User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Consider home subcutaneous immune globulin for refractory dermatomyositis
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
FROM RWCS 2021
Don’t fear patients reading their clinical notes: Opinion
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
What to do if an employee tests positive for COVID-19
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
One-third of health care workers leery of getting COVID-19 vaccine, survey shows
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Moreover, 54% of direct care providers indicated that they would take the vaccine if offered, compared with 60% of noncare providers.
The findings come from what is believed to be the largest survey of health care provider attitudes toward COVID-19 vaccination, published online Jan. 25 in Clinical Infectious Diseases.
“We have shown that self-reported willingness to receive vaccination against COVID-19 differs by age, gender, race and hospital role, with physicians and research scientists showing the highest acceptance,” Jana Shaw, MD, MPH, State University of New York, Syracuse, N.Y, the study’s corresponding author, told this news organization. “Building trust in authorities and confidence in vaccines is a complex and time-consuming process that requires commitment and resources. We have to make those investments as hesitancy can severely undermine vaccination coverage. Because health care providers are members of our communities, it is possible that their views are shared by the public at large. Our findings can assist public health professionals as a starting point of discussion and engagement with communities to ensure that we vaccinate at least 80% of the public to end the pandemic.”
For the study, Dr. Shaw and her colleagues emailed an anonymous survey to 9,565 employees of State University of New York Upstate Medical University, Syracuse, an academic medical center that cares for an estimated 1.8 million people. The survey, which contained questions intended to evaluate attitudes, belief, and willingness to get vaccinated, took place between Nov. 23 and Dec. 5, about a week before the U.S. Food and Drug Administration granted the first emergency use authorization for the Pfizer-BioNTech BNT162b2 mRNA vaccine.
Survey recipients included physicians, nurse practitioners, physician assistants, nurses, pharmacists, medical and nursing students, allied health professionals, and nonclinical ancillary staff.
Of the 9,565 surveys sent, 5,287 responses were collected and used in the final analysis, for a response rate of 55%. The mean age of respondents was 43, 73% were female, 85% were White, 6% were Asian, 5% were Black/African American, and the rest were Native American, Native Hawaiian/Pacific Islander, or from other races. More than half of respondents (59%) reported that they provided direct patient care, and 32% said they provided care for patients with COVID-19.
Of all survey respondents, 58% expressed their intent to receive a COVID-19 vaccine, but this varied by their role in the health care system. For example, in response to the statement, “If a vaccine were offered free of charge, I would take it,” 80% of scientists and physicians agreed that they would, while colleagues in other roles were unsure whether they would take the vaccine, including 34% of registered nurses, 32% of allied health professionals, and 32% of master’s-level clinicians. These differences across roles were significant (P less than .001).
The researchers also found that direct patient care or care for COVID-19 patients was associated with lower vaccination intent. For example, 54% of direct care providers and 62% of non-care providers indicated they would take the vaccine if offered, compared with 52% of those who had provided care for COVID-19 patients vs. 61% of those who had not (P less than .001).
“This was a really surprising finding,” said Dr. Shaw, who is a pediatric infectious diseases physician at SUNY Upstate. “In general, one would expect that perceived severity of disease would lead to a greater desire to get vaccinated. Because our question did not address severity of disease, it is possible that we oversampled respondents who took care of patients with mild disease (i.e., in an outpatient setting). This could have led to an underestimation of disease severity and resulted in lower vaccination intent.”
A focus on rebuilding trust
Survey respondents who agreed or strongly agreed that they would accept a vaccine were older (a mean age of 44 years), compared with those who were not sure or who disagreed (a mean age of 42 vs. 38 years, respectively; P less than .001). In addition, fewer females agreed or strongly agreed that they would accept a vaccine (54% vs. 73% of males), whereas those who self-identified as Black/African American were least likely to want to get vaccinated, compared with those from other ethnic groups (31%, compared with 74% of Asians, 58% of Whites, and 39% of American Indians or Alaska Natives).
“We are deeply aware of the poor decisions scientists made in the past, which led to a prevailing skepticism and ‘feeling like guinea pigs’ among people of color, especially Black adults,” Dr. Shaw said. “Black adults are less likely, compared [with] White adults, to have confidence that scientists act in the public interest. Rebuilding trust will take time and has to start with addressing health care disparities. In addition, we need to acknowledge contributions of Black researchers to science. For example, until recently very few knew that the Moderna vaccine was developed [with the help of] Dr. Kizzmekia Corbett, who is Black.”
