User login
AAD announces diversity initiatives
not only within the academy itself, but also in the profession of dermatology overall.
“Last year’s events surrounding social justice issues and the disproportionate impact of COVID-19 on minority communities underscored an urgent need for the academy to outline a strategy to address gaps in diversity, equity, and inclusion across the academy’s programs, provide better access to dermatologic care, and expand the pipeline for prospective dermatologists,” according to an AAD statement introducing the plan.
“The AAD has long recognized the importance of fostering diversity in the dermatology specialty and increasing dermatologic services to underserved populations as a key strategic goal,” Kanya Ferguson, MD, chair of the AAD’s diversity committee, said in an interview.
“The importance and urgency of furthering these goals have been underscored by the social justice events of 2020 and the disproportionate impact that COVID-19 has had, specifically on Black and Latino communities,” added Dr. Ferguson, of the department of dermatology, at the University of Iowa, Iowa City. “The 3-year plan comprehensively expands current diversity, equity, and inclusion initiatives in an effort to accelerate the Academy’s progress toward its strategic goals.”
“Numerous barriers persist that contribute to the narrowing pipeline in medicine and ultimately in dermatology,” Dr. Ferguson noted. “The AAD’s diversity, equity, and inclusion initiatives, toolkits, and resources aim to address some of these barriers through early exposure, pipeline programming, and mentorship.”
As for the next steps, “the diversity committee will be working hard over the next few years to coordinate the integration and adoption of initiatives throughout the Academy’s activities,” she added. “This work will take a significant amount of collaboration and the committee is excited to move this forward in a meaningful and sustainable way.”
The AAD’s diversity committee headed the development of the plan, unanimously approved by the AAD’s board of directors, which outlines four key goals for the next 3 years, presented in the Diversity in Dermatology plan as follows:
“Promote and facilitate diversity, equity, and inclusion within the AAD.” Steps toward this goal include facilitating diverse representation on AAD committees, councils, and task forces, increasing representation of skin of color session speakers and lecture topics at Academy meetings, and ensuring equity in the selection process for awards including the Leadership Forum, Academic Dermatology Leadership Program, Advanced Leadership Forum, Journal of the AAD Editorial Mentorship Program, and other leadership activities.
- “Ensure dermatologic education and research encompasses health disparities and skin of color, and advocate for Black and Latino patient representation in research.” Steps toward this goal include increasing use of images reflecting the full spectrum of skin types, ensuring that skin of color populations receive information about dermatologic diseases, and supporting underrepresented minority (URM) dermatology physician scientists in leadership and professional development.
- “Expand Academy’s Advocacy Priorities to prioritize addressing health inequities.” Steps toward this goal include prioritizing issues that affect minority and marginalized populations, establishing relationships with relevant congressional leadership, and advocating for patient support groups for diseases that disproportionately impact skin of color patients.
- “Increase the number of practicing dermatologists who are underrepresented minorities and provide leadership and professional development programming.” Steps toward this goal include expanding the AAD mentorship program to include physician scientists, expanding diversity champion programs, expanding outreach to URM college students in STEM majors, and launching an AAD Summer Diversity & Inclusion camp for younger students to promote interest in a medical career.
The AAD diversity committee also has assembled a toolkit of resources designed to help its members learn how to talk about race, be an effective ally, and achieve cultural competency. Additional updated resources include guidelines on mentorship and outreach.
not only within the academy itself, but also in the profession of dermatology overall.
“Last year’s events surrounding social justice issues and the disproportionate impact of COVID-19 on minority communities underscored an urgent need for the academy to outline a strategy to address gaps in diversity, equity, and inclusion across the academy’s programs, provide better access to dermatologic care, and expand the pipeline for prospective dermatologists,” according to an AAD statement introducing the plan.
“The AAD has long recognized the importance of fostering diversity in the dermatology specialty and increasing dermatologic services to underserved populations as a key strategic goal,” Kanya Ferguson, MD, chair of the AAD’s diversity committee, said in an interview.
“The importance and urgency of furthering these goals have been underscored by the social justice events of 2020 and the disproportionate impact that COVID-19 has had, specifically on Black and Latino communities,” added Dr. Ferguson, of the department of dermatology, at the University of Iowa, Iowa City. “The 3-year plan comprehensively expands current diversity, equity, and inclusion initiatives in an effort to accelerate the Academy’s progress toward its strategic goals.”
“Numerous barriers persist that contribute to the narrowing pipeline in medicine and ultimately in dermatology,” Dr. Ferguson noted. “The AAD’s diversity, equity, and inclusion initiatives, toolkits, and resources aim to address some of these barriers through early exposure, pipeline programming, and mentorship.”
As for the next steps, “the diversity committee will be working hard over the next few years to coordinate the integration and adoption of initiatives throughout the Academy’s activities,” she added. “This work will take a significant amount of collaboration and the committee is excited to move this forward in a meaningful and sustainable way.”
The AAD’s diversity committee headed the development of the plan, unanimously approved by the AAD’s board of directors, which outlines four key goals for the next 3 years, presented in the Diversity in Dermatology plan as follows:
“Promote and facilitate diversity, equity, and inclusion within the AAD.” Steps toward this goal include facilitating diverse representation on AAD committees, councils, and task forces, increasing representation of skin of color session speakers and lecture topics at Academy meetings, and ensuring equity in the selection process for awards including the Leadership Forum, Academic Dermatology Leadership Program, Advanced Leadership Forum, Journal of the AAD Editorial Mentorship Program, and other leadership activities.
- “Ensure dermatologic education and research encompasses health disparities and skin of color, and advocate for Black and Latino patient representation in research.” Steps toward this goal include increasing use of images reflecting the full spectrum of skin types, ensuring that skin of color populations receive information about dermatologic diseases, and supporting underrepresented minority (URM) dermatology physician scientists in leadership and professional development.
- “Expand Academy’s Advocacy Priorities to prioritize addressing health inequities.” Steps toward this goal include prioritizing issues that affect minority and marginalized populations, establishing relationships with relevant congressional leadership, and advocating for patient support groups for diseases that disproportionately impact skin of color patients.
- “Increase the number of practicing dermatologists who are underrepresented minorities and provide leadership and professional development programming.” Steps toward this goal include expanding the AAD mentorship program to include physician scientists, expanding diversity champion programs, expanding outreach to URM college students in STEM majors, and launching an AAD Summer Diversity & Inclusion camp for younger students to promote interest in a medical career.
The AAD diversity committee also has assembled a toolkit of resources designed to help its members learn how to talk about race, be an effective ally, and achieve cultural competency. Additional updated resources include guidelines on mentorship and outreach.
not only within the academy itself, but also in the profession of dermatology overall.
“Last year’s events surrounding social justice issues and the disproportionate impact of COVID-19 on minority communities underscored an urgent need for the academy to outline a strategy to address gaps in diversity, equity, and inclusion across the academy’s programs, provide better access to dermatologic care, and expand the pipeline for prospective dermatologists,” according to an AAD statement introducing the plan.
“The AAD has long recognized the importance of fostering diversity in the dermatology specialty and increasing dermatologic services to underserved populations as a key strategic goal,” Kanya Ferguson, MD, chair of the AAD’s diversity committee, said in an interview.
“The importance and urgency of furthering these goals have been underscored by the social justice events of 2020 and the disproportionate impact that COVID-19 has had, specifically on Black and Latino communities,” added Dr. Ferguson, of the department of dermatology, at the University of Iowa, Iowa City. “The 3-year plan comprehensively expands current diversity, equity, and inclusion initiatives in an effort to accelerate the Academy’s progress toward its strategic goals.”
“Numerous barriers persist that contribute to the narrowing pipeline in medicine and ultimately in dermatology,” Dr. Ferguson noted. “The AAD’s diversity, equity, and inclusion initiatives, toolkits, and resources aim to address some of these barriers through early exposure, pipeline programming, and mentorship.”
As for the next steps, “the diversity committee will be working hard over the next few years to coordinate the integration and adoption of initiatives throughout the Academy’s activities,” she added. “This work will take a significant amount of collaboration and the committee is excited to move this forward in a meaningful and sustainable way.”
The AAD’s diversity committee headed the development of the plan, unanimously approved by the AAD’s board of directors, which outlines four key goals for the next 3 years, presented in the Diversity in Dermatology plan as follows:
“Promote and facilitate diversity, equity, and inclusion within the AAD.” Steps toward this goal include facilitating diverse representation on AAD committees, councils, and task forces, increasing representation of skin of color session speakers and lecture topics at Academy meetings, and ensuring equity in the selection process for awards including the Leadership Forum, Academic Dermatology Leadership Program, Advanced Leadership Forum, Journal of the AAD Editorial Mentorship Program, and other leadership activities.
- “Ensure dermatologic education and research encompasses health disparities and skin of color, and advocate for Black and Latino patient representation in research.” Steps toward this goal include increasing use of images reflecting the full spectrum of skin types, ensuring that skin of color populations receive information about dermatologic diseases, and supporting underrepresented minority (URM) dermatology physician scientists in leadership and professional development.
- “Expand Academy’s Advocacy Priorities to prioritize addressing health inequities.” Steps toward this goal include prioritizing issues that affect minority and marginalized populations, establishing relationships with relevant congressional leadership, and advocating for patient support groups for diseases that disproportionately impact skin of color patients.
- “Increase the number of practicing dermatologists who are underrepresented minorities and provide leadership and professional development programming.” Steps toward this goal include expanding the AAD mentorship program to include physician scientists, expanding diversity champion programs, expanding outreach to URM college students in STEM majors, and launching an AAD Summer Diversity & Inclusion camp for younger students to promote interest in a medical career.
The AAD diversity committee also has assembled a toolkit of resources designed to help its members learn how to talk about race, be an effective ally, and achieve cultural competency. Additional updated resources include guidelines on mentorship and outreach.
Headache and COVID-19: Key questions answered
Although coronavirus 19 disease (COVID-19), caused by severe acute respiratory coronavirus 2, is characterized by symptoms that primarily impact the respiratory system, many patients experience neurological manifestations, with headache among leading complaints. Moreover, headache symptoms, including migraine-like headache, can last long after patients recover from COVID-19.
Last November, in an interview with 60 Minutes, Sadie Nagamootoo described her experience. “There are days when I do nothing and cannot get out of bed. The migraines are 10 times worse than a flu headache.”
To help individuals like Nagamootoo and others who experience headache as a result of COVID-19, it is important to understand the data that are emerging and how to incorporate them into practice. Following are answers to important questions that can guide front-line neurologists and other clinicians who are practicing during the pandemic.
Why is headache a symptom of COVID-19? It should come as no surprise that patients with COVID-19 can experience headache. Peng reminds us, in a November 2020 editorial in Cephalalgia, that headache is a common symptom in individuals with acute respiratory disease, representing a physiological response to acute infection. Headache is often the primary reason patients seek treatment.
How is headache associated with COVID-19? It is too early to know with certainty the mechanisms underlying COVID-19 headache, but a possible explanation—according to Uygun and colleagues, writing in the The Journal of Headache and Pain—is that the virus directly invades trigeminal nerve endings in the nasal and oral cavities.
How does headache tend to present in COVID-19? Patricia Pozo-Rosich, MD, PhD, presented on this topic at the American Headache Society’s 2020 Virtual Annual Scientific Meeting in June. In a recent interview with Neurology Reviews, Dr. Pozo-Rosich, head of the Headache & Craniofacial Pain Unit at Vall d’Hebron University Hospital, Barcelona, Spain, noted that “headache seems to have 2 different presentations: 1) migraine-like characteristics that are severe, disabling, and usually start before other COVID symptoms and 2) tension-type headache characteristics, which usually start together with the rest of COVID symptoms.”
Are there symptoms that tend to occur more frequently in patients with COVID-19 and headache? Caronna and colleagues recently published an analysis in Cephalalgia of 130 individuals with COVID-19, showing that loss of smell and/or taste occurred in more than half of patients with headache, compared with fewer than 20% of those without headache. This finding is notable because it has been frequently reported in case reports of patients with COVID-19 and headache.
What does the presence of headache indicate about COVID-19 prognosis? The good news for individuals with COVID-19 who experience headache is that the duration of their COVID-19 illness might very well be shorter. In the Caronna study, COVID-19 duration in individuals with headache was, on average, 1 week shorter (24 days) than in those without headache symptoms (31 days). “We don’t know why,” said Dr. Pozo-Rosich, who is one of the study’s authors. She hypothesizes that it is because of a balance between neuroinflammation and systemic inflammation. “Having an extraordinary initial reaction at the nasal cavity might protect us from having greater systemic inflammation.”
What is the cause of headache from COVID-19? Bolay and colleagues reported in Headache in Spring 2020 that patients developed new-onset, moderate-to-severe, bilateral pulsating or pressing headache toward the frontal area and forehead during the viral phase of disease. The virus activates peripheral trigeminal nerve endings directly or through vasculopathy and/or increased circulating pro-inflammatory cytokines.
