User login
End the Routine Shackling of Incarcerated Inpatients
The police shooting of Jacob Blake, an unarmed Wisconsin man, during an arrest in August 2020, led to more protests in a summer filled with calls against the unequal application of police force. Outrage grew as it was revealed that Blake, paralyzed from his waist down and not yet convicted of a crime, was still handcuffed to his hospital bed while receiving treatment.1 To many this seemed unusually cruel, but to those tasked with caring for incarcerated patients, it is all too familiar. Given the high rates of incarceration in the United States and the increased medical needs of this population, caring for those in custody is unavoidable for many physicians and hospitals. Though safety should be paramount, the universal application of metal handcuffs or leg cuffs by law enforcement officials, a process known as shackling, can lead to a variety of harms and should be abandoned.
BACKGROUND
The United States incarcerates more individuals both in total numbers and per capita than any other country in the world. This is currently believed to be more than two million people on any given day or more than 650 persons per 100,000 population.2 Incarceration occurs in jails, which are locally run facilities holding individuals on short sentences or those not yet convicted who are unable to afford bail before their trials (pretrial), or prisons, which are state and federally run facilities that house those with long sentences. When an incarcerated person experiences a medical emergency requiring hospitalization, they are either treated in the correctional facility or transferred to a local hospital for a higher level of care. Some hospitals are equipped with security measures similar to those of a correctional facility, with secure floors or wings dedicated solely to the care of the incarcerated. Secure units are more commonly seen in hospitals associated with prisons rather than local jails. Other hospitals house incarcerated patients in the same rooms as the public population, and thus movement is restricted by other means.3 Most commonly, this is done with a hard metal shackle resembling a handcuff with one end attached to the leg or wrist and the other end attached to the bed. Some agencies require more restraints, often requiring the use of wrist cuffs and leg cuffs concurrently for the entire duration of a patient’s hospitalization.4 In our experience, agencies apply these restraints universally, regardless of age, illness, mobility, or pretrial status.
Restraint practices are rooted in a concern for practitioner and public safety and bear merit. A patient from a correctional facility is usually guarded by just one officer in lieu of the multiple security measures at a jail or prison facility. Nonsecured hospitals have become sites of multiple escapes by incarcerated inpatients, given the lack of secured doors and the multiple movements during the admission and discharge processes.5 Furthermore, violence against hospital staff is now a focus issue in many hospitals and is no longer accepted as just “part of the job.” In several high-profile incidents, incarcerated inpatients have harmed staff, including one at our own institution, when an incarcerated patient held a makeshift weapon to a student’s throat.6
LEGAL CHALLENGES
The use of shackles during hospital visits has been challenged in US courts and routinely upheld. In one case, an incarcerated patient with renal failure received injuries after his leg edema was so severe that “at one point the shackles themselves were barely visible.”7 Though he was injured, the shackles were determined to have served a penological purpose outside of punishment, such as preventing escape, and the injuries were the result of the patient’s guards not following protocol. British courts have taken a different stance, ruling for an incarcerated patient who challenged the use of cuffs during three outpatient appointments and one inpatient admission.8 While the cuffs in the outpatient setting were deemed acceptable (as they were removed during the medical visit itself), they remained during the duration of the inpatient stay. This was deemed in violation of Title I/Article 3 of the Charter of Fundamental Rights of the European Union, Dignity/The right to integrity of the person. One area in US healthcare where shackling has been roundly condemned is the peripartum shackling of pregnant women. Though courts have had a mixed record to challenges, activism and advocacy have led to the banning of the practice in 23 states, though in most states significant exemptions exist.9 Through the First Step Act of 2018, the federal government banned peripartum shackling for all federal prisoners, but as most incarcerations are under state or local control, a considerable number of incarcerated pregnant women can legally be shackled during their deliveries.
RISKS OF SHACKLING
Legal and safety concerns aside, the shackling of incarcerated patients carries enormous risk. The use of medical restraints in hospitals has decreased over the past few decades, given their proven harms in increasing falls, exacerbating delirium, and increasing the risk of in-hospital death.10 There is no reason to believe that trading a soft medical restraint for a metal leg or wrist cuff would not confer the same risk. Additionally, metal law enforcement cuffs are not designed with patient safety in mind and have been known to cause specific nerve injuries, or handcuff neuropathy. This can occur when placement is too tight or when a patient struggles against them, as could happen with an agitated or delirious patient. The bar for removal, even briefly for an exam, is also much higher than that of a medical restraint, leading to a greater likelihood that certain aspects of the physical exam, such as gait or strength assessment, may not be adequately performed. In one small survey, British physicians reported often performing an exam while the patient was cuffed and with a guard in the room, despite country guidelines against both practices.11
Additionally, marginalized communities are disproportionately incarcerated and have a fraught and tenuous relationship with the healthcare system. Black patients routinely report greater mistrust than White patients in the outcomes of care and the motivations of physicians, in large part due to past and current discrimination and the medical community’s history of experimentation.12 A shackled patient may view a treating physician and hospital as complicit with the practice, rather than seeing the practice as something outside of their control. If a patient’s sole interaction with inpatient medicine involves shackling, it risks damaging whatever fragile physician-patient relationship may exist and could delay or limit care even further.
While the universal application of metal handcuffs or leg cuffs ensures low rates of escape or attacks on workers, it does so at the expense of vulnerable individuals. We have cared for an incarcerated elderly woman arrested for multiple traffic violations, a man with severe autism who slipped through the cracks of mental health diversion protocols and ended up in jail, and an arrested delirious man with severe alcohol withdrawal, all shackled with hard shackles on the wrists, legs, or in the final case, both. Safety and the rights of the vulnerable are not mutually exclusive, and we feel the following measures can protect both.
A WAY FORWARD
First, the universal application of shackles in the hospitalized incarcerated patient should end. If no alternative security measures are available for high-risk patients, correctional facilities must document their necessity as physicians and nurses are required to do for medical restraints. Hospitals should have processes in place for providers who feel unsafe with an unshackled patient or think a patient is unnecessarily shackled, and collegial discussions about shackling with law enforcement should be the norm. If safe to do so, shackles should routinely be removed for physical exams without question. Since law enforcement officials, rather than the hospitals, make the rules for shackling, this will take some degree of physician and administrative advocacy at the hospital level and legislative advocacy at the local and state levels.
Second, vulnerable populations, such as the elderly, those experiencing a mental health crisis, or others at risk for in-hospital delirium, should never be restrained with hard law enforcement cuffs. Restraint procedures should follow standard medical restraint procedures, and soft restraints should be used if at all possible. Given the high rates of psychiatric illness amongst the incarcerated and the role jails play in filling gaps in psychiatric care, medical admissions for those with mental illness are not rare occasions.
Finally, hospitals routinely taking care of an incarcerated population should seek to build secure units, a move that would dramatically reduce the need for shackling. In several cities, the primary referral hospitals for some of the largest jails in the country do not have units with the proper security to allow for freedom of movement, and thus, shackling persists. Creating secure units will take significant investment on the part of hospital and local authorities, but there is potential for decreasing costs due to consolidating supervision, which would lead to better patient outcomes given the above risks.
Advocating for the health of the incarcerated, even those who have not yet been convicted, is typically not a high priority for the general public. As inpatient physicians, we see the impact universal shackling has on some of our most vulnerable patients and should be their voice where they have none. Advocating for and implementing the above procedures will be a step toward improving patient care while maintaining safety.
