User login
Safety remains top parent concern for HPV vaccine
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
FROM PEDIATRICS
Severe rash after COVID-19 vaccination
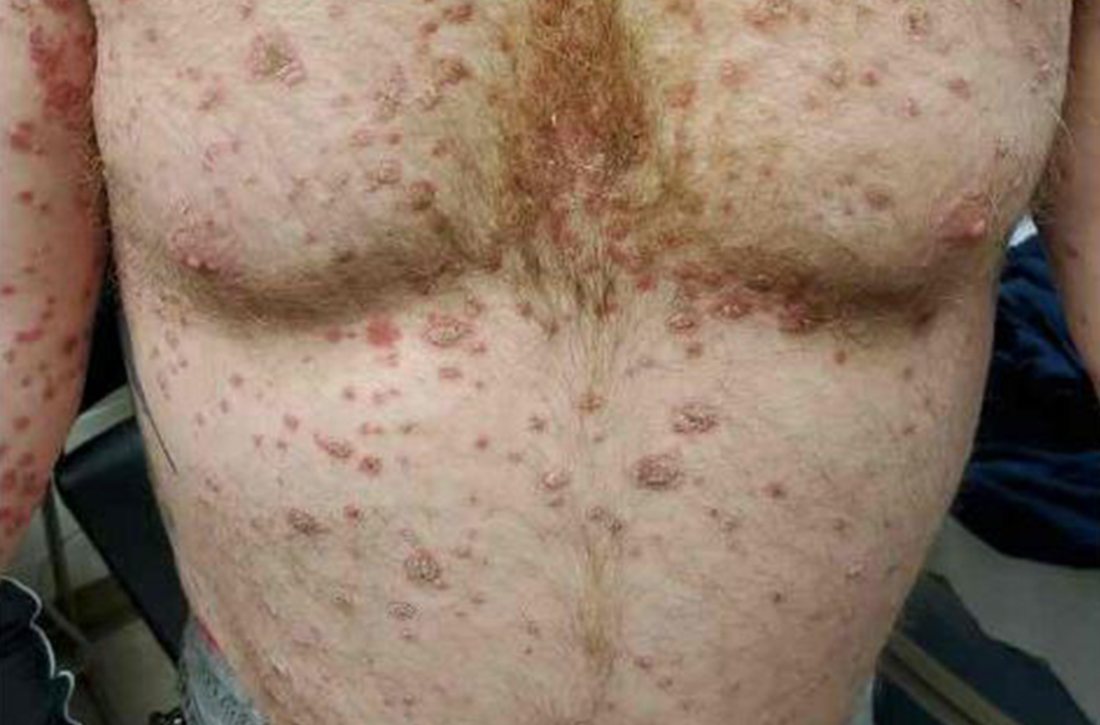
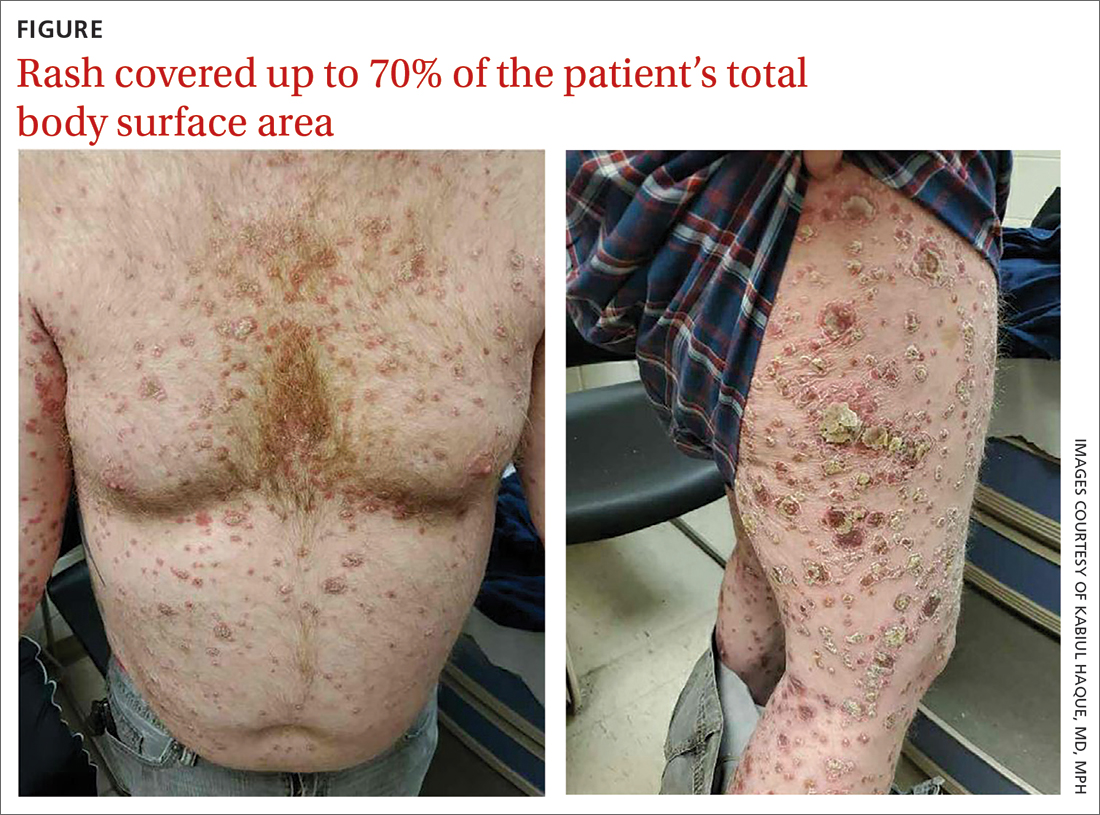
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
Here’s how we can rebuild trust in vaccines
When people ask Paul Offit, MD, what worries him the most about the COVID-19 pandemic, he names two concerns. “One is the lack of socialization and education that came from keeping kids out of school for so long,” Dr. Offit said in a recent interview. “And I think vaccines have suffered.”