The top five main areas of unease that all respondents expressed about a COVID-19 vaccine were concern about adverse events/side effects (47%), efficacy (15%), rushed release (11%), safety (11%), and the research and authorization process (3%).
“I think it is important that fellow clinicians recognize that, in order to boost vaccine confidence we will need careful, individually tailored communication strategies,” Dr. Shaw said. “A consideration should be given to those [strategies] that utilize interpersonal channels that deliver leadership by example and leverage influencers in the institution to encourage wider adoption of vaccination.”
Aaron M. Milstone, MD, MHS, asked to comment on the research, recommended that health care workers advocate for the vaccine and encourage their patients, friends, and loved ones to get vaccinated. “Soon, COVID-19 will have taken more than half a million lives in the U.S.,” said Dr. Milstone, a pediatric epidemiologist at Johns Hopkins University, Baltimore. “Although vaccines can have side effects like fever and muscle aches, and very, very rare more serious side effects, the risks of dying from COVID are much greater than the risk of a serious vaccine reaction. The study’s authors shed light on the ongoing need for leaders of all communities to support the COVID vaccines, not just the scientific community, but religious leaders, political leaders, and community leaders.”
Addressing vaccine hesitancy
Informed by their own survey, Dr. Shaw and her colleagues have developed a plan to address vaccine hesitancy to ensure high vaccine uptake at SUNY Upstate. Those strategies include, but aren’t limited to, institution-wide forums for all employees on COVID-19 vaccine safety, risks, and benefits followed by Q&A sessions, grand rounds for providers summarizing clinical trial data on mRNA vaccines, development of an Ask COVID email line for staff to ask vaccine-related questions, and a detailed vaccine-specific FAQ document.
In addition, SUNY Upstate experts have engaged in numerous media interviews to provide education and updates on the benefits of vaccination to public and staff, stationary vaccine locations, and mobile COVID-19 vaccine carts. “To date, the COVID-19 vaccination process has been well received, and we anticipate strong vaccine uptake,” she said.
Dr. Shaw acknowledged certain limitations of the survey, including its cross-sectional design and the fact that it was conducted in a single health care system in the northeastern United States. “Thus, generalizability to other regions of the U.S. and other countries may be limited,” Dr. Shaw said. “The study was also conducted before EUA [emergency use authorization] was granted to either the Moderna or Pfizer-BioNTech vaccines. It is therefore likely that vaccine acceptance will change over time as more people get vaccinated.”
The authors have disclosed no relevant financial relationships. Dr. Milstone disclosed that he has received a research grant from Merck, but it is not related to vaccines.
A version of this article first appeared on Medscape.com.
Checkpoint inhibitors’ ‘big picture’ safety shown with preexisting autoimmune diseases
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
Patients with advanced melanoma and preexisting autoimmune diseases (AIDs) who were treated with immune checkpoint inhibitors (ICIs) responded well and did not suffer more grade 3 or higher immune-related adverse events than patients without an AID, a new study finds, although some concerns were raised regarding patients with inflammatory bowel disease (IBD).
“To our knowledge, this is the first study to bridge this knowledge gap by presenting ‘real-world’ data on the safety and efficacy of ICI on a national scale,” wrote Monique K. van der Kooij, MD, of Leiden (the Netherlands) University Medical Center and coauthors. The study was published online in Annals of Internal Medicine.
To investigate ICI use and response among this specific subset of melanoma patients, the researchers launched a nationwide cohort study set in the Netherlands. Data were gathered via the Dutch Melanoma Treatment Registry (DMTR), in which 4,367 patients with advanced melanoma were enrolled between July 2013 and July 2018.
Within that cohort, 415 (9.5%) had preexisting AIDs. Nearly 55% had rheumatologic AIDs (n = 227) – which included RA, systemic lupus erythematosus, scleroderma, sarcoidosis, and vasculitis – with the next most frequent being endocrine AID (n = 143) and IBD (n = 55). Patients with AID were older than patients without (67 vs. 63 years) and were more likely to be female (53% vs. 41%).