1. Proctor C. Jacob Blake handcuffed to hospital bed, father says. Chicago Sun-Times. Updated August 27, 2020. Accessed December 29, 2020. chicago.suntimes.com/2020/8/27/21404463/jacob-blake-father-kenosha-police-shooting-hospital-bed-handcuffs
2. Maruschak LM, Minton TD. Correctional populations in the United States, 2017-2018. Bureau of Justice Statistics. August 2020. Accessed September 30, 2020. https://www.bjs.gov/content/pub/pdf/cpus1718.pdf
3. Huh K, Boucher A, Fehr S, McGaffey F, McKillop M, Schiff M. State prisons and the delivery of hospital care: how states set up and finance off-site care for incarcerated individuals. The Pew Charitable Trusts. July 2018. Accessed September 30, 2020. https://www.pewtrusts.org/-/media/assets/2018/07/prisons-and-hospital-care_report.pdf
4. Haber LA, Erickson HP, Ranji SR, Ortiz GM, Pratt LA. Acute care for patients who are incarcerated: a review. JAMA Intern Med. 2019;179(11):1561-1567. https://doi.org/10.1001/jamainternmed.2019.3881
5. Mikow-Porto VA, Smith TA. The IHSSF 2011 Prisoner Escape Study. J Healthc Prot Manage. 2011;27(2):38-58.
6. Lezon D, Blakinger K. Inmate shot by deputy after holding medical student at Ben Taub. Houston Chronicle. October 6, 2016. Accessed December 29, 2020. https://www.chron.com/news/houston-texas/article/Deputy-shoots-suspect-at-Ben-Taub-hopsital-9873972.php
7. Yearwood LT. Pregnant and shackled: why inmates are still giving birth cuffed and bound. The Guardian. January 24, 2020. Accessed December 29, 2020. theguardian.com/us-news/2020/jan/24/shackled-pregnant-women-prisoners-birth
8. Haslar v Megerman, 104 F.3d 178 (8th Cir. 1997).
9. FGP v Serco Plc and SSHD, EWHC 1804 (Admin) (2012).
10. Cleary K, Prescott K. The use of physical restraints in acute and long-term care: an updated review of the evidence, regulations, ethics, and legality. J Acute Care Phys Ther. 2015;6(1):8-15. https://doi.org/10.1097/JAT.0000000000000005
11. Tuite H, Browne K, O’Neill D. Prisoners in general hospitals: doctors’ attitudes and practice. BMJ. 2006;332(7540):548-549. https://doi.org/10.1136/bmj.332.7540.548-b
12. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146-161. https://doi.org/10.1177/1077558700057001S07
The police shooting of Jacob Blake, an unarmed Wisconsin man, during an arrest in August 2020, led to more protests in a summer filled with calls against the unequal application of police force. Outrage grew as it was revealed that Blake, paralyzed from his waist down and not yet convicted of a crime, was still handcuffed to his hospital bed while receiving treatment.1 To many this seemed unusually cruel, but to those tasked with caring for incarcerated patients, it is all too familiar. Given the high rates of incarceration in the United States and the increased medical needs of this population, caring for those in custody is unavoidable for many physicians and hospitals. Though safety should be paramount, the universal application of metal handcuffs or leg cuffs by law enforcement officials, a process known as shackling, can lead to a variety of harms and should be abandoned.
BACKGROUND
The United States incarcerates more individuals both in total numbers and per capita than any other country in the world. This is currently believed to be more than two million people on any given day or more than 650 persons per 100,000 population.2 Incarceration occurs in jails, which are locally run facilities holding individuals on short sentences or those not yet convicted who are unable to afford bail before their trials (pretrial), or prisons, which are state and federally run facilities that house those with long sentences. When an incarcerated person experiences a medical emergency requiring hospitalization, they are either treated in the correctional facility or transferred to a local hospital for a higher level of care. Some hospitals are equipped with security measures similar to those of a correctional facility, with secure floors or wings dedicated solely to the care of the incarcerated. Secure units are more commonly seen in hospitals associated with prisons rather than local jails. Other hospitals house incarcerated patients in the same rooms as the public population, and thus movement is restricted by other means.3 Most commonly, this is done with a hard metal shackle resembling a handcuff with one end attached to the leg or wrist and the other end attached to the bed. Some agencies require more restraints, often requiring the use of wrist cuffs and leg cuffs concurrently for the entire duration of a patient’s hospitalization.4 In our experience, agencies apply these restraints universally, regardless of age, illness, mobility, or pretrial status.
Restraint practices are rooted in a concern for practitioner and public safety and bear merit. A patient from a correctional facility is usually guarded by just one officer in lieu of the multiple security measures at a jail or prison facility. Nonsecured hospitals have become sites of multiple escapes by incarcerated inpatients, given the lack of secured doors and the multiple movements during the admission and discharge processes.5 Furthermore, violence against hospital staff is now a focus issue in many hospitals and is no longer accepted as just “part of the job.” In several high-profile incidents, incarcerated inpatients have harmed staff, including one at our own institution, when an incarcerated patient held a makeshift weapon to a student’s throat.6
LEGAL CHALLENGES
The use of shackles during hospital visits has been challenged in US courts and routinely upheld. In one case, an incarcerated patient with renal failure received injuries after his leg edema was so severe that “at one point the shackles themselves were barely visible.”7 Though he was injured, the shackles were determined to have served a penological purpose outside of punishment, such as preventing escape, and the injuries were the result of the patient’s guards not following protocol. British courts have taken a different stance, ruling for an incarcerated patient who challenged the use of cuffs during three outpatient appointments and one inpatient admission.8 While the cuffs in the outpatient setting were deemed acceptable (as they were removed during the medical visit itself), they remained during the duration of the inpatient stay. This was deemed in violation of Title I/Article 3 of the Charter of Fundamental Rights of the European Union, Dignity/The right to integrity of the person. One area in US healthcare where shackling has been roundly condemned is the peripartum shackling of pregnant women. Though courts have had a mixed record to challenges, activism and advocacy have led to the banning of the practice in 23 states, though in most states significant exemptions exist.9 Through the First Step Act of 2018, the federal government banned peripartum shackling for all federal prisoners, but as most incarcerations are under state or local control, a considerable number of incarcerated pregnant women can legally be shackled during their deliveries.
RISKS OF SHACKLING
Legal and safety concerns aside, the shackling of incarcerated patients carries enormous risk. The use of medical restraints in hospitals has decreased over the past few decades, given their proven harms in increasing falls, exacerbating delirium, and increasing the risk of in-hospital death.10 There is no reason to believe that trading a soft medical restraint for a metal leg or wrist cuff would not confer the same risk. Additionally, metal law enforcement cuffs are not designed with patient safety in mind and have been known to cause specific nerve injuries, or handcuff neuropathy. This can occur when placement is too tight or when a patient struggles against them, as could happen with an agitated or delirious patient. The bar for removal, even briefly for an exam, is also much higher than that of a medical restraint, leading to a greater likelihood that certain aspects of the physical exam, such as gait or strength assessment, may not be adequately performed. In one small survey, British physicians reported often performing an exam while the patient was cuffed and with a guard in the room, despite country guidelines against both practices.11
Additionally, marginalized communities are disproportionately incarcerated and have a fraught and tenuous relationship with the healthcare system. Black patients routinely report greater mistrust than White patients in the outcomes of care and the motivations of physicians, in large part due to past and current discrimination and the medical community’s history of experimentation.12 A shackled patient may view a treating physician and hospital as complicit with the practice, rather than seeing the practice as something outside of their control. If a patient’s sole interaction with inpatient medicine involves shackling, it risks damaging whatever fragile physician-patient relationship may exist and could delay or limit care even further.
While the universal application of metal handcuffs or leg cuffs ensures low rates of escape or attacks on workers, it does so at the expense of vulnerable individuals. We have cared for an incarcerated elderly woman arrested for multiple traffic violations, a man with severe autism who slipped through the cracks of mental health diversion protocols and ended up in jail, and an arrested delirious man with severe alcohol withdrawal, all shackled with hard shackles on the wrists, legs, or in the final case, both. Safety and the rights of the vulnerable are not mutually exclusive, and we feel the following measures can protect both.
A WAY FORWARD
First, the universal application of shackles in the hospitalized incarcerated patient should end. If no alternative security measures are available for high-risk patients, correctional facilities must document their necessity as physicians and nurses are required to do for medical restraints. Hospitals should have processes in place for providers who feel unsafe with an unshackled patient or think a patient is unnecessarily shackled, and collegial discussions about shackling with law enforcement should be the norm. If safe to do so, shackles should routinely be removed for physical exams without question. Since law enforcement officials, rather than the hospitals, make the rules for shackling, this will take some degree of physician and administrative advocacy at the hospital level and legislative advocacy at the local and state levels.