Dr. Offit is director of the Vaccine Education Center and a professor of pediatrics at Children’s Hospital of Philadelphia. He has watched with alarm as the American public appears to be losing faith in the lifesaving vaccines the public health community has worked hard to promote. The Centers for Disease Control and Prevention estimates that the proportion of kids entering kindergarten who have received state-required vaccines dipped to 94% in the 2020-2021 school year – a full point less than the year before the pandemic – then dropped by another percentage point, to 93%, the following year.
Although a couple of percentage points may sound trivial, were only 93% of kindergarteners to receive the vaccine against measles, mumps, and rubella (MMR), approximately 250,000 vulnerable 5-year-olds could spark the next big outbreak, such as the recent measles outbreaks in Ohio and Minnesota.
Dr. Offit is one of many public health officials and clinicians who are working to reverse the concerning trends in pediatric vaccinations.
“I just don’t want to see an outbreak of something that we could have avoided because we were not protected enough,” Judith Shlay, MD, associate director of the Public Health Institute at Denver Health, said.
Official stumbles in part to blame
Disruptions in health care from the COVID-19 pandemic certainly played a role in the decline. Parents were afraid to expose their children to other sick kids, providers shifted to a telehealth model, and routine preventive care was difficult to access.
But Dr. Offit also blamed erosion of trust on mistakes made by government and public health institutions for the alarming trend. “I think that health care professionals have lost some level of trust in the Food and Drug Administration and CDC.”
He cited as an example poor messaging during a large outbreak in Massachusetts in summer 2021, when the CDC published a report that highlighted the high proportion of COVID-19 cases among vaccinated people. Health officials called those cases “breakthrough” infections, although most were mild or asymptomatic.
Dr. Offit said the CDC should have focused the message instead on the low rate (1%) of hospitalizations and the low number of deaths from the infections. Instead, they had to walk back their promise that vaccinated people didn’t need to wear masks. At other times, the Biden administration pressured public health officials by promising to make booster shots available to the American public when the FDA and CDC felt they lacked evidence to recommend the injections.
Rupali Limaye, PhD, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University, Baltimore, studies vaccine behavior and decision-making. She would go a step further in characterizing the roots of worsening vaccine hesitancy.
“In the last 20 years, we’ve seen there’s less and less trust in health care providers in general,” Dr. Limaye said. “More people are turning to their social networks or social contacts for that kind of information.” In the maelstrom of the COVID-19 pandemic, digital social networks facilitated the spread of misinformation about COVID-19 faster than scientists could unravel the mysteries of the disease.
“There’s always been this underlying hesitancy for some people about vaccines,” Dr. Shlay said. But she has noticed more resistance to the COVID-19 vaccine from parents nervous about the new mRNA technology. “There was a lot of politicization of the vaccine, even though the mRNA vaccine technology has been around for a long time,” she said.
Multipronged approaches
Dr. Shlay is committed to restoring childhood vaccination uptake to prepandemic levels now that clinics are open again. To do so, she is relying on a combination of quality improvement strategies and outreach to undervaccinated populations.
Denver Health, for instance, offers vaccinations at any inpatient or outpatient visit – not just well-child visits – with the help of alerts built into their electronic health records that notify clinicians if a patient is due for a vaccine.
COVID-19 revealed marked health inequities in underserved communities as Black, Hispanic, and people from other minority communities experienced higher rates of COVID-19 cases and deaths, compared with White people. The Public Health Institute, which is part of Denver Health, has responded with vaccine outreach teams that go to schools, shelters, churches, and community-based organizations to vaccinate children. They focus their efforts on areas where immunization rates are low. Health centers in schools throughout Colorado vaccinate students, and the Public Health Institute partners with Denver-area public schools to provide vaccines to students in schools that don’t have such centers. (They also provide dental care and behavioral health services.)
But it is unlikely that restoring clinic operations and making vaccines more accessible will fill the gap. After 3 years of fear and mistrust, parents are still nervous about routine shots. To help clinicians facilitate conversations about vaccination, Denver Health trains providers in communication techniques using motivational interviewing (MI), a collaborative goal-oriented approach that encourages changes in health behaviors.
Dr. Shlay, who stressed the value of persistence, advised, “Through motivational interviewing, discussing things, talking about it, you can actually address most of the concerns.”
Giving parents a boost in the right direction
That spirit drives the work of Boost Oregon, a parent-led nonprofit organization founded in 2015 that helps parents make science-based decisions for themselves and their families. Even before the pandemic, primary care providers needed better strategies for addressing parents who had concerns about vaccines and found themselves failing in the effort while trying to see 20 patients a day.
For families that have questions about vaccines, Boost Oregon holds community meetings in which parents meet with clinicians, share their concerns with other parents, and get answers to their questions in a nonjudgmental way. The 1- to 2-hour sessions enable deeper discussions of the issues than many clinicians can manage in a 20-minute patient visit.
Boost Oregon also trains providers in communication techniques using MI. Ryan Hassan, MD, a pediatrician in private practice who serves as the medical director for the organization, has made the approach an integral part of his day. A key realization for him about the use of MI is that if providers want to build trust with parents, they need to accept that their role is not simply to educate but also to listen.