What else is important to be aware of regarding headache evolution in individuals with COVID-19? The bad news for many of these individuals is that, although their COVID-19 illness might dissipate more quickly, headaches could linger. Moreover, many will be experiencing chronic headache for the first time in their life. Caronna reported that that one third of follow-up patients who reported headache were experiencing persistent disabling headache daily after 6 weeks, and more than half had no history of recurrent headache.
What is the recommended treatment for headache associated with COVID-19? Dr. Pozo-Rosich recommends starting with a nonsteroidal anti-inflammatory medication. Eventually, steroids might be indicated, “especially if the disease progresses.”
It is important for neurologists to be aware of new-onset headache associated with anosmia early in the disease. Test for the virus in such a patient; hopefully, their course will be shorter, milder, and non-respiratory.
Although coronavirus 19 disease (COVID-19), caused by severe acute respiratory coronavirus 2, is characterized by symptoms that primarily impact the respiratory system, many patients experience neurological manifestations, with headache among leading complaints. Moreover, headache symptoms, including migraine-like headache, can last long after patients recover from COVID-19.
Last November, in an interview with 60 Minutes, Sadie Nagamootoo described her experience. “There are days when I do nothing and cannot get out of bed. The migraines are 10 times worse than a flu headache.”
To help individuals like Nagamootoo and others who experience headache as a result of COVID-19, it is important to understand the data that are emerging and how to incorporate them into practice. Following are answers to important questions that can guide front-line neurologists and other clinicians who are practicing during the pandemic.
Why is headache a symptom of COVID-19? It should come as no surprise that patients with COVID-19 can experience headache. Peng reminds us, in a November 2020 editorial in Cephalalgia, that headache is a common symptom in individuals with acute respiratory disease, representing a physiological response to acute infection. Headache is often the primary reason patients seek treatment.
How is headache associated with COVID-19? It is too early to know with certainty the mechanisms underlying COVID-19 headache, but a possible explanation—according to Uygun and colleagues, writing in the The Journal of Headache and Pain—is that the virus directly invades trigeminal nerve endings in the nasal and oral cavities.
How does headache tend to present in COVID-19? Patricia Pozo-Rosich, MD, PhD, presented on this topic at the American Headache Society’s 2020 Virtual Annual Scientific Meeting in June. In a recent interview with Neurology Reviews, Dr. Pozo-Rosich, head of the Headache & Craniofacial Pain Unit at Vall d’Hebron University Hospital, Barcelona, Spain, noted that “headache seems to have 2 different presentations: 1) migraine-like characteristics that are severe, disabling, and usually start before other COVID symptoms and 2) tension-type headache characteristics, which usually start together with the rest of COVID symptoms.”
Are there symptoms that tend to occur more frequently in patients with COVID-19 and headache? Caronna and colleagues recently published an analysis in Cephalalgia of 130 individuals with COVID-19, showing that loss of smell and/or taste occurred in more than half of patients with headache, compared with fewer than 20% of those without headache. This finding is notable because it has been frequently reported in case reports of patients with COVID-19 and headache.
What does the presence of headache indicate about COVID-19 prognosis? The good news for individuals with COVID-19 who experience headache is that the duration of their COVID-19 illness might very well be shorter. In the Caronna study, COVID-19 duration in individuals with headache was, on average, 1 week shorter (24 days) than in those without headache symptoms (31 days). “We don’t know why,” said Dr. Pozo-Rosich, who is one of the study’s authors. She hypothesizes that it is because of a balance between neuroinflammation and systemic inflammation. “Having an extraordinary initial reaction at the nasal cavity might protect us from having greater systemic inflammation.”
What is the cause of headache from COVID-19? Bolay and colleagues reported in Headache in Spring 2020 that patients developed new-onset, moderate-to-severe, bilateral pulsating or pressing headache toward the frontal area and forehead during the viral phase of disease. The virus activates peripheral trigeminal nerve endings directly or through vasculopathy and/or increased circulating pro-inflammatory cytokines.
What else is important to be aware of regarding headache evolution in individuals with COVID-19? The bad news for many of these individuals is that, although their COVID-19 illness might dissipate more quickly, headaches could linger. Moreover, many will be experiencing chronic headache for the first time in their life. Caronna reported that that one third of follow-up patients who reported headache were experiencing persistent disabling headache daily after 6 weeks, and more than half had no history of recurrent headache.
What is the recommended treatment for headache associated with COVID-19? Dr. Pozo-Rosich recommends starting with a nonsteroidal anti-inflammatory medication. Eventually, steroids might be indicated, “especially if the disease progresses.”
It is important for neurologists to be aware of new-onset headache associated with anosmia early in the disease. Test for the virus in such a patient; hopefully, their course will be shorter, milder, and non-respiratory.
Although coronavirus 19 disease (COVID-19), caused by severe acute respiratory coronavirus 2, is characterized by symptoms that primarily impact the respiratory system, many patients experience neurological manifestations, with headache among leading complaints. Moreover, headache symptoms, including migraine-like headache, can last long after patients recover from COVID-19.
Last November, in an interview with 60 Minutes, Sadie Nagamootoo described her experience. “There are days when I do nothing and cannot get out of bed. The migraines are 10 times worse than a flu headache.”
To help individuals like Nagamootoo and others who experience headache as a result of COVID-19, it is important to understand the data that are emerging and how to incorporate them into practice. Following are answers to important questions that can guide front-line neurologists and other clinicians who are practicing during the pandemic.
Why is headache a symptom of COVID-19? It should come as no surprise that patients with COVID-19 can experience headache. Peng reminds us, in a November 2020 editorial in Cephalalgia, that headache is a common symptom in individuals with acute respiratory disease, representing a physiological response to acute infection. Headache is often the primary reason patients seek treatment.
How is headache associated with COVID-19? It is too early to know with certainty the mechanisms underlying COVID-19 headache, but a possible explanation—according to Uygun and colleagues, writing in the The Journal of Headache and Pain—is that the virus directly invades trigeminal nerve endings in the nasal and oral cavities.
How does headache tend to present in COVID-19? Patricia Pozo-Rosich, MD, PhD, presented on this topic at the American Headache Society’s 2020 Virtual Annual Scientific Meeting in June. In a recent interview with Neurology Reviews, Dr. Pozo-Rosich, head of the Headache & Craniofacial Pain Unit at Vall d’Hebron University Hospital, Barcelona, Spain, noted that “headache seems to have 2 different presentations: 1) migraine-like characteristics that are severe, disabling, and usually start before other COVID symptoms and 2) tension-type headache characteristics, which usually start together with the rest of COVID symptoms.”
Are there symptoms that tend to occur more frequently in patients with COVID-19 and headache? Caronna and colleagues recently published an analysis in Cephalalgia of 130 individuals with COVID-19, showing that loss of smell and/or taste occurred in more than half of patients with headache, compared with fewer than 20% of those without headache. This finding is notable because it has been frequently reported in case reports of patients with COVID-19 and headache.
What does the presence of headache indicate about COVID-19 prognosis? The good news for individuals with COVID-19 who experience headache is that the duration of their COVID-19 illness might very well be shorter. In the Caronna study, COVID-19 duration in individuals with headache was, on average, 1 week shorter (24 days) than in those without headache symptoms (31 days). “We don’t know why,” said Dr. Pozo-Rosich, who is one of the study’s authors. She hypothesizes that it is because of a balance between neuroinflammation and systemic inflammation. “Having an extraordinary initial reaction at the nasal cavity might protect us from having greater systemic inflammation.”
What is the cause of headache from COVID-19? Bolay and colleagues reported in Headache in Spring 2020 that patients developed new-onset, moderate-to-severe, bilateral pulsating or pressing headache toward the frontal area and forehead during the viral phase of disease. The virus activates peripheral trigeminal nerve endings directly or through vasculopathy and/or increased circulating pro-inflammatory cytokines.
What else is important to be aware of regarding headache evolution in individuals with COVID-19? The bad news for many of these individuals is that, although their COVID-19 illness might dissipate more quickly, headaches could linger. Moreover, many will be experiencing chronic headache for the first time in their life. Caronna reported that that one third of follow-up patients who reported headache were experiencing persistent disabling headache daily after 6 weeks, and more than half had no history of recurrent headache.
What is the recommended treatment for headache associated with COVID-19? Dr. Pozo-Rosich recommends starting with a nonsteroidal anti-inflammatory medication. Eventually, steroids might be indicated, “especially if the disease progresses.”
It is important for neurologists to be aware of new-onset headache associated with anosmia early in the disease. Test for the virus in such a patient; hopefully, their course will be shorter, milder, and non-respiratory.
Outcomes have improved for PAH in connective tissue disease
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
Survival rates for patients with pulmonary arterial hypertension associated with connective tissue diseases have improved significantly in recent years, and there is growing evidence that treatments for idiopathic pulmonary arterial hypertension can also benefit this group.
In an article published online Feb. 3, 2021, in Arthritis & Rheumatology, researchers report the outcomes of a meta-analysis to explore the effect of more modern pulmonary arterial hypertension treatments on patients with conditions such as systemic sclerosis.
First author Dinesh Khanna, MBBS, MSc, of the division of rheumatology at the University of Michigan, Ann Arbor, said in an interview that connective tissue disease–associated pulmonary arterial hypertension (CTD-PAH) was a leading cause of death, but earlier clinical trials had found poor outcomes in patients with CTD, compared with those with idiopathic PAH.
“Recent clinical trial data show that aggressive, up-front PAH treatments have better outcomes in those with CTD-PAH, and we wanted to explore these observations carefully in a systematic review and meta-analysis,” Dr. Khanna said.
The analysis included 11 randomized, controlled trials, involving 4,329 patients with PAH (1,267 with CTD), and 19 registries with a total of 9,739 patients with PAH, including 4,008 with CTD. Trials were required to report long-term clinical outcomes with a median enrollment time of greater than 6 months, and outcomes measured between 3-6 months after the patients started treatment.
Patients with CTDs had an older mean age and a lower 6-minute walk distance than did those with idiopathic PAH.
Five randomized, controlled trials – involving 3,172 patients, 941 of whom had a CTD – found that additional PAH treatment was associated with a 36% reduction in the risk of morbidity or mortality events, compared with controls both in the overall PAH group and in those with CTD.
Additional therapy was also associated with a 34.6-meter increase in 6-minute walk distance in the general PAH population, and a 20.4-meter increase in those with CTD.
The authors commented that the smaller improvement in 6-minute walk distance among patients with CTD may be influenced by comorbidities such as musculoskeletal involvement that would be independent of their cardiopulmonary function.
Differential patient survival among PAH etiologies
“Our meta-analysis of RCTs demonstrated that patients with CTD-PAH derive a clinically significant benefit from currently available PAH therapies which, in many patients, comprised the addition of a drug targeting a second or third pathway involved in the pathophysiology of PAH,” the authors wrote.
When researchers analyzed data from nine registries that included a wide range of PAH etiologies, they found the overall survival rates were lower among patients with CTD, compared with the overall population. The analysis also suggested that patients with systemic sclerosis and PAH had lower survival rates than did those with systemic lupus erythematosus.
Dr. Khanna said this may relate to different pathophysiology of PAH in patients with CTDs, but could also be a reflection of other differences, such as older age and the involvement of other comorbidities, including lung fibrosis and heart involvement.
Data across all 19 registries also showed that survival rates among those with CTD were higher in registries where more than 50% of the registry study period was during or after 2010, compared with registries where 50% or more of the study period was before 2010.
The authors suggested the differences in survival rates may relate to increased screening for PAH, particularly among people with CTDs. They noted that increased screening leads to earlier diagnosis, which could introduce a lead-time bias such that later registries would have younger participants with less severe disease. However, their analysis found that the later registries had older patients but also with less severe disease, and they suggested that it wasn’t possible to determine if lead-time bias was playing a role in their results.
Improvements in treatment options could also account for differences in survival over time, although the authors commented that only six registries in the study included patients from 2015 or later, when currently available treatments came into use and early combination therapy was used more.
“These data also support the 2018 World Symposium on Pulmonary Hypertension recommendations to initiate up-front combination pulmonary arterial hypertension therapy in majority of cases with CTD-PAH,” Dr. Khanna said.
‘Still have to be aggressive at identifying the high-risk patients’
Commenting on the findings, Virginia Steen, MD, of the division of rheumatology at Georgetown University, Washington, said clinicians were finally seeing some significant changes over time in scleroderma-associated PAH.
“Although some of it may be just early diagnosis, I think that the combination of early diagnosis and more aggressive treatment with combination medication is definitely making a difference,” Dr. Steen said in an interview. “The bottom line is that we as rheumatologists still have to be aggressive at identifying the high-risk patients, making an early diagnosis, and working with our pulmonary hypertension colleagues and aggressively treating these patients so we can make a long-term difference.”