Second, vulnerable populations, such as the elderly, those experiencing a mental health crisis, or others at risk for in-hospital delirium, should never be restrained with hard law enforcement cuffs. Restraint procedures should follow standard medical restraint procedures, and soft restraints should be used if at all possible. Given the high rates of psychiatric illness amongst the incarcerated and the role jails play in filling gaps in psychiatric care, medical admissions for those with mental illness are not rare occasions.
Finally, hospitals routinely taking care of an incarcerated population should seek to build secure units, a move that would dramatically reduce the need for shackling. In several cities, the primary referral hospitals for some of the largest jails in the country do not have units with the proper security to allow for freedom of movement, and thus, shackling persists. Creating secure units will take significant investment on the part of hospital and local authorities, but there is potential for decreasing costs due to consolidating supervision, which would lead to better patient outcomes given the above risks.
Advocating for the health of the incarcerated, even those who have not yet been convicted, is typically not a high priority for the general public. As inpatient physicians, we see the impact universal shackling has on some of our most vulnerable patients and should be their voice where they have none. Advocating for and implementing the above procedures will be a step toward improving patient care while maintaining safety.
The police shooting of Jacob Blake, an unarmed Wisconsin man, during an arrest in August 2020, led to more protests in a summer filled with calls against the unequal application of police force. Outrage grew as it was revealed that Blake, paralyzed from his waist down and not yet convicted of a crime, was still handcuffed to his hospital bed while receiving treatment.1 To many this seemed unusually cruel, but to those tasked with caring for incarcerated patients, it is all too familiar. Given the high rates of incarceration in the United States and the increased medical needs of this population, caring for those in custody is unavoidable for many physicians and hospitals. Though safety should be paramount, the universal application of metal handcuffs or leg cuffs by law enforcement officials, a process known as shackling, can lead to a variety of harms and should be abandoned.
BACKGROUND
The United States incarcerates more individuals both in total numbers and per capita than any other country in the world. This is currently believed to be more than two million people on any given day or more than 650 persons per 100,000 population.2 Incarceration occurs in jails, which are locally run facilities holding individuals on short sentences or those not yet convicted who are unable to afford bail before their trials (pretrial), or prisons, which are state and federally run facilities that house those with long sentences. When an incarcerated person experiences a medical emergency requiring hospitalization, they are either treated in the correctional facility or transferred to a local hospital for a higher level of care. Some hospitals are equipped with security measures similar to those of a correctional facility, with secure floors or wings dedicated solely to the care of the incarcerated. Secure units are more commonly seen in hospitals associated with prisons rather than local jails. Other hospitals house incarcerated patients in the same rooms as the public population, and thus movement is restricted by other means.3 Most commonly, this is done with a hard metal shackle resembling a handcuff with one end attached to the leg or wrist and the other end attached to the bed. Some agencies require more restraints, often requiring the use of wrist cuffs and leg cuffs concurrently for the entire duration of a patient’s hospitalization.4 In our experience, agencies apply these restraints universally, regardless of age, illness, mobility, or pretrial status.
Restraint practices are rooted in a concern for practitioner and public safety and bear merit. A patient from a correctional facility is usually guarded by just one officer in lieu of the multiple security measures at a jail or prison facility. Nonsecured hospitals have become sites of multiple escapes by incarcerated inpatients, given the lack of secured doors and the multiple movements during the admission and discharge processes.5 Furthermore, violence against hospital staff is now a focus issue in many hospitals and is no longer accepted as just “part of the job.” In several high-profile incidents, incarcerated inpatients have harmed staff, including one at our own institution, when an incarcerated patient held a makeshift weapon to a student’s throat.6
LEGAL CHALLENGES
The use of shackles during hospital visits has been challenged in US courts and routinely upheld. In one case, an incarcerated patient with renal failure received injuries after his leg edema was so severe that “at one point the shackles themselves were barely visible.”7 Though he was injured, the shackles were determined to have served a penological purpose outside of punishment, such as preventing escape, and the injuries were the result of the patient’s guards not following protocol. British courts have taken a different stance, ruling for an incarcerated patient who challenged the use of cuffs during three outpatient appointments and one inpatient admission.8 While the cuffs in the outpatient setting were deemed acceptable (as they were removed during the medical visit itself), they remained during the duration of the inpatient stay. This was deemed in violation of Title I/Article 3 of the Charter of Fundamental Rights of the European Union, Dignity/The right to integrity of the person. One area in US healthcare where shackling has been roundly condemned is the peripartum shackling of pregnant women. Though courts have had a mixed record to challenges, activism and advocacy have led to the banning of the practice in 23 states, though in most states significant exemptions exist.9 Through the First Step Act of 2018, the federal government banned peripartum shackling for all federal prisoners, but as most incarcerations are under state or local control, a considerable number of incarcerated pregnant women can legally be shackled during their deliveries.
RISKS OF SHACKLING
Legal and safety concerns aside, the shackling of incarcerated patients carries enormous risk. The use of medical restraints in hospitals has decreased over the past few decades, given their proven harms in increasing falls, exacerbating delirium, and increasing the risk of in-hospital death.10 There is no reason to believe that trading a soft medical restraint for a metal leg or wrist cuff would not confer the same risk. Additionally, metal law enforcement cuffs are not designed with patient safety in mind and have been known to cause specific nerve injuries, or handcuff neuropathy. This can occur when placement is too tight or when a patient struggles against them, as could happen with an agitated or delirious patient. The bar for removal, even briefly for an exam, is also much higher than that of a medical restraint, leading to a greater likelihood that certain aspects of the physical exam, such as gait or strength assessment, may not be adequately performed. In one small survey, British physicians reported often performing an exam while the patient was cuffed and with a guard in the room, despite country guidelines against both practices.11
Additionally, marginalized communities are disproportionately incarcerated and have a fraught and tenuous relationship with the healthcare system. Black patients routinely report greater mistrust than White patients in the outcomes of care and the motivations of physicians, in large part due to past and current discrimination and the medical community’s history of experimentation.12 A shackled patient may view a treating physician and hospital as complicit with the practice, rather than seeing the practice as something outside of their control. If a patient’s sole interaction with inpatient medicine involves shackling, it risks damaging whatever fragile physician-patient relationship may exist and could delay or limit care even further.
While the universal application of metal handcuffs or leg cuffs ensures low rates of escape or attacks on workers, it does so at the expense of vulnerable individuals. We have cared for an incarcerated elderly woman arrested for multiple traffic violations, a man with severe autism who slipped through the cracks of mental health diversion protocols and ended up in jail, and an arrested delirious man with severe alcohol withdrawal, all shackled with hard shackles on the wrists, legs, or in the final case, both. Safety and the rights of the vulnerable are not mutually exclusive, and we feel the following measures can protect both.
A WAY FORWARD
First, the universal application of shackles in the hospitalized incarcerated patient should end. If no alternative security measures are available for high-risk patients, correctional facilities must document their necessity as physicians and nurses are required to do for medical restraints. Hospitals should have processes in place for providers who feel unsafe with an unshackled patient or think a patient is unnecessarily shackled, and collegial discussions about shackling with law enforcement should be the norm. If safe to do so, shackles should routinely be removed for physical exams without question. Since law enforcement officials, rather than the hospitals, make the rules for shackling, this will take some degree of physician and administrative advocacy at the hospital level and legislative advocacy at the local and state levels.
Second, vulnerable populations, such as the elderly, those experiencing a mental health crisis, or others at risk for in-hospital delirium, should never be restrained with hard law enforcement cuffs. Restraint procedures should follow standard medical restraint procedures, and soft restraints should be used if at all possible. Given the high rates of psychiatric illness amongst the incarcerated and the role jails play in filling gaps in psychiatric care, medical admissions for those with mental illness are not rare occasions.