“Even if it’s the wildest conspiracy theory I’ve ever heard, that is my opportunity to show them that I’m listening and to empathize,” Dr. Hassan said.
His next step, a central tenet of MI, is to make reflective statements that summarize the parent’s concerns, demonstrate empathy, and help him get to the heart of their concerns. He then tailors his message to their issues.
Dr. Hassan tells people who are learning the technique to acknowledge that patients have the autonomy to make their own decisions. Coercing them into a decision is unhelpful and potentially counterproductive. “You can’t change anyone else’s mind,” he said. “You have to help them change their own mind.”
Dr. Limaye reinforced that message. Overwhelmed by conflicting messages on the internet, people are just trying to find answers. She trains providers not to dismiss patients’ concerns, because dismissal erodes trust.
“When you’re dealing with misinformation and conspiracy, to me, one thing to keep in mind is that it’s the long game,” Dr. Limaye said, “You’re not going to be able to sway them in one conversation.”
Can the powers of social media be harnessed for pro-vaccine messaging? Dr. Limaye has studied social media strategies to promote vaccine acceptance and has identified several elements that can be useful for swaying opinions about vaccine.
One is the messenger – as people trust their physicians less, “it’s important to find influencers that people might trust to actually spread a message,” she said. Another factor is that as society has become more polarized, interaction with the leadership of groups that hold influence has become key. To promote vaccine acceptance, for example, leaders of moms’ groups on Facebook could be equipped with evidence-based information.
“It’s important for us to reach out and engage with those that are leaders in those groups, because they kind of hold the power,” Dr. Limaye said.
Framing the message is critical. Dr. Limaye has found that personal narratives can be persuasive and that to influence vaccine behavior, it is necessary to tailor the approach to the specific audience. Danish researchers, for example, in 2017 launched a campaign to increase uptake of HPV vaccinations among teenagers. The researchers provided facts about the safety and effectiveness of the vaccine, cited posts by clinicians about the importance of immunization against the virus, and relayed personal stories, such as one about a father who chose to vaccinate his daughter and another about a blogger’s encounter with a woman with cervical cancer. The researchers found that the highest engagement rates were achieved through personal content and that such content generated the highest proportion of positive comments.
According to Dr. Limaye, to change behavior, social media messaging must address the issues of risk perception and self-efficacy. For risk perception regarding vaccines, a successful message needs to address the parents’ questions about whether their child is at risk for catching a disease, such as measles or pertussis, and if they are, whether the child will wind up in the hospital.
Self-efficacy is the belief that one can accomplish a task. An effective message would provide information on where to find free or low-cost vaccines and would identify locations that are easy to reach and that have expanded hours for working parents, Dr. Limaye said.
What’s the best approach for boosting vaccination rates in the post-pandemic era? In the 1850s, Massachusetts enacted the first vaccine mandate in the United States to prevent smallpox, and by the 1900s, similar laws had been passed in almost half of states. But recent polls suggest that support for vaccine mandates is dwindling. In a poll by the Kaiser Family Foundation last fall, 71% of adults said that healthy children should be required to be vaccinated against measles before entering school, which was down from 82% in a similar poll in 2019.
So perhaps a better approach for promoting vaccine confidence in the 21st century would involve wider use of MI by clinicians and more focus by public health agencies taking advantage of the potential power of social media. As Dr. Offit put it, “I think trust is the key thing.”
Dr. Offit, Dr. Limaye, Dr. Shlay, and Dr. Hassan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When people ask Paul Offit, MD, what worries him the most about the COVID-19 pandemic, he names two concerns. “One is the lack of socialization and education that came from keeping kids out of school for so long,” Dr. Offit said in a recent interview. “And I think vaccines have suffered.”
Dr. Offit is director of the Vaccine Education Center and a professor of pediatrics at Children’s Hospital of Philadelphia. He has watched with alarm as the American public appears to be losing faith in the lifesaving vaccines the public health community has worked hard to promote. The Centers for Disease Control and Prevention estimates that the proportion of kids entering kindergarten who have received state-required vaccines dipped to 94% in the 2020-2021 school year – a full point less than the year before the pandemic – then dropped by another percentage point, to 93%, the following year.
Although a couple of percentage points may sound trivial, were only 93% of kindergarteners to receive the vaccine against measles, mumps, and rubella (MMR), approximately 250,000 vulnerable 5-year-olds could spark the next big outbreak, such as the recent measles outbreaks in Ohio and Minnesota.
Dr. Offit is one of many public health officials and clinicians who are working to reverse the concerning trends in pediatric vaccinations.
“I just don’t want to see an outbreak of something that we could have avoided because we were not protected enough,” Judith Shlay, MD, associate director of the Public Health Institute at Denver Health, said.
Official stumbles in part to blame
Disruptions in health care from the COVID-19 pandemic certainly played a role in the decline. Parents were afraid to expose their children to other sick kids, providers shifted to a telehealth model, and routine preventive care was difficult to access.
But Dr. Offit also blamed erosion of trust on mistakes made by government and public health institutions for the alarming trend. “I think that health care professionals have lost some level of trust in the Food and Drug Administration and CDC.”
He cited as an example poor messaging during a large outbreak in Massachusetts in summer 2021, when the CDC published a report that highlighted the high proportion of COVID-19 cases among vaccinated people. Health officials called those cases “breakthrough” infections, although most were mild or asymptomatic.
Dr. Offit said the CDC should have focused the message instead on the low rate (1%) of hospitalizations and the low number of deaths from the infections. Instead, they had to walk back their promise that vaccinated people didn’t need to wear masks. At other times, the Biden administration pressured public health officials by promising to make booster shots available to the American public when the FDA and CDC felt they lacked evidence to recommend the injections.