The authors of an accompanying editorial said the meta-analysis’ findings showed the positive impact of early combination therapy and early diagnosis through proactive screening.
“It is notable because the present analysis again confirms that outcomes are worse in CTD-PAH than in idiopathic or familial forms of PAH, the impact of treatments should no longer be regarded as insignificant,” the editorial’s authors wrote. “This is a practice changing observation, especially now that many of the drugs are available in generic formulations and so the cost of modern PAH treatment has fallen at the same time as its true value is convincingly demonstrated.”
They also argued there was strong evidence for the value of combination therapies, both for PAH-targeted drugs used in combination and concurrent use of immunosuppression and drugs specifically for PAH in some patients with CTD-PAH.
However, they pointed out that not all treatments for idiopathic PAH were suitable for patients with CTDs, highlighting the example of anticoagulation that can improve survival in the first but worsen it in the second.
The study was funded by Actelion. Six authors declared funding and grants from the pharmaceutical sector, including the study sponsor, and three authors were employees of Actelion.
FROM ARTHRITIS & RHEUMATOLOGY
Consider home subcutaneous immune globulin for refractory dermatomyositis
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
Home-based subcutaneous immune globulin therapy is a promising alternative to intravenous immune globulin therapy for patients with refractory dermatomyositis or polymyositis, Anna Postolova, MD, MPH, declared at the 2021 Rheumatology Winter Clinical Symposium.
“This is really exciting. I think in the years to come we may see a change to having our patients be able to do immune globulin therapy at home,” said Dr. Postolova, a rheumatologist and allergist/immunologist at Stanford (Calif.) Health Care.
“The technology is there. I think our patients might feel more comfortable getting immune globulin at home,” she said. “I would love to switch more patients from IVIg to SCIg [subcutaneous immune globulin] in my practice.”
A few caveats: SCIg remains off label for treatment of dermatomyositis (DM) or polymyositis (PM). Its approved indication is as replacement therapy in patients with primary or secondary immunodeficiency diseases. IVIg is approved for this indication, but is also approved for DM/PM refractory to high-dose corticosteroids and immunosuppressants. Yet SCIg is clearly effective for these autoimmune inflammatory diseases, albeit to date the supporting evidence comes chiefly from observational studies and anecdotal experience.
“I don’t know if insurers will cover it, but they should because it’s obviously a lot cheaper to do it at home,” she noted.
SCIg advantages
SCIg offers compelling advantages over IVIg in addition to its substantially lower cost. These include far fewer systemic side effects, shorter infusion time, greater bioavailability, and better quality of life. Patients self-administer SCIg at home, avoiding the inconvenience of IVIg therapy, which entails travel time for once-monthly hospitalization or long hours spent in an infusion center, she explained.
French investigators recently documented a previously unappreciated further advantage of home-based SCIg. They convened a focus group of patients with DM or PM experienced with both IVIg and home SCIg and determined that participants uniformly preferred home SCIg. The patients cited a new and welcome feeling of autonomy and control.
“All patients with experience of IVIg and SCIg expressed a clear preference for SCIg, which was described to be easy, less disruptive for daily life, well tolerated, and less time-consuming. Preference was mainly related to a restoration of autonomy. Home-based self-administration reinforced the feeling of independence,” according to the investigators.
Available products
Six preparations of SCIg are commercially available. Most are in 10% concentration, as are all IVIg products. However, a 20% formulation of SCIg known as Hizentra allows for a smaller infusion volume and quicker completion of a treatment session. And one SCIg product, HyQvia, uses recombinant human hyaluronidase-facilitated 10% immune globulin, allowing home infusion of large volumes of sustained-release immune globulin on a once-monthly basis.
The relatively recent introduction of home SCIg for treatment of autoimmune inflammatory diseases, including DM, PM, and chronic inflammatory demyelinating polyneuropathy, has been pioneered mainly by European investigators. The treatment is often given by programmable mechanical pump once weekly. Italian investigators have reported efficacy in DM using 0.2 g/kg per week, which is about half the monthly total dose of IVIg employed. The infusion rate is 10-40 mL/hour, with a volume of around 35 mL per injection site.
Alternatively, SCIg can be delivered by rapid push infusions of smaller volumes with a syringe two or three times per week; that’s the regimen that was used at 2 g/kg over the course of a month by patients in the French focus group study, who didn’t mind the more frequent dosing.
“As they have had severe long-lasting symptoms, SCIg was perceived as a curative rather than a preventive therapy,” according to the French investigators.
More than 40% of patients experience adverse reactions to IVIg. These often involve headaches, nausea, back or abdominal pain, arthralgias, and/or difficulty breathing. Thromboembolic events and acute renal failure occur occasionally. For this reason, many physicians give a prophylactic dose of corticosteroids an hour before a patient’s first dose of IVIg. These systemic side effects are so rare with SCIg that Dr. Postolova has never pretreated with steroids, even though the main reason she resorts to the home therapy is a patient’s track record of poor tolerance of IVIg. The lower abdomen and thigh are the most commonly used subcutaneous infusion sites. Mild local infusion site reactions are fairly common.
Formulating IVIg and SCIg is a complex process that entails plasma procurement and pooling, fractionation, and purification. It takes 10,000-60,000 plasma donations to make one lot of IVIg. Donations are accepted only from repeated donors. Samples are held for 6 months and tested for infectious agents. However, efforts are underway to develop bioengineered recombinant immune globulin products that don’t require donated plasma. These products are being designed to capture and enhance the most important mechanisms of benefit of plasma-derived immunoglobulins using Fc fragments that target key receptors, rather than relying on full-length immune globulin. The goal is enhanced efficacy at much lower doses than with IVIg or SCIg.
Dr. Postolova reported having no financial conflicts regarding her presentation.
FROM RWCS 2021
Ergonomic consultation spares endoscopists a pain in the neck
Assessment of position and posture by a physical therapist can help reduce and prevent injury in endoscopists, based on data from a pilot study of eight individuals.
Musculoskeletal injuries among endoscopists are gaining more attention: One technical review indicated that the “prevalence of musculoskeletal pain or injuries ranged from 29% to 89% of gastroenterologists.” While data on avoiding musculoskeletal injury related to endoscopy are limited, recognition of the role of ergonomics is increasing, Stacy A. Markwell, a physical therapist in Chapel Hill, N.C., and colleagues, wrote in a study published in Gastrointestinal Endoscopy.
The mental concentration required along with the physical demands on manipulating the scope have been shown to negatively impact posture, the researchers noted.
The researchers reviewed data from eight endoscopists who were aged 32-71 years; they had a range of clinical experience and were performing 6-30 colonoscopies and 3-21 upper endoscopies per week.
These endoscopists volunteered for an ergonomic intervention involving use of an individualized wellness plan. They completed the Nordic Musculoskeletal Questionnaire to evaluate musculoskeletal complaints during the past 12 months and the past 7 days. Three of the eight participants reported pain at work at initial assessment, which often worsened over the course of the day, and five mentioned fatigue while working. They specified 22 pain sites, mainly in the neck and back. In addition, participants were photographed to evaluate posture in a static position and self-selected “tired” positions.
“When frequent or consistent posturing resulted in suboptimal joint alignment, muscle length, loading at end range of muscle or joints, and/or prolonged static active positioning, participants were photographed to provide personalized feedback for wellness education,” the researchers wrote.
The physical therapist used information from the evaluation and photographs to develop individual plans to improve the ergonomics of the endoscopic suite with adjustments to the location of the bed and positioning of chairs, standing surfaces, and monitors and keyboards. In addition to adjusting the endoscopic suite, the physical therapist developed individual wellness plans including exercises to relieve pain and improve posture, as well as pain education to help clinicians recognize and manage pain and fatigue.
By the end of the study, in a follow-up 6-12 months after the wellness intervention, 63% of pain sites (14 of 22) reported by participants were reduced in intensity or resolved, 32% were unchanged (7 of 22), and 4% increased (1 of 22).
Overall, seven of the eight participants said that the pictures of their posture along with the movement analysis was helpful, and three participants asked for reassessment by the physical therapist. In this study, the average cost of the wellness program was $500.
“All endoscopists reported that the wellness plan was helpful, with procedure suite and posture recommendations being the most beneficial,” the researchers reported. “Upon gaining insight with visualization of their posture and movement during endoscopy, participants’ understanding and motivation to make corrections was intensified.”
The study findings were limited by several factors including the small size, use of a single physical therapist, short follow-up, lack of controls, and use of a single site, the researchers noted. However, “our study provides a detailed, pragmatic, and reproducible framework for performing an individualized physical therapist–directed comprehensive assessment and personalized wellness plan in the workplace to help meet the challenges of ergonomics in endoscopy.”
Recognition of the value of ergonomics is rising
“Endoscopy related injury and disability is a known hazard of our profession,” said Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, in an interview. “Any studies to assess and, more importantly, offer ways to prevent such injury are immediately relevant. In this context, ergonomics for endoscopy is an increasing area of research.”
Dr. Ketwaroo said that the study results were not surprising. “I agree with authors that there is a paucity of general ergonomic training and assessment. Specific individualized wellness plans are rare. Developing an individual plan based on observation by physical therapists, and taking into account baseline injury or predisposition to injury would be expected to be more high yield for preventing injury and improving performance
“I believe the main take-home message from the study is that an individualized ergonomic plan based on assessment and feedback by physical therapists appears promising for optimizing endoscopic performance to minimize injury and reduce fatigue,” Dr. Ketwaroo said. However, “long-term studies in much larger samples will be needed to document objective findings of reduced injury or fatigue.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Assessment of position and posture by a physical therapist can help reduce and prevent injury in endoscopists, based on data from a pilot study of eight individuals.
Musculoskeletal injuries among endoscopists are gaining more attention: One technical review indicated that the “prevalence of musculoskeletal pain or injuries ranged from 29% to 89% of gastroenterologists.” While data on avoiding musculoskeletal injury related to endoscopy are limited, recognition of the role of ergonomics is increasing, Stacy A. Markwell, a physical therapist in Chapel Hill, N.C., and colleagues, wrote in a study published in Gastrointestinal Endoscopy.
The mental concentration required along with the physical demands on manipulating the scope have been shown to negatively impact posture, the researchers noted.
The researchers reviewed data from eight endoscopists who were aged 32-71 years; they had a range of clinical experience and were performing 6-30 colonoscopies and 3-21 upper endoscopies per week.
These endoscopists volunteered for an ergonomic intervention involving use of an individualized wellness plan. They completed the Nordic Musculoskeletal Questionnaire to evaluate musculoskeletal complaints during the past 12 months and the past 7 days. Three of the eight participants reported pain at work at initial assessment, which often worsened over the course of the day, and five mentioned fatigue while working. They specified 22 pain sites, mainly in the neck and back. In addition, participants were photographed to evaluate posture in a static position and self-selected “tired” positions.
“When frequent or consistent posturing resulted in suboptimal joint alignment, muscle length, loading at end range of muscle or joints, and/or prolonged static active positioning, participants were photographed to provide personalized feedback for wellness education,” the researchers wrote.
The physical therapist used information from the evaluation and photographs to develop individual plans to improve the ergonomics of the endoscopic suite with adjustments to the location of the bed and positioning of chairs, standing surfaces, and monitors and keyboards. In addition to adjusting the endoscopic suite, the physical therapist developed individual wellness plans including exercises to relieve pain and improve posture, as well as pain education to help clinicians recognize and manage pain and fatigue.
By the end of the study, in a follow-up 6-12 months after the wellness intervention, 63% of pain sites (14 of 22) reported by participants were reduced in intensity or resolved, 32% were unchanged (7 of 22), and 4% increased (1 of 22).
Overall, seven of the eight participants said that the pictures of their posture along with the movement analysis was helpful, and three participants asked for reassessment by the physical therapist. In this study, the average cost of the wellness program was $500.
“All endoscopists reported that the wellness plan was helpful, with procedure suite and posture recommendations being the most beneficial,” the researchers reported. “Upon gaining insight with visualization of their posture and movement during endoscopy, participants’ understanding and motivation to make corrections was intensified.”
The study findings were limited by several factors including the small size, use of a single physical therapist, short follow-up, lack of controls, and use of a single site, the researchers noted. However, “our study provides a detailed, pragmatic, and reproducible framework for performing an individualized physical therapist–directed comprehensive assessment and personalized wellness plan in the workplace to help meet the challenges of ergonomics in endoscopy.”
Recognition of the value of ergonomics is rising
“Endoscopy related injury and disability is a known hazard of our profession,” said Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, in an interview. “Any studies to assess and, more importantly, offer ways to prevent such injury are immediately relevant. In this context, ergonomics for endoscopy is an increasing area of research.”