Finally, hospitals routinely taking care of an incarcerated population should seek to build secure units, a move that would dramatically reduce the need for shackling. In several cities, the primary referral hospitals for some of the largest jails in the country do not have units with the proper security to allow for freedom of movement, and thus, shackling persists. Creating secure units will take significant investment on the part of hospital and local authorities, but there is potential for decreasing costs due to consolidating supervision, which would lead to better patient outcomes given the above risks.
Advocating for the health of the incarcerated, even those who have not yet been convicted, is typically not a high priority for the general public. As inpatient physicians, we see the impact universal shackling has on some of our most vulnerable patients and should be their voice where they have none. Advocating for and implementing the above procedures will be a step toward improving patient care while maintaining safety.
1. Proctor C. Jacob Blake handcuffed to hospital bed, father says. Chicago Sun-Times. Updated August 27, 2020. Accessed December 29, 2020. chicago.suntimes.com/2020/8/27/21404463/jacob-blake-father-kenosha-police-shooting-hospital-bed-handcuffs
2. Maruschak LM, Minton TD. Correctional populations in the United States, 2017-2018. Bureau of Justice Statistics. August 2020. Accessed September 30, 2020. https://www.bjs.gov/content/pub/pdf/cpus1718.pdf
3. Huh K, Boucher A, Fehr S, McGaffey F, McKillop M, Schiff M. State prisons and the delivery of hospital care: how states set up and finance off-site care for incarcerated individuals. The Pew Charitable Trusts. July 2018. Accessed September 30, 2020. https://www.pewtrusts.org/-/media/assets/2018/07/prisons-and-hospital-care_report.pdf
4. Haber LA, Erickson HP, Ranji SR, Ortiz GM, Pratt LA. Acute care for patients who are incarcerated: a review. JAMA Intern Med. 2019;179(11):1561-1567. https://doi.org/10.1001/jamainternmed.2019.3881
5. Mikow-Porto VA, Smith TA. The IHSSF 2011 Prisoner Escape Study. J Healthc Prot Manage. 2011;27(2):38-58.
6. Lezon D, Blakinger K. Inmate shot by deputy after holding medical student at Ben Taub. Houston Chronicle. October 6, 2016. Accessed December 29, 2020. https://www.chron.com/news/houston-texas/article/Deputy-shoots-suspect-at-Ben-Taub-hopsital-9873972.php
7. Yearwood LT. Pregnant and shackled: why inmates are still giving birth cuffed and bound. The Guardian. January 24, 2020. Accessed December 29, 2020. theguardian.com/us-news/2020/jan/24/shackled-pregnant-women-prisoners-birth
8. Haslar v Megerman, 104 F.3d 178 (8th Cir. 1997).
9. FGP v Serco Plc and SSHD, EWHC 1804 (Admin) (2012).
10. Cleary K, Prescott K. The use of physical restraints in acute and long-term care: an updated review of the evidence, regulations, ethics, and legality. J Acute Care Phys Ther. 2015;6(1):8-15. https://doi.org/10.1097/JAT.0000000000000005
11. Tuite H, Browne K, O’Neill D. Prisoners in general hospitals: doctors’ attitudes and practice. BMJ. 2006;332(7540):548-549. https://doi.org/10.1136/bmj.332.7540.548-b
12. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146-161. https://doi.org/10.1177/1077558700057001S07
1. Proctor C. Jacob Blake handcuffed to hospital bed, father says. Chicago Sun-Times. Updated August 27, 2020. Accessed December 29, 2020. chicago.suntimes.com/2020/8/27/21404463/jacob-blake-father-kenosha-police-shooting-hospital-bed-handcuffs
2. Maruschak LM, Minton TD. Correctional populations in the United States, 2017-2018. Bureau of Justice Statistics. August 2020. Accessed September 30, 2020. https://www.bjs.gov/content/pub/pdf/cpus1718.pdf
3. Huh K, Boucher A, Fehr S, McGaffey F, McKillop M, Schiff M. State prisons and the delivery of hospital care: how states set up and finance off-site care for incarcerated individuals. The Pew Charitable Trusts. July 2018. Accessed September 30, 2020. https://www.pewtrusts.org/-/media/assets/2018/07/prisons-and-hospital-care_report.pdf
4. Haber LA, Erickson HP, Ranji SR, Ortiz GM, Pratt LA. Acute care for patients who are incarcerated: a review. JAMA Intern Med. 2019;179(11):1561-1567. https://doi.org/10.1001/jamainternmed.2019.3881
5. Mikow-Porto VA, Smith TA. The IHSSF 2011 Prisoner Escape Study. J Healthc Prot Manage. 2011;27(2):38-58.
6. Lezon D, Blakinger K. Inmate shot by deputy after holding medical student at Ben Taub. Houston Chronicle. October 6, 2016. Accessed December 29, 2020. https://www.chron.com/news/houston-texas/article/Deputy-shoots-suspect-at-Ben-Taub-hopsital-9873972.php
7. Yearwood LT. Pregnant and shackled: why inmates are still giving birth cuffed and bound. The Guardian. January 24, 2020. Accessed December 29, 2020. theguardian.com/us-news/2020/jan/24/shackled-pregnant-women-prisoners-birth
8. Haslar v Megerman, 104 F.3d 178 (8th Cir. 1997).
9. FGP v Serco Plc and SSHD, EWHC 1804 (Admin) (2012).
10. Cleary K, Prescott K. The use of physical restraints in acute and long-term care: an updated review of the evidence, regulations, ethics, and legality. J Acute Care Phys Ther. 2015;6(1):8-15. https://doi.org/10.1097/JAT.0000000000000005
11. Tuite H, Browne K, O’Neill D. Prisoners in general hospitals: doctors’ attitudes and practice. BMJ. 2006;332(7540):548-549. https://doi.org/10.1136/bmj.332.7540.548-b
12. LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146-161. https://doi.org/10.1177/1077558700057001S07
© 2021 Society of Hospital Medicine
AHA/ACC guidance on ethics, professionalism in cardiovascular care
The American Heart Association and the American College of Cardiology have issued a new report on medical ethics and professionalism in cardiovascular medicine.
The report addresses a variety of topics including diversity, equity, inclusion, and belonging; racial, ethnic and gender inequities; conflicts of interest; clinician well-being; data privacy; social justice; and modern health care delivery systems.
The 54-page report is based on the proceedings of the joint 2020 Consensus Conference on Professionalism and Ethics, held Oct. 19 and 20, 2020. It was published online May 11 in Circulation and the Journal of the American College of Cardiology .
The 2020 consensus conference on professionalism and ethics came at a time even more fraught than the eras of the three previous meetings on the same topics, held in 1989, 1997, and 2004, the writing group notes.
“We have seen the COVID-19 pandemic challenge the physical and economic health of the entire country, coupled with a series of national tragedies that have awakened the call for social justice,” conference cochair C. Michael Valentine, MD, said in a news release.
“There is no better time than now to review, evaluate, and take a fresh perspective on medical ethics and professionalism,” said Dr. Valentine, professor of medicine at the Heart and Vascular Center, University of Virginia, Charlottesville.
“We hope this report will provide cardiovascular professionals and health systems with the recommendations and tools they need to address conflicts of interest; racial, ethnic, and gender inequities; and improve diversity, inclusion, and wellness among our workforce,” Dr. Valentine added. “The majority of our members are now employed and must be engaged as the leaders for change in cardiovascular care.”
Road map to improve diversity, achieve allyship
The writing committee was made up of a diverse group of cardiologists, internists, and associated health care professionals and laypeople and was organized into five task forces, each addressing a specific topic: conflicts of interest; diversity, equity, inclusion, and belonging; clinician well-being; patient autonomy, privacy, and social justice in health care; and modern health care delivery.
The report serves as a road map to achieve equity, inclusion, and belonging among cardiovascular professionals and calls for ongoing assessment of the professional culture and climate, focused on improving diversity and achieving effective allyship, the writing group says.
The report proposes continuous training to address individual, structural, and systemic racism, sexism, homophobia, classism, and ableism.
It offers recommendations for championing equity in patient care that include an annual review of practice records to look for differences in patient treatment by race, ethnicity, zip code, and primary language.