The ICIs used in the study included anti-CTLA4 (ipilimumab), anti–programmed death 1 (PD-1) (nivolumab or pembrolizumab), or a combination of nivolumab and ipilimumab. Of the patients with AID, 55% (n = 228) were treated with ICI, compared with 58% of patients without AID. A total of 87 AID patients were treated with anti-CTLA4, 187 received anti-PD-1, and 34 received the combination. The combination was not readily available in the Netherlands until 2017, the authors stated, acknowledging that it may be wise to revisit its effects in the coming years.
Incidence of immune-related adverse events
The incidence of immune-related adverse events (irAEs) grade 3 and above for patients with and without AID who were given anti-CTLA4 was 30%. The incidence rate of irAEs was also similar for patients with (17%; 95% confidence interval, 12%-23%) and without (13%; 95% CI, 12%-15%) AID on anti-PD-1. Patients with AIDs who took anti-PD-1 therapy discontinued it more often because of toxicity than did the patients without AIDs.
The combination group had irAE incidence rates of 44% (95% CI, 27%-62%) for patients with AID, compared with 48% (95% CI, 43%-53%) for patients without AIDs. Overall, no patients with AIDs on ICIs died of toxicity, compared with three deaths among patients without AID on anti-CTLA4, five deaths among patients on anti-PD-1, and one patient on the combination.
Patients with IBD had a notably higher risk of anti-PD-1–induced colitis (19%; 95% CI, 7%-37%), compared with patients with other AIDs (3%; 95% CI, 0%-6%) and patients without AIDs (2%; 95% CI, 2%-3%). IBD patients were also more likely than all other groups on ICIs to stop treatment because of toxicity, leading the researchers to note that “close monitoring in patients with IBD is advised.”
Overall survival after diagnosis was similar in patients with AIDs (median, 13 months; 95% CI, 10-16 months) and without (median, 14 months; 95% CI, 13-15 months), as was the objective response rate to anti-CTLA4 treatment (10% vs. 16%), anti-PD-1 treatment (40% vs. 44%), and combination therapy (39% vs. 43%).
Study largely bypasses the effects of checkpoint inhibitors on RA patients
“For detail, you can’t look to this study,” Anne R. Bass, MD, of the division of rheumatology at the Hospital for Special Surgery in New York, said in an interview. “But for a big-picture look at ‘how safe are checkpoint inhibitors,’ I think it’s an important one.”
Dr. Bass noted that the investigators lumped certain elements together and bypassed others, including their focus on grade 3 or higher adverse events. That was a decision the authors themselves recognized as a potential limitation of their research.
“Understandably, they were worried about life-threatening adverse events, and that’s fine,” she said. But for patients with arthritis who flare, their events are usually grade 2 or even grade 1 and therefore not captured or analyzed in the study. “This does not really address the risk of flare in an RA patient.”
She also questioned their grouping of AIDs, with a bevy of rheumatic diseases categorized as one cluster and the “other” group being particularly broad in its inclusion of “all AIDs not listed” – though only eight patients were placed into that group.
That said, the researchers relied on an oncology database, not one aimed at AID or adverse events. “The numbers are so much bigger than any other study in this area that’s been done,” she said. “It’s both a strength and a weakness of this kind of database.”
Indeed, the authors considered their use of nationwide, population-based data from the DMTR a benefit, calling it “a strength of our approach.”
The DMTR was funded by a grant from the Netherlands Organization for Health Research and Development and sponsored by Bristol-Myers Squibb, Novartis, Roche Nederland, Merck Sharp & Dohme, and Pierre Fabre via the Dutch Institute for Clinical Auditing.
