidence of these tumors, with a rate that continues to increase at an APC of 3.2%.2
EC incidence is increasing with a particularly concerning rise in those who report Black race, but are these same disparities being seen in EC mortality? Unfortunately, drastic disparities are seen in survival data for Black women afflicted with EC. Black patients are more likely to be diagnosed with advanced or metastatic EC and less likely to be diagnosed with localized tumors. While being diagnosed with a more advanced stage of disease does affect survival in EC, Black patients have worse survival regardless of stage of disease at the time of diagnosis.1 As discussed earlier, the more aggressive type II tumors are composed of nonendometrioid histologies and are more common in Black women. This could lead to the false assumption that these higher-risk tumors are why Black women are disproportionately dying from EC; however, when examining survival by histologic subtype, Black women are more frequently dying from the lower-risk endometrioid EC regardless of stage of disease. The same disparate survival outcomes are also seen in nonendometrioid histologies.2 Thus, Black patients have the lowest survival rates irrespective of stage at diagnosis or histologic subtype.
The disparities seen in EC mortality are not new. They can be seen in data for over 30 years and are only widening. While there has been an increase in mortality rates from EC across all races and ethnicities from 2015 to 2019 compared with 1990 to 1994, the mortality rate ratio for Black women compared with White women has increased from 1.83 in 1990-1994 to 1.98 in 2015-2019.3 In the early 1990s, the risk of death from ovarian cancer was twice that of EC. The mortality of EC is now similar to that of ovarian cancer. This threshold in mortality ratio of EC to ovarian cancer has already been seen in Black women, who have experienced greater mortality in EC compared with ovarian cancer since 2005. In fact, the EC mortality of Black women in 2019 was similar to the mortality of White women with ovarian cancer nearly 30 years ago.3
Decades of data have demonstrated the glaring racial disparities seen in EC, and yet, no significant progress has been made in addressing this inequity. Oncology research is now beginning to move beyond describing these differences to a strategy of achieving equitable cancer care. While the study frameworks and novel investigations aimed at addressing the disparities in EC is outside the scope of this article, disparities in clinical trial enrollment continue to exist.
A recent example can be seen in the practice-changing KEYNOTE-775 trial, which led to the Food and Drug Administration approval of lenvatinib plus pembrolizumab in EC treatment.4 A total of 827 patients with EC that progressed or recurred following treatment with platinum-based chemotherapy were enrolled in this multinational, multicenter trial. Thirty-one (3.7%) of the patients enrolled were Black. Of those who were enrolled in the United States, 14% were Black. The authors report that this proportion of Black patients in the United States is consistent with 2020 census data, which reported 13.4% of people identified as Black. However, using census data as a benchmark for equitable enrollment is inappropriate. Certain demographic groups are historically more difficult to count, and the COVID-19 pandemic exacerbated the challenge in obtaining an accurate count through job loss, government distrust, and access restrictions resulting in an estimated net undercount of 2.45% in those who report Black race.5 Composition of trial enrollment should mirror the population that will be affected by the study results. As advanced EC disproportionately affects Black patients, their enrollment must be higher in these pivotal trials. How else are we to know if these novel therapeutics will work in the population that is most afflicted by EC?
Future studies must account for socioeconomic factors while acknowledging the role of social determinants of health. It is imperative that we use the knowledge that race is a social construct created to control access to power and that there are biologic responses to environmental stresses, including that of racism, affecting health and disease. Changes at every level, from individual practitioners up to federal policies, will need to be enacted or else the unacceptable status quo will continue.
Dr. Burkett is a clinical fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina at Chapel Hill.
References
1. Siegel RL et al. CA Cancer J Clin. 2022;72:7-33.
2. Clarke MA et al. J Clin Oncol. 2019;37:1895-908.
3. Giaquinto AN et al. Obstet Gynecol. 2022;139:440-2.
4. Makker V et al. N Engl J Med. 2022;386:437-48.
5. Elliott D et al. Simulating the 2020 Census: Miscounts and the fairness of outcomes. Urban Institute; 2021.
Endometrial cancer (EC) is the most common gynecologic malignancy and is the fourth most common cancer seen in U.S. women. It is the only major cancer that has continued to see a rise in incidence and mortality for the past 2 decades, and it is anticipated that nearly 66,000 new cases of EC will be diagnosed this year with 12,550 deaths.1 Given that the well-established risk factors for developing EC including obesity, diabetes, and insulin resistance, the obesity epidemic is indisputably playing a significant role in the increasing incidence.
Historically, White women were thought to have the highest incidence of EC; however, this incidence rate did not account for hysterectomy prevalence, which can vary widely by numerous factors including age, race, ethnicity, and geographic region. When correcting EC incidence rates for prevalence of hysterectomy, Black women have had the highest incidence of EC since 2007, and rates continue to climb.2 In fact, the average annual percent change (APC) in EC incidence from 2000 to 2015 was stable for White women at 0.2% while Black women had a near order of magnitude greater APC at 2.1%.2
Differing incidence rates of EC can also be seen by histologic subtype. Endometrioid EC is the more common and less lethal histology of EC that often coincides with the type I classification of EC. These tumors are estrogen driven; therefore, they are associated with conditions resulting in excess estrogen (for example, anovulation, obesity, and hyperlipidemia). Nonendometrioid histologies, primarily composed of serous tumors, are more rare, are typically more aggressive, are not estrogen driven, and are commonly classified as type II tumors. Racial differences between type I and type II tumors are seen with White women more commonly being diagnosed with type I tumors while Black women more typically have type II tumors. White women have the greatest incidence rate of endometrioid EC with an APC that remained relatively unchanged from 2000 to 2015. Black women’s APC in incidence rate of endometrioid EC has increased during this same period at 1.3%. For nonendometrioid tumors, an increasing incidence is seen in all races and ethnicities; however, Black women have a much higher incidence of these tumors, with a rate that continues to increase at an APC of 3.2%.2
EC incidence is increasing with a particularly concerning rise in those who report Black race, but are these same disparities being seen in EC mortality? Unfortunately, drastic disparities are seen in survival data for Black women afflicted with EC. Black patients are more likely to be diagnosed with advanced or metastatic EC and less likely to be diagnosed with localized tumors. While being diagnosed with a more advanced stage of disease does affect survival in EC, Black patients have worse survival regardless of stage of disease at the time of diagnosis.1 As discussed earlier, the more aggressive type II tumors are composed of nonendometrioid histologies and are more common in Black women. This could lead to the false assumption that these higher-risk tumors are why Black women are disproportionately dying from EC; however, when examining survival by histologic subtype, Black women are more frequently dying from the lower-risk endometrioid EC regardless of stage of disease. The same disparate survival outcomes are also seen in nonendometrioid histologies.2 Thus, Black patients have the lowest survival rates irrespective of stage at diagnosis or histologic subtype.
The disparities seen in EC mortality are not new. They can be seen in data for over 30 years and are only widening. While there has been an increase in mortality rates from EC across all races and ethnicities from 2015 to 2019 compared with 1990 to 1994, the mortality rate ratio for Black women compared with White women has increased from 1.83 in 1990-1994 to 1.98 in 2015-2019.3 In the early 1990s, the risk of death from ovarian cancer was twice that of EC. The mortality of EC is now similar to that of ovarian cancer. This threshold in mortality ratio of EC to ovarian cancer has already been seen in Black women, who have experienced greater mortality in EC compared with ovarian cancer since 2005. In fact, the EC mortality of Black women in 2019 was similar to the mortality of White women with ovarian cancer nearly 30 years ago.3
Decades of data have demonstrated the glaring racial disparities seen in EC, and yet, no significant progress has been made in addressing this inequity. Oncology research is now beginning to move beyond describing these differences to a strategy of achieving equitable cancer care. While the study frameworks and novel investigations aimed at addressing the disparities in EC is outside the scope of this article, disparities in clinical trial enrollment continue to exist.
A recent example can be seen in the practice-changing KEYNOTE-775 trial, which led to the Food and Drug Administration approval of lenvatinib plus pembrolizumab in EC treatment.4 A total of 827 patients with EC that progressed or recurred following treatment with platinum-based chemotherapy were enrolled in this multinational, multicenter trial. Thirty-one (3.7%) of the patients enrolled were Black. Of those who were enrolled in the United States, 14% were Black. The authors report that this proportion of Black patients in the United States is consistent with 2020 census data, which reported 13.4% of people identified as Black. However, using census data as a benchmark for equitable enrollment is inappropriate. Certain demographic groups are historically more difficult to count, and the COVID-19 pandemic exacerbated the challenge in obtaining an accurate count through job loss, government distrust, and access restrictions resulting in an estimated net undercount of 2.45% in those who report Black race.5 Composition of trial enrollment should mirror the population that will be affected by the study results. As advanced EC disproportionately affects Black patients, their enrollment must be higher in these pivotal trials. How else are we to know if these novel therapeutics will work in the population that is most afflicted by EC?