Rupali Limaye, PhD, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University, Baltimore, studies vaccine behavior and decision-making. She would go a step further in characterizing the roots of worsening vaccine hesitancy.
“In the last 20 years, we’ve seen there’s less and less trust in health care providers in general,” Dr. Limaye said. “More people are turning to their social networks or social contacts for that kind of information.” In the maelstrom of the COVID-19 pandemic, digital social networks facilitated the spread of misinformation about COVID-19 faster than scientists could unravel the mysteries of the disease.
“There’s always been this underlying hesitancy for some people about vaccines,” Dr. Shlay said. But she has noticed more resistance to the COVID-19 vaccine from parents nervous about the new mRNA technology. “There was a lot of politicization of the vaccine, even though the mRNA vaccine technology has been around for a long time,” she said.
Multipronged approaches
Dr. Shlay is committed to restoring childhood vaccination uptake to prepandemic levels now that clinics are open again. To do so, she is relying on a combination of quality improvement strategies and outreach to undervaccinated populations.
Denver Health, for instance, offers vaccinations at any inpatient or outpatient visit – not just well-child visits – with the help of alerts built into their electronic health records that notify clinicians if a patient is due for a vaccine.
COVID-19 revealed marked health inequities in underserved communities as Black, Hispanic, and people from other minority communities experienced higher rates of COVID-19 cases and deaths, compared with White people. The Public Health Institute, which is part of Denver Health, has responded with vaccine outreach teams that go to schools, shelters, churches, and community-based organizations to vaccinate children. They focus their efforts on areas where immunization rates are low. Health centers in schools throughout Colorado vaccinate students, and the Public Health Institute partners with Denver-area public schools to provide vaccines to students in schools that don’t have such centers. (They also provide dental care and behavioral health services.)
But it is unlikely that restoring clinic operations and making vaccines more accessible will fill the gap. After 3 years of fear and mistrust, parents are still nervous about routine shots. To help clinicians facilitate conversations about vaccination, Denver Health trains providers in communication techniques using motivational interviewing (MI), a collaborative goal-oriented approach that encourages changes in health behaviors.
Dr. Shlay, who stressed the value of persistence, advised, “Through motivational interviewing, discussing things, talking about it, you can actually address most of the concerns.”
Giving parents a boost in the right direction
That spirit drives the work of Boost Oregon, a parent-led nonprofit organization founded in 2015 that helps parents make science-based decisions for themselves and their families. Even before the pandemic, primary care providers needed better strategies for addressing parents who had concerns about vaccines and found themselves failing in the effort while trying to see 20 patients a day.
For families that have questions about vaccines, Boost Oregon holds community meetings in which parents meet with clinicians, share their concerns with other parents, and get answers to their questions in a nonjudgmental way. The 1- to 2-hour sessions enable deeper discussions of the issues than many clinicians can manage in a 20-minute patient visit.
Boost Oregon also trains providers in communication techniques using MI. Ryan Hassan, MD, a pediatrician in private practice who serves as the medical director for the organization, has made the approach an integral part of his day. A key realization for him about the use of MI is that if providers want to build trust with parents, they need to accept that their role is not simply to educate but also to listen.
“Even if it’s the wildest conspiracy theory I’ve ever heard, that is my opportunity to show them that I’m listening and to empathize,” Dr. Hassan said.
His next step, a central tenet of MI, is to make reflective statements that summarize the parent’s concerns, demonstrate empathy, and help him get to the heart of their concerns. He then tailors his message to their issues.
Dr. Hassan tells people who are learning the technique to acknowledge that patients have the autonomy to make their own decisions. Coercing them into a decision is unhelpful and potentially counterproductive. “You can’t change anyone else’s mind,” he said. “You have to help them change their own mind.”
Dr. Limaye reinforced that message. Overwhelmed by conflicting messages on the internet, people are just trying to find answers. She trains providers not to dismiss patients’ concerns, because dismissal erodes trust.
“When you’re dealing with misinformation and conspiracy, to me, one thing to keep in mind is that it’s the long game,” Dr. Limaye said, “You’re not going to be able to sway them in one conversation.”
Can the powers of social media be harnessed for pro-vaccine messaging? Dr. Limaye has studied social media strategies to promote vaccine acceptance and has identified several elements that can be useful for swaying opinions about vaccine.
One is the messenger – as people trust their physicians less, “it’s important to find influencers that people might trust to actually spread a message,” she said. Another factor is that as society has become more polarized, interaction with the leadership of groups that hold influence has become key. To promote vaccine acceptance, for example, leaders of moms’ groups on Facebook could be equipped with evidence-based information.
“It’s important for us to reach out and engage with those that are leaders in those groups, because they kind of hold the power,” Dr. Limaye said.
Framing the message is critical. Dr. Limaye has found that personal narratives can be persuasive and that to influence vaccine behavior, it is necessary to tailor the approach to the specific audience. Danish researchers, for example, in 2017 launched a campaign to increase uptake of HPV vaccinations among teenagers. The researchers provided facts about the safety and effectiveness of the vaccine, cited posts by clinicians about the importance of immunization against the virus, and relayed personal stories, such as one about a father who chose to vaccinate his daughter and another about a blogger’s encounter with a woman with cervical cancer. The researchers found that the highest engagement rates were achieved through personal content and that such content generated the highest proportion of positive comments.
According to Dr. Limaye, to change behavior, social media messaging must address the issues of risk perception and self-efficacy. For risk perception regarding vaccines, a successful message needs to address the parents’ questions about whether their child is at risk for catching a disease, such as measles or pertussis, and if they are, whether the child will wind up in the hospital.