FROM ANNALS OF INTERNAL MEDICINE
Cumulative exposure to high-potency topical steroid doses drives osteoporosis fractures
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
FROM JAMA DERMATOLOGY
Survey: Most patients support teledermatology
Many medical practices turned to telemedicine when the pandemic shut down the economy last spring, but what do dermatology patients think about the socially distant approach?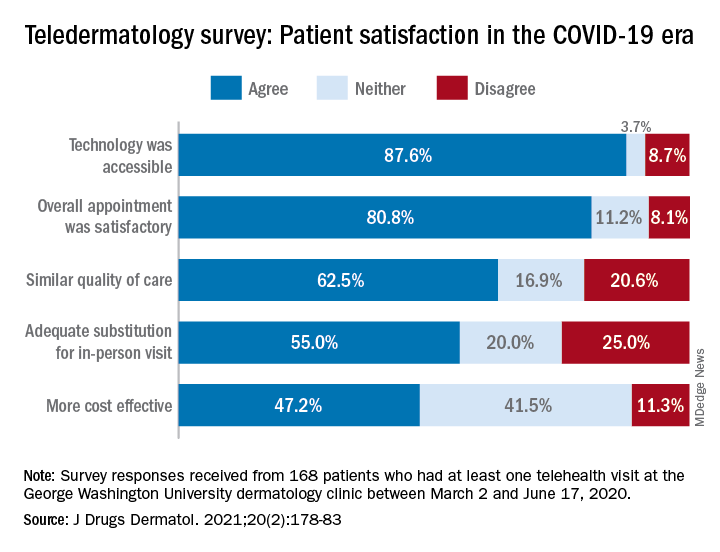
and 80% said that they would consider another such visit in the future, according to a survey conducted at George Washington University in Washington.
Although “telehealth is not without its drawbacks … it is clear from this study that the majority of patients feel positively towards teledermatology during the COVID-19 pandemic and [believe it] can be a suitable alternative for patients who are unable to meet with their providers in person,” Samuel Yeroushalmi, Sarah H. Millan, and associates at the university said in the Journal of Drugs in Dermatology.
When presented with a set of statements about the telehealth experience, the 168 survey respondents largely agreed that the overall appointment was satisfactory (80.8%), that minimal barriers were present (78.1%), and that the quality of care was similar to an in-person visit (62.5%), the investigators said.
Other factors, however, were not as well supported. Less than half (47.2%) of the respondents agreed that the telehealth appointments were more cost effective, and just over half (54.7%) agreed that they provided an adequate skin exam, they reported.
Of the set of 14 statements given to the patients – all of whom had at least one telehealth visit with the GW clinic between March 2 and June 17, 2020 – the one on the adequacy of the skin exam provided the largest share of disagreement at 27.1%, Mr. Yeroushalmi and Ms. Millan, medical students at the university and coauthors.
The lack of physical touch was mentioned most often (26.8%) when respondents were asked about their reasons for disliking telehealth visits, followed by the feeling that they had received an inadequate assessment (15.7%), they said.
Despite these drawbacks, “the convenience and efficacy of telehealth as well as its ability to maintain separation while social distancing recommendations are in place make it an effective way for dermatologists to continue to provide quality and safe care during the pandemics as well as during potential future public health crises,” the investigators concluded.
Many medical practices turned to telemedicine when the pandemic shut down the economy last spring, but what do dermatology patients think about the socially distant approach?
and 80% said that they would consider another such visit in the future, according to a survey conducted at George Washington University in Washington.
Although “telehealth is not without its drawbacks … it is clear from this study that the majority of patients feel positively towards teledermatology during the COVID-19 pandemic and [believe it] can be a suitable alternative for patients who are unable to meet with their providers in person,” Samuel Yeroushalmi, Sarah H. Millan, and associates at the university said in the Journal of Drugs in Dermatology.
When presented with a set of statements about the telehealth experience, the 168 survey respondents largely agreed that the overall appointment was satisfactory (80.8%), that minimal barriers were present (78.1%), and that the quality of care was similar to an in-person visit (62.5%), the investigators said.
Other factors, however, were not as well supported. Less than half (47.2%) of the respondents agreed that the telehealth appointments were more cost effective, and just over half (54.7%) agreed that they provided an adequate skin exam, they reported.
Of the set of 14 statements given to the patients – all of whom had at least one telehealth visit with the GW clinic between March 2 and June 17, 2020 – the one on the adequacy of the skin exam provided the largest share of disagreement at 27.1%, Mr. Yeroushalmi and Ms. Millan, medical students at the university and coauthors.
The lack of physical touch was mentioned most often (26.8%) when respondents were asked about their reasons for disliking telehealth visits, followed by the feeling that they had received an inadequate assessment (15.7%), they said.