Future studies must account for socioeconomic factors while acknowledging the role of social determinants of health. It is imperative that we use the knowledge that race is a social construct created to control access to power and that there are biologic responses to environmental stresses, including that of racism, affecting health and disease. Changes at every level, from individual practitioners up to federal policies, will need to be enacted or else the unacceptable status quo will continue.
Dr. Burkett is a clinical fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina at Chapel Hill.
References
1. Siegel RL et al. CA Cancer J Clin. 2022;72:7-33.
2. Clarke MA et al. J Clin Oncol. 2019;37:1895-908.
3. Giaquinto AN et al. Obstet Gynecol. 2022;139:440-2.
4. Makker V et al. N Engl J Med. 2022;386:437-48.
5. Elliott D et al. Simulating the 2020 Census: Miscounts and the fairness of outcomes. Urban Institute; 2021.
Moderate activity versus sweat equity
It’s no secret that the fitness level of all age groups in our country is poor. A recent study in Pediatrics sharpens the focus on the question of how we might address the problem in the teenage population. Based in England, the investigators placed wrist accelerometers on their 13- and 14-year-old subjects who were then assessed using shuttle runs at progressively faster speeds.
The researchers found that the participants’ cardiorespiratory fitness improved as the subjects’ time doing vigorous activity increased up to 20 minutes and then plateaued. The study authors could not prove that the vigorous activity caused the increased in fitness. However, they were impressed by the plateau phenomenon and suggest that this might suggest a change in the recommendations by the World Health Organization and U.S. Department of Health & Human Services which currently call for 60 minutes of moderate to vigorous physical activity per day for adolescents
At first blush a shift down to 20 minutes of vigorous activity would appear to be workable and achievable. This would be particularly true for public school systems that are already struggling to get any kind of activity shoehorned into their schedules that are already crammed in an attempt to address mandated academic achievement goals. Freeing up an additional 40 minutes of the school day and yielding improved cardiorespiratory fitness sounds like a win-win.
But, let’s take a deep breath and for a few moments return to the world of reality. First, how many school systems are providing that 60 minutes of moderate activity (let’s forget the vigorous piece for the moment) included in the current WHO/HHS recommendations? Next, let’s take a look at what “vigorous” activity means. There are variety of definitions but in general they include sweating, flushing, and dyspnea to the point of having difficulty speaking.
Let’s just focus on the “sweating” part. To me that sounds like an activity that would require some wardrobe alteration at a minimum and very likely a locker room and a shower. Those can be fightin’ words for many teenagers. Even if a school can provide adequate locker room and shower infrastructure change-ups and showers are time-gobbling activities. And, more realistically, what are the chances of getting body image–challenged adolescents to willingly take advantage of them? You don’t have to talk to very many adults before you will hear stories of discomfort and embarrassment resulting from forced locker room and shower experiences. When I was a teenager the only way you could flunk physical education was to refuse to go in the locker room and “change up.” I think or at least hope that physical educators are more sensitive to the fragility of their adolescents students. But, the bottom line is that creating a curriculum that will improve cardiorespiratory fitness is fraught with challenges most school systems can’t address. It’s sad but true.
So, where does that leave us? This new study from England may be helpful for families who are caught in a time crunch and looking improve their fitness or for the physical educator who would like to help his/her motivated students get on a healthier track. But, this study should not prompt us to throw up our hands and toss out the current recommendations of an hour of moderate activity. As unrealistic as it may be for most school systems it allows for the injection of physical activity into academic settings where creative educators can offer things like walking lectures and field trips. It all boils down to the fact that some activity is better than none at all with or without the sweat equity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
It’s no secret that the fitness level of all age groups in our country is poor. A recent study in Pediatrics sharpens the focus on the question of how we might address the problem in the teenage population. Based in England, the investigators placed wrist accelerometers on their 13- and 14-year-old subjects who were then assessed using shuttle runs at progressively faster speeds.
The researchers found that the participants’ cardiorespiratory fitness improved as the subjects’ time doing vigorous activity increased up to 20 minutes and then plateaued. The study authors could not prove that the vigorous activity caused the increased in fitness. However, they were impressed by the plateau phenomenon and suggest that this might suggest a change in the recommendations by the World Health Organization and U.S. Department of Health & Human Services which currently call for 60 minutes of moderate to vigorous physical activity per day for adolescents
At first blush a shift down to 20 minutes of vigorous activity would appear to be workable and achievable. This would be particularly true for public school systems that are already struggling to get any kind of activity shoehorned into their schedules that are already crammed in an attempt to address mandated academic achievement goals. Freeing up an additional 40 minutes of the school day and yielding improved cardiorespiratory fitness sounds like a win-win.
But, let’s take a deep breath and for a few moments return to the world of reality. First, how many school systems are providing that 60 minutes of moderate activity (let’s forget the vigorous piece for the moment) included in the current WHO/HHS recommendations? Next, let’s take a look at what “vigorous” activity means. There are variety of definitions but in general they include sweating, flushing, and dyspnea to the point of having difficulty speaking.
Let’s just focus on the “sweating” part. To me that sounds like an activity that would require some wardrobe alteration at a minimum and very likely a locker room and a shower. Those can be fightin’ words for many teenagers. Even if a school can provide adequate locker room and shower infrastructure change-ups and showers are time-gobbling activities. And, more realistically, what are the chances of getting body image–challenged adolescents to willingly take advantage of them? You don’t have to talk to very many adults before you will hear stories of discomfort and embarrassment resulting from forced locker room and shower experiences. When I was a teenager the only way you could flunk physical education was to refuse to go in the locker room and “change up.” I think or at least hope that physical educators are more sensitive to the fragility of their adolescents students. But, the bottom line is that creating a curriculum that will improve cardiorespiratory fitness is fraught with challenges most school systems can’t address. It’s sad but true.
So, where does that leave us? This new study from England may be helpful for families who are caught in a time crunch and looking improve their fitness or for the physical educator who would like to help his/her motivated students get on a healthier track. But, this study should not prompt us to throw up our hands and toss out the current recommendations of an hour of moderate activity. As unrealistic as it may be for most school systems it allows for the injection of physical activity into academic settings where creative educators can offer things like walking lectures and field trips. It all boils down to the fact that some activity is better than none at all with or without the sweat equity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
It’s no secret that the fitness level of all age groups in our country is poor. A recent study in Pediatrics sharpens the focus on the question of how we might address the problem in the teenage population. Based in England, the investigators placed wrist accelerometers on their 13- and 14-year-old subjects who were then assessed using shuttle runs at progressively faster speeds.
The researchers found that the participants’ cardiorespiratory fitness improved as the subjects’ time doing vigorous activity increased up to 20 minutes and then plateaued. The study authors could not prove that the vigorous activity caused the increased in fitness. However, they were impressed by the plateau phenomenon and suggest that this might suggest a change in the recommendations by the World Health Organization and U.S. Department of Health & Human Services which currently call for 60 minutes of moderate to vigorous physical activity per day for adolescents
At first blush a shift down to 20 minutes of vigorous activity would appear to be workable and achievable. This would be particularly true for public school systems that are already struggling to get any kind of activity shoehorned into their schedules that are already crammed in an attempt to address mandated academic achievement goals. Freeing up an additional 40 minutes of the school day and yielding improved cardiorespiratory fitness sounds like a win-win.
But, let’s take a deep breath and for a few moments return to the world of reality. First, how many school systems are providing that 60 minutes of moderate activity (let’s forget the vigorous piece for the moment) included in the current WHO/HHS recommendations? Next, let’s take a look at what “vigorous” activity means. There are variety of definitions but in general they include sweating, flushing, and dyspnea to the point of having difficulty speaking.
Let’s just focus on the “sweating” part. To me that sounds like an activity that would require some wardrobe alteration at a minimum and very likely a locker room and a shower. Those can be fightin’ words for many teenagers. Even if a school can provide adequate locker room and shower infrastructure change-ups and showers are time-gobbling activities. And, more realistically, what are the chances of getting body image–challenged adolescents to willingly take advantage of them? You don’t have to talk to very many adults before you will hear stories of discomfort and embarrassment resulting from forced locker room and shower experiences. When I was a teenager the only way you could flunk physical education was to refuse to go in the locker room and “change up.” I think or at least hope that physical educators are more sensitive to the fragility of their adolescents students. But, the bottom line is that creating a curriculum that will improve cardiorespiratory fitness is fraught with challenges most school systems can’t address. It’s sad but true.
So, where does that leave us? This new study from England may be helpful for families who are caught in a time crunch and looking improve their fitness or for the physical educator who would like to help his/her motivated students get on a healthier track. But, this study should not prompt us to throw up our hands and toss out the current recommendations of an hour of moderate activity. As unrealistic as it may be for most school systems it allows for the injection of physical activity into academic settings where creative educators can offer things like walking lectures and field trips. It all boils down to the fact that some activity is better than none at all with or without the sweat equity.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Adolescent female with rash on the arms and posterior legs
Erythema annulare centrifugum
A thorough body examination failed to reveal any other rashes or lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. A potassium hydroxide analysis of skin scrapings was negative for fungal elements. Punch biopsy of the skin on the left arm revealed focal intermittent parakeratosis, mildly acanthotic and spongiotic epidermis, and a tight superficial perivascular chronic dermatitis consisting of lymphocytes and histiocytes (Figures). Given these findings, a diagnosis of erythema annulare centrifugum (EAC) was rendered.