Dr. Ketwaroo said that the study results were not surprising. “I agree with authors that there is a paucity of general ergonomic training and assessment. Specific individualized wellness plans are rare. Developing an individual plan based on observation by physical therapists, and taking into account baseline injury or predisposition to injury would be expected to be more high yield for preventing injury and improving performance
“I believe the main take-home message from the study is that an individualized ergonomic plan based on assessment and feedback by physical therapists appears promising for optimizing endoscopic performance to minimize injury and reduce fatigue,” Dr. Ketwaroo said. However, “long-term studies in much larger samples will be needed to document objective findings of reduced injury or fatigue.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Assessment of position and posture by a physical therapist can help reduce and prevent injury in endoscopists, based on data from a pilot study of eight individuals.
Musculoskeletal injuries among endoscopists are gaining more attention: One technical review indicated that the “prevalence of musculoskeletal pain or injuries ranged from 29% to 89% of gastroenterologists.” While data on avoiding musculoskeletal injury related to endoscopy are limited, recognition of the role of ergonomics is increasing, Stacy A. Markwell, a physical therapist in Chapel Hill, N.C., and colleagues, wrote in a study published in Gastrointestinal Endoscopy.
The mental concentration required along with the physical demands on manipulating the scope have been shown to negatively impact posture, the researchers noted.
The researchers reviewed data from eight endoscopists who were aged 32-71 years; they had a range of clinical experience and were performing 6-30 colonoscopies and 3-21 upper endoscopies per week.
These endoscopists volunteered for an ergonomic intervention involving use of an individualized wellness plan. They completed the Nordic Musculoskeletal Questionnaire to evaluate musculoskeletal complaints during the past 12 months and the past 7 days. Three of the eight participants reported pain at work at initial assessment, which often worsened over the course of the day, and five mentioned fatigue while working. They specified 22 pain sites, mainly in the neck and back. In addition, participants were photographed to evaluate posture in a static position and self-selected “tired” positions.
“When frequent or consistent posturing resulted in suboptimal joint alignment, muscle length, loading at end range of muscle or joints, and/or prolonged static active positioning, participants were photographed to provide personalized feedback for wellness education,” the researchers wrote.
The physical therapist used information from the evaluation and photographs to develop individual plans to improve the ergonomics of the endoscopic suite with adjustments to the location of the bed and positioning of chairs, standing surfaces, and monitors and keyboards. In addition to adjusting the endoscopic suite, the physical therapist developed individual wellness plans including exercises to relieve pain and improve posture, as well as pain education to help clinicians recognize and manage pain and fatigue.
By the end of the study, in a follow-up 6-12 months after the wellness intervention, 63% of pain sites (14 of 22) reported by participants were reduced in intensity or resolved, 32% were unchanged (7 of 22), and 4% increased (1 of 22).
Overall, seven of the eight participants said that the pictures of their posture along with the movement analysis was helpful, and three participants asked for reassessment by the physical therapist. In this study, the average cost of the wellness program was $500.
“All endoscopists reported that the wellness plan was helpful, with procedure suite and posture recommendations being the most beneficial,” the researchers reported. “Upon gaining insight with visualization of their posture and movement during endoscopy, participants’ understanding and motivation to make corrections was intensified.”
The study findings were limited by several factors including the small size, use of a single physical therapist, short follow-up, lack of controls, and use of a single site, the researchers noted. However, “our study provides a detailed, pragmatic, and reproducible framework for performing an individualized physical therapist–directed comprehensive assessment and personalized wellness plan in the workplace to help meet the challenges of ergonomics in endoscopy.”
Recognition of the value of ergonomics is rising
“Endoscopy related injury and disability is a known hazard of our profession,” said Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, in an interview. “Any studies to assess and, more importantly, offer ways to prevent such injury are immediately relevant. In this context, ergonomics for endoscopy is an increasing area of research.”
Dr. Ketwaroo said that the study results were not surprising. “I agree with authors that there is a paucity of general ergonomic training and assessment. Specific individualized wellness plans are rare. Developing an individual plan based on observation by physical therapists, and taking into account baseline injury or predisposition to injury would be expected to be more high yield for preventing injury and improving performance
“I believe the main take-home message from the study is that an individualized ergonomic plan based on assessment and feedback by physical therapists appears promising for optimizing endoscopic performance to minimize injury and reduce fatigue,” Dr. Ketwaroo said. However, “long-term studies in much larger samples will be needed to document objective findings of reduced injury or fatigue.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
FROM GASTROINTESTINAL ENDOSCOPY
Tocilizumab may improve lung function in early systemic sclerosis
Treatment with tocilizumab (Actemra) could stabilize or improve lung function in people with early interstitial lung disease associated with systemic sclerosis (SSc-ILD), a new study has found.
A paper published online Feb. 3 in Arthritis & Rheumatology presents the results of a post hoc analysis of data from a phase 3, placebo-controlled, double-blind trial of subcutaneous tocilizumab in patients with SSc and progressive skin disease, which included high-resolution chest CT to assess lung involvement and fibrosis.
Tocilizumab is a monoclonal antibody that targets interleukin-6 and is currently approved for the treatment of immune-mediated diseases such as rheumatoid arthritis, giant cell arteritis, cytokine release syndrome, and systemic and polyarticular course juvenile idiopathic arthritis.
Two previous studies of tocilizumab in patients with early, diffuse cutaneous SSc had also found that the treatment was associated with preservation of lung function but did not characterize that effect using radiography.
Of the 210 participants in the trial, called focuSSced, 136 were found to have interstitial lung disease at baseline and were randomized to 162 mg tocilizumab weekly or placebo for 48 weeks.
At baseline, around three-quarters of those with interstitial lung disease had moderate to severe lung involvement, defined as ground glass opacities, honeycombing, and fibrotic reticulation across at least 20% of the whole lung.
Those in the tocilizumab group showed a 0.1% mean decline in forced vital capacity (FVC) over the 48-week study, while those in the placebo group had a mean decline of 6.3%.
When stratified by severity of lung involvement, those with mild lung disease group treated with tocilizumab had a 4.1% decline in FVC, compared with a 10% decline in the placebo group; those with moderate disease in the treatment group had an 0.7% mean increase in FVC, compared with a 5.7% decrease in the placebo group, and those with severe lung involvement in the treatment arm had a 2.1% increase in FVC, compared with a 6.7% decrease in the placebo arm.
Those treated with tocilizumab also showed a statistically significant 1.8% improvement in the amount of lung involvement, which was largely seen in those with more extensive lung involvement at baseline. Those with more than 20% of the lung affected had a significant 4.9% reduction in lung area affected, while those in the placebo arm showed a significant increase in fibrosis.
First author David Roofeh, MD, of the University of Michigan Scleroderma Program, and colleagues wrote that most patients with SSc will develop interstitial lung disease – particularly those with early, diffuse cutaneous SSc and elevated markers such as C-reactive protein.
“Patients with these high-risk features, especially those with disease in the initial phase of development, represent an important target for early intervention as ILD is largely irreversible in SSc,” the authors wrote.
Findings from a specific patient population may not be generalizable
Commenting on the findings, Lorinda Chung, MD, of Stanford (Calif.) University, said in an interview that the study demonstrated that tocilizumab could prevent radiographic progression of ILD in early diffuse SSc patients with mild to severe lung disease and evidence of active skin disease, as well as elevated inflammatory markers.
“This was a very specific patient population who was studied in the focuSSced clinical trial, and this paper only evaluated a subset of these patients,” Dr. Chung said. “The results may not be generalizable to all SSc-ILD patients and further studies are needed.”
The authors suggested that the patients with progressive skin disease and elevated acute phase reactants may represent a group in the immunoinflammatory phase of the disease rather than the advanced fibrotic stage, and that this might be a “window of therapeutic opportunity to preserve lung function.”
Dr. Chung noted that the radiographic improvement induced by tocilizumab treatment was greatest in those with the most radiographic disease at baseline.
“This may reflect tocilizumab’s impact on decreasing inflammation, but we are not provided the data on the effects of tocilizumab on the individual components of the QILD [quantitative ILD: summation of ground glass opacities, honeycombing, and fibrotic reticulation],” she said.
The study’s authors also made a point about the utility of screening patients with high-resolution chest CT to detect early signs of ILD.
“Our data demonstrate the value of obtaining HRCT at the time of diagnosis: PFTs [pulmonary function tests] are not sensitive enough to accurately assess the presence of ILD and delays in treatment initiation may lead to irreversible disease,” they wrote.
Describing the results as ‘hypothesis-generating’ owing to the post hoc nature of the analysis, the authors said that FVC was an indirect measure of the flow-resistive properties of the lung, and that other aspects of SSc – such as hide-bound chest thickness – could cause thoracic restriction.
Two authors were funded by the National Institutes of Health. Six authors declared grants, funding, and other support from the pharmaceutical sector, including Roche, which sponsored the original focuSSced trial.
Treatment with tocilizumab (Actemra) could stabilize or improve lung function in people with early interstitial lung disease associated with systemic sclerosis (SSc-ILD), a new study has found.
A paper published online Feb. 3 in Arthritis & Rheumatology presents the results of a post hoc analysis of data from a phase 3, placebo-controlled, double-blind trial of subcutaneous tocilizumab in patients with SSc and progressive skin disease, which included high-resolution chest CT to assess lung involvement and fibrosis.
Tocilizumab is a monoclonal antibody that targets interleukin-6 and is currently approved for the treatment of immune-mediated diseases such as rheumatoid arthritis, giant cell arteritis, cytokine release syndrome, and systemic and polyarticular course juvenile idiopathic arthritis.
Two previous studies of tocilizumab in patients with early, diffuse cutaneous SSc had also found that the treatment was associated with preservation of lung function but did not characterize that effect using radiography.
Of the 210 participants in the trial, called focuSSced, 136 were found to have interstitial lung disease at baseline and were randomized to 162 mg tocilizumab weekly or placebo for 48 weeks.
At baseline, around three-quarters of those with interstitial lung disease had moderate to severe lung involvement, defined as ground glass opacities, honeycombing, and fibrotic reticulation across at least 20% of the whole lung.
Those in the tocilizumab group showed a 0.1% mean decline in forced vital capacity (FVC) over the 48-week study, while those in the placebo group had a mean decline of 6.3%.
When stratified by severity of lung involvement, those with mild lung disease group treated with tocilizumab had a 4.1% decline in FVC, compared with a 10% decline in the placebo group; those with moderate disease in the treatment group had an 0.7% mean increase in FVC, compared with a 5.7% decrease in the placebo group, and those with severe lung involvement in the treatment arm had a 2.1% increase in FVC, compared with a 6.7% decrease in the placebo arm.
Those treated with tocilizumab also showed a statistically significant 1.8% improvement in the amount of lung involvement, which was largely seen in those with more extensive lung involvement at baseline. Those with more than 20% of the lung affected had a significant 4.9% reduction in lung area affected, while those in the placebo arm showed a significant increase in fibrosis.
First author David Roofeh, MD, of the University of Michigan Scleroderma Program, and colleagues wrote that most patients with SSc will develop interstitial lung disease – particularly those with early, diffuse cutaneous SSc and elevated markers such as C-reactive protein.
“Patients with these high-risk features, especially those with disease in the initial phase of development, represent an important target for early intervention as ILD is largely irreversible in SSc,” the authors wrote.
Findings from a specific patient population may not be generalizable
Commenting on the findings, Lorinda Chung, MD, of Stanford (Calif.) University, said in an interview that the study demonstrated that tocilizumab could prevent radiographic progression of ILD in early diffuse SSc patients with mild to severe lung disease and evidence of active skin disease, as well as elevated inflammatory markers.
“This was a very specific patient population who was studied in the focuSSced clinical trial, and this paper only evaluated a subset of these patients,” Dr. Chung said. “The results may not be generalizable to all SSc-ILD patients and further studies are needed.”
The authors suggested that the patients with progressive skin disease and elevated acute phase reactants may represent a group in the immunoinflammatory phase of the disease rather than the advanced fibrotic stage, and that this might be a “window of therapeutic opportunity to preserve lung function.”
Dr. Chung noted that the radiographic improvement induced by tocilizumab treatment was greatest in those with the most radiographic disease at baseline.
“This may reflect tocilizumab’s impact on decreasing inflammation, but we are not provided the data on the effects of tocilizumab on the individual components of the QILD [quantitative ILD: summation of ground glass opacities, honeycombing, and fibrotic reticulation],” she said.
The study’s authors also made a point about the utility of screening patients with high-resolution chest CT to detect early signs of ILD.
“Our data demonstrate the value of obtaining HRCT at the time of diagnosis: PFTs [pulmonary function tests] are not sensitive enough to accurately assess the presence of ILD and delays in treatment initiation may lead to irreversible disease,” they wrote.
Describing the results as ‘hypothesis-generating’ owing to the post hoc nature of the analysis, the authors said that FVC was an indirect measure of the flow-resistive properties of the lung, and that other aspects of SSc – such as hide-bound chest thickness – could cause thoracic restriction.
Two authors were funded by the National Institutes of Health. Six authors declared grants, funding, and other support from the pharmaceutical sector, including Roche, which sponsored the original focuSSced trial.