The report calls for a foundation of training in allyship and antiracism as part of medical school course requirements and experiences: A required course on social justice, race, and racism as part of the first-year curriculum; school programs and professional organizations supporting students, trainees, and members in allyship and antiracism action; and facilitating immersion and partnership with surrounding communities.
“As much as 80% of a person’s health is determined by the social and economic conditions of their environment,” consensus cochair Ivor Benjamin, MD, said in the release.
“To achieve social justice and mitigate health disparities, we must go to the margins and shift our discussions to be inclusive of populations such as rural and marginalized groups from the perspective of health equity lens for all,” said Dr. Benjamin, professor of medicine, Medical College of Wisconsin, Milwaukee.
The report also highlights the need for psychosocial support of the cardiovascular community and recommends that health care organizations prioritize regular assessment of clinicians’ well-being and engagement.
It also recommends addressing the well-being of trainees in postgraduate training programs and calls for an ombudsman program that allows for confidential reporting of mistreatment and access to support.
The report also highlights additional opportunities to:
- improve the efficiency of health information technology, such as electronic health records, and reduce the administrative burden
- identify and assist clinicians who experience mental health conditions, , or
- emphasize patient autonomy using shared decision-making and patient-centered care that is supportive of the individual patient’s values
- increase privacy protections for patient data used in research
- maintain integrity as new ways of delivering care, such as telemedicine, team-based care approaches, and physician-owned specialty centers emerge
- perform routine audits of electronic health records to promote optimal patient care, as well as ethical medical practice
- expand and make mandatory the reporting of intellectual or associational interests in addition to relationships with industry
The report’s details and recommendations will be presented and discussed Saturday, May 15, at 8:00 AM ET, during ACC.21. The session is titled Diversity and Equity: The Means to Expand Inclusion and Belonging.
The AHA will present a live webinar and six-episode podcast series (available on demand) to highlight the report’s details, dialogue, and actionable steps for cardiovascular and health care professionals, researchers, and educators.
This research had no commercial funding. The list of 40 volunteer committee members and coauthors, including their disclosures, are listed in the original report.
A version of this article first appeared on Medscape.com.
The American Heart Association and the American College of Cardiology have issued a new report on medical ethics and professionalism in cardiovascular medicine.
The report addresses a variety of topics including diversity, equity, inclusion, and belonging; racial, ethnic and gender inequities; conflicts of interest; clinician well-being; data privacy; social justice; and modern health care delivery systems.
The 54-page report is based on the proceedings of the joint 2020 Consensus Conference on Professionalism and Ethics, held Oct. 19 and 20, 2020. It was published online May 11 in Circulation and the Journal of the American College of Cardiology .
The 2020 consensus conference on professionalism and ethics came at a time even more fraught than the eras of the three previous meetings on the same topics, held in 1989, 1997, and 2004, the writing group notes.
“We have seen the COVID-19 pandemic challenge the physical and economic health of the entire country, coupled with a series of national tragedies that have awakened the call for social justice,” conference cochair C. Michael Valentine, MD, said in a news release.
“There is no better time than now to review, evaluate, and take a fresh perspective on medical ethics and professionalism,” said Dr. Valentine, professor of medicine at the Heart and Vascular Center, University of Virginia, Charlottesville.
“We hope this report will provide cardiovascular professionals and health systems with the recommendations and tools they need to address conflicts of interest; racial, ethnic, and gender inequities; and improve diversity, inclusion, and wellness among our workforce,” Dr. Valentine added. “The majority of our members are now employed and must be engaged as the leaders for change in cardiovascular care.”
Road map to improve diversity, achieve allyship
The writing committee was made up of a diverse group of cardiologists, internists, and associated health care professionals and laypeople and was organized into five task forces, each addressing a specific topic: conflicts of interest; diversity, equity, inclusion, and belonging; clinician well-being; patient autonomy, privacy, and social justice in health care; and modern health care delivery.
The report serves as a road map to achieve equity, inclusion, and belonging among cardiovascular professionals and calls for ongoing assessment of the professional culture and climate, focused on improving diversity and achieving effective allyship, the writing group says.
The report proposes continuous training to address individual, structural, and systemic racism, sexism, homophobia, classism, and ableism.
It offers recommendations for championing equity in patient care that include an annual review of practice records to look for differences in patient treatment by race, ethnicity, zip code, and primary language.
The report calls for a foundation of training in allyship and antiracism as part of medical school course requirements and experiences: A required course on social justice, race, and racism as part of the first-year curriculum; school programs and professional organizations supporting students, trainees, and members in allyship and antiracism action; and facilitating immersion and partnership with surrounding communities.
“As much as 80% of a person’s health is determined by the social and economic conditions of their environment,” consensus cochair Ivor Benjamin, MD, said in the release.
“To achieve social justice and mitigate health disparities, we must go to the margins and shift our discussions to be inclusive of populations such as rural and marginalized groups from the perspective of health equity lens for all,” said Dr. Benjamin, professor of medicine, Medical College of Wisconsin, Milwaukee.
The report also highlights the need for psychosocial support of the cardiovascular community and recommends that health care organizations prioritize regular assessment of clinicians’ well-being and engagement.
It also recommends addressing the well-being of trainees in postgraduate training programs and calls for an ombudsman program that allows for confidential reporting of mistreatment and access to support.
The report also highlights additional opportunities to:
- improve the efficiency of health information technology, such as electronic health records, and reduce the administrative burden
- identify and assist clinicians who experience mental health conditions, , or
- emphasize patient autonomy using shared decision-making and patient-centered care that is supportive of the individual patient’s values
- increase privacy protections for patient data used in research
- maintain integrity as new ways of delivering care, such as telemedicine, team-based care approaches, and physician-owned specialty centers emerge
- perform routine audits of electronic health records to promote optimal patient care, as well as ethical medical practice
- expand and make mandatory the reporting of intellectual or associational interests in addition to relationships with industry
The report’s details and recommendations will be presented and discussed Saturday, May 15, at 8:00 AM ET, during ACC.21. The session is titled Diversity and Equity: The Means to Expand Inclusion and Belonging.
The AHA will present a live webinar and six-episode podcast series (available on demand) to highlight the report’s details, dialogue, and actionable steps for cardiovascular and health care professionals, researchers, and educators.
This research had no commercial funding. The list of 40 volunteer committee members and coauthors, including their disclosures, are listed in the original report.
A version of this article first appeared on Medscape.com.
The American Heart Association and the American College of Cardiology have issued a new report on medical ethics and professionalism in cardiovascular medicine.
The report addresses a variety of topics including diversity, equity, inclusion, and belonging; racial, ethnic and gender inequities; conflicts of interest; clinician well-being; data privacy; social justice; and modern health care delivery systems.
The 54-page report is based on the proceedings of the joint 2020 Consensus Conference on Professionalism and Ethics, held Oct. 19 and 20, 2020. It was published online May 11 in Circulation and the Journal of the American College of Cardiology .
The 2020 consensus conference on professionalism and ethics came at a time even more fraught than the eras of the three previous meetings on the same topics, held in 1989, 1997, and 2004, the writing group notes.
“We have seen the COVID-19 pandemic challenge the physical and economic health of the entire country, coupled with a series of national tragedies that have awakened the call for social justice,” conference cochair C. Michael Valentine, MD, said in a news release.
“There is no better time than now to review, evaluate, and take a fresh perspective on medical ethics and professionalism,” said Dr. Valentine, professor of medicine at the Heart and Vascular Center, University of Virginia, Charlottesville.
“We hope this report will provide cardiovascular professionals and health systems with the recommendations and tools they need to address conflicts of interest; racial, ethnic, and gender inequities; and improve diversity, inclusion, and wellness among our workforce,” Dr. Valentine added. “The majority of our members are now employed and must be engaged as the leaders for change in cardiovascular care.”
Road map to improve diversity, achieve allyship
The writing committee was made up of a diverse group of cardiologists, internists, and associated health care professionals and laypeople and was organized into five task forces, each addressing a specific topic: conflicts of interest; diversity, equity, inclusion, and belonging; clinician well-being; patient autonomy, privacy, and social justice in health care; and modern health care delivery.