EAC is a rare, reactive skin rash characterized by redness (erythema) and ring-shaped lesions (annulare) that slowly spread from the center (centrifugum). The lesions present with a characteristic trailing scale on the inner border of the erythematous ring. Lesions may be asymptomatic or mildly pruritic and commonly involve the trunk, buttocks, hips, and upper legs. It is important to note that its duration is highly variable, ranging from weeks to decades, with most cases persisting for 9 months. EAC typically affects young or middle-aged adults but can occur at any age.
Although the etiology of EAC is unknown, it is believed to be a hypersensitivity reaction to a foreign antigen. Cutaneous fungal infections are commonly reported as triggers as well as other viral infections, medications, malignancy, underlying systemic disease, and certain foods. Treatment depends on the underlying condition and removing the implicated agent. However, most cases of EAC are idiopathic and self-limiting. It is possible that our patient’s prior history of tinea capitis could have triggered the skin lesions suggestive of EAC, but interestingly, these lesions did not go away after the fungal infection was cleared and have continued to recur. For patients with refractory lesions or treatment of patients without an identifiable cause, the use of oral antimicrobials has been proposed. Medications such as azithromycin, erythromycin, fluconazole, and metronidazole have been reported to be helpful in some patients with refractory EAC. Our patient wanted to continue topical treatment with betamethasone as needed and may consider antimicrobial therapy if the lesions continue to recur.
Tinea corporis refers to a superficial fungal infection of the skin. It may present as one or more asymmetrical, annular, pruritic plaques with a raised scaly leading edge rather than the trailing scale seen with EAC. Diagnosis is made by KOH examination of skin scrapings. Common risk factors include close contact with an infected person or animal, warm, moist environments, sharing personal items, and prolonged use of systemic corticosteroids. Our patient’s KOH analysis of skin scrapings was negative for fungal elements.
Erythema marginatum is a rare skin rash commonly seen with acute rheumatic fever secondary to streptococcal infection. It presents as annular erythematous lesions on the trunk and proximal extremities that are exacerbated by heat. It is often associated with active carditis related to rheumatic fever. This self-limited rash usually resolves in 2-3 days. Our patient was asymptomatic without involvement of other organs.
Like EAC, granuloma annulare is a benign chronic skin condition that presents with ring-shaped lesions. Its etiology is unknown, and lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The distinguishing feature of granuloma annulare from other annular lesions is its absence of scale.
Urticaria multiforme is an allergic hypersensitivity reaction commonly linked to viral infections, medications, and immunizations. Clinical features include blanchable annular/polycyclic lesions with a central purplish or dusky hue. Diagnostic pearls include the presence of pruritus, dermatographism, and individual lesions that resolve within 24 hours, all of which were not found in our patient’s case.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Laborada and Dr. Matiz have no relevant financial disclosures.
References
1. Paller A and Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 4th ed. Philadelphia: Elsevier Saunders, 2011.
2. McDaniel B and Cook C. “Erythema annulare centrifugum” 2021 Aug 27. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2022 Jan. PMID: 29494101.
3. Leung AK e al. Drugs Context. 2020 Jul 20;9:5-6.
4. Majmundar VD and Nagalli S. “Erythema marginatum” 2022 May 8. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2022 Jan.
5. Piette EW and Rosenbach M. J Am Acad Dermatol. 2016 Sep;75(3):467-79.
6. Barros M et al. BMJ Case Rep. 2021 Jan 28;14(1):e241011.
Erythema annulare centrifugum
A thorough body examination failed to reveal any other rashes or lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. A potassium hydroxide analysis of skin scrapings was negative for fungal elements. Punch biopsy of the skin on the left arm revealed focal intermittent parakeratosis, mildly acanthotic and spongiotic epidermis, and a tight superficial perivascular chronic dermatitis consisting of lymphocytes and histiocytes (Figures). Given these findings, a diagnosis of erythema annulare centrifugum (EAC) was rendered.
EAC is a rare, reactive skin rash characterized by redness (erythema) and ring-shaped lesions (annulare) that slowly spread from the center (centrifugum). The lesions present with a characteristic trailing scale on the inner border of the erythematous ring. Lesions may be asymptomatic or mildly pruritic and commonly involve the trunk, buttocks, hips, and upper legs. It is important to note that its duration is highly variable, ranging from weeks to decades, with most cases persisting for 9 months. EAC typically affects young or middle-aged adults but can occur at any age.
Although the etiology of EAC is unknown, it is believed to be a hypersensitivity reaction to a foreign antigen. Cutaneous fungal infections are commonly reported as triggers as well as other viral infections, medications, malignancy, underlying systemic disease, and certain foods. Treatment depends on the underlying condition and removing the implicated agent. However, most cases of EAC are idiopathic and self-limiting. It is possible that our patient’s prior history of tinea capitis could have triggered the skin lesions suggestive of EAC, but interestingly, these lesions did not go away after the fungal infection was cleared and have continued to recur. For patients with refractory lesions or treatment of patients without an identifiable cause, the use of oral antimicrobials has been proposed. Medications such as azithromycin, erythromycin, fluconazole, and metronidazole have been reported to be helpful in some patients with refractory EAC. Our patient wanted to continue topical treatment with betamethasone as needed and may consider antimicrobial therapy if the lesions continue to recur.
Tinea corporis refers to a superficial fungal infection of the skin. It may present as one or more asymmetrical, annular, pruritic plaques with a raised scaly leading edge rather than the trailing scale seen with EAC. Diagnosis is made by KOH examination of skin scrapings. Common risk factors include close contact with an infected person or animal, warm, moist environments, sharing personal items, and prolonged use of systemic corticosteroids. Our patient’s KOH analysis of skin scrapings was negative for fungal elements.
Erythema marginatum is a rare skin rash commonly seen with acute rheumatic fever secondary to streptococcal infection. It presents as annular erythematous lesions on the trunk and proximal extremities that are exacerbated by heat. It is often associated with active carditis related to rheumatic fever. This self-limited rash usually resolves in 2-3 days. Our patient was asymptomatic without involvement of other organs.
Like EAC, granuloma annulare is a benign chronic skin condition that presents with ring-shaped lesions. Its etiology is unknown, and lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The distinguishing feature of granuloma annulare from other annular lesions is its absence of scale.
Urticaria multiforme is an allergic hypersensitivity reaction commonly linked to viral infections, medications, and immunizations. Clinical features include blanchable annular/polycyclic lesions with a central purplish or dusky hue. Diagnostic pearls include the presence of pruritus, dermatographism, and individual lesions that resolve within 24 hours, all of which were not found in our patient’s case.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Laborada and Dr. Matiz have no relevant financial disclosures.
References
1. Paller A and Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 4th ed. Philadelphia: Elsevier Saunders, 2011.
2. McDaniel B and Cook C. “Erythema annulare centrifugum” 2021 Aug 27. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2022 Jan. PMID: 29494101.
3. Leung AK e al. Drugs Context. 2020 Jul 20;9:5-6.
4. Majmundar VD and Nagalli S. “Erythema marginatum” 2022 May 8. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2022 Jan.
5. Piette EW and Rosenbach M. J Am Acad Dermatol. 2016 Sep;75(3):467-79.
6. Barros M et al. BMJ Case Rep. 2021 Jan 28;14(1):e241011.
Erythema annulare centrifugum
A thorough body examination failed to reveal any other rashes or lesions suggestive of a fungal infection. A blood count and urinalysis were within normal limits. She had no lymphadenopathy or hepatosplenomegaly. A potassium hydroxide analysis of skin scrapings was negative for fungal elements. Punch biopsy of the skin on the left arm revealed focal intermittent parakeratosis, mildly acanthotic and spongiotic epidermis, and a tight superficial perivascular chronic dermatitis consisting of lymphocytes and histiocytes (Figures). Given these findings, a diagnosis of erythema annulare centrifugum (EAC) was rendered.
EAC is a rare, reactive skin rash characterized by redness (erythema) and ring-shaped lesions (annulare) that slowly spread from the center (centrifugum). The lesions present with a characteristic trailing scale on the inner border of the erythematous ring. Lesions may be asymptomatic or mildly pruritic and commonly involve the trunk, buttocks, hips, and upper legs. It is important to note that its duration is highly variable, ranging from weeks to decades, with most cases persisting for 9 months. EAC typically affects young or middle-aged adults but can occur at any age.