Self-efficacy is the belief that one can accomplish a task. An effective message would provide information on where to find free or low-cost vaccines and would identify locations that are easy to reach and that have expanded hours for working parents, Dr. Limaye said.
What’s the best approach for boosting vaccination rates in the post-pandemic era? In the 1850s, Massachusetts enacted the first vaccine mandate in the United States to prevent smallpox, and by the 1900s, similar laws had been passed in almost half of states. But recent polls suggest that support for vaccine mandates is dwindling. In a poll by the Kaiser Family Foundation last fall, 71% of adults said that healthy children should be required to be vaccinated against measles before entering school, which was down from 82% in a similar poll in 2019.
So perhaps a better approach for promoting vaccine confidence in the 21st century would involve wider use of MI by clinicians and more focus by public health agencies taking advantage of the potential power of social media. As Dr. Offit put it, “I think trust is the key thing.”
Dr. Offit, Dr. Limaye, Dr. Shlay, and Dr. Hassan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When people ask Paul Offit, MD, what worries him the most about the COVID-19 pandemic, he names two concerns. “One is the lack of socialization and education that came from keeping kids out of school for so long,” Dr. Offit said in a recent interview. “And I think vaccines have suffered.”
Dr. Offit is director of the Vaccine Education Center and a professor of pediatrics at Children’s Hospital of Philadelphia. He has watched with alarm as the American public appears to be losing faith in the lifesaving vaccines the public health community has worked hard to promote. The Centers for Disease Control and Prevention estimates that the proportion of kids entering kindergarten who have received state-required vaccines dipped to 94% in the 2020-2021 school year – a full point less than the year before the pandemic – then dropped by another percentage point, to 93%, the following year.
Although a couple of percentage points may sound trivial, were only 93% of kindergarteners to receive the vaccine against measles, mumps, and rubella (MMR), approximately 250,000 vulnerable 5-year-olds could spark the next big outbreak, such as the recent measles outbreaks in Ohio and Minnesota.
Dr. Offit is one of many public health officials and clinicians who are working to reverse the concerning trends in pediatric vaccinations.
“I just don’t want to see an outbreak of something that we could have avoided because we were not protected enough,” Judith Shlay, MD, associate director of the Public Health Institute at Denver Health, said.
Official stumbles in part to blame
Disruptions in health care from the COVID-19 pandemic certainly played a role in the decline. Parents were afraid to expose their children to other sick kids, providers shifted to a telehealth model, and routine preventive care was difficult to access.
But Dr. Offit also blamed erosion of trust on mistakes made by government and public health institutions for the alarming trend. “I think that health care professionals have lost some level of trust in the Food and Drug Administration and CDC.”
He cited as an example poor messaging during a large outbreak in Massachusetts in summer 2021, when the CDC published a report that highlighted the high proportion of COVID-19 cases among vaccinated people. Health officials called those cases “breakthrough” infections, although most were mild or asymptomatic.
Dr. Offit said the CDC should have focused the message instead on the low rate (1%) of hospitalizations and the low number of deaths from the infections. Instead, they had to walk back their promise that vaccinated people didn’t need to wear masks. At other times, the Biden administration pressured public health officials by promising to make booster shots available to the American public when the FDA and CDC felt they lacked evidence to recommend the injections.
Rupali Limaye, PhD, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University, Baltimore, studies vaccine behavior and decision-making. She would go a step further in characterizing the roots of worsening vaccine hesitancy.
“In the last 20 years, we’ve seen there’s less and less trust in health care providers in general,” Dr. Limaye said. “More people are turning to their social networks or social contacts for that kind of information.” In the maelstrom of the COVID-19 pandemic, digital social networks facilitated the spread of misinformation about COVID-19 faster than scientists could unravel the mysteries of the disease.
“There’s always been this underlying hesitancy for some people about vaccines,” Dr. Shlay said. But she has noticed more resistance to the COVID-19 vaccine from parents nervous about the new mRNA technology. “There was a lot of politicization of the vaccine, even though the mRNA vaccine technology has been around for a long time,” she said.
Multipronged approaches
Dr. Shlay is committed to restoring childhood vaccination uptake to prepandemic levels now that clinics are open again. To do so, she is relying on a combination of quality improvement strategies and outreach to undervaccinated populations.
Denver Health, for instance, offers vaccinations at any inpatient or outpatient visit – not just well-child visits – with the help of alerts built into their electronic health records that notify clinicians if a patient is due for a vaccine.
COVID-19 revealed marked health inequities in underserved communities as Black, Hispanic, and people from other minority communities experienced higher rates of COVID-19 cases and deaths, compared with White people. The Public Health Institute, which is part of Denver Health, has responded with vaccine outreach teams that go to schools, shelters, churches, and community-based organizations to vaccinate children. They focus their efforts on areas where immunization rates are low. Health centers in schools throughout Colorado vaccinate students, and the Public Health Institute partners with Denver-area public schools to provide vaccines to students in schools that don’t have such centers. (They also provide dental care and behavioral health services.)
But it is unlikely that restoring clinic operations and making vaccines more accessible will fill the gap. After 3 years of fear and mistrust, parents are still nervous about routine shots. To help clinicians facilitate conversations about vaccination, Denver Health trains providers in communication techniques using motivational interviewing (MI), a collaborative goal-oriented approach that encourages changes in health behaviors.
Dr. Shlay, who stressed the value of persistence, advised, “Through motivational interviewing, discussing things, talking about it, you can actually address most of the concerns.”