The report serves as a road map to achieve equity, inclusion, and belonging among cardiovascular professionals and calls for ongoing assessment of the professional culture and climate, focused on improving diversity and achieving effective allyship, the writing group says.
The report proposes continuous training to address individual, structural, and systemic racism, sexism, homophobia, classism, and ableism.
It offers recommendations for championing equity in patient care that include an annual review of practice records to look for differences in patient treatment by race, ethnicity, zip code, and primary language.
The report calls for a foundation of training in allyship and antiracism as part of medical school course requirements and experiences: A required course on social justice, race, and racism as part of the first-year curriculum; school programs and professional organizations supporting students, trainees, and members in allyship and antiracism action; and facilitating immersion and partnership with surrounding communities.
“As much as 80% of a person’s health is determined by the social and economic conditions of their environment,” consensus cochair Ivor Benjamin, MD, said in the release.
“To achieve social justice and mitigate health disparities, we must go to the margins and shift our discussions to be inclusive of populations such as rural and marginalized groups from the perspective of health equity lens for all,” said Dr. Benjamin, professor of medicine, Medical College of Wisconsin, Milwaukee.
The report also highlights the need for psychosocial support of the cardiovascular community and recommends that health care organizations prioritize regular assessment of clinicians’ well-being and engagement.
It also recommends addressing the well-being of trainees in postgraduate training programs and calls for an ombudsman program that allows for confidential reporting of mistreatment and access to support.
The report also highlights additional opportunities to:
- improve the efficiency of health information technology, such as electronic health records, and reduce the administrative burden
- identify and assist clinicians who experience mental health conditions, , or
- emphasize patient autonomy using shared decision-making and patient-centered care that is supportive of the individual patient’s values
- increase privacy protections for patient data used in research
- maintain integrity as new ways of delivering care, such as telemedicine, team-based care approaches, and physician-owned specialty centers emerge
- perform routine audits of electronic health records to promote optimal patient care, as well as ethical medical practice
- expand and make mandatory the reporting of intellectual or associational interests in addition to relationships with industry
The report’s details and recommendations will be presented and discussed Saturday, May 15, at 8:00 AM ET, during ACC.21. The session is titled Diversity and Equity: The Means to Expand Inclusion and Belonging.
The AHA will present a live webinar and six-episode podcast series (available on demand) to highlight the report’s details, dialogue, and actionable steps for cardiovascular and health care professionals, researchers, and educators.
This research had no commercial funding. The list of 40 volunteer committee members and coauthors, including their disclosures, are listed in the original report.
A version of this article first appeared on Medscape.com.
High-dose methotrexate of no CNS benefit for patients with high-risk DLBCL
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
FROM BLOOD ADVANCES
New guidance for those fully vaccinated against COVID-19
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
Use your court awareness to go faster in practice
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Addressing today’s racial health inequities requires understanding their roots
The health disparities seen in today’s high rates of Black infant and maternal morbidity and mortality are rooted in health inequities and generational stress dating back centuries in the United States, but today’s obstetricians can make changes in their own practices to address this inequity, according to Haywood L. Brown, MD, professor of ob.gyn. and associate dean of diversity at the Morsani College of Medicine and vice president of institutional equity at the University of South Florida, Tampa.
Dr. Brown delivered his remarks during the Benson and Pamela Harer Seminar on History at the annual meeting of the American College of Obstetricians and Gynecologists on May 2. His talk focused on the origins of perinatal and maternal health inequities and how those original factors play out today in increased maternal and neonatal morbidity and mortality among Black women and their babies.
“Racial and ethnic disparities and inequity in maternal and child health are prevalent and persistent. We have to move beyond the documentation,” Dr. Brown told attendees. “We have to adopt uniform care standards, recognizing our own biases and understanding that the contribution of social determinants of health are important in the care and outcome of women. And we have to work on decreasing the stress of women who give birth.”
Evelyn Nicole Mitchell, MD, faculty chair of the ob.gyn. diversity and inclusion committee at the University of Southern California, found Dr. Brown’s talk compelling and hopes it opens the eyes of others who attended.
“You really have to understand the why behind the problems we have, and it really goes back to slavery and this historical distrust that’s been here from the beginning,” Dr. Mitchell said in an interview. “I hope this allows people to open their eyes and think about this situation from their patients’ shoes, to really put their guard down and explore, ‘how can I contribute to fixing this system that has been here from the beginning?’ I think a lot of people get defensive and think: ‘Oh, I’m not a racist. I just don’t want to talk about this,’ but it’s about a system being racist.” The question then, Dr. Mitchell said, is: “So how do I contribute to that system?”
Dr. Brown frequently returned to the theme of high stress levels in Black mothers contributing to poorer outcomes, such as preterm birth. That stress arises originally from the generational stress brought on by racism and oppression over the centuries but has been compounded by poverty, racial injustice, lack of access to adequate nutrition, lower education levels, environmental factors, and other determinants of health.
“The bottom line, as Dr. Brown said, is that we need to decrease the stress level of Black mothers giving birth,” Dr. Mitchell said. “How can I, as a provider, decrease the stress level of my patients? Well, No. 1, I can identify and eliminate implicit bias that I may harbor.”
Slavery husbandry laid the groundwork for today
The most surprising aspect of Dr. Brown’s lecture for Dr. Mitchell was the fact that enslaved women received a measure of protection that other enslaved people did not to “ensure that they were healthy and that they were able to reproduce in the future,” Dr. Mitchell said. “It was for the wrong reasons – to keep slavery going – but in a sense they were prioritizing Black women to take advantage of their reproductive capacity, compared to nowadays where Black women are facing severe disparities.”
To safeguard enslaved women’s fecundity, plantation owners attempted to reduce stressors in the women’s lives, such as allowing them to cohabitate with a husband and nuclear family, though sexual assault and abuse still occurred. The owners also tracked the enslaved girls’ menstrual cycles after menarche to maximize their “breeding” potential, especially between the ages of 15 and 24. Slave owners delegated older enslaved women as maternity caregivers and midwives, leading to the passing down of midwifery skills through generations of Black American women.
“Pregnant women received the best medical care on the plantation because of the premium placed on reproduction,” Dr. Brown said. Wealthier planters called in doctors for complicated deliveries, which provided J. Marian Sims the ability conduct surgical experiments on Betsey, Lucy, and Anarcha to treat vesicovaginal fistula since fistula “limited her ability to do the maximum work she could in the house or on the plantation,” Dr. Brown said.
After slavery ended, health care access did not improve for Black people. In 1920, there was approximately 1 Black physician for every 3,000 Black people, compared with 1 in 500 for the White population, and grannie midwives continued to be the primary birthing attendants for Black women. Over the next several decades, however, both maternal and infant mortality across all races began steeply dropping. Reasons for the drop included the incorporation of the American Board of Obstetrics and Gynecology in 1930, a shift from home births to hospital births, and the legalization of abortion, which led to an 89% decline in deaths from septic illegal abortions from 1950 to 1973.
Still, Black maternal and infant mortality remained higher than White, and the poverty gap further exacerbated outcomes.
“Substandard maternity care really is the origin of many of the Black maternal and infant morbidity and mortality” complications, such as low birth weight, small for gestational age, growth restriction, and intrauterine starvation, “which we now believe are the origin of things like hypertension, diabetes, and obesity,” Dr. Brown said.
Today, inequities persist because of the systemic racism throughout this history.
“As we talk about health disparities, prematurity, growth restriction, and maternal morbidity, the fetal origins for adult disease in diabetes and hypertension and obesity have generational implications over the last 400 years,” Dr. Brown said. “Generational stress and stresses in lack women from slavery to present times are some of the origins of the things that we see today, including segregation, economic inequities, eugenic sterilizations, the quality of education, and of course, systemic racism on health care access and quality.”
It is this long arc of history that Dr. Mitchell hopes attendees will begin to grasp.
“If you don’t understand all that and have that depth, there’s no way for you to truly understand the problems that are going on and how to solve them,” Dr. Mitchell said. She hopes that especially those who have been more “resistant to accepting these truths” can start to see the big picture. “Hopefully, they can look at it as a systemic problem and then focus on how they can change the system.”