Although the etiology of EAC is unknown, it is believed to be a hypersensitivity reaction to a foreign antigen. Cutaneous fungal infections are commonly reported as triggers as well as other viral infections, medications, malignancy, underlying systemic disease, and certain foods. Treatment depends on the underlying condition and removing the implicated agent. However, most cases of EAC are idiopathic and self-limiting. It is possible that our patient’s prior history of tinea capitis could have triggered the skin lesions suggestive of EAC, but interestingly, these lesions did not go away after the fungal infection was cleared and have continued to recur. For patients with refractory lesions or treatment of patients without an identifiable cause, the use of oral antimicrobials has been proposed. Medications such as azithromycin, erythromycin, fluconazole, and metronidazole have been reported to be helpful in some patients with refractory EAC. Our patient wanted to continue topical treatment with betamethasone as needed and may consider antimicrobial therapy if the lesions continue to recur.
Tinea corporis refers to a superficial fungal infection of the skin. It may present as one or more asymmetrical, annular, pruritic plaques with a raised scaly leading edge rather than the trailing scale seen with EAC. Diagnosis is made by KOH examination of skin scrapings. Common risk factors include close contact with an infected person or animal, warm, moist environments, sharing personal items, and prolonged use of systemic corticosteroids. Our patient’s KOH analysis of skin scrapings was negative for fungal elements.
Erythema marginatum is a rare skin rash commonly seen with acute rheumatic fever secondary to streptococcal infection. It presents as annular erythematous lesions on the trunk and proximal extremities that are exacerbated by heat. It is often associated with active carditis related to rheumatic fever. This self-limited rash usually resolves in 2-3 days. Our patient was asymptomatic without involvement of other organs.
Like EAC, granuloma annulare is a benign chronic skin condition that presents with ring-shaped lesions. Its etiology is unknown, and lesions may be asymptomatic or mildly pruritic. Localized granuloma annulare typically presents as reddish-brown papules or plaques on the fingers, hands, elbows, dorsal feet, or ankles. The distinguishing feature of granuloma annulare from other annular lesions is its absence of scale.
Urticaria multiforme is an allergic hypersensitivity reaction commonly linked to viral infections, medications, and immunizations. Clinical features include blanchable annular/polycyclic lesions with a central purplish or dusky hue. Diagnostic pearls include the presence of pruritus, dermatographism, and individual lesions that resolve within 24 hours, all of which were not found in our patient’s case.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Ms. Laborada and Dr. Matiz have no relevant financial disclosures.
References
1. Paller A and Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. 4th ed. Philadelphia: Elsevier Saunders, 2011.
2. McDaniel B and Cook C. “Erythema annulare centrifugum” 2021 Aug 27. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2022 Jan. PMID: 29494101.
3. Leung AK e al. Drugs Context. 2020 Jul 20;9:5-6.
4. Majmundar VD and Nagalli S. “Erythema marginatum” 2022 May 8. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2022 Jan.
5. Piette EW and Rosenbach M. J Am Acad Dermatol. 2016 Sep;75(3):467-79.
6. Barros M et al. BMJ Case Rep. 2021 Jan 28;14(1):e241011.
A review of systems was noncontributory. She was not taking any other medications or vitamin supplements. There were no pets at home and no other affected family members. Physical exam was notable for scattered, pink, annular plaques with central clearing, faint brownish pigmentation, and fine scale.
Monkeypox: What’s a pediatrician to do?
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Is hepatitis C an STI?
A 32-year-old woman had sex with a man she met while on vacation 6 weeks ago. She was intoxicated at the time and does not know much about the person. She recalls having engaged in vaginal intercourse without a condom. She does not have any symptoms.
She previously received baseline lab testing per Centers for Disease Control and Prevention guidelines 2 years ago with a negative HIV test and negative hepatitis C test. She asks for testing for STIs. What would you recommend?
A. HIV, hepatitis C, gonorrhea, chlamydia, and human papillomavirus
B. HIV, hepatitis C, gonorrhea, chlamydia, and herpes simplex virus
C. HIV, hepatitis C, gonorrhea, and chlamydia
D. HIV, gonorrhea, and chlamydia
E. Gonorrhea and chlamydia
HIV risk estimate
The most practical answer is E, check for gonorrhea and chlamydia. Many protocols in place for evaluating people for STIs will test for hepatitis C as well as HIV with single exposures. In this column, we will look at the lack of evidence of heterosexual sexual transmission of hepatitis C.
In regards to HIV risk, the estimated risk of transmission male to female from an HIV-infected individual is 0.08% per sexual encounter.1 The prevalence in the United States – where HIV occurs in about 0.5% of the adult population – was used to estimate the risk of a person with unknown HIV status acquiring HIV. The calculated risk from one sexual encounter would be 0.0004 (1 in 250,000).
Studies of hepatitis C transmission
Tahan and colleagues did a prospective study of 600 heterosexual couples where one partner had hepatitis C and the other didn’t. Over a mean of 3 years of follow-up, none of the seronegative spouses developed hepatitis C.2
Terrault and colleagues completed a cross-sectional study of hepatitis C virus (HCV)–positive individuals and their monogamous heterosexual partners to evaluate risk of sexual transmission of HCV.3 Based on 8,377 person-years of follow-up, the estimated maximum transmission rate was 0.07%/year, which was about 1/190,000 sexual contacts. No specific sexual practices were associated with transmission. The authors of this study concurred with CDC recommendations that persons with HCV infection in long-term monogamous relationships need not change their sexual practices.4
Vandelli and colleagues followed 776 heterosexual partners of HCV-infected individuals over 10 years.5 None of the couples reported condom use. Over the follow up period, three HCV infections occurred, but based on discordance of the typing of viral isolates, sexual transmission was excluded.
Jin and colleagues completed a systematic review of studies looking at possible sexual transmission of HCV in gay and bisexual men.6 HIV-positive men had a HCV incidence of 6.4 per 1,000 person-years, compared with 0.4 per 1000 person-years in HIV-negative men. The authors discussed several possible causes for increased transmission risk in HIV-infected individuals including coexisting STIs and higher HCV viral load in semen of HIV-infected individuals, as well as lower immunity.
Summary
In hepatitis C–discordant heterosexual couples, hepatitis C does not appear to be sexually transmitted.
The risk of sexual transmission of hepatitis C to non–HIV-infected individuals appears to be exceedingly low.
Many thanks to Hunter Handsfield, MD, for suggesting this topic and sharing supporting articles.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
1. Boily MC et al. Lancet Infect Dis. 2009 Feb;9(2):118-29.
2. Tahan V et al. Am J Gastroenterol. 2005;100:821-4.
3. Terrault NA et al. Hepatology. 2013;57:881-9
4. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-38.
5. Vandelli C et al. Am J Gastroenterol. 2004;99:855-9.
6. Jin F et al. Sexual Health.2017;14:28-41.
A 32-year-old woman had sex with a man she met while on vacation 6 weeks ago. She was intoxicated at the time and does not know much about the person. She recalls having engaged in vaginal intercourse without a condom. She does not have any symptoms.
She previously received baseline lab testing per Centers for Disease Control and Prevention guidelines 2 years ago with a negative HIV test and negative hepatitis C test. She asks for testing for STIs. What would you recommend?
A. HIV, hepatitis C, gonorrhea, chlamydia, and human papillomavirus
B. HIV, hepatitis C, gonorrhea, chlamydia, and herpes simplex virus
C. HIV, hepatitis C, gonorrhea, and chlamydia
D. HIV, gonorrhea, and chlamydia
E. Gonorrhea and chlamydia
HIV risk estimate
The most practical answer is E, check for gonorrhea and chlamydia. Many protocols in place for evaluating people for STIs will test for hepatitis C as well as HIV with single exposures. In this column, we will look at the lack of evidence of heterosexual sexual transmission of hepatitis C.
In regards to HIV risk, the estimated risk of transmission male to female from an HIV-infected individual is 0.08% per sexual encounter.1 The prevalence in the United States – where HIV occurs in about 0.5% of the adult population – was used to estimate the risk of a person with unknown HIV status acquiring HIV. The calculated risk from one sexual encounter would be 0.0004 (1 in 250,000).
Studies of hepatitis C transmission
Tahan and colleagues did a prospective study of 600 heterosexual couples where one partner had hepatitis C and the other didn’t. Over a mean of 3 years of follow-up, none of the seronegative spouses developed hepatitis C.2
Terrault and colleagues completed a cross-sectional study of hepatitis C virus (HCV)–positive individuals and their monogamous heterosexual partners to evaluate risk of sexual transmission of HCV.3 Based on 8,377 person-years of follow-up, the estimated maximum transmission rate was 0.07%/year, which was about 1/190,000 sexual contacts. No specific sexual practices were associated with transmission. The authors of this study concurred with CDC recommendations that persons with HCV infection in long-term monogamous relationships need not change their sexual practices.4
Vandelli and colleagues followed 776 heterosexual partners of HCV-infected individuals over 10 years.5 None of the couples reported condom use. Over the follow up period, three HCV infections occurred, but based on discordance of the typing of viral isolates, sexual transmission was excluded.