Giving parents a boost in the right direction
That spirit drives the work of Boost Oregon, a parent-led nonprofit organization founded in 2015 that helps parents make science-based decisions for themselves and their families. Even before the pandemic, primary care providers needed better strategies for addressing parents who had concerns about vaccines and found themselves failing in the effort while trying to see 20 patients a day.
For families that have questions about vaccines, Boost Oregon holds community meetings in which parents meet with clinicians, share their concerns with other parents, and get answers to their questions in a nonjudgmental way. The 1- to 2-hour sessions enable deeper discussions of the issues than many clinicians can manage in a 20-minute patient visit.
Boost Oregon also trains providers in communication techniques using MI. Ryan Hassan, MD, a pediatrician in private practice who serves as the medical director for the organization, has made the approach an integral part of his day. A key realization for him about the use of MI is that if providers want to build trust with parents, they need to accept that their role is not simply to educate but also to listen.
“Even if it’s the wildest conspiracy theory I’ve ever heard, that is my opportunity to show them that I’m listening and to empathize,” Dr. Hassan said.
His next step, a central tenet of MI, is to make reflective statements that summarize the parent’s concerns, demonstrate empathy, and help him get to the heart of their concerns. He then tailors his message to their issues.
Dr. Hassan tells people who are learning the technique to acknowledge that patients have the autonomy to make their own decisions. Coercing them into a decision is unhelpful and potentially counterproductive. “You can’t change anyone else’s mind,” he said. “You have to help them change their own mind.”
Dr. Limaye reinforced that message. Overwhelmed by conflicting messages on the internet, people are just trying to find answers. She trains providers not to dismiss patients’ concerns, because dismissal erodes trust.
“When you’re dealing with misinformation and conspiracy, to me, one thing to keep in mind is that it’s the long game,” Dr. Limaye said, “You’re not going to be able to sway them in one conversation.”
Can the powers of social media be harnessed for pro-vaccine messaging? Dr. Limaye has studied social media strategies to promote vaccine acceptance and has identified several elements that can be useful for swaying opinions about vaccine.
One is the messenger – as people trust their physicians less, “it’s important to find influencers that people might trust to actually spread a message,” she said. Another factor is that as society has become more polarized, interaction with the leadership of groups that hold influence has become key. To promote vaccine acceptance, for example, leaders of moms’ groups on Facebook could be equipped with evidence-based information.
“It’s important for us to reach out and engage with those that are leaders in those groups, because they kind of hold the power,” Dr. Limaye said.
Framing the message is critical. Dr. Limaye has found that personal narratives can be persuasive and that to influence vaccine behavior, it is necessary to tailor the approach to the specific audience. Danish researchers, for example, in 2017 launched a campaign to increase uptake of HPV vaccinations among teenagers. The researchers provided facts about the safety and effectiveness of the vaccine, cited posts by clinicians about the importance of immunization against the virus, and relayed personal stories, such as one about a father who chose to vaccinate his daughter and another about a blogger’s encounter with a woman with cervical cancer. The researchers found that the highest engagement rates were achieved through personal content and that such content generated the highest proportion of positive comments.
According to Dr. Limaye, to change behavior, social media messaging must address the issues of risk perception and self-efficacy. For risk perception regarding vaccines, a successful message needs to address the parents’ questions about whether their child is at risk for catching a disease, such as measles or pertussis, and if they are, whether the child will wind up in the hospital.
Self-efficacy is the belief that one can accomplish a task. An effective message would provide information on where to find free or low-cost vaccines and would identify locations that are easy to reach and that have expanded hours for working parents, Dr. Limaye said.
What’s the best approach for boosting vaccination rates in the post-pandemic era? In the 1850s, Massachusetts enacted the first vaccine mandate in the United States to prevent smallpox, and by the 1900s, similar laws had been passed in almost half of states. But recent polls suggest that support for vaccine mandates is dwindling. In a poll by the Kaiser Family Foundation last fall, 71% of adults said that healthy children should be required to be vaccinated against measles before entering school, which was down from 82% in a similar poll in 2019.
So perhaps a better approach for promoting vaccine confidence in the 21st century would involve wider use of MI by clinicians and more focus by public health agencies taking advantage of the potential power of social media. As Dr. Offit put it, “I think trust is the key thing.”
Dr. Offit, Dr. Limaye, Dr. Shlay, and Dr. Hassan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
New drugs in primary care: Lessons learned from COVID-19
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
AT INTERNAL MEDICINE 2023
FDA approves first RSV vaccine for older adults
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older,the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older,the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older,the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
‘Exciting’ results for cancer vaccine plus pembro in melanoma
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
according to the latest data from the KEYNOTE-942 trial.
This recurrence-free survival benefit corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with the immunotherapy alone.
The randomized phase 2b trial is the first to show a positive result for a cancer vaccine in a randomized trial. The results, if confirmed in further studies, hold promise for treating other solid tumors with sensitivity to the programmed death-1 (PD-1) protein, investigators said.
“KEYNOTE-942 is the first randomized study to demonstrate improvement in recurrence-free survival in melanoma, or in any cancer in my view, with an individualized neoantigen vaccine approach,” trial investigator Jeffrey S. Weber, MD, PhD, of NYU Langone Perlmutter Cancer Center in New York, said during an oral abstract session at the annual meeting of the American Association for Cancer Research.
“I have every confidence that this strategy will be expanded to other histologies that are PD-1 sensitive, such as non–small cell lung cancer, renal cell cancer, hepatocellular cancer, gastroesophageal cancer, et cetera,” Dr. Weber said.