Dr Brown is a contributor to UpToDate and the Merck Manual and serves on the advisory boards of Merck for Mothers Global Women’s Health and BabyScripts. Dr. Mitchell has no disclosures.
The health disparities seen in today’s high rates of Black infant and maternal morbidity and mortality are rooted in health inequities and generational stress dating back centuries in the United States, but today’s obstetricians can make changes in their own practices to address this inequity, according to Haywood L. Brown, MD, professor of ob.gyn. and associate dean of diversity at the Morsani College of Medicine and vice president of institutional equity at the University of South Florida, Tampa.
Dr. Brown delivered his remarks during the Benson and Pamela Harer Seminar on History at the annual meeting of the American College of Obstetricians and Gynecologists on May 2. His talk focused on the origins of perinatal and maternal health inequities and how those original factors play out today in increased maternal and neonatal morbidity and mortality among Black women and their babies.
“Racial and ethnic disparities and inequity in maternal and child health are prevalent and persistent. We have to move beyond the documentation,” Dr. Brown told attendees. “We have to adopt uniform care standards, recognizing our own biases and understanding that the contribution of social determinants of health are important in the care and outcome of women. And we have to work on decreasing the stress of women who give birth.”
Evelyn Nicole Mitchell, MD, faculty chair of the ob.gyn. diversity and inclusion committee at the University of Southern California, found Dr. Brown’s talk compelling and hopes it opens the eyes of others who attended.
“You really have to understand the why behind the problems we have, and it really goes back to slavery and this historical distrust that’s been here from the beginning,” Dr. Mitchell said in an interview. “I hope this allows people to open their eyes and think about this situation from their patients’ shoes, to really put their guard down and explore, ‘how can I contribute to fixing this system that has been here from the beginning?’ I think a lot of people get defensive and think: ‘Oh, I’m not a racist. I just don’t want to talk about this,’ but it’s about a system being racist.” The question then, Dr. Mitchell said, is: “So how do I contribute to that system?”
Dr. Brown frequently returned to the theme of high stress levels in Black mothers contributing to poorer outcomes, such as preterm birth. That stress arises originally from the generational stress brought on by racism and oppression over the centuries but has been compounded by poverty, racial injustice, lack of access to adequate nutrition, lower education levels, environmental factors, and other determinants of health.
“The bottom line, as Dr. Brown said, is that we need to decrease the stress level of Black mothers giving birth,” Dr. Mitchell said. “How can I, as a provider, decrease the stress level of my patients? Well, No. 1, I can identify and eliminate implicit bias that I may harbor.”
Slavery husbandry laid the groundwork for today
The most surprising aspect of Dr. Brown’s lecture for Dr. Mitchell was the fact that enslaved women received a measure of protection that other enslaved people did not to “ensure that they were healthy and that they were able to reproduce in the future,” Dr. Mitchell said. “It was for the wrong reasons – to keep slavery going – but in a sense they were prioritizing Black women to take advantage of their reproductive capacity, compared to nowadays where Black women are facing severe disparities.”
To safeguard enslaved women’s fecundity, plantation owners attempted to reduce stressors in the women’s lives, such as allowing them to cohabitate with a husband and nuclear family, though sexual assault and abuse still occurred. The owners also tracked the enslaved girls’ menstrual cycles after menarche to maximize their “breeding” potential, especially between the ages of 15 and 24. Slave owners delegated older enslaved women as maternity caregivers and midwives, leading to the passing down of midwifery skills through generations of Black American women.
“Pregnant women received the best medical care on the plantation because of the premium placed on reproduction,” Dr. Brown said. Wealthier planters called in doctors for complicated deliveries, which provided J. Marian Sims the ability conduct surgical experiments on Betsey, Lucy, and Anarcha to treat vesicovaginal fistula since fistula “limited her ability to do the maximum work she could in the house or on the plantation,” Dr. Brown said.
After slavery ended, health care access did not improve for Black people. In 1920, there was approximately 1 Black physician for every 3,000 Black people, compared with 1 in 500 for the White population, and grannie midwives continued to be the primary birthing attendants for Black women. Over the next several decades, however, both maternal and infant mortality across all races began steeply dropping. Reasons for the drop included the incorporation of the American Board of Obstetrics and Gynecology in 1930, a shift from home births to hospital births, and the legalization of abortion, which led to an 89% decline in deaths from septic illegal abortions from 1950 to 1973.
Still, Black maternal and infant mortality remained higher than White, and the poverty gap further exacerbated outcomes.
“Substandard maternity care really is the origin of many of the Black maternal and infant morbidity and mortality” complications, such as low birth weight, small for gestational age, growth restriction, and intrauterine starvation, “which we now believe are the origin of things like hypertension, diabetes, and obesity,” Dr. Brown said.
Today, inequities persist because of the systemic racism throughout this history.
“As we talk about health disparities, prematurity, growth restriction, and maternal morbidity, the fetal origins for adult disease in diabetes and hypertension and obesity have generational implications over the last 400 years,” Dr. Brown said. “Generational stress and stresses in lack women from slavery to present times are some of the origins of the things that we see today, including segregation, economic inequities, eugenic sterilizations, the quality of education, and of course, systemic racism on health care access and quality.”
It is this long arc of history that Dr. Mitchell hopes attendees will begin to grasp.
“If you don’t understand all that and have that depth, there’s no way for you to truly understand the problems that are going on and how to solve them,” Dr. Mitchell said. She hopes that especially those who have been more “resistant to accepting these truths” can start to see the big picture. “Hopefully, they can look at it as a systemic problem and then focus on how they can change the system.”
Dr Brown is a contributor to UpToDate and the Merck Manual and serves on the advisory boards of Merck for Mothers Global Women’s Health and BabyScripts. Dr. Mitchell has no disclosures.
The health disparities seen in today’s high rates of Black infant and maternal morbidity and mortality are rooted in health inequities and generational stress dating back centuries in the United States, but today’s obstetricians can make changes in their own practices to address this inequity, according to Haywood L. Brown, MD, professor of ob.gyn. and associate dean of diversity at the Morsani College of Medicine and vice president of institutional equity at the University of South Florida, Tampa.
Dr. Brown delivered his remarks during the Benson and Pamela Harer Seminar on History at the annual meeting of the American College of Obstetricians and Gynecologists on May 2. His talk focused on the origins of perinatal and maternal health inequities and how those original factors play out today in increased maternal and neonatal morbidity and mortality among Black women and their babies.
“Racial and ethnic disparities and inequity in maternal and child health are prevalent and persistent. We have to move beyond the documentation,” Dr. Brown told attendees. “We have to adopt uniform care standards, recognizing our own biases and understanding that the contribution of social determinants of health are important in the care and outcome of women. And we have to work on decreasing the stress of women who give birth.”
Evelyn Nicole Mitchell, MD, faculty chair of the ob.gyn. diversity and inclusion committee at the University of Southern California, found Dr. Brown’s talk compelling and hopes it opens the eyes of others who attended.
“You really have to understand the why behind the problems we have, and it really goes back to slavery and this historical distrust that’s been here from the beginning,” Dr. Mitchell said in an interview. “I hope this allows people to open their eyes and think about this situation from their patients’ shoes, to really put their guard down and explore, ‘how can I contribute to fixing this system that has been here from the beginning?’ I think a lot of people get defensive and think: ‘Oh, I’m not a racist. I just don’t want to talk about this,’ but it’s about a system being racist.” The question then, Dr. Mitchell said, is: “So how do I contribute to that system?”
Dr. Brown frequently returned to the theme of high stress levels in Black mothers contributing to poorer outcomes, such as preterm birth. That stress arises originally from the generational stress brought on by racism and oppression over the centuries but has been compounded by poverty, racial injustice, lack of access to adequate nutrition, lower education levels, environmental factors, and other determinants of health.