Despite these drawbacks, “the convenience and efficacy of telehealth as well as its ability to maintain separation while social distancing recommendations are in place make it an effective way for dermatologists to continue to provide quality and safe care during the pandemics as well as during potential future public health crises,” the investigators concluded.
Many medical practices turned to telemedicine when the pandemic shut down the economy last spring, but what do dermatology patients think about the socially distant approach?
and 80% said that they would consider another such visit in the future, according to a survey conducted at George Washington University in Washington.
Although “telehealth is not without its drawbacks … it is clear from this study that the majority of patients feel positively towards teledermatology during the COVID-19 pandemic and [believe it] can be a suitable alternative for patients who are unable to meet with their providers in person,” Samuel Yeroushalmi, Sarah H. Millan, and associates at the university said in the Journal of Drugs in Dermatology.
When presented with a set of statements about the telehealth experience, the 168 survey respondents largely agreed that the overall appointment was satisfactory (80.8%), that minimal barriers were present (78.1%), and that the quality of care was similar to an in-person visit (62.5%), the investigators said.
Other factors, however, were not as well supported. Less than half (47.2%) of the respondents agreed that the telehealth appointments were more cost effective, and just over half (54.7%) agreed that they provided an adequate skin exam, they reported.
Of the set of 14 statements given to the patients – all of whom had at least one telehealth visit with the GW clinic between March 2 and June 17, 2020 – the one on the adequacy of the skin exam provided the largest share of disagreement at 27.1%, Mr. Yeroushalmi and Ms. Millan, medical students at the university and coauthors.
The lack of physical touch was mentioned most often (26.8%) when respondents were asked about their reasons for disliking telehealth visits, followed by the feeling that they had received an inadequate assessment (15.7%), they said.
Despite these drawbacks, “the convenience and efficacy of telehealth as well as its ability to maintain separation while social distancing recommendations are in place make it an effective way for dermatologists to continue to provide quality and safe care during the pandemics as well as during potential future public health crises,” the investigators concluded.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Psoriasis registry study finds normal pregnancy outcomes
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
FROM JAMA DERMATOLOGY
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
A 35-year-old male who takes antiseizure medications, has asymptomatic lesions on his nose and cheeks, present since birth
although up to 75% of cases may be caused by a spontaneous mutation. It is caused by mutations in the TSC1 gene on chromosome 9q34–encoding hamartin or the TSC2 gene on chromosome I6pl3–encoding tuberin. Patients present at birth and males and females are affected equally.
There are multiple skin findings in TS that may herald the diagnosis. The earliest findings are hypopigmented macules, found in 85% of patients. They may be in an ash-leaf shape or confetti pattern. Adenoma sebaceum, or angiofibromas, are present on the forehead, nose, and cheeks, and often present in childhood. Periungual angiofibromas called Koenen tumors tend to occur at puberty. Connective-tissue nevi called Shagreen plaques, or collagenomas, may be present, which is what our patient exhibits on his back. The lumbosacral region is the most common area for these to appear in the first decade of life.
TS can affect other organ systems in the body. Seizures, neuropsychiatric diseases, and mental deficiency are common. Cortical tumors, gliomas, and astrocytomas may develop in the brain. Congenital retinal hamartomas (phakomas) occur. Renal cysts and angiomyolipomas may occur in the kidneys. In the lungs, patients may develop lymphangiomyomatosis. Rhabdomyomas can occur in the heart in infancy and may regress spontaneously over time. Bony changes such as cysts and sclerosis may occur.
Treatment and monitoring of TS requires a multidisciplinary approach with neurology, pulmonology, cardiology, ophthalmology, orthopedics, and dermatology. Cosmetic treatment for angiofibromas includes CO2 laser, shaving, and dermabrasion. Topical rapamycin use has been described in the literature to improve the appearance of angiofibromas. Our patient has been using rapamycin 1% cream for more than 5 years and has had a substantial reduction in the size and number of angiofibromas.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References:
Spitz J. Genodermatoses. A Clinical Guide to Genetic Skin Disorders. Philadelphia: Lippincott Williams & Wilkins, 2005.
James W et al. Andrews’ Diseases of the Skin. Philadelphia: Saunders, 2006.
Bolognia JL et al. Dermatology. London: Mosby Elsevier, 2008.
although up to 75% of cases may be caused by a spontaneous mutation. It is caused by mutations in the TSC1 gene on chromosome 9q34–encoding hamartin or the TSC2 gene on chromosome I6pl3–encoding tuberin. Patients present at birth and males and females are affected equally.
There are multiple skin findings in TS that may herald the diagnosis. The earliest findings are hypopigmented macules, found in 85% of patients. They may be in an ash-leaf shape or confetti pattern. Adenoma sebaceum, or angiofibromas, are present on the forehead, nose, and cheeks, and often present in childhood. Periungual angiofibromas called Koenen tumors tend to occur at puberty. Connective-tissue nevi called Shagreen plaques, or collagenomas, may be present, which is what our patient exhibits on his back. The lumbosacral region is the most common area for these to appear in the first decade of life.
TS can affect other organ systems in the body. Seizures, neuropsychiatric diseases, and mental deficiency are common. Cortical tumors, gliomas, and astrocytomas may develop in the brain. Congenital retinal hamartomas (phakomas) occur. Renal cysts and angiomyolipomas may occur in the kidneys. In the lungs, patients may develop lymphangiomyomatosis. Rhabdomyomas can occur in the heart in infancy and may regress spontaneously over time. Bony changes such as cysts and sclerosis may occur.
Treatment and monitoring of TS requires a multidisciplinary approach with neurology, pulmonology, cardiology, ophthalmology, orthopedics, and dermatology. Cosmetic treatment for angiofibromas includes CO2 laser, shaving, and dermabrasion. Topical rapamycin use has been described in the literature to improve the appearance of angiofibromas. Our patient has been using rapamycin 1% cream for more than 5 years and has had a substantial reduction in the size and number of angiofibromas.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References:
Spitz J. Genodermatoses. A Clinical Guide to Genetic Skin Disorders. Philadelphia: Lippincott Williams & Wilkins, 2005.
James W et al. Andrews’ Diseases of the Skin. Philadelphia: Saunders, 2006.
Bolognia JL et al. Dermatology. London: Mosby Elsevier, 2008.
although up to 75% of cases may be caused by a spontaneous mutation. It is caused by mutations in the TSC1 gene on chromosome 9q34–encoding hamartin or the TSC2 gene on chromosome I6pl3–encoding tuberin. Patients present at birth and males and females are affected equally.
There are multiple skin findings in TS that may herald the diagnosis. The earliest findings are hypopigmented macules, found in 85% of patients. They may be in an ash-leaf shape or confetti pattern. Adenoma sebaceum, or angiofibromas, are present on the forehead, nose, and cheeks, and often present in childhood. Periungual angiofibromas called Koenen tumors tend to occur at puberty. Connective-tissue nevi called Shagreen plaques, or collagenomas, may be present, which is what our patient exhibits on his back. The lumbosacral region is the most common area for these to appear in the first decade of life.
TS can affect other organ systems in the body. Seizures, neuropsychiatric diseases, and mental deficiency are common. Cortical tumors, gliomas, and astrocytomas may develop in the brain. Congenital retinal hamartomas (phakomas) occur. Renal cysts and angiomyolipomas may occur in the kidneys. In the lungs, patients may develop lymphangiomyomatosis. Rhabdomyomas can occur in the heart in infancy and may regress spontaneously over time. Bony changes such as cysts and sclerosis may occur.
Treatment and monitoring of TS requires a multidisciplinary approach with neurology, pulmonology, cardiology, ophthalmology, orthopedics, and dermatology. Cosmetic treatment for angiofibromas includes CO2 laser, shaving, and dermabrasion. Topical rapamycin use has been described in the literature to improve the appearance of angiofibromas. Our patient has been using rapamycin 1% cream for more than 5 years and has had a substantial reduction in the size and number of angiofibromas.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References:
Spitz J. Genodermatoses. A Clinical Guide to Genetic Skin Disorders. Philadelphia: Lippincott Williams & Wilkins, 2005.
James W et al. Andrews’ Diseases of the Skin. Philadelphia: Saunders, 2006.
Bolognia JL et al. Dermatology. London: Mosby Elsevier, 2008.