Jin and colleagues completed a systematic review of studies looking at possible sexual transmission of HCV in gay and bisexual men.6 HIV-positive men had a HCV incidence of 6.4 per 1,000 person-years, compared with 0.4 per 1000 person-years in HIV-negative men. The authors discussed several possible causes for increased transmission risk in HIV-infected individuals including coexisting STIs and higher HCV viral load in semen of HIV-infected individuals, as well as lower immunity.
Summary
In hepatitis C–discordant heterosexual couples, hepatitis C does not appear to be sexually transmitted.
The risk of sexual transmission of hepatitis C to non–HIV-infected individuals appears to be exceedingly low.
Many thanks to Hunter Handsfield, MD, for suggesting this topic and sharing supporting articles.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
1. Boily MC et al. Lancet Infect Dis. 2009 Feb;9(2):118-29.
2. Tahan V et al. Am J Gastroenterol. 2005;100:821-4.
3. Terrault NA et al. Hepatology. 2013;57:881-9
4. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-38.
5. Vandelli C et al. Am J Gastroenterol. 2004;99:855-9.
6. Jin F et al. Sexual Health.2017;14:28-41.
A 32-year-old woman had sex with a man she met while on vacation 6 weeks ago. She was intoxicated at the time and does not know much about the person. She recalls having engaged in vaginal intercourse without a condom. She does not have any symptoms.
She previously received baseline lab testing per Centers for Disease Control and Prevention guidelines 2 years ago with a negative HIV test and negative hepatitis C test. She asks for testing for STIs. What would you recommend?
A. HIV, hepatitis C, gonorrhea, chlamydia, and human papillomavirus
B. HIV, hepatitis C, gonorrhea, chlamydia, and herpes simplex virus
C. HIV, hepatitis C, gonorrhea, and chlamydia
D. HIV, gonorrhea, and chlamydia
E. Gonorrhea and chlamydia
HIV risk estimate
The most practical answer is E, check for gonorrhea and chlamydia. Many protocols in place for evaluating people for STIs will test for hepatitis C as well as HIV with single exposures. In this column, we will look at the lack of evidence of heterosexual sexual transmission of hepatitis C.
In regards to HIV risk, the estimated risk of transmission male to female from an HIV-infected individual is 0.08% per sexual encounter.1 The prevalence in the United States – where HIV occurs in about 0.5% of the adult population – was used to estimate the risk of a person with unknown HIV status acquiring HIV. The calculated risk from one sexual encounter would be 0.0004 (1 in 250,000).
Studies of hepatitis C transmission
Tahan and colleagues did a prospective study of 600 heterosexual couples where one partner had hepatitis C and the other didn’t. Over a mean of 3 years of follow-up, none of the seronegative spouses developed hepatitis C.2
Terrault and colleagues completed a cross-sectional study of hepatitis C virus (HCV)–positive individuals and their monogamous heterosexual partners to evaluate risk of sexual transmission of HCV.3 Based on 8,377 person-years of follow-up, the estimated maximum transmission rate was 0.07%/year, which was about 1/190,000 sexual contacts. No specific sexual practices were associated with transmission. The authors of this study concurred with CDC recommendations that persons with HCV infection in long-term monogamous relationships need not change their sexual practices.4
Vandelli and colleagues followed 776 heterosexual partners of HCV-infected individuals over 10 years.5 None of the couples reported condom use. Over the follow up period, three HCV infections occurred, but based on discordance of the typing of viral isolates, sexual transmission was excluded.
Jin and colleagues completed a systematic review of studies looking at possible sexual transmission of HCV in gay and bisexual men.6 HIV-positive men had a HCV incidence of 6.4 per 1,000 person-years, compared with 0.4 per 1000 person-years in HIV-negative men. The authors discussed several possible causes for increased transmission risk in HIV-infected individuals including coexisting STIs and higher HCV viral load in semen of HIV-infected individuals, as well as lower immunity.
Summary
In hepatitis C–discordant heterosexual couples, hepatitis C does not appear to be sexually transmitted.
The risk of sexual transmission of hepatitis C to non–HIV-infected individuals appears to be exceedingly low.
Many thanks to Hunter Handsfield, MD, for suggesting this topic and sharing supporting articles.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
1. Boily MC et al. Lancet Infect Dis. 2009 Feb;9(2):118-29.
2. Tahan V et al. Am J Gastroenterol. 2005;100:821-4.
3. Terrault NA et al. Hepatology. 2013;57:881-9
4. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-38.
5. Vandelli C et al. Am J Gastroenterol. 2004;99:855-9.
6. Jin F et al. Sexual Health.2017;14:28-41.
Why do young men target schools for violent attacks? And what can we do about it?
Schools are intended to be a safe place to acquire knowledge, try out ideas, practice socializing, and build a foundation for adulthood. Many schools fulfill this mission for most children, but for children at both extremes of ability their school experience does not suffice.
When asked, “If you had the choice, would you rather stay home or go to school?” my patients almost universally prefer school. They all know that school is where they should be; they want to be normal, accepted by peers, getting ready for the world’s coming demands, and validation that they will make it as adults. Endorsement otherwise is a warning sign.
When such important tasks of childhood are thwarted children may despair, withdraw, give up, or a small number become furious. These may profoundly resent the children who are experiencing success when they could not. They may hate the teachers and the place where they experienced failure and humiliation. Lack of a positive connection to school characterizes children who are violent toward schools as well as those who drop out.
Schools may fail to support the basic needs of children for many reasons. Schools may avoid physical violence but fail to protect the children’s self-esteem. I have heard stories of teachers calling on children to perform who are clearly struggling or shy, insulting incorrect answers, calling names, putting names on the board, reading out failed grades, posting grades publicly, even allowing peers to mock students. Teachers may deny or disregard parent complaints, or even worsen treatment of the child. Although children may at times falsify complaints, children’s and parents’ reports must be taken seriously and remain anonymous. When we hear of such toxic situations for our patients, we can get details and contact school administrators without naming the child, as often the family feels they can’t. Repeated humiliation may require not only remediation, but consequences. We can advocate for a change in classroom or request a 504 Plan if emotional health is affected.
All children learn best and experience success and even joy when the tasks they face are at or slightly beyond their skill level. But with the wide range of abilities, especially for boys, education may need to be individualized. This is very difficult in larger classrooms with fewer resources, too few adult helpers, inexperienced teachers, or high levels of student misbehavior. Basing teacher promotion mainly on standardized test results makes individualizing instruction even less likely. Smaller class size is better; even the recommended (less than 20) or regulated (less than 30) class sizes are associated with suboptimal achievement, compared with smaller ones. Some ways to attain smaller class size include split days or alternate-day sessions, although these also have disadvantages.
While we can advocate for these changes, we can also encourage parents to promote academic skills by talking to and reading to their children of all ages, trying Reach Out and Read for young children, providing counting games, board games, and math songs! Besides screening for attention-deficit/hyperactivity disorder, we can use standard paragraphs and math problems (for example, WRAT, Einstein) to check skills when performance is low or behavior is a problem the school denies. When concerned, we can write letters for parents to sign requesting testing and an individualized education plan to determine need for tutoring or special education.
While Federal legislation requiring the “least restrictive environment” for education was intended to avoid sidelining differently able children, some can’t learn in a regular class. Conversely, if instruction in a special class is adjusted to the child with the lowest skills, minimal learning may occur for others. Although we can speak with the teacher about “this child’s abilities among those in his class” we can first suggest that the parent visit class to observe. Outside tutoring or home schooling may help a child move up to a regular class.
Sometimes a child’s learning is hampered by classrooms with numerous children misbehaving; this is also a reason for resentment. We can inform school administrators about methods such as The Good Behavior Game (paxis.org) that can improve behavior and connection for the whole class.
While a social “pecking order” is universal, it is unacceptable for children to be allowed to humiliate or hurt a peer, or damage their reputation. While this moral teaching should occur at home, it needs to continue at school where peers are forced into groups they did not choose. Screening for bullying at pediatric visits is now a universal recommendation as 30% report being bullied. We need to ask all children about “mean kids in school” or gang involvement for older children.
Parents can support their children experiencing cyberbullying and switch them to a “dumb phone” with no texting option, limited phone time, or no phone at all. Policies against bullying coming from school administrators are most effective but we can inform schools about the STOPit app for children to report bullying anonymously as well as education for students to stand together against a bully (stopbullying.gov). A Lunch Bunch for younger children or a buddy system for older ones can be requested to help them make friends.
With diverse child aptitudes, schools need to offer students alternative opportunities for self-expression and contribution. We can ask about a child’s strengths and suggest related extracurriculars activities in school or outside, including volunteering. Participation on teams or in clubs must not be blocked for those with poor grades. Perhaps tying participation to tutoring would satisfy the school’s desire to motivate instead. Parents can be encouraged to advocate for music, art, and drama classes – programs that are often victims of budget cuts – that can create the essential school connection.