Invited discussant Margaret Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, called the results “exciting,” especially in light of previous results in cancer vaccine trials. “Despite hundreds of formulations and dozens of studies, cancer vaccines have been disappointing so far, and have largely failed to have a meaningful impact in oncology,” she said.
A promising personalized vaccine
The mRNA vaccine is individually tailored and encodes up to 34 patient-specific tumor neoantigens. The vaccine also acts as an adjuvant to strengthen the immune response.
Dr. Weber said that the “mRNA 4157 is what one would call an individualized neoantigen therapy. It will target an individual patient’s unique tumor mutations, and the revelation over the last 5-10 years, is that, for better or worse, virtually all the neoantigens are unique to an individual patient. There are very, very few true universal neoantigens, or at least universal neoantigens that could have clinical utility.”
The vaccines are developed from tumor biopsy tissues that then undergo whole exome and RNA sequencing to identify single nucleotide variants that are present in the tumor but not in normal tissue.
The findings are then fed into a computer algorithm that identifies potential neoepitope peptides that would bind well to the patient’s human leukocyte antigen (HLA) type and could evoke strong T-cell responses.
“Once they’re chosen, you concatenate the sequences together into a single-strand mRNA vaccine, it’s packaged with nanoparticles to encapsulate it, and there you have your mRNA vaccine,” Dr. Weber explained.
In the KEYNOTE-942 trial, the investigators randomly assigned patients with completely resected high-risk cutaneous melanoma on a 2:1 basis to receive mRNA-4157 via intramuscular injection every 3 weeks for a total of nine doses, plus intravenous pembrolizumab every 3 weeks for 18 cycles (107 patients) or pembrolizumab alone (50 patients). Median follow-up was 101 weeks in the combination group and 105 weeks in the pembrolizumab group.
Overall, the 18-month recurrence-free survival rates were 78.6% in the combination arm and 62.2% in the pembrolizumab arm. The recurrence-free survival rates corresponded to a 44% reduced risk of recurrence or death in patients who received the personalized vaccine plus pembrolizumab compared with those who received only pembrolizumab (hazard ratio [HR] for recurrence, 0.561; P =.0266).
Grade 3 or greater adverse events occurred in 25% of patients in the combination group and 18% of patients in the pembrolizumab group. The most common grade 3 event associated with the vaccine was fatigue. No grade 4 adverse events or deaths were associated with the vaccine, and the addition of the vaccine to pembrolizumab did not appear to increase risk for immune-mediated adverse events.
In a subanalysis, Dr. Weber and colleagues explored the relationship between tumor mutational burden and recurrence-free survival. Higher tumor mutational burden may mean more neoepitopes to target, which is helpful when developing personalized neoantigen vaccines, explained coinvestigator Ryan Sullivan, MD, associate director of the melanoma program at Mass General Cancer Center, Boston, who presented the subanalysis results.
The investigators performed whole exome and whole transcriptome sequencing of baseline tumor biopsy samples to determine the mutational burden of tumors and defined a high mutational burden as 10 or more mutations per megabase.
Overall, in the combination group, patients with a higher tumor mutational burden at baseline showed improved outcomes (HR, 0.652; 95% confidence interval [CI], 0.284-1.494), as did patients with a lower tumor mutational burden (HR, 0.586; 95% CI, 0.243-1.415).
The authors found the same was true for patients with high vs. low tumor inflammation scores (high: HR, 0.576; 95% CI, 0.209-1.591 vs. low: HR, 0.528; 95% CI, 0.253-1.101) and higher PD-L1 expression (PD-L1 positive: HR, 0.485; 95% CI, 0.226-1.039 vs. PD-L1 negative: HR, 0.162; 95% CI, 0.038-0.685).
The hazard ratios crossed 1, which suggest that the combination was similarly effective in all patient subsets, said Dr. Sullivan.
Dr. Callahan also highlighted that the P value was based on a one-side log-rank test, “a relatively low bar to jump over” and that there were slight imbalances in both PD-1 expression status and tumor mutational burden – both of which favored the vaccine group and may be associated with better recurrence-free survival.
The 16% difference in recurrence-free survival seen with the combination vs. pembrolizumab alone, if confirmed in further studies, “is clinically meaningful for high-risk patients,” said Dr. Callahan. “The authors are to be congratulated for presenting the first randomized study of a neoantigen vaccine with a clinical efficacy primary endpoint, and this is a trial that incorporates many of the lessons we’ve learned along the years.”
Dr. Sullivan also commented on the promising results. “The field of cancer vaccines is a wasteland of failed clinical trials after some initial promising data, so to have something like this where it does appear that this vaccine strategy works is good not only for patients with melanoma but for those people who have dedicated their lives to trying to develop cancer vaccines,” he said in an interview.
KEYNOTE-942 was funded by Moderna with collaboration from Merck. Dr. Weber has financial relationships with Merck, Moderna, and other companies. Dr. Sullivan has served as a paid consultant for Merck and has received research funding from the company. Dr. Callahan disclosed a consulting/advisory role with Moderna, Merck, and others.
A version of this article first appeared on Medscape.com.
FROM AACR 2023
CDC backs FDA’s call for second COVID booster for those at high risk
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
Cancer, heart disease vaccines may be ready by 2030, Moderna says
The announcement is yet another sign of what many are calling “the golden age” of vaccine development, which is largely credited to the pandemic’s use of mRNA technology to create COVID-19 vaccines.
“I think what we have learned in recent months is that if you ever thought that mRNA was just for infectious diseases, or just for COVID, the evidence now is that that’s absolutely not the case,” Moderna Chief Medical Officer Paul Burton, MD, PhD, told The Guardian. “It can be applied to all sorts of disease areas; we are in cancer, infectious disease, cardiovascular disease, autoimmune diseases, rare disease. We have studies in all of those areas, and they have all shown tremendous promise.”