Treatment with tocilizumab (Actemra) could stabilize or improve lung function in people with early interstitial lung disease associated with systemic sclerosis (SSc-ILD), a new study has found.
A paper published online Feb. 3 in Arthritis & Rheumatology presents the results of a post hoc analysis of data from a phase 3, placebo-controlled, double-blind trial of subcutaneous tocilizumab in patients with SSc and progressive skin disease, which included high-resolution chest CT to assess lung involvement and fibrosis.
Tocilizumab is a monoclonal antibody that targets interleukin-6 and is currently approved for the treatment of immune-mediated diseases such as rheumatoid arthritis, giant cell arteritis, cytokine release syndrome, and systemic and polyarticular course juvenile idiopathic arthritis.
Two previous studies of tocilizumab in patients with early, diffuse cutaneous SSc had also found that the treatment was associated with preservation of lung function but did not characterize that effect using radiography.
Of the 210 participants in the trial, called focuSSced, 136 were found to have interstitial lung disease at baseline and were randomized to 162 mg tocilizumab weekly or placebo for 48 weeks.
At baseline, around three-quarters of those with interstitial lung disease had moderate to severe lung involvement, defined as ground glass opacities, honeycombing, and fibrotic reticulation across at least 20% of the whole lung.
Those in the tocilizumab group showed a 0.1% mean decline in forced vital capacity (FVC) over the 48-week study, while those in the placebo group had a mean decline of 6.3%.
When stratified by severity of lung involvement, those with mild lung disease group treated with tocilizumab had a 4.1% decline in FVC, compared with a 10% decline in the placebo group; those with moderate disease in the treatment group had an 0.7% mean increase in FVC, compared with a 5.7% decrease in the placebo group, and those with severe lung involvement in the treatment arm had a 2.1% increase in FVC, compared with a 6.7% decrease in the placebo arm.
Those treated with tocilizumab also showed a statistically significant 1.8% improvement in the amount of lung involvement, which was largely seen in those with more extensive lung involvement at baseline. Those with more than 20% of the lung affected had a significant 4.9% reduction in lung area affected, while those in the placebo arm showed a significant increase in fibrosis.
First author David Roofeh, MD, of the University of Michigan Scleroderma Program, and colleagues wrote that most patients with SSc will develop interstitial lung disease – particularly those with early, diffuse cutaneous SSc and elevated markers such as C-reactive protein.
“Patients with these high-risk features, especially those with disease in the initial phase of development, represent an important target for early intervention as ILD is largely irreversible in SSc,” the authors wrote.
Findings from a specific patient population may not be generalizable
Commenting on the findings, Lorinda Chung, MD, of Stanford (Calif.) University, said in an interview that the study demonstrated that tocilizumab could prevent radiographic progression of ILD in early diffuse SSc patients with mild to severe lung disease and evidence of active skin disease, as well as elevated inflammatory markers.
“This was a very specific patient population who was studied in the focuSSced clinical trial, and this paper only evaluated a subset of these patients,” Dr. Chung said. “The results may not be generalizable to all SSc-ILD patients and further studies are needed.”
The authors suggested that the patients with progressive skin disease and elevated acute phase reactants may represent a group in the immunoinflammatory phase of the disease rather than the advanced fibrotic stage, and that this might be a “window of therapeutic opportunity to preserve lung function.”
Dr. Chung noted that the radiographic improvement induced by tocilizumab treatment was greatest in those with the most radiographic disease at baseline.
“This may reflect tocilizumab’s impact on decreasing inflammation, but we are not provided the data on the effects of tocilizumab on the individual components of the QILD [quantitative ILD: summation of ground glass opacities, honeycombing, and fibrotic reticulation],” she said.
The study’s authors also made a point about the utility of screening patients with high-resolution chest CT to detect early signs of ILD.
“Our data demonstrate the value of obtaining HRCT at the time of diagnosis: PFTs [pulmonary function tests] are not sensitive enough to accurately assess the presence of ILD and delays in treatment initiation may lead to irreversible disease,” they wrote.
Describing the results as ‘hypothesis-generating’ owing to the post hoc nature of the analysis, the authors said that FVC was an indirect measure of the flow-resistive properties of the lung, and that other aspects of SSc – such as hide-bound chest thickness – could cause thoracic restriction.
Two authors were funded by the National Institutes of Health. Six authors declared grants, funding, and other support from the pharmaceutical sector, including Roche, which sponsored the original focuSSced trial.
FROM ARTHRITIS & RHEUMATOLOGY
Prognostic gene signature identifies high- vs. low-risk DLBCL patients
according to the results of a database analysis.
A total of 33 genes formed the signature that could be transformed into a risk score, according to a study by Santosh Khanal, a senior bioinformatics scientist at Children’s Mercy Kansas City (Mo.), and colleagues published in Cancer Genetics.
Their study used gene expression and clinical parameters from the Lymphoma/Leukemia Molecular Profiling Project from 233 patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy to identify genes whose expression was associated with overall survival (OS). They refined the information to develop prognostic gene signature that could be used to calculate risk scores for each individual and predict OS.
Significant separation
The researchers initially found 61 genes individually associated with OS that had a nonadjusted P ≤ .001 using the univariate Cox regression model. The 61 genes were then assessed using multivariate Cox analysis to identify a minimal set of genes that could predict OS, resulting in a minimal set of 33 genes that were used to develop a survival risk score for each individual.
The OS of the high-risk group was significantly reduced, compared with the low-risk group (hazard ratio, 0.046; P < .0001). Upon stratification of individuals by risk score into quartiles, patients in the lowest quartile risk score had a 100% probability of survival, while individuals in the highest quartile had a 9.2% OS by year 5.
In order to validate their results, the researchers calculated risk scores using their prognostic gene set in three additional published DLBCL studies. For all three studies, individuals with low risk score had significantly better OS, “indicating the robustness of the gene signature for multiple external datasets,” according to the researchers.
The top biological pathways and processes that were significantly overrepresented in the 33-gene set were the thioester biosynthetic process (P = .00005), cellular response to hormone stimulus (P = .002), G protein–coupled receptor ligand binding (P = .003), and myeloid cell activation involved in immune responses (P = 0.006).
“As new therapies for lymphoma become available, including new immunotherapies and personalized medicine approaches such as [chimeric antigen receptor] T cells, it will be important to identify candidate individuals that are at high risk and may benefit from experimental therapeutic approaches compared with individuals who will have lower risk of death with current therapies,” the researchers concluded.
The authors reported that they had no competing interests.
according to the results of a database analysis.
A total of 33 genes formed the signature that could be transformed into a risk score, according to a study by Santosh Khanal, a senior bioinformatics scientist at Children’s Mercy Kansas City (Mo.), and colleagues published in Cancer Genetics.
Their study used gene expression and clinical parameters from the Lymphoma/Leukemia Molecular Profiling Project from 233 patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy to identify genes whose expression was associated with overall survival (OS). They refined the information to develop prognostic gene signature that could be used to calculate risk scores for each individual and predict OS.
Significant separation
The researchers initially found 61 genes individually associated with OS that had a nonadjusted P ≤ .001 using the univariate Cox regression model. The 61 genes were then assessed using multivariate Cox analysis to identify a minimal set of genes that could predict OS, resulting in a minimal set of 33 genes that were used to develop a survival risk score for each individual.
The OS of the high-risk group was significantly reduced, compared with the low-risk group (hazard ratio, 0.046; P < .0001). Upon stratification of individuals by risk score into quartiles, patients in the lowest quartile risk score had a 100% probability of survival, while individuals in the highest quartile had a 9.2% OS by year 5.
In order to validate their results, the researchers calculated risk scores using their prognostic gene set in three additional published DLBCL studies. For all three studies, individuals with low risk score had significantly better OS, “indicating the robustness of the gene signature for multiple external datasets,” according to the researchers.
The top biological pathways and processes that were significantly overrepresented in the 33-gene set were the thioester biosynthetic process (P = .00005), cellular response to hormone stimulus (P = .002), G protein–coupled receptor ligand binding (P = .003), and myeloid cell activation involved in immune responses (P = 0.006).
“As new therapies for lymphoma become available, including new immunotherapies and personalized medicine approaches such as [chimeric antigen receptor] T cells, it will be important to identify candidate individuals that are at high risk and may benefit from experimental therapeutic approaches compared with individuals who will have lower risk of death with current therapies,” the researchers concluded.
The authors reported that they had no competing interests.
according to the results of a database analysis.
A total of 33 genes formed the signature that could be transformed into a risk score, according to a study by Santosh Khanal, a senior bioinformatics scientist at Children’s Mercy Kansas City (Mo.), and colleagues published in Cancer Genetics.
Their study used gene expression and clinical parameters from the Lymphoma/Leukemia Molecular Profiling Project from 233 patients who received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy to identify genes whose expression was associated with overall survival (OS). They refined the information to develop prognostic gene signature that could be used to calculate risk scores for each individual and predict OS.
Significant separation
The researchers initially found 61 genes individually associated with OS that had a nonadjusted P ≤ .001 using the univariate Cox regression model. The 61 genes were then assessed using multivariate Cox analysis to identify a minimal set of genes that could predict OS, resulting in a minimal set of 33 genes that were used to develop a survival risk score for each individual.
The OS of the high-risk group was significantly reduced, compared with the low-risk group (hazard ratio, 0.046; P < .0001). Upon stratification of individuals by risk score into quartiles, patients in the lowest quartile risk score had a 100% probability of survival, while individuals in the highest quartile had a 9.2% OS by year 5.
In order to validate their results, the researchers calculated risk scores using their prognostic gene set in three additional published DLBCL studies. For all three studies, individuals with low risk score had significantly better OS, “indicating the robustness of the gene signature for multiple external datasets,” according to the researchers.
The top biological pathways and processes that were significantly overrepresented in the 33-gene set were the thioester biosynthetic process (P = .00005), cellular response to hormone stimulus (P = .002), G protein–coupled receptor ligand binding (P = .003), and myeloid cell activation involved in immune responses (P = 0.006).
“As new therapies for lymphoma become available, including new immunotherapies and personalized medicine approaches such as [chimeric antigen receptor] T cells, it will be important to identify candidate individuals that are at high risk and may benefit from experimental therapeutic approaches compared with individuals who will have lower risk of death with current therapies,” the researchers concluded.
The authors reported that they had no competing interests.
FROM CANCER GENETICS
Don’t fear patients reading their clinical notes: Opinion
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Introducing Point-Counterpoint Perspectives in the Journal of Hospital Medicine
Providing high-quality, efficient, and evidence-based healthcare is a complicated and complex process. The right approach or path forward is not always clear. In medicine, decision-making inherently involves uncertainty; evidence may be lacking, or values or context may differ, and thus, reasonable clinicians may choose to make different decisions based on the same data.
In this spirit of fostering education and healthy debate to improve understanding of challenges relevant to the field of hospital medicine, we are pleased to introduce our Point-Counterpoint series within the Perspectives in Hospital Medicine section of the journal. Point-Counterpoint Perspectives presents a debate by content experts. Each provides an interpretation of evidence regarding patient management or other controversial issues relating to hospital-based care. The format consists of an overview of the topic with an original point followed by a counterpoint response and, finally, a rebuttal (Table). We ask contributors to be as outspoken in their points and counterpoints as the evidence allows in order to fully elaborate the questions and uncertainties that may otherwise be familiar only to experts in the field or to those in other disciplines.
Our inaugural point-counterpoint articles address whether healthcare workers should receive priority for scarce drugs and therapies during the coronavirus disease 2019 (COVID-19) pandemic. The intermittent shortage of medical supplies and protective equipment has made it not only difficult but also at times dangerous for healthcare workers to care for infected patients.1 The risks of developing COVID-19 and fear of transmitting it to loved ones has led to stress, fatigue, and burnout among healthcare workers, leading some to quit and even attempt suicide. The downstream effects of this stress may adversely affect patients and exacerbate staffing challenges in an already taxed healthcare system.2 Do we have a special obligation to those on the front lines? We are grateful to Drs Kirk R Daffner, Armand Antommaria, and Ndidi I Unaka, for addressing this controversial topic.3-5
1. Lagu T, Artenstein AW, Werner RM. Fool me twice: the role for hospitals and health systems in fixing the broken PPE supply chain. J Hosp Med. 2020;15(9):570-571. https://doi.org/10.12788/jhm.3489
2. Ali SS. Why some nurses have quit during the coronavirus pandemic. NBC News. May,10, 2020. Accessed January 18, 2021. https://www.nbcnews.com/news/us-news/why-some-nurses-have-quit-during-coronavirus-pandemic-n1201796
3. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. 2021;16(3):180-181. https://doi.org/10.12788/jhm.3596
4. Antommaria A, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical resources is impractical and unjust. J Hosp Med. 2021;16(3):182-183. https://doi.org/10.12788/jhm.3597
5. Daffner KR. Rebuttal: accounting for the community’s reciprocal obligations during a pandemic. J Hosp Med. 2021;16(3):184. https://doi.org/10.12788/jhm.3600
Providing high-quality, efficient, and evidence-based healthcare is a complicated and complex process. The right approach or path forward is not always clear. In medicine, decision-making inherently involves uncertainty; evidence may be lacking, or values or context may differ, and thus, reasonable clinicians may choose to make different decisions based on the same data.