Students in many areas lack access to classes in trades early enough in their education. The requirements for English or math may be out of reach and result in students dropping out before trade classes are an option. We may identify our patients who may do better with a trade education and advise families to request transfer to a high school offering this.
The best connection a child can have to a school is an adult who values them. The child may identify a preferred teacher to us so that we, or the parent, can call to ask them to provide special attention. Facilitating times for students to get to know teachers may require alteration in bus schedules, lunch times, study halls, or breaks, or keeping the school open longer outside class hours. While more mental health providers are clearly needed, sometimes it is the groundskeeper, the secretary, or the lunch helper who can make the best connection with a child.
As pediatricians, we must listen to struggling youth, acknowledge their pain, and model this empathy for their parents who may be obsessing over grades. Problem-solving about how to get accommodations, informal or formal, can inspire hope. We can coach parents and youth to meet respectfully with the school about issues to avoid labeling the child as a problem.
As pediatricians, our recommendations for school funding and policies may carry extra weight. We may share ideas through talks at PTA meetings, serve on school boards, or endorse leaders planning greater resources for schools to optimize each child’s experience and connection to school.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Schools are intended to be a safe place to acquire knowledge, try out ideas, practice socializing, and build a foundation for adulthood. Many schools fulfill this mission for most children, but for children at both extremes of ability their school experience does not suffice.
When asked, “If you had the choice, would you rather stay home or go to school?” my patients almost universally prefer school. They all know that school is where they should be; they want to be normal, accepted by peers, getting ready for the world’s coming demands, and validation that they will make it as adults. Endorsement otherwise is a warning sign.
When such important tasks of childhood are thwarted children may despair, withdraw, give up, or a small number become furious. These may profoundly resent the children who are experiencing success when they could not. They may hate the teachers and the place where they experienced failure and humiliation. Lack of a positive connection to school characterizes children who are violent toward schools as well as those who drop out.
Schools may fail to support the basic needs of children for many reasons. Schools may avoid physical violence but fail to protect the children’s self-esteem. I have heard stories of teachers calling on children to perform who are clearly struggling or shy, insulting incorrect answers, calling names, putting names on the board, reading out failed grades, posting grades publicly, even allowing peers to mock students. Teachers may deny or disregard parent complaints, or even worsen treatment of the child. Although children may at times falsify complaints, children’s and parents’ reports must be taken seriously and remain anonymous. When we hear of such toxic situations for our patients, we can get details and contact school administrators without naming the child, as often the family feels they can’t. Repeated humiliation may require not only remediation, but consequences. We can advocate for a change in classroom or request a 504 Plan if emotional health is affected.
All children learn best and experience success and even joy when the tasks they face are at or slightly beyond their skill level. But with the wide range of abilities, especially for boys, education may need to be individualized. This is very difficult in larger classrooms with fewer resources, too few adult helpers, inexperienced teachers, or high levels of student misbehavior. Basing teacher promotion mainly on standardized test results makes individualizing instruction even less likely. Smaller class size is better; even the recommended (less than 20) or regulated (less than 30) class sizes are associated with suboptimal achievement, compared with smaller ones. Some ways to attain smaller class size include split days or alternate-day sessions, although these also have disadvantages.
While we can advocate for these changes, we can also encourage parents to promote academic skills by talking to and reading to their children of all ages, trying Reach Out and Read for young children, providing counting games, board games, and math songs! Besides screening for attention-deficit/hyperactivity disorder, we can use standard paragraphs and math problems (for example, WRAT, Einstein) to check skills when performance is low or behavior is a problem the school denies. When concerned, we can write letters for parents to sign requesting testing and an individualized education plan to determine need for tutoring or special education.
While Federal legislation requiring the “least restrictive environment” for education was intended to avoid sidelining differently able children, some can’t learn in a regular class. Conversely, if instruction in a special class is adjusted to the child with the lowest skills, minimal learning may occur for others. Although we can speak with the teacher about “this child’s abilities among those in his class” we can first suggest that the parent visit class to observe. Outside tutoring or home schooling may help a child move up to a regular class.
Sometimes a child’s learning is hampered by classrooms with numerous children misbehaving; this is also a reason for resentment. We can inform school administrators about methods such as The Good Behavior Game (paxis.org) that can improve behavior and connection for the whole class.
While a social “pecking order” is universal, it is unacceptable for children to be allowed to humiliate or hurt a peer, or damage their reputation. While this moral teaching should occur at home, it needs to continue at school where peers are forced into groups they did not choose. Screening for bullying at pediatric visits is now a universal recommendation as 30% report being bullied. We need to ask all children about “mean kids in school” or gang involvement for older children.
Parents can support their children experiencing cyberbullying and switch them to a “dumb phone” with no texting option, limited phone time, or no phone at all. Policies against bullying coming from school administrators are most effective but we can inform schools about the STOPit app for children to report bullying anonymously as well as education for students to stand together against a bully (stopbullying.gov). A Lunch Bunch for younger children or a buddy system for older ones can be requested to help them make friends.
With diverse child aptitudes, schools need to offer students alternative opportunities for self-expression and contribution. We can ask about a child’s strengths and suggest related extracurriculars activities in school or outside, including volunteering. Participation on teams or in clubs must not be blocked for those with poor grades. Perhaps tying participation to tutoring would satisfy the school’s desire to motivate instead. Parents can be encouraged to advocate for music, art, and drama classes – programs that are often victims of budget cuts – that can create the essential school connection.
Students in many areas lack access to classes in trades early enough in their education. The requirements for English or math may be out of reach and result in students dropping out before trade classes are an option. We may identify our patients who may do better with a trade education and advise families to request transfer to a high school offering this.
The best connection a child can have to a school is an adult who values them. The child may identify a preferred teacher to us so that we, or the parent, can call to ask them to provide special attention. Facilitating times for students to get to know teachers may require alteration in bus schedules, lunch times, study halls, or breaks, or keeping the school open longer outside class hours. While more mental health providers are clearly needed, sometimes it is the groundskeeper, the secretary, or the lunch helper who can make the best connection with a child.
As pediatricians, we must listen to struggling youth, acknowledge their pain, and model this empathy for their parents who may be obsessing over grades. Problem-solving about how to get accommodations, informal or formal, can inspire hope. We can coach parents and youth to meet respectfully with the school about issues to avoid labeling the child as a problem.
As pediatricians, our recommendations for school funding and policies may carry extra weight. We may share ideas through talks at PTA meetings, serve on school boards, or endorse leaders planning greater resources for schools to optimize each child’s experience and connection to school.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Schools are intended to be a safe place to acquire knowledge, try out ideas, practice socializing, and build a foundation for adulthood. Many schools fulfill this mission for most children, but for children at both extremes of ability their school experience does not suffice.
When asked, “If you had the choice, would you rather stay home or go to school?” my patients almost universally prefer school. They all know that school is where they should be; they want to be normal, accepted by peers, getting ready for the world’s coming demands, and validation that they will make it as adults. Endorsement otherwise is a warning sign.
When such important tasks of childhood are thwarted children may despair, withdraw, give up, or a small number become furious. These may profoundly resent the children who are experiencing success when they could not. They may hate the teachers and the place where they experienced failure and humiliation. Lack of a positive connection to school characterizes children who are violent toward schools as well as those who drop out.
Schools may fail to support the basic needs of children for many reasons. Schools may avoid physical violence but fail to protect the children’s self-esteem. I have heard stories of teachers calling on children to perform who are clearly struggling or shy, insulting incorrect answers, calling names, putting names on the board, reading out failed grades, posting grades publicly, even allowing peers to mock students. Teachers may deny or disregard parent complaints, or even worsen treatment of the child. Although children may at times falsify complaints, children’s and parents’ reports must be taken seriously and remain anonymous. When we hear of such toxic situations for our patients, we can get details and contact school administrators without naming the child, as often the family feels they can’t. Repeated humiliation may require not only remediation, but consequences. We can advocate for a change in classroom or request a 504 Plan if emotional health is affected.
All children learn best and experience success and even joy when the tasks they face are at or slightly beyond their skill level. But with the wide range of abilities, especially for boys, education may need to be individualized. This is very difficult in larger classrooms with fewer resources, too few adult helpers, inexperienced teachers, or high levels of student misbehavior. Basing teacher promotion mainly on standardized test results makes individualizing instruction even less likely. Smaller class size is better; even the recommended (less than 20) or regulated (less than 30) class sizes are associated with suboptimal achievement, compared with smaller ones. Some ways to attain smaller class size include split days or alternate-day sessions, although these also have disadvantages.
While we can advocate for these changes, we can also encourage parents to promote academic skills by talking to and reading to their children of all ages, trying Reach Out and Read for young children, providing counting games, board games, and math songs! Besides screening for attention-deficit/hyperactivity disorder, we can use standard paragraphs and math problems (for example, WRAT, Einstein) to check skills when performance is low or behavior is a problem the school denies. When concerned, we can write letters for parents to sign requesting testing and an individualized education plan to determine need for tutoring or special education.