The U.S. Food and Drug Administration recently designated two new Moderna vaccines as breakthrough therapies: a shot that prevents respiratory syncytial virus (RSV) in older people and a shot that helps prevent the recurrence of melanoma. The FDA’s breakthrough designation is given when a new treatment’s early trial results are substantially better than an existing therapy.
The mRNA vaccine technology that made headlines for its role in COVID-19 vaccines works by teaching the body how to make a specific protein to help the immune system prevent or target a certain disease.
Dr. Burton anticipates that mRNA technology will result in breakthroughs such as a cancer vaccine that can be personalized based on the features of a specific tumor.
“I think we will have mRNA-based therapies for rare diseases that were previously undruggable, and I think that 10 years from now, we will be approaching a world where you truly can identify the genetic cause of a disease and, with relative simplicity, go and edit that out and repair it using mRNA-based technology,” he said.
The Moderna executive made the statements before its annual update on its vaccine pipeline projects, which the company calls “Vaccines Day.” The Massachusetts-based drugmaker said it has given someone the first dose of a “next-generation” COVID-19 vaccine in a phase III trial, has made progress on a Lyme disease shot, and is developing a vaccine for the highly contagious norovirus.
In all, Moderna expects “six major vaccine product launches in the next few years,” the company said in a statement, adding that it expects the COVID-19 booster market alone to be valued at $15 billion.
A version of this article first appeared on WebMD.com.
The announcement is yet another sign of what many are calling “the golden age” of vaccine development, which is largely credited to the pandemic’s use of mRNA technology to create COVID-19 vaccines.
“I think what we have learned in recent months is that if you ever thought that mRNA was just for infectious diseases, or just for COVID, the evidence now is that that’s absolutely not the case,” Moderna Chief Medical Officer Paul Burton, MD, PhD, told The Guardian. “It can be applied to all sorts of disease areas; we are in cancer, infectious disease, cardiovascular disease, autoimmune diseases, rare disease. We have studies in all of those areas, and they have all shown tremendous promise.”
The U.S. Food and Drug Administration recently designated two new Moderna vaccines as breakthrough therapies: a shot that prevents respiratory syncytial virus (RSV) in older people and a shot that helps prevent the recurrence of melanoma. The FDA’s breakthrough designation is given when a new treatment’s early trial results are substantially better than an existing therapy.
The mRNA vaccine technology that made headlines for its role in COVID-19 vaccines works by teaching the body how to make a specific protein to help the immune system prevent or target a certain disease.
Dr. Burton anticipates that mRNA technology will result in breakthroughs such as a cancer vaccine that can be personalized based on the features of a specific tumor.
“I think we will have mRNA-based therapies for rare diseases that were previously undruggable, and I think that 10 years from now, we will be approaching a world where you truly can identify the genetic cause of a disease and, with relative simplicity, go and edit that out and repair it using mRNA-based technology,” he said.
The Moderna executive made the statements before its annual update on its vaccine pipeline projects, which the company calls “Vaccines Day.” The Massachusetts-based drugmaker said it has given someone the first dose of a “next-generation” COVID-19 vaccine in a phase III trial, has made progress on a Lyme disease shot, and is developing a vaccine for the highly contagious norovirus.
In all, Moderna expects “six major vaccine product launches in the next few years,” the company said in a statement, adding that it expects the COVID-19 booster market alone to be valued at $15 billion.
A version of this article first appeared on WebMD.com.
The announcement is yet another sign of what many are calling “the golden age” of vaccine development, which is largely credited to the pandemic’s use of mRNA technology to create COVID-19 vaccines.
“I think what we have learned in recent months is that if you ever thought that mRNA was just for infectious diseases, or just for COVID, the evidence now is that that’s absolutely not the case,” Moderna Chief Medical Officer Paul Burton, MD, PhD, told The Guardian. “It can be applied to all sorts of disease areas; we are in cancer, infectious disease, cardiovascular disease, autoimmune diseases, rare disease. We have studies in all of those areas, and they have all shown tremendous promise.”
The U.S. Food and Drug Administration recently designated two new Moderna vaccines as breakthrough therapies: a shot that prevents respiratory syncytial virus (RSV) in older people and a shot that helps prevent the recurrence of melanoma. The FDA’s breakthrough designation is given when a new treatment’s early trial results are substantially better than an existing therapy.
The mRNA vaccine technology that made headlines for its role in COVID-19 vaccines works by teaching the body how to make a specific protein to help the immune system prevent or target a certain disease.
Dr. Burton anticipates that mRNA technology will result in breakthroughs such as a cancer vaccine that can be personalized based on the features of a specific tumor.
“I think we will have mRNA-based therapies for rare diseases that were previously undruggable, and I think that 10 years from now, we will be approaching a world where you truly can identify the genetic cause of a disease and, with relative simplicity, go and edit that out and repair it using mRNA-based technology,” he said.
The Moderna executive made the statements before its annual update on its vaccine pipeline projects, which the company calls “Vaccines Day.” The Massachusetts-based drugmaker said it has given someone the first dose of a “next-generation” COVID-19 vaccine in a phase III trial, has made progress on a Lyme disease shot, and is developing a vaccine for the highly contagious norovirus.
In all, Moderna expects “six major vaccine product launches in the next few years,” the company said in a statement, adding that it expects the COVID-19 booster market alone to be valued at $15 billion.
A version of this article first appeared on WebMD.com.
Parents of patients with rheumatic disease, MIS-C strongly hesitant of COVID vaccination
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023