In this spirit of fostering education and healthy debate to improve understanding of challenges relevant to the field of hospital medicine, we are pleased to introduce our Point-Counterpoint series within the Perspectives in Hospital Medicine section of the journal. Point-Counterpoint Perspectives presents a debate by content experts. Each provides an interpretation of evidence regarding patient management or other controversial issues relating to hospital-based care. The format consists of an overview of the topic with an original point followed by a counterpoint response and, finally, a rebuttal (Table). We ask contributors to be as outspoken in their points and counterpoints as the evidence allows in order to fully elaborate the questions and uncertainties that may otherwise be familiar only to experts in the field or to those in other disciplines.

Our inaugural point-counterpoint articles address whether healthcare workers should receive priority for scarce drugs and therapies during the coronavirus disease 2019 (COVID-19) pandemic. The intermittent shortage of medical supplies and protective equipment has made it not only difficult but also at times dangerous for healthcare workers to care for infected patients.1 The risks of developing COVID-19 and fear of transmitting it to loved ones has led to stress, fatigue, and burnout among healthcare workers, leading some to quit and even attempt suicide. The downstream effects of this stress may adversely affect patients and exacerbate staffing challenges in an already taxed healthcare system.2 Do we have a special obligation to those on the front lines? We are grateful to Drs Kirk R Daffner, Armand Antommaria, and Ndidi I Unaka, for addressing this controversial topic.3-5
Providing high-quality, efficient, and evidence-based healthcare is a complicated and complex process. The right approach or path forward is not always clear. In medicine, decision-making inherently involves uncertainty; evidence may be lacking, or values or context may differ, and thus, reasonable clinicians may choose to make different decisions based on the same data.
In this spirit of fostering education and healthy debate to improve understanding of challenges relevant to the field of hospital medicine, we are pleased to introduce our Point-Counterpoint series within the Perspectives in Hospital Medicine section of the journal. Point-Counterpoint Perspectives presents a debate by content experts. Each provides an interpretation of evidence regarding patient management or other controversial issues relating to hospital-based care. The format consists of an overview of the topic with an original point followed by a counterpoint response and, finally, a rebuttal (Table). We ask contributors to be as outspoken in their points and counterpoints as the evidence allows in order to fully elaborate the questions and uncertainties that may otherwise be familiar only to experts in the field or to those in other disciplines.

Our inaugural point-counterpoint articles address whether healthcare workers should receive priority for scarce drugs and therapies during the coronavirus disease 2019 (COVID-19) pandemic. The intermittent shortage of medical supplies and protective equipment has made it not only difficult but also at times dangerous for healthcare workers to care for infected patients.1 The risks of developing COVID-19 and fear of transmitting it to loved ones has led to stress, fatigue, and burnout among healthcare workers, leading some to quit and even attempt suicide. The downstream effects of this stress may adversely affect patients and exacerbate staffing challenges in an already taxed healthcare system.2 Do we have a special obligation to those on the front lines? We are grateful to Drs Kirk R Daffner, Armand Antommaria, and Ndidi I Unaka, for addressing this controversial topic.3-5
1. Lagu T, Artenstein AW, Werner RM. Fool me twice: the role for hospitals and health systems in fixing the broken PPE supply chain. J Hosp Med. 2020;15(9):570-571. https://doi.org/10.12788/jhm.3489
2. Ali SS. Why some nurses have quit during the coronavirus pandemic. NBC News. May,10, 2020. Accessed January 18, 2021. https://www.nbcnews.com/news/us-news/why-some-nurses-have-quit-during-coronavirus-pandemic-n1201796
3. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. 2021;16(3):180-181. https://doi.org/10.12788/jhm.3596
4. Antommaria A, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical resources is impractical and unjust. J Hosp Med. 2021;16(3):182-183. https://doi.org/10.12788/jhm.3597
5. Daffner KR. Rebuttal: accounting for the community’s reciprocal obligations during a pandemic. J Hosp Med. 2021;16(3):184. https://doi.org/10.12788/jhm.3600
1. Lagu T, Artenstein AW, Werner RM. Fool me twice: the role for hospitals and health systems in fixing the broken PPE supply chain. J Hosp Med. 2020;15(9):570-571. https://doi.org/10.12788/jhm.3489
2. Ali SS. Why some nurses have quit during the coronavirus pandemic. NBC News. May,10, 2020. Accessed January 18, 2021. https://www.nbcnews.com/news/us-news/why-some-nurses-have-quit-during-coronavirus-pandemic-n1201796
3. Daffner KR. Point: healthcare providers should receive treatment priority during a pandemic. J Hosp Med. 2021;16(3):180-181. https://doi.org/10.12788/jhm.3596
4. Antommaria A, Unaka NI. Counterpoint: prioritizing healthcare workers for scarce critical resources is impractical and unjust. J Hosp Med. 2021;16(3):182-183. https://doi.org/10.12788/jhm.3597
5. Daffner KR. Rebuttal: accounting for the community’s reciprocal obligations during a pandemic. J Hosp Med. 2021;16(3):184. https://doi.org/10.12788/jhm.3600
© 2021 Society of Hospital Medicine
Readmissions Following Hospitalization for Infection in Children With or Without Medical Complexity
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.
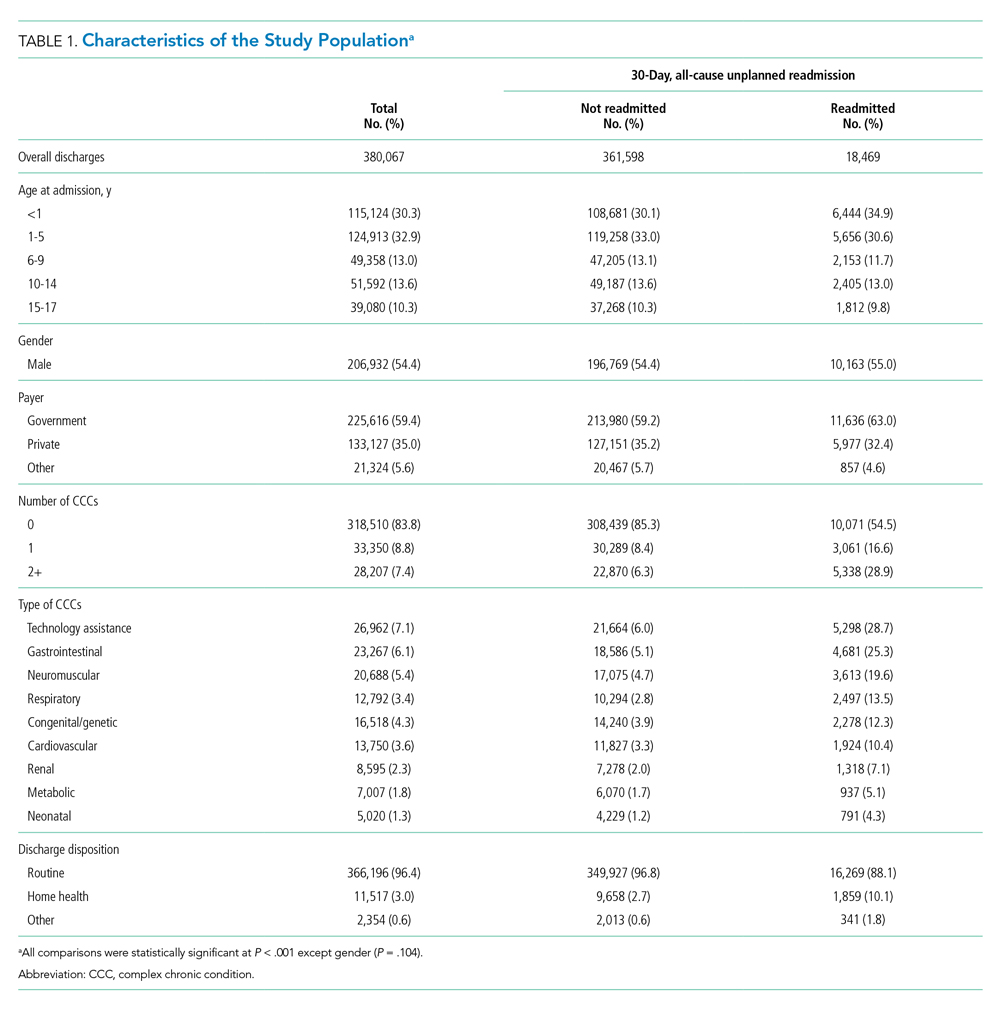
Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.
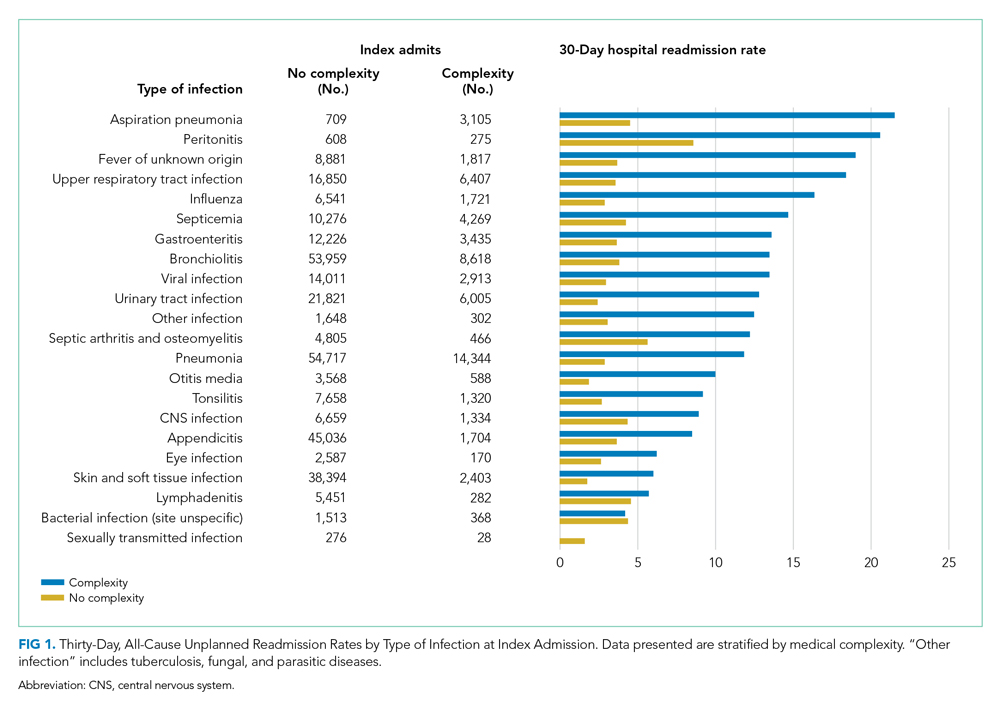
Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.
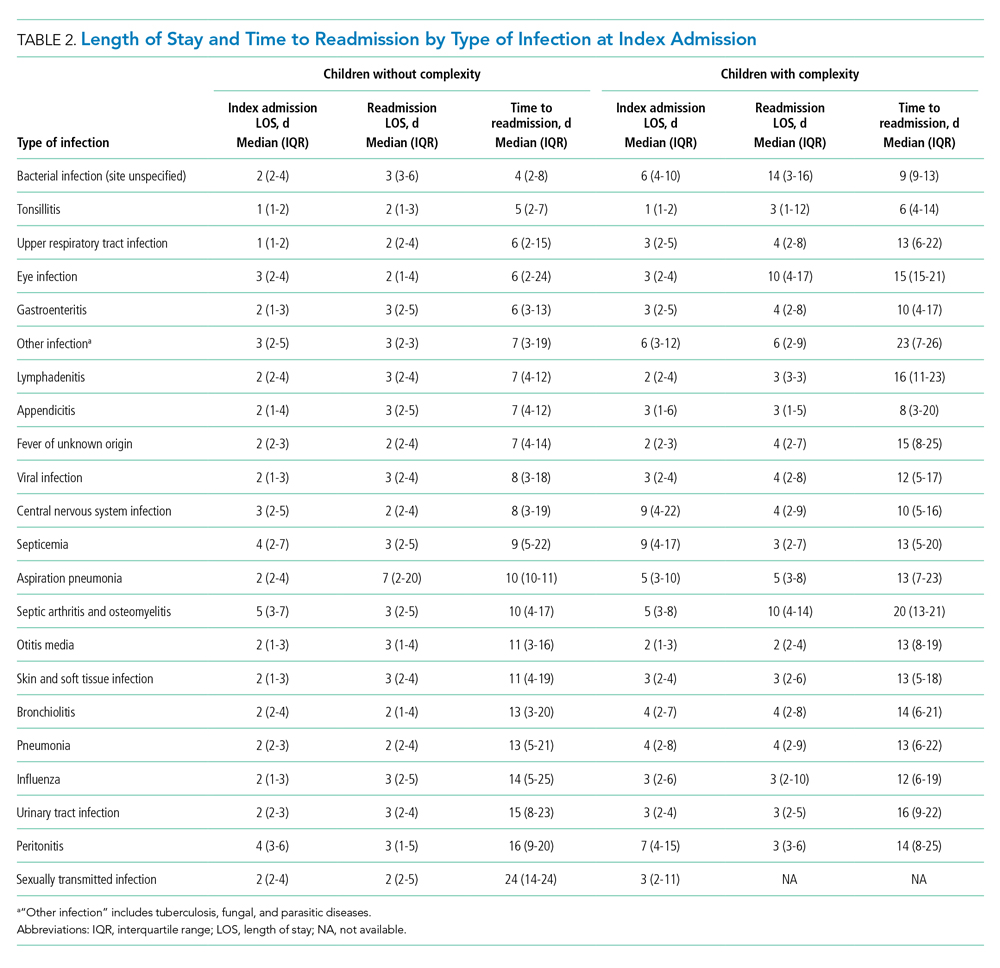
Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.