While Federal legislation requiring the “least restrictive environment” for education was intended to avoid sidelining differently able children, some can’t learn in a regular class. Conversely, if instruction in a special class is adjusted to the child with the lowest skills, minimal learning may occur for others. Although we can speak with the teacher about “this child’s abilities among those in his class” we can first suggest that the parent visit class to observe. Outside tutoring or home schooling may help a child move up to a regular class.
Sometimes a child’s learning is hampered by classrooms with numerous children misbehaving; this is also a reason for resentment. We can inform school administrators about methods such as The Good Behavior Game (paxis.org) that can improve behavior and connection for the whole class.
While a social “pecking order” is universal, it is unacceptable for children to be allowed to humiliate or hurt a peer, or damage their reputation. While this moral teaching should occur at home, it needs to continue at school where peers are forced into groups they did not choose. Screening for bullying at pediatric visits is now a universal recommendation as 30% report being bullied. We need to ask all children about “mean kids in school” or gang involvement for older children.
Parents can support their children experiencing cyberbullying and switch them to a “dumb phone” with no texting option, limited phone time, or no phone at all. Policies against bullying coming from school administrators are most effective but we can inform schools about the STOPit app for children to report bullying anonymously as well as education for students to stand together against a bully (stopbullying.gov). A Lunch Bunch for younger children or a buddy system for older ones can be requested to help them make friends.
With diverse child aptitudes, schools need to offer students alternative opportunities for self-expression and contribution. We can ask about a child’s strengths and suggest related extracurriculars activities in school or outside, including volunteering. Participation on teams or in clubs must not be blocked for those with poor grades. Perhaps tying participation to tutoring would satisfy the school’s desire to motivate instead. Parents can be encouraged to advocate for music, art, and drama classes – programs that are often victims of budget cuts – that can create the essential school connection.
Students in many areas lack access to classes in trades early enough in their education. The requirements for English or math may be out of reach and result in students dropping out before trade classes are an option. We may identify our patients who may do better with a trade education and advise families to request transfer to a high school offering this.
The best connection a child can have to a school is an adult who values them. The child may identify a preferred teacher to us so that we, or the parent, can call to ask them to provide special attention. Facilitating times for students to get to know teachers may require alteration in bus schedules, lunch times, study halls, or breaks, or keeping the school open longer outside class hours. While more mental health providers are clearly needed, sometimes it is the groundskeeper, the secretary, or the lunch helper who can make the best connection with a child.
As pediatricians, we must listen to struggling youth, acknowledge their pain, and model this empathy for their parents who may be obsessing over grades. Problem-solving about how to get accommodations, informal or formal, can inspire hope. We can coach parents and youth to meet respectfully with the school about issues to avoid labeling the child as a problem.
As pediatricians, our recommendations for school funding and policies may carry extra weight. We may share ideas through talks at PTA meetings, serve on school boards, or endorse leaders planning greater resources for schools to optimize each child’s experience and connection to school.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
When suffering defies diagnosis
I still remember the woman who came to my office that day, years ago. She was struggling and uncomfortable, and she wanted “something” for stress. She described her life, and to me, it sounded stressful. She lived in a blended family and she described the chaos that one might expect to find in a household with four teens, their friends, their activities, and all it took to keep the household going. I spent 2 hours evaluating the patient, and I could not find a diagnosis that fit this problem nor – I believed – a pill that would fix it. She didn’t “meet criteria” for a psychiatric disorder, but she insisted she was uncomfortable and she wanted to try medication. I admit, I relented and I gave her a prescription for fluoxetine.
When she returned a few weeks later, my patient said she felt better, and what I remember decades later was her statement: “Now I can see dishes in the sink and be okay with it.” Perhaps she had downplayed her anxiety during our first meeting, but what I took from this was that some people are uncomfortable in ways that our lexicon does not capture, and sometimes medication helps with this discomfort.
The APA’s Diagnostic and Statistical Manual of Mental Disorders attempts to capture the problems of emotional and behavioral distress and classify them into discrete syndromes that can be validated and reliably diagnosed by different evaluators. Our disorders are syndromic; they are defined by clusters of symptoms that occur together, and not by a single symptom, lab value, or radiologic finding. The DSM is rewritten periodically so that what is or is not a disorder can bend with new discoveries and with a changing culture. And for better or for worse, when there is an available medication that can alleviate a problem, this may influence what once was a variant of normal into becoming a disorder.
Our illnesses often lie along a spectrum, so there is no precise point where someone who is easily distracted is a person with attention deficit disorder as opposed to being a mentally healthy person who is easily distracted, or a shy person is someone with social anxiety disorder. whether they want to address this with medications, and whether their distress warrants taking a chance that they might have side effects or an adverse reaction to a medication.
When we look at our criteria, sometimes we fall short. One needs to have at least five symptoms out of nine options, to be present for 2 weeks to be diagnosed with major depression, yet I don’t know a single psychiatrist who would not offer medication to a patient who ascribed to feeling profoundly sad with thoughts of suicide in the absence of other symptoms of depression. These issues have come to the forefront with the recent inclusion of prolonged grief in the DSM, as a disorder that is distinct from both normal grieving and from major depression.
In recent weeks, mass murder has been on everyone’s mind as we mourn those lost in Uvalde, Buffalo, and unfortunately, in so many other places. Absolutely no one thinks that someone who shoots strangers is “normal” or emotionally well. Yet psychiatry is often tasked with figuring out if someone is mad (mentally ill), bad (evil), or both. We don’t have a clear path for how to treat and manage people who commit horrendous acts of violence unless they meet criteria for another illness. Yet no one would argue that a person who informs others that he is thinking of killing strangers is in need of some type of intervention, regardless of his motive. We struggle too, with how to manage people who have more regular angry outbursts or emotional dysregulation. Perhaps we diagnose intermittent explosive disorder, or irritability caused by a mood disorder, but we don’t always know how to help people to control their tempers and modulate their emotions. And our semantics to describe psychic pain and anguish are surprisingly limited – sometimes we can only assume that someone who lashes out must be in turmoil.
Psychiatry continues to struggle with our relationship with human suffering. Suffering is part of life, not necessarily a sign of illness, and in his iconic memoir, “Man’s Search for Meaning,” psychiatrist Viktor Frankl, MD, wrote of the atrocities he endured in a Nazi concentration camp. It was through his suffering that Dr. Frankl found meaning and he used these harrowing experiences to fuel positive emotions later in life. Dr. Frankl wrote: “If there is a meaning in life at all, then there must be a meaning in suffering. Suffering is an ineradicable part of life, even as fate and death. Without suffering and death, human life cannot be complete.”
Suffering may be the kindling for acts of violence, or for profound creativity. Would we have music, art, cinema, poetry, or fiction if no one ever suffered? Yet suffering and emotional torment are often what leads people to seek treatment, and what leads us, as healers, to offer any range of therapies. For years, suicide rates have been rising, as have overdose death. And now, in addition to these “deaths of despair,” we are hearing about skyrocketing rates of depression and anxiety in our world that is so full of reasons to be sad and anxious. Access to treatment is limited by so many things, and it is not always clear when one needs psychiatric interventions or when problems will heal on their own, leaving scars or not.
I wrote this article in response to the hundreds of comments that were placed on an article I wrote after the horrors at Uvalde and Buffalo: “Don’t Equate Mass Shootings with Mental Illness.” Many of the commenters suggested I believe the shooter was perfectly sane, and that I am naive (or worse). Many wrote in with their own thoughts about what causes people to become mass murderers. One commenter wrote: “To suggest that random killers do not have mental health issues and their behavior is normal is ridiculous.” I don’t believe that I ever suggested that such behavior was normal, but – for many of these crimes – we as a society have decided to treat the behavior as criminal and not as the product of our current concept of mental disorders. Obviously, people who are well, who are emotionally at peace and comfortable in their own skin, don’t kill strangers.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore.
I still remember the woman who came to my office that day, years ago. She was struggling and uncomfortable, and she wanted “something” for stress. She described her life, and to me, it sounded stressful. She lived in a blended family and she described the chaos that one might expect to find in a household with four teens, their friends, their activities, and all it took to keep the household going. I spent 2 hours evaluating the patient, and I could not find a diagnosis that fit this problem nor – I believed – a pill that would fix it. She didn’t “meet criteria” for a psychiatric disorder, but she insisted she was uncomfortable and she wanted to try medication. I admit, I relented and I gave her a prescription for fluoxetine.
When she returned a few weeks later, my patient said she felt better, and what I remember decades later was her statement: “Now I can see dishes in the sink and be okay with it.” Perhaps she had downplayed her anxiety during our first meeting, but what I took from this was that some people are uncomfortable in ways that our lexicon does not capture, and sometimes medication helps with this discomfort.