“The bottom line, as Dr. Brown said, is that we need to decrease the stress level of Black mothers giving birth,” Dr. Mitchell said. “How can I, as a provider, decrease the stress level of my patients? Well, No. 1, I can identify and eliminate implicit bias that I may harbor.”
Slavery husbandry laid the groundwork for today
The most surprising aspect of Dr. Brown’s lecture for Dr. Mitchell was the fact that enslaved women received a measure of protection that other enslaved people did not to “ensure that they were healthy and that they were able to reproduce in the future,” Dr. Mitchell said. “It was for the wrong reasons – to keep slavery going – but in a sense they were prioritizing Black women to take advantage of their reproductive capacity, compared to nowadays where Black women are facing severe disparities.”
To safeguard enslaved women’s fecundity, plantation owners attempted to reduce stressors in the women’s lives, such as allowing them to cohabitate with a husband and nuclear family, though sexual assault and abuse still occurred. The owners also tracked the enslaved girls’ menstrual cycles after menarche to maximize their “breeding” potential, especially between the ages of 15 and 24. Slave owners delegated older enslaved women as maternity caregivers and midwives, leading to the passing down of midwifery skills through generations of Black American women.
“Pregnant women received the best medical care on the plantation because of the premium placed on reproduction,” Dr. Brown said. Wealthier planters called in doctors for complicated deliveries, which provided J. Marian Sims the ability conduct surgical experiments on Betsey, Lucy, and Anarcha to treat vesicovaginal fistula since fistula “limited her ability to do the maximum work she could in the house or on the plantation,” Dr. Brown said.
After slavery ended, health care access did not improve for Black people. In 1920, there was approximately 1 Black physician for every 3,000 Black people, compared with 1 in 500 for the White population, and grannie midwives continued to be the primary birthing attendants for Black women. Over the next several decades, however, both maternal and infant mortality across all races began steeply dropping. Reasons for the drop included the incorporation of the American Board of Obstetrics and Gynecology in 1930, a shift from home births to hospital births, and the legalization of abortion, which led to an 89% decline in deaths from septic illegal abortions from 1950 to 1973.
Still, Black maternal and infant mortality remained higher than White, and the poverty gap further exacerbated outcomes.
“Substandard maternity care really is the origin of many of the Black maternal and infant morbidity and mortality” complications, such as low birth weight, small for gestational age, growth restriction, and intrauterine starvation, “which we now believe are the origin of things like hypertension, diabetes, and obesity,” Dr. Brown said.
Today, inequities persist because of the systemic racism throughout this history.
“As we talk about health disparities, prematurity, growth restriction, and maternal morbidity, the fetal origins for adult disease in diabetes and hypertension and obesity have generational implications over the last 400 years,” Dr. Brown said. “Generational stress and stresses in lack women from slavery to present times are some of the origins of the things that we see today, including segregation, economic inequities, eugenic sterilizations, the quality of education, and of course, systemic racism on health care access and quality.”
It is this long arc of history that Dr. Mitchell hopes attendees will begin to grasp.
“If you don’t understand all that and have that depth, there’s no way for you to truly understand the problems that are going on and how to solve them,” Dr. Mitchell said. She hopes that especially those who have been more “resistant to accepting these truths” can start to see the big picture. “Hopefully, they can look at it as a systemic problem and then focus on how they can change the system.”
Dr Brown is a contributor to UpToDate and the Merck Manual and serves on the advisory boards of Merck for Mothers Global Women’s Health and BabyScripts. Dr. Mitchell has no disclosures.
FROM ACOG 2021
Is NPH associated with fewer adverse events than analog basal insulin for adults with T2D?
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
EVIDENCE-BASED ANSWER:
NO. Insulin glargine may lead to less patient-reported, symptomatic, and nocturnal hypoglycemia, although overall, there may not be a difference in the risk for severe hypoglycemia or hypoglycemia-related emergency department (ED) visits and hospitalizations (strength of recommendation [SOR]: B, systematic review of randomized controlled trials [RCTs], individual RCTs, and observational study).
A woman with scaling, and painful, crusted, erythematous papules and pustules on her face
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Lesions in pelvis may be ‘tip of the iceberg’ in endometriosis
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
FROM ACOG 2021
Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 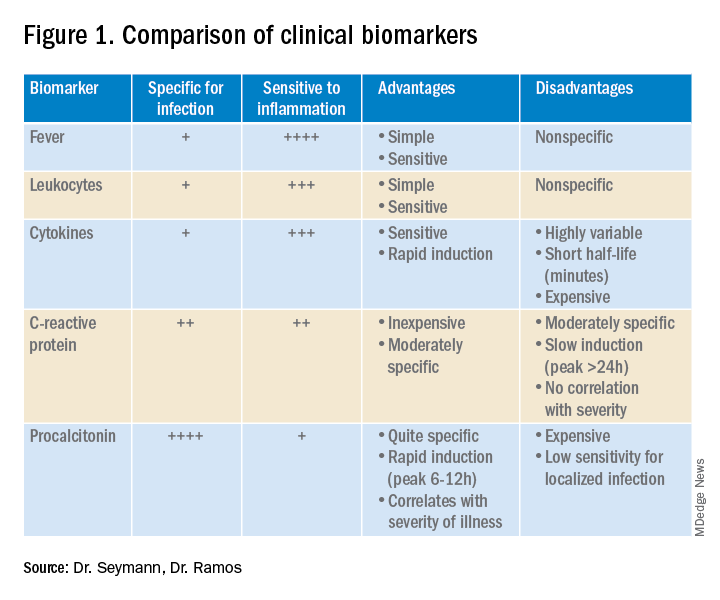
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
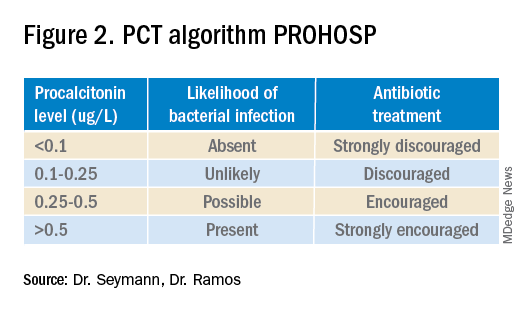
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.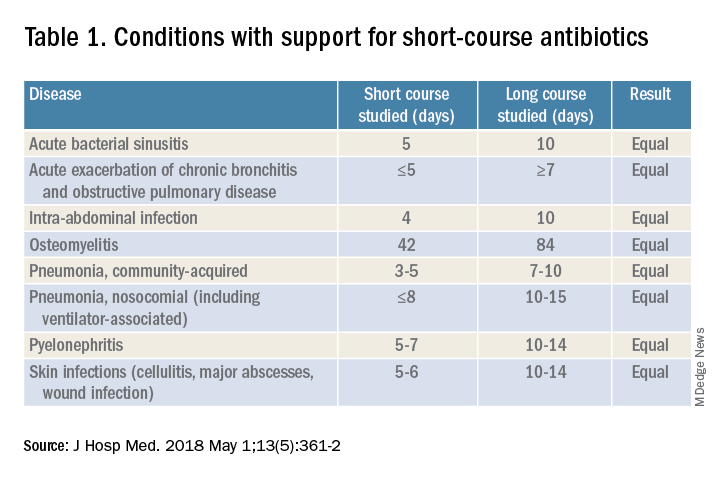
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.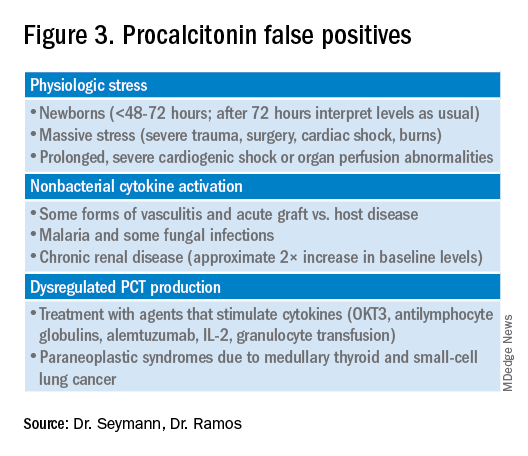
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.