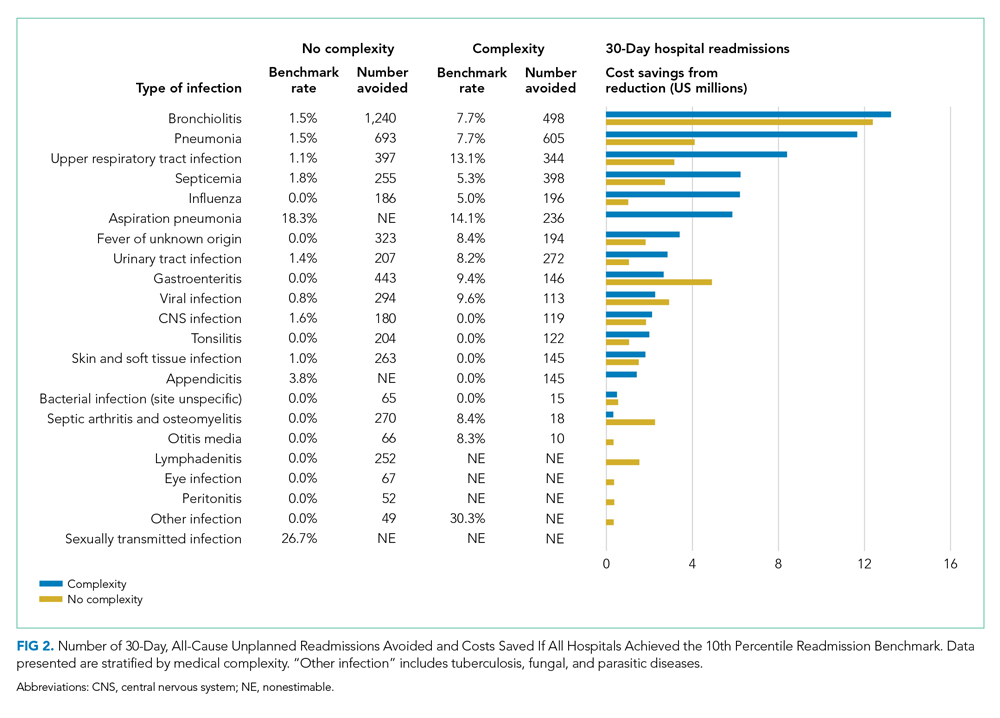
Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.

Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.

Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.

Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.

Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
Hospitalizations for infections are common in children, with respiratory illnesses, including pneumonia and bronchiolitis, among the most prevalent indications for hospitalization.1,2 Infections are also among the most frequent indications for all-cause readmissions and for potentially preventable readmissions in children.3-5 Beyond hospital resource use, infection hospitalizations and readmissions represent a considerable cause of life disruption for patients and their families.6,7 While emerging evidence supports shortened durations of parenteral antibiotics before transitioning to oral therapy for some infections (eg, pyelonephritis, osteomyelitis),8-10 other infections may require extended treatment courses for weeks. The risk of adverse outcomes (eg, complications of medical treatment, readmission risk) and burdens placed on patients and their families may therefore differ across infection types and extend well beyond the immediate hospitalization.
Although infections are common and pediatric providers are expected to have proficiency in managing infections, substantial variation in the management of common pediatric infections exists and is associated with adverse hospitalization outcomes, including increased readmission risk and healthcare costs.11-18 Potentially avoidable resource use associated with hospital readmission from infection has led to adoption of hospital-level readmission metrics as indicators of the quality of healthcare delivery. For example, the Pediatric Quality Measures Program, established by the Children’s Health Insurance Program Reauthorization Act of 2009, has prioritized measurement of readmissions following hospitalization for lower respiratory tract infection.19 With government agencies increasingly using readmission metrics to assess quality of healthcare delivery, developing metrics that focus on these resource-intensive conditions is essential.
Because infections are a common and costly indication for hospital resource use and because substantial variation in management has been observed, promoting a broader understanding of infection-specific readmission rates is important for prioritizing readmission-reduction opportunities in children. This study’s objectives were the following: (1) to describe the prevalence and characteristics of infection hospitalizations in children and their associated readmissions and (2) to estimate the number of readmissions avoided and costs saved if all hospitals achieved the 10th percentile of the hospitals’ risk-adjusted readmission rate (ie, readmission benchmark).
METHODS
Study Design and Data Source
We performed a retrospective cohort analysis using the 2014 Agency for Healthcare Research and Quality (AHRQ) Nationwide Readmissions Database (NRD).20 The 2014 NRD is an administrative database that contains information on inpatient stays from January 1, 2014, to December 31, 2014, for all payers and allows for weighted national estimates of readmissions for all US individuals. Data within NRD are aggregated from 22 geographically diverse states representing approximately one-half of the US population. NRD contains deidentified patient-level data with unique verified patient identifiers to track individuals within and across hospitals in a state. However, AHRQ guidelines specify that NRD cannot be used for reporting hospital-specific readmission rates. Thus, for the current study, the Inpatient Essentials (Children’s Hospital Association), or IE, database was used to measure hospital-level readmission rates and to distinguish benchmark readmission rates for individual infection diagnoses.21 The IE database is composed of 90 children’s hospitals distributed throughout all regions of the United States. The inclusion of free-standing children’s hospitals and children’s hospitals within adult hospitals allows for comparisons and benchmarking across hospitals on multiple metrics, including readmissions.
Study Population
Children 0 to 17 years of age with a primary diagnosis at the index admission for infection between January 1, 2014, and November 30, 2014, were included. The end date of November 30, 2014, allowed for a full 30-day readmission window for all index admissions. We excluded index admissions that resulted in transfer to another acute care hospital or in-hospital mortality. Additionally, we excluded index admissions of children who had hematologic or immunologic conditions, malignancy, or history of bone marrow and solid-organ transplant, using the classification system for complex chronic conditions (CCCs) from Feudtner et al.22 Due to the high likelihood of immunosuppression in patients with these conditions, children may have nuanced experiences with illness severity, trajectory, and treatment associated with infection that place them at increased risk for nonpreventable readmission.
Main Exposure
The main exposure was infection type during the index admission. Condition-specific index admissions were identified using AHRQ’s Clinical Classifications Software (CCS) categories.23 CCS is a classification schema that categorizes the greater than 14,000 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes and 3,900 ICD-9-CM procedure codes into clinically meaningful categories of 295 diagnosis (including mental health codes and E-codes) and 231 procedural groupings. Twenty-two groupings indicative of infection were distinguished and used for the current study. Examples of infections included aspiration pneumonia, pneumonia, bronchiolitis, and sexually transmitted infection. We combined related CCS categories when possible for ease of interpretation and presentation of data (Appendix Table 1).
Main Outcome Measure
The main outcome measure was 30-day hospital readmission. Readmission was defined as all-cause, unplanned admission within 30 days following discharge from a preceding hospitalization. Planned hospital readmissions were identified and excluded using methods from AHRQ’s Pediatric All-Condition Readmission Measure.24 We defined a same-cause return as a return with the same CCS infection category as the index admission. Costs associated with readmissions were estimated from charges using hospital-specific cost-to-charge ratios provided with NRD.
Patient Demographic and Clinical Characteristics
Patient demographic characteristics included age at index admission (<1 year, 1-5 years, 6-9 years, 10-14 years, and 15-18 years), sex, payer (ie, government, private, other), and discharge disposition (ie, routine, home health, other). We assessed all patients for medical complexity, as defined by the presence of at least one CCC, and we reported the categories of CCCs by organ system involved.22 Otherwise, patients were identified as without medical complexity.
Statistical Analysis
We summarized continuous variables with medians and interquartile ranges and categorical variables with frequencies and percentages. To develop benchmark readmission rates for each infection type, we used generalized linear mixed models with random intercepts for each hospital in the IE database. For each infection type, the benchmark readmission rate was defined as the 10th percentile of hospitals’ risk-adjusted readmission rates. The 10th percentile was chosen to identify the best performing 10% of hospitals (ie, hospitals with the lowest readmission rates). Because children with medical complexity account for a large proportion of hospital resource use and are at high risk for readmission,4,25 we developed benchmarks stratified by presence/absence of a CCC (ie, with complexity vs without complexity). Models were adjusted for severity of illness using the Hospitalization Resource Intensity Score for Kids (H-RISK),26 a scoring system that assigns relative weights for each All Patient Refined Diagnosis-Related Group (3M Corporation) and severity of illness level, and each hospital’s risk-adjusted readmission rate was determined.
With use of weights to achieve national estimates of index admissions and readmissions, we determined the number of potentially avoidable readmissions by calculating the number of readmissions observed in the NRD that would not occur if all hospitals achieved readmission rates equal to the 10th percentile. Avoidable costs were estimated by multiplying the number of potentially avoidable readmissions by the mean cost of a readmission for infections of that type. Estimates of avoidable readmissions and costs were stratified by medical complexity. In addition to describing estimates at the 10th percentile benchmark, we similarly developed estimates of potentially avoidable readmissions and avoidable costs for the 5th and 25th percentiles, which are presented within Appendix Table 2 (children without complexity) and Appendix Table 3 (children with complexity).
All statistical analyses were performed using SAS version 9.4 (SAS Institute), and P values <.001 were considered statistically significant due to the large sample size. The Office of Research Integrity at Children’s Mercy Hospital deemed this study exempt from institutional board review.
RESULTS
Characteristics of the Study Population
The study included 380,067 index admissions for infection and an accompanying 18,469 unplanned all-cause readmissions over the 30 days following discharge (readmission rate, 4.9%; Table 1). Children ages 1 to 5 years accounted for the largest percentage (32.9%) of index hospitalizations, followed by infants younger than 1 year (30.3%). The readmission rate by age group was highest for infants younger than 1 year, compared with rates among all other age groups (5.6% among infants < 1 year vs 4.4%-4.7% for other age groups; P < .001). In the overall cohort, 16.2% of admissions included patients with a CCC. Children with medical complexity had higher readmission rates than those without medical complexity (no CCC, 3.2%; 1 CCC, 9.2%; 2+ CCCs, 18.9%). A greater percentage of children experiencing a readmission had government insurance (63.0% vs 59.2%; P < .001) and received home health nursing (10.1% vs 2.7%; P < .001) following the index encounter.

Children Without Complexity
Index Admissions and 30-day Readmissions
Among patients without medical complexity, index admissions occurred most frequently for pneumonia (n = 54,717), bronchiolitis (n = 53,959), and appendicitis (n = 45,036) (Figure 1). The median length of stay (LOS) for index admissions ranged from 1 to 5 days (Table 2), with septic arthritis and osteomyelitis having the longest median LOS at 5 (IQR, 3-7) days.

Thirty-day readmission rates varied substantially by infection at the index admission (range, 1.5% for sexually transmitted infection to 8.6% for peritonitis) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 7 days (Table 2), while the median number of days to readmission varied substantially by infection type (range, 4 days for bacterial infection [site unspecified] to 24 days for sexually transmitted infections). Among the top five indications for admission for children without complexity, 14.9% to 52.8% of readmissions were for the same cause as the index admission; however, many additional returns were likely related to the index admission (Appendix Table 4). For example, among other return reasons, an additional 992 (61.7%) readmissions following appendicitis hospitalizations were for complications of surgical procedures or medical care, peritonitis, intestinal obstruction, and abdominal pain.

Impact of Achieving Readmission Benchmarks
Among children without complexity, readmission benchmarks (ie, the 10th percentile of readmission rates across hospitals) ranged from 0% to 26.7% (Figure 2). An estimated 54.7% of readmissions (n = 5,507) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $44.5 million in savings. Pneumonia, bronchiolitis, gastroenteritis, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if a 10th percentile benchmark was achieved.

Children With Medical Complexity
Index Admissions and 30-day Readmissions
Among patients with complexity, index admissions occurred most frequently for pneumonia (n = 14,344), bronchiolitis (n = 8,618), and upper respiratory tract infection (n = 6,407) (Figure 1). The median LOS for index admissions ranged from 1 to 9 days (Table 2), with septicemia and CNS infections having the longest median LOS at 9 days.
Thirty-day readmission rates varied substantially by the type of infection at the index admission (range, 0% for sexually transmitted infection to 21.6% for aspiration pneumonia) (Figure 1). The median LOS for 30-day readmissions varied from 2 to 14 days (Table 2), and the median number of days to readmission varied substantially by infection type (range, 6 days for tonsillitis to 23 days for other infection). Among the top five indications for admission for medically complex children, 8% to 40.4% of readmissions were for the same cause as the index admission (Appendix Table 4). As with the children without complexity, additional returns were likely related to the index admission.
Impact of Achieving Readmission Benchmarks
Among children with medical complexity, readmission benchmarks ranged from 0% to 30.3% (Figure 2). An estimated 42.6% of readmissions (n = 3,576) could potentially be reduced if hospitals achieved infection-specific benchmark readmission rates, which could result in an estimated $70.8 million in savings. Pneumonia, bronchiolitis, septicemia, and upper respiratory tract infections were among conditions with the greatest potential reductions in readmissions and costs if the benchmarks were achieved.
DISCUSSION
The current study uncovered new findings regarding unplanned readmissions following index infection hospitalizations for children. In particular, readmission rates and time to readmission varied substantially by infection subtype. The study also identified priority infections and unique considerations for children with CCCs, all of which may help maximize the value of readmission metrics aimed at advancing hospital quality and reducing costs for infection hospitalizations in children. If all hospitals achieved the readmission rates of the best performing hospitals, 42.6% to 54.7% fewer readmissions could be realized with associated cost savings.
Nationally, studies have reported 30-day, all-cause unplanned readmission rates of 6.2% to 10.3%.5,27 In our current study we observed an overall readmission rate of 4.9% across all infectious conditions; however, readmission rates varied substantially by condition, with upper and lower respiratory tract infections, septicemia, and gastroenteritis among infections with the greatest number of potentially avoidable readmissions based on achievement of readmission benchmarks. Currently, pediatric-specific all-cause and lower respiratory tract infection readmission metrics have been developed with the aim of improving quality of care and reducing healthcare expenditures.28 Future readmission studies and metrics may prioritize conditions such as septicemia, gastroenteritis, and other respiratory tract infections. Our current study demonstrated that many readmissions following infection hospitalizations were associated with the same CCS category or within a related CCS category (eg, complications of surgical procedures or medical care, appendicitis, peritonitis, intestinal obstruction, and abdominal pain constituted the top five indications for readmission for appendicitis, whereas respiratory illnesses constituted the top five indications for readmissions for pneumonia). While this current study cannot clarify this relationship further, and the “avoidability” of unplanned readmissions is debated,29-31 our findings suggest that future investigations might focus on identifying whether condition-specific interventions during the index admission could mitigate some readmissions.
While the LOS of the index admission and the readmission were similar for most infection subtypes, we observed substantial variability in the temporal risk for readmission by infection subtype. Our observations complement studies exploring the timing of readmissions for other pediatric conditions.32-34 In particular, our findings further highlight that the composition and interaction of factors influencing infection readmissions may vary by condition. Infections represent a diverse group of conditions, with treatment courses that vary in need for parenteral antibiotics, ability to tailor treatment based on organism and susceptibilities, and length of treatment. While treatment for some infections may be accomplished, or nearly accomplished, prior to discharge, other infections (eg, osteomyelitis) may require prolonged treatment, shifting the burden of management and monitoring to patients and their families, which along with the timeliness and adequacy of outpatient follow-up, may modify an individual’s readmission risk. Consequently, a “one-size fits all” approach to discharge counseling may not be successful across all conditions. Our study lays the groundwork for how these temporal relationships may be used by clinicians to counsel families regarding the likely readmission timeframe, based on infection, and to establish follow-up appointments ahead of the infection-specific “readmission window,” which may allow outpatient providers to intervene when readmission risk is greatest.
Consistent with prior literature, children with medical complexity in our study had increased frequency of 30-day, all-cause unplanned readmissions and LOS, compared with peers who did not have complexity.5 Readmission rates following hospitalizations for aspiration pneumonia were comparable to those reported by Thompson et al in their study examining rates of pneumonia in children with neurologic impairment.35 In our current study, nearly 96% of readmissions following aspiration pneumonia hospitalizations were for children with medical complexity, and more than 58% of these readmissions were for aspiration pneumonia or respiratory illness. Future investigations should seek to explore factors contributing to readmissions in children with medical complexity and to evaluate whether interventions such as postdischarge coaching or discharge bundles could assist with reductions in healthcare resource use.36,37
Despite a lack of clear association between readmissions and quality of care for children,38 readmissions rates continue to be used as a quality measure for hospitalized patients. Within our present study, we found that achieving benchmark readmission rates for infection hospitalizations could lead to substantial reductions in readmissions; however, these reductions were seen across this relatively similar group of infection diagnoses, and hospitals may face greater challenges when attempting to achieve a 10th percentile readmission benchmark across a more expansive set of diagnoses. Despite these challenges, understanding the intricacies of readmissions may lead to improved readmission metrics and the systematic identification of avoidable readmissions, the goal of which is to enhance the quality of healthcare for hospitalized children.
Our findings should be interpreted in the context of several limitations. We defined our readmission benchmark at the 10th percentile using the IE database. Because an estimated 70% of hospitalizations for children occur within general hospitals,39 we believe that our use of the IE database allowed for increased generalizability, though the broadening of our findings to nonacademic hospital settings may be less reliable. While we describe the 10th percentile readmission benchmark here, alternative benchmarks would result in different estimates of avoidable readmissions and associated readmission costs (Appendix Tables 2 and 3). The IE and NRD databases do not distinguish intensive care use. We tried to address this limitation through model adjustments using H-RISK, which is particularly helpful for adjusting for NICU admissions through use of the 27 All Patient Refined Diagnosis-Related Groups for neonatal conditions. Additionally, the NRD uses state-level data to derive national estimates and is not equipped to measure readmissions to hospitals in a different state, distinguish patient deaths occurring after discharge, or to examine the specific indication for readmission (ie, unable to assess if the readmission is related to a complication of the treatment plan, progression of the illness course, or for an unrelated reason). While sociodemographic and socioeconomic factors may affect readmissions, the NRD does not contain information on patients’ race/ethnicity, family/social attributes, or postdischarge follow-up health services, which are known to influence the need for readmission.
Despite these limitations, this study highlights future areas for research and potential opportunities for reducing readmission following infection hospitalizations. First, identifying hospital- and systems-based factors that contribute to readmission reductions at the best-performing hospitals may identify opportunities for hospitals with the highest readmission rates to achieve the rates of the best-performing hospitals. Second, conditions such as upper and lower respiratory tract infections, along with septicemia and gastroenteritis, may serve as prime targets for future investigation based on the estimated number of avoidable readmissions and potential cost savings associated with these conditions. Finally, future investigations that explore discharge counseling and follow-up tailored to the infection-specific temporal risk and patient complexity may identify opportunities for further readmission reductions.
CONCLUSION
Our study provides a broad look at readmissions following infection hospitalizations and highlights substantial variation in readmissions based on infection type. To improve hospital resource use for infections, future preventive measures could prioritize children with complex chronic conditions and those with specific diagnoses (eg, upper and lower respiratory tract infections).
Disclaimer
This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, NIH or the US government.
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
1. Keren R, Luan X, Localio R, et al; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155-1164. https://doi.org/10.1001/archpediatrics.2012.1266
2. Van Horne B, Netherton E, Helton J, Fu M, Greeley C. The scope and trends of pediatric hospitalizations in Texas, 2004-2010. Hosp Pediatr. 2015;5(7):390-398. https://doi.org/10.1542/hpeds.2014-0105
3. Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100-109. https://doi.org/10.1542/peds.2014-0331
4. Gay JC, Hain PD, Grantham JA, Saville BR. Epidemiology of 15-day readmissions to a children’s hospital. Pediatrics. 2011;127(6):e1505-e1512. https://doi.org/10.1542/peds.2010-1737
5. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351
6. Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics. 2006;118(suppl 3):S203-S218. https://doi.org/10.1542/peds.2006-0951b
7. Rennick JE, Johnston CC, Dougherty G, Platt R, Ritchie JA. Children’s psychological responses after critical illness and exposure to invasive technology. J Dev Behav Pediatr. 2002;23(3):133-144. https://doi.org/10.1097/00004703-200206000-00002
8. Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev. 2003;(1):CD003966. https://doi.org/10.1002/14651858.cd003966
9. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. 2014;33(2):136-142. https://doi.org/10.1097/inf.0000000000000023
10. Keren R, Shah SS, Srivastava R, et al; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
11. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McCulloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
12. Neubauer HC, Hall M, Wallace SS, Cruz AT, Queen MA, Foradori DM, Aronson PL, Markham JL, Nead JA, Hester GZ, McCulloh RJ, Lopez MA. Variation in diagnostic test use and associated outcomes in staphylococcal scalded skin syndrome at children’s hospitals. Hosp Pediatr. 2018;8(9):530-537. https://doi.org/10.1542/hpeds.2018-0032
13. Aronson PL, Thurm C, Alpern ER, et al; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382
14. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. https://doi.org/10.1542/peds.2013-0179
15. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/inf.0b013e31825f2b10
16. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062
17. Knapp JF, Simon SD, Sharma V. Variation and trends in ED use of radiographs for asthma, bronchiolitis, and croup in children. Pediatrics. 2013;132(2):245-252. https://doi.org/10.1542/peds.2012-2830
18. Rice-Townsend S, Barnes JN, Hall M, Baxter JL, Rangel SJ. Variation in practice and resource utilization associated with the diagnosis and management of appendicitis at freestanding children’s hospitals: implications for value-based comparative analysis. Ann Surg. 2014;259(6):1228-1234. https://doi.org/10.1097/SLA.0000000000000246
19. Pediatric Quality Measures Program (PQMP). Agency for Healthcare Research and Quality. Accessed March 1, 2019. https://www.ahrq.gov/pqmp/index.html
20. NRD Database Documentation. Accessed June 1, 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp
21. Inpatient Essentials. Children’s Hospitals Association. Accessed August 1, 2018. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Inpatient-Essentials
22. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
23. Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project. March 2017. Accessed August 2, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
24. NQF: Quality Positioning System. National Quality Forum. Accessed September 3, 2018. http://www.qualityforum.org/QPS/QPSTool.aspx
25. Berry JG, Ash AS, Cohen E, Hasan F, Feudtner C, Hall M. Contributions of children with multiple chronic conditions to pediatric hospitalizations in the United States: a retrospective cohort analysis. Hosp Pediatr. 2017;7(7):365-372. https://doi.org/10.1542/hpeds.2016-0179
26. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of Hospitalization Resource Intensity Scores for Kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
27. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e122. https://doi.org/10.1016/j.jpeds.2015.11.051
28. NQF: Pediatric Measures Final Report. National Quality Forum. June 2016. Accessed January 24, 2019. https://www.qualityforum.org/Publications/2016/06/Pediatric_Measures_Final_Report.aspx
29. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
30. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
31. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
32. Bucholz EM, Gay JC, Hall M, Harris M, Berry JG. Timing and causes of common pediatric readmissions. J Pediatr. 2018;200:240-248.e1. https://doi.org/10.1016/j.jpeds.2018.04.044
33. Morse RB, Hall M, Fieldston ES, et al. Children’s hospitals with shorter lengths of stay do not have higher readmission rates. J Pediatr. 2013;163(4):1034-8.e1. https://doi.org/10.1016/j.jpeds.2013.03.083
34. Kenyon CC, Melvin PR, Chiang VW, Elliott MN, Schuster MA, Berry JG. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003
35. Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. https://doi.org/10.1542/peds.2015-1612
36. Coller RJ, Klitzner TS, Lerner CF, et al. Complex Care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics. 2018;142(2):e20174278. https://doi.org/10.1542/peds.2017-4278
37. Stephens JR, Kimple KS, Steiner MJ, Berry JG. Discharge interventions and modifiable risk factors for preventing hospital readmissions in children with medical complexity. Rev Recent Clin Trials. 2017;12(4):290-297. https://doi.org/10.2174/1574887112666170816144455
38. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
39. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
© 2021 Society of Hospital Medicine