The APA’s Diagnostic and Statistical Manual of Mental Disorders attempts to capture the problems of emotional and behavioral distress and classify them into discrete syndromes that can be validated and reliably diagnosed by different evaluators. Our disorders are syndromic; they are defined by clusters of symptoms that occur together, and not by a single symptom, lab value, or radiologic finding. The DSM is rewritten periodically so that what is or is not a disorder can bend with new discoveries and with a changing culture. And for better or for worse, when there is an available medication that can alleviate a problem, this may influence what once was a variant of normal into becoming a disorder.
Our illnesses often lie along a spectrum, so there is no precise point where someone who is easily distracted is a person with attention deficit disorder as opposed to being a mentally healthy person who is easily distracted, or a shy person is someone with social anxiety disorder. whether they want to address this with medications, and whether their distress warrants taking a chance that they might have side effects or an adverse reaction to a medication.
When we look at our criteria, sometimes we fall short. One needs to have at least five symptoms out of nine options, to be present for 2 weeks to be diagnosed with major depression, yet I don’t know a single psychiatrist who would not offer medication to a patient who ascribed to feeling profoundly sad with thoughts of suicide in the absence of other symptoms of depression. These issues have come to the forefront with the recent inclusion of prolonged grief in the DSM, as a disorder that is distinct from both normal grieving and from major depression.
In recent weeks, mass murder has been on everyone’s mind as we mourn those lost in Uvalde, Buffalo, and unfortunately, in so many other places. Absolutely no one thinks that someone who shoots strangers is “normal” or emotionally well. Yet psychiatry is often tasked with figuring out if someone is mad (mentally ill), bad (evil), or both. We don’t have a clear path for how to treat and manage people who commit horrendous acts of violence unless they meet criteria for another illness. Yet no one would argue that a person who informs others that he is thinking of killing strangers is in need of some type of intervention, regardless of his motive. We struggle too, with how to manage people who have more regular angry outbursts or emotional dysregulation. Perhaps we diagnose intermittent explosive disorder, or irritability caused by a mood disorder, but we don’t always know how to help people to control their tempers and modulate their emotions. And our semantics to describe psychic pain and anguish are surprisingly limited – sometimes we can only assume that someone who lashes out must be in turmoil.
Psychiatry continues to struggle with our relationship with human suffering. Suffering is part of life, not necessarily a sign of illness, and in his iconic memoir, “Man’s Search for Meaning,” psychiatrist Viktor Frankl, MD, wrote of the atrocities he endured in a Nazi concentration camp. It was through his suffering that Dr. Frankl found meaning and he used these harrowing experiences to fuel positive emotions later in life. Dr. Frankl wrote: “If there is a meaning in life at all, then there must be a meaning in suffering. Suffering is an ineradicable part of life, even as fate and death. Without suffering and death, human life cannot be complete.”
Suffering may be the kindling for acts of violence, or for profound creativity. Would we have music, art, cinema, poetry, or fiction if no one ever suffered? Yet suffering and emotional torment are often what leads people to seek treatment, and what leads us, as healers, to offer any range of therapies. For years, suicide rates have been rising, as have overdose death. And now, in addition to these “deaths of despair,” we are hearing about skyrocketing rates of depression and anxiety in our world that is so full of reasons to be sad and anxious. Access to treatment is limited by so many things, and it is not always clear when one needs psychiatric interventions or when problems will heal on their own, leaving scars or not.
I wrote this article in response to the hundreds of comments that were placed on an article I wrote after the horrors at Uvalde and Buffalo: “Don’t Equate Mass Shootings with Mental Illness.” Many of the commenters suggested I believe the shooter was perfectly sane, and that I am naive (or worse). Many wrote in with their own thoughts about what causes people to become mass murderers. One commenter wrote: “To suggest that random killers do not have mental health issues and their behavior is normal is ridiculous.” I don’t believe that I ever suggested that such behavior was normal, but – for many of these crimes – we as a society have decided to treat the behavior as criminal and not as the product of our current concept of mental disorders. Obviously, people who are well, who are emotionally at peace and comfortable in their own skin, don’t kill strangers.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore.
I still remember the woman who came to my office that day, years ago. She was struggling and uncomfortable, and she wanted “something” for stress. She described her life, and to me, it sounded stressful. She lived in a blended family and she described the chaos that one might expect to find in a household with four teens, their friends, their activities, and all it took to keep the household going. I spent 2 hours evaluating the patient, and I could not find a diagnosis that fit this problem nor – I believed – a pill that would fix it. She didn’t “meet criteria” for a psychiatric disorder, but she insisted she was uncomfortable and she wanted to try medication. I admit, I relented and I gave her a prescription for fluoxetine.
When she returned a few weeks later, my patient said she felt better, and what I remember decades later was her statement: “Now I can see dishes in the sink and be okay with it.” Perhaps she had downplayed her anxiety during our first meeting, but what I took from this was that some people are uncomfortable in ways that our lexicon does not capture, and sometimes medication helps with this discomfort.
The APA’s Diagnostic and Statistical Manual of Mental Disorders attempts to capture the problems of emotional and behavioral distress and classify them into discrete syndromes that can be validated and reliably diagnosed by different evaluators. Our disorders are syndromic; they are defined by clusters of symptoms that occur together, and not by a single symptom, lab value, or radiologic finding. The DSM is rewritten periodically so that what is or is not a disorder can bend with new discoveries and with a changing culture. And for better or for worse, when there is an available medication that can alleviate a problem, this may influence what once was a variant of normal into becoming a disorder.
Our illnesses often lie along a spectrum, so there is no precise point where someone who is easily distracted is a person with attention deficit disorder as opposed to being a mentally healthy person who is easily distracted, or a shy person is someone with social anxiety disorder. whether they want to address this with medications, and whether their distress warrants taking a chance that they might have side effects or an adverse reaction to a medication.
When we look at our criteria, sometimes we fall short. One needs to have at least five symptoms out of nine options, to be present for 2 weeks to be diagnosed with major depression, yet I don’t know a single psychiatrist who would not offer medication to a patient who ascribed to feeling profoundly sad with thoughts of suicide in the absence of other symptoms of depression. These issues have come to the forefront with the recent inclusion of prolonged grief in the DSM, as a disorder that is distinct from both normal grieving and from major depression.
In recent weeks, mass murder has been on everyone’s mind as we mourn those lost in Uvalde, Buffalo, and unfortunately, in so many other places. Absolutely no one thinks that someone who shoots strangers is “normal” or emotionally well. Yet psychiatry is often tasked with figuring out if someone is mad (mentally ill), bad (evil), or both. We don’t have a clear path for how to treat and manage people who commit horrendous acts of violence unless they meet criteria for another illness. Yet no one would argue that a person who informs others that he is thinking of killing strangers is in need of some type of intervention, regardless of his motive. We struggle too, with how to manage people who have more regular angry outbursts or emotional dysregulation. Perhaps we diagnose intermittent explosive disorder, or irritability caused by a mood disorder, but we don’t always know how to help people to control their tempers and modulate their emotions. And our semantics to describe psychic pain and anguish are surprisingly limited – sometimes we can only assume that someone who lashes out must be in turmoil.
Psychiatry continues to struggle with our relationship with human suffering. Suffering is part of life, not necessarily a sign of illness, and in his iconic memoir, “Man’s Search for Meaning,” psychiatrist Viktor Frankl, MD, wrote of the atrocities he endured in a Nazi concentration camp. It was through his suffering that Dr. Frankl found meaning and he used these harrowing experiences to fuel positive emotions later in life. Dr. Frankl wrote: “If there is a meaning in life at all, then there must be a meaning in suffering. Suffering is an ineradicable part of life, even as fate and death. Without suffering and death, human life cannot be complete.”
Suffering may be the kindling for acts of violence, or for profound creativity. Would we have music, art, cinema, poetry, or fiction if no one ever suffered? Yet suffering and emotional torment are often what leads people to seek treatment, and what leads us, as healers, to offer any range of therapies. For years, suicide rates have been rising, as have overdose death. And now, in addition to these “deaths of despair,” we are hearing about skyrocketing rates of depression and anxiety in our world that is so full of reasons to be sad and anxious. Access to treatment is limited by so many things, and it is not always clear when one needs psychiatric interventions or when problems will heal on their own, leaving scars or not.
I wrote this article in response to the hundreds of comments that were placed on an article I wrote after the horrors at Uvalde and Buffalo: “Don’t Equate Mass Shootings with Mental Illness.” Many of the commenters suggested I believe the shooter was perfectly sane, and that I am naive (or worse). Many wrote in with their own thoughts about what causes people to become mass murderers. One commenter wrote: “To suggest that random killers do not have mental health issues and their behavior is normal is ridiculous.” I don’t believe that I ever suggested that such behavior was normal, but – for many of these crimes – we as a society have decided to treat the behavior as criminal and not as the product of our current concept of mental disorders. Obviously, people who are well, who are emotionally at peace and comfortable in their own skin, don’t kill strangers.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore.