User login
Female cardiac advantage essentially lost after MI
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
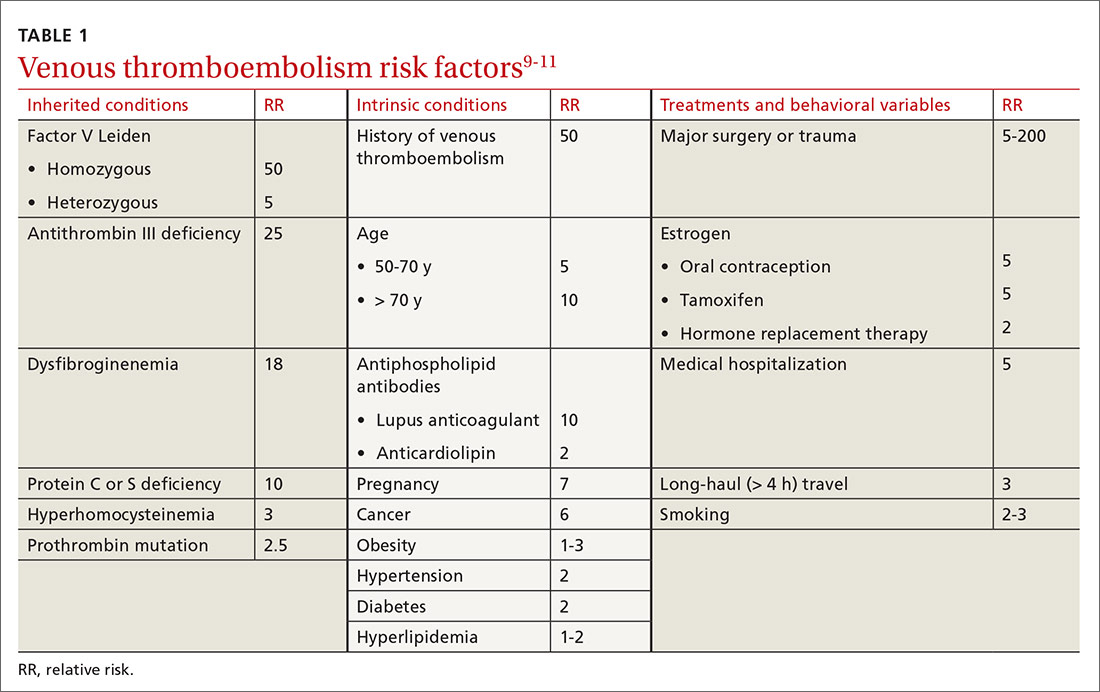
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Biomarkers for Disease Activity in RRMS Reported at ACTRIMS/ECTRIMS 2020
In relapsing-remitting multiple sclerosis (RRMS), MRI has provided a key indication of disease presence and activity. With the availability of serum neurofilament (sNfL) assays, disease activity can be correlated with sNfL levels.
Dr Tobias Derfuss, from University Hospital Basel in Basel, Switzerland, discusses emerging research reported at the ACTRIMS/ECTRIMS 2020 Virtual Meeting, focusing on the use of sNfL as a biomarker for monitoring treatment response and disease activity in RRMS.
Dr Derfuss highlights one study in which longitudinal observations showed that high levels of sNfL at baseline are associated with a high risk for gadolinium-enhancing lesions; the study authors suggest that quarterly monitoring may be adequate for surveillance of subclinical disease.
INFORMATION FROM INDUSTRY
Resources
Have You Seen the Head-to-Head Efficacy Data for ZEPOSIA® (ozanimod)?
Clinical Trial Safety Findings for an S1P Therapy
Discover How to Start Appropriate Patients on an S1P
US-ZEP-20-0997 10/20
In another study, higher sNfL levels at baseline were linked to a higher risk for T2 lesions and a more pronounced brain atrophy rate, but disability progression was not correlated to baseline sNfL levels.
Finally, Dr Derfuss reports on a real-world, large cohort study supporting the value of sNfL to capture and predict disability progression independent of relapses.
Tobias J. Derfuss, MD, Professor, Head of Outpatient Clinic, Department of Neurology, University Hospital Board, Basel, Switzerland
Tobias J. Derfuss, MD, has disclosed the following relevant financial relationships: Received financial compensation for his activities in advisory boards, steering committees, data safety monitoring boards, and consultation for: Novartis; Merck; Biogen; Celgene; Actelion; Mitsubishi Pharma; MedDay; Roche; Sanofi Genzyme. Received research grant from: Novartis; Biogen; Roche; Swiss National Science Foundation; European Union; Swiss MS Society. Spouse is an employee of and holds stock options in: Novartis
In relapsing-remitting multiple sclerosis (RRMS), MRI has provided a key indication of disease presence and activity. With the availability of serum neurofilament (sNfL) assays, disease activity can be correlated with sNfL levels.
Dr Tobias Derfuss, from University Hospital Basel in Basel, Switzerland, discusses emerging research reported at the ACTRIMS/ECTRIMS 2020 Virtual Meeting, focusing on the use of sNfL as a biomarker for monitoring treatment response and disease activity in RRMS.
Dr Derfuss highlights one study in which longitudinal observations showed that high levels of sNfL at baseline are associated with a high risk for gadolinium-enhancing lesions; the study authors suggest that quarterly monitoring may be adequate for surveillance of subclinical disease.
INFORMATION FROM INDUSTRY
Resources
Have You Seen the Head-to-Head Efficacy Data for ZEPOSIA® (ozanimod)?
Clinical Trial Safety Findings for an S1P Therapy
Discover How to Start Appropriate Patients on an S1P
US-ZEP-20-0997 10/20
In another study, higher sNfL levels at baseline were linked to a higher risk for T2 lesions and a more pronounced brain atrophy rate, but disability progression was not correlated to baseline sNfL levels.
Finally, Dr Derfuss reports on a real-world, large cohort study supporting the value of sNfL to capture and predict disability progression independent of relapses.
Tobias J. Derfuss, MD, Professor, Head of Outpatient Clinic, Department of Neurology, University Hospital Board, Basel, Switzerland
Tobias J. Derfuss, MD, has disclosed the following relevant financial relationships: Received financial compensation for his activities in advisory boards, steering committees, data safety monitoring boards, and consultation for: Novartis; Merck; Biogen; Celgene; Actelion; Mitsubishi Pharma; MedDay; Roche; Sanofi Genzyme. Received research grant from: Novartis; Biogen; Roche; Swiss National Science Foundation; European Union; Swiss MS Society. Spouse is an employee of and holds stock options in: Novartis
In relapsing-remitting multiple sclerosis (RRMS), MRI has provided a key indication of disease presence and activity. With the availability of serum neurofilament (sNfL) assays, disease activity can be correlated with sNfL levels.
Dr Tobias Derfuss, from University Hospital Basel in Basel, Switzerland, discusses emerging research reported at the ACTRIMS/ECTRIMS 2020 Virtual Meeting, focusing on the use of sNfL as a biomarker for monitoring treatment response and disease activity in RRMS.
Dr Derfuss highlights one study in which longitudinal observations showed that high levels of sNfL at baseline are associated with a high risk for gadolinium-enhancing lesions; the study authors suggest that quarterly monitoring may be adequate for surveillance of subclinical disease.
INFORMATION FROM INDUSTRY
Resources
Have You Seen the Head-to-Head Efficacy Data for ZEPOSIA® (ozanimod)?
Clinical Trial Safety Findings for an S1P Therapy
Discover How to Start Appropriate Patients on an S1P
US-ZEP-20-0997 10/20
In another study, higher sNfL levels at baseline were linked to a higher risk for T2 lesions and a more pronounced brain atrophy rate, but disability progression was not correlated to baseline sNfL levels.
Finally, Dr Derfuss reports on a real-world, large cohort study supporting the value of sNfL to capture and predict disability progression independent of relapses.
Tobias J. Derfuss, MD, Professor, Head of Outpatient Clinic, Department of Neurology, University Hospital Board, Basel, Switzerland
Tobias J. Derfuss, MD, has disclosed the following relevant financial relationships: Received financial compensation for his activities in advisory boards, steering committees, data safety monitoring boards, and consultation for: Novartis; Merck; Biogen; Celgene; Actelion; Mitsubishi Pharma; MedDay; Roche; Sanofi Genzyme. Received research grant from: Novartis; Biogen; Roche; Swiss National Science Foundation; European Union; Swiss MS Society. Spouse is an employee of and holds stock options in: Novartis

Access to care: A nurse practitioner’s plea
Having been a reader of Pediatric News for years, I want to bring to light access-to-care issues involving COVID-19 medical facility restrictions for pediatric patients and their parents.
On March 27, 2020, I received a phone call from the Department of Human Services pleading with me to take a medically fragile child who was entering the foster care system that day. He had very specific needs, and they had no one available who could medically meet those needs. The week prior was my kids’ scheduled spring break; the week I got the call was the week that I was voluntarily furloughed from my job as a pediatric nurse practitioner so that I could stay home with my kids as their school would not be reopening for the year, and someone had to be with them. I was already home with my 3-year-old and 6-year-old, so why not add another?
Leo (name changed for privacy) came to me with a multitude of diagnoses, to say the least. Not only did he require physical, speech, and occupational therapy twice weekly, but he often had appointments with 10 different specialists at the local children’s hospital. The first few weeks he was in my care, we had almost daily visits to either therapists or specialists. Keeping up with these types of appointments in a normal world is difficult ... I was getting the crash course on how to navigate all of it in the COVID-19 world.
So now, I am the primary caregiver during the day for my two children and our medically fragile foster child who has multiple medical appointments a week. Our local children’s hospital allowed only the caregiver to accompany him to his visits. In theory this sounds great, right? Fewer people in a facility equals less exposure, less risk, and fewer COVID-19 infections.
But what about the negative consequences of these hospital policies? I have two other children I was caring for. I couldn’t take them to their grandparents’ house because people over age 65 years are at risk of having COVID-19 complications. I had been furloughed, so our income was half what it typically was. Regardless,
Now imagine if I were a single mom who had three kids and a lesser paying job. Schools are closed and she’s forced to work from home and homeschool her children. Or worse, she’s been laid off and living on unemployment. Do you think she is going to have the time or finances available to hire a babysitter so that she can take her medically fragile child in for his cardiology follow-up? Because not only does she have to pay the copays and whatever insurance doesn’t cover, but now she has to fork over $50 for child care. If you don’t know the answer already, it’s no, she does not have the time or the finances. So her child misses a cardiology appointment, which means that his meds weren’t increased according to his growth, which means his pulmonary hypertension is not controlled, which worsens his heart failure ... you get my drift.
Fast forward to Sept. 22, 2020. I had a cardiology appointment at our local heart hospital for myself. It’s 2020, people, I’ve been having some palpitations that I needed checked out and was going in to have a heart monitor patch placed. I had my 4-year-old son with me because he is on a hybrid schedule where we homeschool 2 days a week. We entered the building wearing masks, and I was immediately stopped by security and informed that, according to the COVID-19 policy for their hospital, children under 16 are not allowed to enter the building. After some discussion, I was ultimately refused care because my son was with me that day. Refused care because I had a masked 4-year-old with a normal temperature at my side.
These policies are not working. We are in health care. It should not matter what pandemic is on the table, we should not be refusing patients access to care based on who is by their side that day. We knew the risks when we entered our profession, and we know the proper measures to protect ourselves. Our patients also know the risks and can protect themselves accordingly.
So this is my plea to all medical facilities out there: Stop. Stop telling people their loved ones can’t accompany them to appointments. Stop telling caregivers to wait in their cars while their elderly, demented mothers have their annual physicals. Stop telling moms they need to leave their other children at home. This is now a huge access-to-care issue nationwide and it needs to stop. Excess deaths in our nation are soaring, and it’s not just because people don’t want to seek medical attention; it’s because medical facilities are making it almost impossible to seek help for many. People are dying, and it’s not only from COVID-19. This is on us as health care providers, and we need to step up to the plate and do what is right.
Ms. Baxendale is a nurse practitioner in Mustang, Okla. Email her at pdnews@mdedge.com.
Having been a reader of Pediatric News for years, I want to bring to light access-to-care issues involving COVID-19 medical facility restrictions for pediatric patients and their parents.
On March 27, 2020, I received a phone call from the Department of Human Services pleading with me to take a medically fragile child who was entering the foster care system that day. He had very specific needs, and they had no one available who could medically meet those needs. The week prior was my kids’ scheduled spring break; the week I got the call was the week that I was voluntarily furloughed from my job as a pediatric nurse practitioner so that I could stay home with my kids as their school would not be reopening for the year, and someone had to be with them. I was already home with my 3-year-old and 6-year-old, so why not add another?
Leo (name changed for privacy) came to me with a multitude of diagnoses, to say the least. Not only did he require physical, speech, and occupational therapy twice weekly, but he often had appointments with 10 different specialists at the local children’s hospital. The first few weeks he was in my care, we had almost daily visits to either therapists or specialists. Keeping up with these types of appointments in a normal world is difficult ... I was getting the crash course on how to navigate all of it in the COVID-19 world.
So now, I am the primary caregiver during the day for my two children and our medically fragile foster child who has multiple medical appointments a week. Our local children’s hospital allowed only the caregiver to accompany him to his visits. In theory this sounds great, right? Fewer people in a facility equals less exposure, less risk, and fewer COVID-19 infections.
But what about the negative consequences of these hospital policies? I have two other children I was caring for. I couldn’t take them to their grandparents’ house because people over age 65 years are at risk of having COVID-19 complications. I had been furloughed, so our income was half what it typically was. Regardless,
Now imagine if I were a single mom who had three kids and a lesser paying job. Schools are closed and she’s forced to work from home and homeschool her children. Or worse, she’s been laid off and living on unemployment. Do you think she is going to have the time or finances available to hire a babysitter so that she can take her medically fragile child in for his cardiology follow-up? Because not only does she have to pay the copays and whatever insurance doesn’t cover, but now she has to fork over $50 for child care. If you don’t know the answer already, it’s no, she does not have the time or the finances. So her child misses a cardiology appointment, which means that his meds weren’t increased according to his growth, which means his pulmonary hypertension is not controlled, which worsens his heart failure ... you get my drift.
Fast forward to Sept. 22, 2020. I had a cardiology appointment at our local heart hospital for myself. It’s 2020, people, I’ve been having some palpitations that I needed checked out and was going in to have a heart monitor patch placed. I had my 4-year-old son with me because he is on a hybrid schedule where we homeschool 2 days a week. We entered the building wearing masks, and I was immediately stopped by security and informed that, according to the COVID-19 policy for their hospital, children under 16 are not allowed to enter the building. After some discussion, I was ultimately refused care because my son was with me that day. Refused care because I had a masked 4-year-old with a normal temperature at my side.
These policies are not working. We are in health care. It should not matter what pandemic is on the table, we should not be refusing patients access to care based on who is by their side that day. We knew the risks when we entered our profession, and we know the proper measures to protect ourselves. Our patients also know the risks and can protect themselves accordingly.
So this is my plea to all medical facilities out there: Stop. Stop telling people their loved ones can’t accompany them to appointments. Stop telling caregivers to wait in their cars while their elderly, demented mothers have their annual physicals. Stop telling moms they need to leave their other children at home. This is now a huge access-to-care issue nationwide and it needs to stop. Excess deaths in our nation are soaring, and it’s not just because people don’t want to seek medical attention; it’s because medical facilities are making it almost impossible to seek help for many. People are dying, and it’s not only from COVID-19. This is on us as health care providers, and we need to step up to the plate and do what is right.
Ms. Baxendale is a nurse practitioner in Mustang, Okla. Email her at pdnews@mdedge.com.
Having been a reader of Pediatric News for years, I want to bring to light access-to-care issues involving COVID-19 medical facility restrictions for pediatric patients and their parents.
On March 27, 2020, I received a phone call from the Department of Human Services pleading with me to take a medically fragile child who was entering the foster care system that day. He had very specific needs, and they had no one available who could medically meet those needs. The week prior was my kids’ scheduled spring break; the week I got the call was the week that I was voluntarily furloughed from my job as a pediatric nurse practitioner so that I could stay home with my kids as their school would not be reopening for the year, and someone had to be with them. I was already home with my 3-year-old and 6-year-old, so why not add another?
Leo (name changed for privacy) came to me with a multitude of diagnoses, to say the least. Not only did he require physical, speech, and occupational therapy twice weekly, but he often had appointments with 10 different specialists at the local children’s hospital. The first few weeks he was in my care, we had almost daily visits to either therapists or specialists. Keeping up with these types of appointments in a normal world is difficult ... I was getting the crash course on how to navigate all of it in the COVID-19 world.
So now, I am the primary caregiver during the day for my two children and our medically fragile foster child who has multiple medical appointments a week. Our local children’s hospital allowed only the caregiver to accompany him to his visits. In theory this sounds great, right? Fewer people in a facility equals less exposure, less risk, and fewer COVID-19 infections.
But what about the negative consequences of these hospital policies? I have two other children I was caring for. I couldn’t take them to their grandparents’ house because people over age 65 years are at risk of having COVID-19 complications. I had been furloughed, so our income was half what it typically was. Regardless,
Now imagine if I were a single mom who had three kids and a lesser paying job. Schools are closed and she’s forced to work from home and homeschool her children. Or worse, she’s been laid off and living on unemployment. Do you think she is going to have the time or finances available to hire a babysitter so that she can take her medically fragile child in for his cardiology follow-up? Because not only does she have to pay the copays and whatever insurance doesn’t cover, but now she has to fork over $50 for child care. If you don’t know the answer already, it’s no, she does not have the time or the finances. So her child misses a cardiology appointment, which means that his meds weren’t increased according to his growth, which means his pulmonary hypertension is not controlled, which worsens his heart failure ... you get my drift.
Fast forward to Sept. 22, 2020. I had a cardiology appointment at our local heart hospital for myself. It’s 2020, people, I’ve been having some palpitations that I needed checked out and was going in to have a heart monitor patch placed. I had my 4-year-old son with me because he is on a hybrid schedule where we homeschool 2 days a week. We entered the building wearing masks, and I was immediately stopped by security and informed that, according to the COVID-19 policy for their hospital, children under 16 are not allowed to enter the building. After some discussion, I was ultimately refused care because my son was with me that day. Refused care because I had a masked 4-year-old with a normal temperature at my side.
These policies are not working. We are in health care. It should not matter what pandemic is on the table, we should not be refusing patients access to care based on who is by their side that day. We knew the risks when we entered our profession, and we know the proper measures to protect ourselves. Our patients also know the risks and can protect themselves accordingly.
So this is my plea to all medical facilities out there: Stop. Stop telling people their loved ones can’t accompany them to appointments. Stop telling caregivers to wait in their cars while their elderly, demented mothers have their annual physicals. Stop telling moms they need to leave their other children at home. This is now a huge access-to-care issue nationwide and it needs to stop. Excess deaths in our nation are soaring, and it’s not just because people don’t want to seek medical attention; it’s because medical facilities are making it almost impossible to seek help for many. People are dying, and it’s not only from COVID-19. This is on us as health care providers, and we need to step up to the plate and do what is right.
Ms. Baxendale is a nurse practitioner in Mustang, Okla. Email her at pdnews@mdedge.com.
Neurofibromatosis type 1: More than skin deep
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder that is estimated to occur in 1:2500 births and to have a prevalence of 1:2000 to 1:4000.1,2 It was first described in 1882 by Friedrich Daniel Von Recklinghausen, who identified patients and their relatives with signs of neuroectodermal abnormalities (café-au-lait macules [CALMs], axillary and inguinal freckling, and neurofibromas).
NF1 may begin insidiously in childhood and evolves as the patient ages. It is associated with intracranial, intraspinal, and intraorbital neoplasms, although other organs and tissues can also be involved.
The family physician might be the first one to recognize the signs of this condition during a well-child exam and is in a unique position to coordinate a multidisciplinary approach to care.
A mutated allele and early manifestations on the skin
NF1 has been attributed to genetic mosaicism and is classified as segmental, generalized, or (less frequently) gonadal. The disorder results from germline mutations in the NF1 tumor-suppressor gene on chromosome 17, known to codify the cytoplasmic protein called neurofibromin.3 The penetrance of NF1 is complete, which means that 100% of patients with the mutated allele will develop the disease.
Patients typically have symptoms by the third decade of life, although many will show signs of the disease in early childhood. CALMs are the earliest expression of NF1. They manifest in the first 2 years of life and are found in almost all affected patients. The lesions are well defined and measure 10 to 40 mm. They are typically light brown, although they may darken with sun exposure.
Histologically, the lesions will show macromelanosomes and high concentrations of melanin but do not represent an increased risk for malignancy.4 Not all isolated CALMs are a sign of NF1. While children younger than 29 months with 6 or more CALMs have a high risk for NF1 (80.4%; 95% confidence interval [CI], 74.6% to 86.2%), those who are older than 29 months with at least 1 atypical CALM or fewer than 6 CALMs have just a 0.9% (95% CI, 0% to 2.6%) risk for constitutional NF1.5
Freckles are also observed in 90% of patients with NF1; these tend to develop after the third year of life. The breast and trunk are the most commonly affected areas in adults. The pathophysiology is unknown, but this freckling is believed to be related to skin friction, high humidity, and ambient temperature.6
Continue to: Neurofibromas are benign...
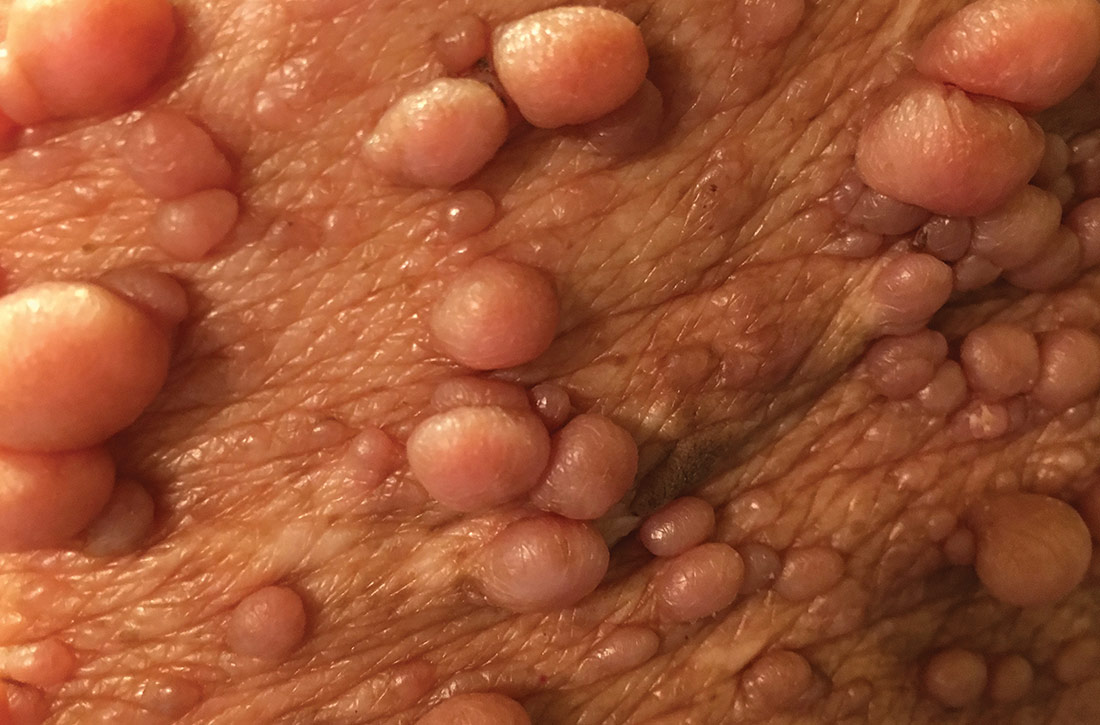
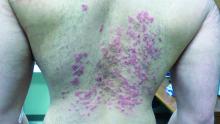
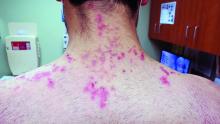
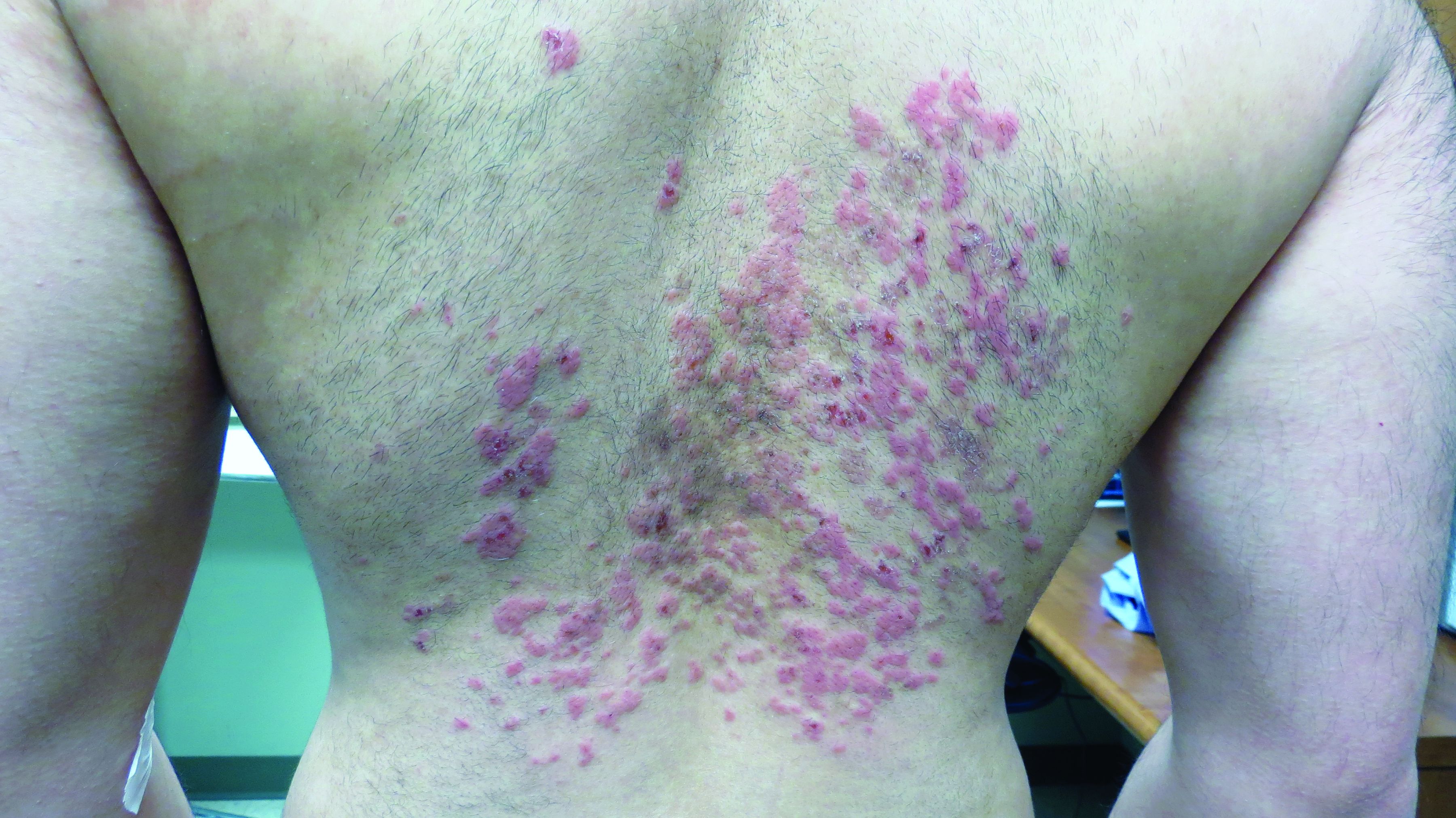
Neurofibromas are benign subcutaneous palpable lesions that grow within peripheral nerve tissue, including spinal, subcutaneous, plexiform, or dermal encapsulated nerves. Originating in Schwann cells, they are composed of fibroblasts, mast cells, macrophages, endothelial cells, and other perineural cells. Some patients show disfiguration when hundreds of these masses are present (FIGURE). These tumors increase in number as the patient ages or during pregnancy, which is thought to be secondary to hormonal changes.7 They are sometimes painful and can be pruritic. Their appearance can also cause patient distress.
The diagnosis is a clinical one
Suspicion for NF1 should be high in patients presenting with the dermatologic findings described, although CALMs and freckling are not exclusive to NF1. Diagnostic criteria for NF1, which distinguish it from other conditions, were first outlined in a National Institutes of Health Consensus Development Conference Statement in 1987.8 The list of criteria has subsequently been expanded.
While the presence of at least 2 criteria is required for diagnosis,2 NF1 should be suspected in individuals who have any of the following findings8,9:
- the presence of at least 6 CALMs that are > 5 mm in prepubertal children and > 15 mm in adults
- 2 or more neurofibromas of any type, or at least one plexiform neurofibroma
- axillary or groin freckling
- optic pathway glioma
- 2 or more Lisch nodules (iris hamartomas seen on slit-lamp examination)
- bony dysplasia (sphenoid wing dysplasia, bowing of long bone ± pseudarthrosis)
- first-degree relative with NF1.
What you’ll see as the disease progresses
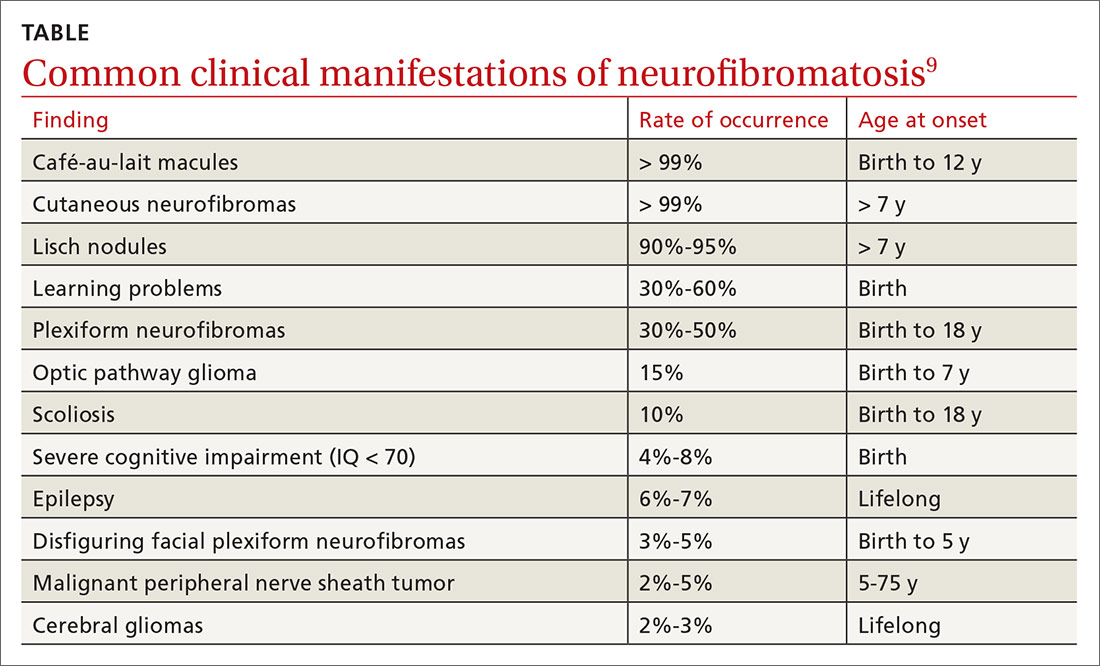
NF1 can affect a variety of systems, and potential complications of the disease are numerous and varied (see TABLE9). Here is some of what you may see as the patient’s disease progresses to various organ systems:
Learning disabilities and other cognitive and behavioral problems, such as attention-deficit/hyperactivity disorder, may affect up to 70% of children with NF1. Additionally, children with NF1 have visual/spatial problems, impaired visual motor integration, and language deficits.10 The etiology of cognitive impairment in NF1 is unknown.11
Continue to: Hypertension
Hypertension is common and may contribute to premature death in patients with NF1. Up to 27% of patients will have significant cardiovascular anomalies, including pulmonary valve stenosis, hypertrophic cardiomyopathy in patients with complete deletions of the NF1 gene, intracardiac neurofibromas, renal artery stenosis, coarctation of the aorta, and cerebral infarctions.12 Renal artery stenosis occurs in approximately 2% of the NF1 population, and the diagnosis should be considered in hypertensive children, young adults, pregnant women, older individuals with refractory hypertension, and those with an abdominal bruit.13
Psychological issues. The disfigurement caused by neurofibromas and the uncertainty of an unpredictable disease course can cause psychological manifestations for patients with NF1. Anxiety and depression are common. Not surprisingly, patients with more severe disease report more adverse psychological effects.
Orthopedic deformities. Spinal deformities are the most common skeletal manifestation of NF1, with an incidence estimated from 10% to 25% in various studies. Bone mineral density, as measured by age- and gender-adjusted Z-scores, is significantly lower in NF1 patients than in the general population.14 Children may develop bowing of the long bones, particularly the tibia, and pseudarthrosis, a false joint in a long bone. Children with NF1 need yearly assessment of the spine. Patients with clinical evidence of scoliosis should be referred to Orthopedics for further evaluation.
Eye issues. A majority of adult patients develop neurofibroma-like nodules in the iris known as Lisch nodules. The nodules are not thought to cause any ophthalmologic complications. Patients may also develop palpebral neurofibroma, which may become large and sporadically show malignant transformation. Optic nerve glioma may cause strabismus and proptosis, and a large number of patients will also develop glaucoma and globe enlargement.15
Gastrointestinal lesions and cancer. Neurofibromas can grow in the stomach, liver, mesentery, retroperitoneum, and bowel. Adenocarcinoma developed in 23% of patients.16 Gastrointestinal tract bleeding, pseudo-obstruction, and protein-losing enteropathy also may occur.17
Continue to: Central nervous system manifestations
Central nervous system manifestations. Neurological manifestations have been observed in 55% of patients with NF1.18 These include headache, hydrocephalus, epilepsy, lacunar stroke, white matter disease, intraspinal neurofibroma, facial palsy, radiculopathy, and polyneuropathy. Tumors include optic pathway tumors, meningioma, and cerebral glioma. Glioma is the predominant tumor type in NF1 and occurs in all parts of the nervous system, with a predilection for the optic pathways, brainstem, and cerebellum.18
Malignant peripheral nerve sheath tumors. There is an 8% to 13% lifetime risk for malignant peripheral nerve sheath tumors (MPNST), predominantly in individuals between the ages of 20 and 35.19,20 Any change in neurofibroma from soft to hard, or a rapid increase in the size, is suspicious for MPNST. Other symptoms include persistent pain lasting for longer than a month, pain that disturbs sleep, and new neurological deficits. These cancers can be hard to detect, leading to poor prognosis secondary to metastasis.19,20 The greatest risk factors for MPNST are pain associated with a mass and the presence of cutaneous and subcutaneous neurofibromas.21
Treatment is symptom based, but there is a new option
Treatment is individualized to the patient’s symptoms. Neurofibromas that are disfiguring, disruptive, or malignant may be surgically removed.
In April 2020, the US Food and Drug Administration (FDA) approved selumetinib (Koselugo) for the treatment of pediatric patients (ages ≥ 2 years) with NF1 who have symptomatic, inoperable plexiform neurofibromas (PNs).22 In a clinical trial, patients received selumetinib 25 mg/m2 orally twice a day until they demonstrated disease progression or experienced “unacceptable” adverse events.22,23 The overall response rate was 66%, defined as “the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3 to 6 months.”22
Of note, all patients had a partial, not complete, response. Common adverse effects included vomiting, rash, abdominal pain, diarrhea, and nausea.23 Selumetinib may also cause more serious adverse effects, including cardiomyopathy and ocular toxicity. Prior to treatment initiation and at regular intervals during treatment, patients should undergo cardiac and ophthalmic evaluation.22,23 Selumetinib was granted priority review and orphan drug status by the FDA.22
Continue to: You play a key role in ongoing monitoring
You play a key role in ongoing monitoring
In light of the condition’s heterogeneity, the goals of care include early recognition and treatment of complications, especially neoplasms; optimization of quality of life; and identification and treatment of comorbidities. Family physicians are well positioned to monitor patients with NF1 for age-specific disease manifestations and potential complications.9 All patients require:
- an annual physical examination by a physician who is familiar with the individual and with the disease
- annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- regular blood pressure monitoring
- other studies (eg, MRI) only as indicated on the basis of clinically apparent signs or symptoms
- monitoring by an appropriate specialist if there are abnormalities of the central nervous, skeletal, or cardiovascular systems
- referral to a neurologist for any unexplained neurological signs and symptoms. Referral should be urgent if there are acute symptoms of progressive sensory disturbance, motor deficit and incoordination, or sphincter disturbances since these might indicate an intracranial lesion or spinal cord compression. Headaches on waking, morning vomiting, and altered consciousness are suggestive of raised intracranial pressure.
Children with NF1 benefit from coordinated care between the FP and a pediatrician or other specialist familiar with the disease. In addition to providing usual well care, perform regular assessment of development and school performance. Pay careful attention to the cardiovascular system (particularly blood pressure) and evaluate for scoliosis.
Young adults should be continually monitored for all complications, especially hypertension. This population requires continued education about NF1 and its possible complications and may benefit from counseling about disease inheritance. Screen for anxiety and depression; offer psychological support.
Adults require monitoring based on patient preference and disease severity. For this population, blood pressure should be measured annually, or more frequently if the patient’s values indicate borderline hypertension. Provide education about complications, especially MPNSTs and spinal cord compression. Patients who have abnormalities of the central nervous, skeletal, or cardiovascular systems should be monitored by an appropriate specialist. If desired, the patient may be referred to a geneticist, especially if he or she expresses concern about inheritance. Cutaneous neurofibromas can be removed if they cause discomfort, although removal occasionally results in neurological deficit.
CORRESPONDENCE
T. Grant Phillips, MD, Associate Director, UPMC Altoona Family Physicians Residency, 501 Howard Avenue, Altoona, PA 16601-4899; phillipstg2@upmc.edu
1. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019;103:1035-1054.
2. Miller DT, Freedenberg D, Schorry E, et al; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143:e20190660.
3. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 200l;61:1-14.
4. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-844.
5. Ben-Shachar S, Dubov T, Toledano-Alhadef H, et al. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J Am Acad Dermatol. 2017;76:1077-1083.e3.
6. Friedman JM. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. www.ncbi.nlm.nih.gov/books/NBK1109. Accessed Septemeber 28, 2020.
7. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A:1624-1633.
8. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD, July 13-15, 1987. Neurofibromatosis. 1988;1:172-178. https://consensus.nih.gov/1987/1987Neurofibramatosis064html.htm. Accessed Septemeber 28, 2020.
9. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
10. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6:185-194.
11. North KN, Riccardi VM, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121-1127.
12. Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
13. Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: a report of the NF1 Cardiovascular Task Force. Genet Med. 2003;4:105-111.
14. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
15. Abdolrahimzadeh B, Piraino DC, Albanese G, et al. Neurofibromatosis: an update of ophthalmic characteristics and applications of optical coherence tomography. Clin Ophthalmol. 2016;10:851-860.
16. Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105.
17. Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J Gastroenterol. 2008;14:80-82.
18. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(pt 3):473-481.
19. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res. 2002;62:1573-1577.
20. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311-314.
21. King AA, Debaun MR, Riccardi VM, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392.
22. US Food and Drug Administration. FDA approves first therapy for children with debilitating and disfiguring rare disease [news release]. April 10, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed September 28, 2020.
23. Koselugo (selumetinib) [product information]. Wilmington, DC: AstraZeneca Pharmaceuticals LP; April 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed September 24, 2020.
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder that is estimated to occur in 1:2500 births and to have a prevalence of 1:2000 to 1:4000.1,2 It was first described in 1882 by Friedrich Daniel Von Recklinghausen, who identified patients and their relatives with signs of neuroectodermal abnormalities (café-au-lait macules [CALMs], axillary and inguinal freckling, and neurofibromas).
NF1 may begin insidiously in childhood and evolves as the patient ages. It is associated with intracranial, intraspinal, and intraorbital neoplasms, although other organs and tissues can also be involved.
The family physician might be the first one to recognize the signs of this condition during a well-child exam and is in a unique position to coordinate a multidisciplinary approach to care.
A mutated allele and early manifestations on the skin
NF1 has been attributed to genetic mosaicism and is classified as segmental, generalized, or (less frequently) gonadal. The disorder results from germline mutations in the NF1 tumor-suppressor gene on chromosome 17, known to codify the cytoplasmic protein called neurofibromin.3 The penetrance of NF1 is complete, which means that 100% of patients with the mutated allele will develop the disease.
Patients typically have symptoms by the third decade of life, although many will show signs of the disease in early childhood. CALMs are the earliest expression of NF1. They manifest in the first 2 years of life and are found in almost all affected patients. The lesions are well defined and measure 10 to 40 mm. They are typically light brown, although they may darken with sun exposure.
Histologically, the lesions will show macromelanosomes and high concentrations of melanin but do not represent an increased risk for malignancy.4 Not all isolated CALMs are a sign of NF1. While children younger than 29 months with 6 or more CALMs have a high risk for NF1 (80.4%; 95% confidence interval [CI], 74.6% to 86.2%), those who are older than 29 months with at least 1 atypical CALM or fewer than 6 CALMs have just a 0.9% (95% CI, 0% to 2.6%) risk for constitutional NF1.5
Freckles are also observed in 90% of patients with NF1; these tend to develop after the third year of life. The breast and trunk are the most commonly affected areas in adults. The pathophysiology is unknown, but this freckling is believed to be related to skin friction, high humidity, and ambient temperature.6
Continue to: Neurofibromas are benign...
Neurofibromas are benign subcutaneous palpable lesions that grow within peripheral nerve tissue, including spinal, subcutaneous, plexiform, or dermal encapsulated nerves. Originating in Schwann cells, they are composed of fibroblasts, mast cells, macrophages, endothelial cells, and other perineural cells. Some patients show disfiguration when hundreds of these masses are present (FIGURE). These tumors increase in number as the patient ages or during pregnancy, which is thought to be secondary to hormonal changes.7 They are sometimes painful and can be pruritic. Their appearance can also cause patient distress.
The diagnosis is a clinical one
Suspicion for NF1 should be high in patients presenting with the dermatologic findings described, although CALMs and freckling are not exclusive to NF1. Diagnostic criteria for NF1, which distinguish it from other conditions, were first outlined in a National Institutes of Health Consensus Development Conference Statement in 1987.8 The list of criteria has subsequently been expanded.
While the presence of at least 2 criteria is required for diagnosis,2 NF1 should be suspected in individuals who have any of the following findings8,9:
- the presence of at least 6 CALMs that are > 5 mm in prepubertal children and > 15 mm in adults
- 2 or more neurofibromas of any type, or at least one plexiform neurofibroma
- axillary or groin freckling
- optic pathway glioma
- 2 or more Lisch nodules (iris hamartomas seen on slit-lamp examination)
- bony dysplasia (sphenoid wing dysplasia, bowing of long bone ± pseudarthrosis)
- first-degree relative with NF1.
What you’ll see as the disease progresses
NF1 can affect a variety of systems, and potential complications of the disease are numerous and varied (see TABLE9). Here is some of what you may see as the patient’s disease progresses to various organ systems:
Learning disabilities and other cognitive and behavioral problems, such as attention-deficit/hyperactivity disorder, may affect up to 70% of children with NF1. Additionally, children with NF1 have visual/spatial problems, impaired visual motor integration, and language deficits.10 The etiology of cognitive impairment in NF1 is unknown.11
Continue to: Hypertension
Hypertension is common and may contribute to premature death in patients with NF1. Up to 27% of patients will have significant cardiovascular anomalies, including pulmonary valve stenosis, hypertrophic cardiomyopathy in patients with complete deletions of the NF1 gene, intracardiac neurofibromas, renal artery stenosis, coarctation of the aorta, and cerebral infarctions.12 Renal artery stenosis occurs in approximately 2% of the NF1 population, and the diagnosis should be considered in hypertensive children, young adults, pregnant women, older individuals with refractory hypertension, and those with an abdominal bruit.13
Psychological issues. The disfigurement caused by neurofibromas and the uncertainty of an unpredictable disease course can cause psychological manifestations for patients with NF1. Anxiety and depression are common. Not surprisingly, patients with more severe disease report more adverse psychological effects.
Orthopedic deformities. Spinal deformities are the most common skeletal manifestation of NF1, with an incidence estimated from 10% to 25% in various studies. Bone mineral density, as measured by age- and gender-adjusted Z-scores, is significantly lower in NF1 patients than in the general population.14 Children may develop bowing of the long bones, particularly the tibia, and pseudarthrosis, a false joint in a long bone. Children with NF1 need yearly assessment of the spine. Patients with clinical evidence of scoliosis should be referred to Orthopedics for further evaluation.
Eye issues. A majority of adult patients develop neurofibroma-like nodules in the iris known as Lisch nodules. The nodules are not thought to cause any ophthalmologic complications. Patients may also develop palpebral neurofibroma, which may become large and sporadically show malignant transformation. Optic nerve glioma may cause strabismus and proptosis, and a large number of patients will also develop glaucoma and globe enlargement.15
Gastrointestinal lesions and cancer. Neurofibromas can grow in the stomach, liver, mesentery, retroperitoneum, and bowel. Adenocarcinoma developed in 23% of patients.16 Gastrointestinal tract bleeding, pseudo-obstruction, and protein-losing enteropathy also may occur.17
Continue to: Central nervous system manifestations
Central nervous system manifestations. Neurological manifestations have been observed in 55% of patients with NF1.18 These include headache, hydrocephalus, epilepsy, lacunar stroke, white matter disease, intraspinal neurofibroma, facial palsy, radiculopathy, and polyneuropathy. Tumors include optic pathway tumors, meningioma, and cerebral glioma. Glioma is the predominant tumor type in NF1 and occurs in all parts of the nervous system, with a predilection for the optic pathways, brainstem, and cerebellum.18
Malignant peripheral nerve sheath tumors. There is an 8% to 13% lifetime risk for malignant peripheral nerve sheath tumors (MPNST), predominantly in individuals between the ages of 20 and 35.19,20 Any change in neurofibroma from soft to hard, or a rapid increase in the size, is suspicious for MPNST. Other symptoms include persistent pain lasting for longer than a month, pain that disturbs sleep, and new neurological deficits. These cancers can be hard to detect, leading to poor prognosis secondary to metastasis.19,20 The greatest risk factors for MPNST are pain associated with a mass and the presence of cutaneous and subcutaneous neurofibromas.21
Treatment is symptom based, but there is a new option
Treatment is individualized to the patient’s symptoms. Neurofibromas that are disfiguring, disruptive, or malignant may be surgically removed.
In April 2020, the US Food and Drug Administration (FDA) approved selumetinib (Koselugo) for the treatment of pediatric patients (ages ≥ 2 years) with NF1 who have symptomatic, inoperable plexiform neurofibromas (PNs).22 In a clinical trial, patients received selumetinib 25 mg/m2 orally twice a day until they demonstrated disease progression or experienced “unacceptable” adverse events.22,23 The overall response rate was 66%, defined as “the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3 to 6 months.”22
Of note, all patients had a partial, not complete, response. Common adverse effects included vomiting, rash, abdominal pain, diarrhea, and nausea.23 Selumetinib may also cause more serious adverse effects, including cardiomyopathy and ocular toxicity. Prior to treatment initiation and at regular intervals during treatment, patients should undergo cardiac and ophthalmic evaluation.22,23 Selumetinib was granted priority review and orphan drug status by the FDA.22
Continue to: You play a key role in ongoing monitoring
You play a key role in ongoing monitoring
In light of the condition’s heterogeneity, the goals of care include early recognition and treatment of complications, especially neoplasms; optimization of quality of life; and identification and treatment of comorbidities. Family physicians are well positioned to monitor patients with NF1 for age-specific disease manifestations and potential complications.9 All patients require:
- an annual physical examination by a physician who is familiar with the individual and with the disease
- annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- regular blood pressure monitoring
- other studies (eg, MRI) only as indicated on the basis of clinically apparent signs or symptoms
- monitoring by an appropriate specialist if there are abnormalities of the central nervous, skeletal, or cardiovascular systems
- referral to a neurologist for any unexplained neurological signs and symptoms. Referral should be urgent if there are acute symptoms of progressive sensory disturbance, motor deficit and incoordination, or sphincter disturbances since these might indicate an intracranial lesion or spinal cord compression. Headaches on waking, morning vomiting, and altered consciousness are suggestive of raised intracranial pressure.
Children with NF1 benefit from coordinated care between the FP and a pediatrician or other specialist familiar with the disease. In addition to providing usual well care, perform regular assessment of development and school performance. Pay careful attention to the cardiovascular system (particularly blood pressure) and evaluate for scoliosis.
Young adults should be continually monitored for all complications, especially hypertension. This population requires continued education about NF1 and its possible complications and may benefit from counseling about disease inheritance. Screen for anxiety and depression; offer psychological support.
Adults require monitoring based on patient preference and disease severity. For this population, blood pressure should be measured annually, or more frequently if the patient’s values indicate borderline hypertension. Provide education about complications, especially MPNSTs and spinal cord compression. Patients who have abnormalities of the central nervous, skeletal, or cardiovascular systems should be monitored by an appropriate specialist. If desired, the patient may be referred to a geneticist, especially if he or she expresses concern about inheritance. Cutaneous neurofibromas can be removed if they cause discomfort, although removal occasionally results in neurological deficit.
CORRESPONDENCE
T. Grant Phillips, MD, Associate Director, UPMC Altoona Family Physicians Residency, 501 Howard Avenue, Altoona, PA 16601-4899; phillipstg2@upmc.edu
Neurofibromatosis type 1 (NF1) is an autosomal dominant inherited disorder that is estimated to occur in 1:2500 births and to have a prevalence of 1:2000 to 1:4000.1,2 It was first described in 1882 by Friedrich Daniel Von Recklinghausen, who identified patients and their relatives with signs of neuroectodermal abnormalities (café-au-lait macules [CALMs], axillary and inguinal freckling, and neurofibromas).
NF1 may begin insidiously in childhood and evolves as the patient ages. It is associated with intracranial, intraspinal, and intraorbital neoplasms, although other organs and tissues can also be involved.
The family physician might be the first one to recognize the signs of this condition during a well-child exam and is in a unique position to coordinate a multidisciplinary approach to care.
A mutated allele and early manifestations on the skin
NF1 has been attributed to genetic mosaicism and is classified as segmental, generalized, or (less frequently) gonadal. The disorder results from germline mutations in the NF1 tumor-suppressor gene on chromosome 17, known to codify the cytoplasmic protein called neurofibromin.3 The penetrance of NF1 is complete, which means that 100% of patients with the mutated allele will develop the disease.
Patients typically have symptoms by the third decade of life, although many will show signs of the disease in early childhood. CALMs are the earliest expression of NF1. They manifest in the first 2 years of life and are found in almost all affected patients. The lesions are well defined and measure 10 to 40 mm. They are typically light brown, although they may darken with sun exposure.
Histologically, the lesions will show macromelanosomes and high concentrations of melanin but do not represent an increased risk for malignancy.4 Not all isolated CALMs are a sign of NF1. While children younger than 29 months with 6 or more CALMs have a high risk for NF1 (80.4%; 95% confidence interval [CI], 74.6% to 86.2%), those who are older than 29 months with at least 1 atypical CALM or fewer than 6 CALMs have just a 0.9% (95% CI, 0% to 2.6%) risk for constitutional NF1.5
Freckles are also observed in 90% of patients with NF1; these tend to develop after the third year of life. The breast and trunk are the most commonly affected areas in adults. The pathophysiology is unknown, but this freckling is believed to be related to skin friction, high humidity, and ambient temperature.6
Continue to: Neurofibromas are benign...
Neurofibromas are benign subcutaneous palpable lesions that grow within peripheral nerve tissue, including spinal, subcutaneous, plexiform, or dermal encapsulated nerves. Originating in Schwann cells, they are composed of fibroblasts, mast cells, macrophages, endothelial cells, and other perineural cells. Some patients show disfiguration when hundreds of these masses are present (FIGURE). These tumors increase in number as the patient ages or during pregnancy, which is thought to be secondary to hormonal changes.7 They are sometimes painful and can be pruritic. Their appearance can also cause patient distress.
The diagnosis is a clinical one
Suspicion for NF1 should be high in patients presenting with the dermatologic findings described, although CALMs and freckling are not exclusive to NF1. Diagnostic criteria for NF1, which distinguish it from other conditions, were first outlined in a National Institutes of Health Consensus Development Conference Statement in 1987.8 The list of criteria has subsequently been expanded.
While the presence of at least 2 criteria is required for diagnosis,2 NF1 should be suspected in individuals who have any of the following findings8,9:
- the presence of at least 6 CALMs that are > 5 mm in prepubertal children and > 15 mm in adults
- 2 or more neurofibromas of any type, or at least one plexiform neurofibroma
- axillary or groin freckling
- optic pathway glioma
- 2 or more Lisch nodules (iris hamartomas seen on slit-lamp examination)
- bony dysplasia (sphenoid wing dysplasia, bowing of long bone ± pseudarthrosis)
- first-degree relative with NF1.
What you’ll see as the disease progresses
NF1 can affect a variety of systems, and potential complications of the disease are numerous and varied (see TABLE9). Here is some of what you may see as the patient’s disease progresses to various organ systems:
Learning disabilities and other cognitive and behavioral problems, such as attention-deficit/hyperactivity disorder, may affect up to 70% of children with NF1. Additionally, children with NF1 have visual/spatial problems, impaired visual motor integration, and language deficits.10 The etiology of cognitive impairment in NF1 is unknown.11
Continue to: Hypertension
Hypertension is common and may contribute to premature death in patients with NF1. Up to 27% of patients will have significant cardiovascular anomalies, including pulmonary valve stenosis, hypertrophic cardiomyopathy in patients with complete deletions of the NF1 gene, intracardiac neurofibromas, renal artery stenosis, coarctation of the aorta, and cerebral infarctions.12 Renal artery stenosis occurs in approximately 2% of the NF1 population, and the diagnosis should be considered in hypertensive children, young adults, pregnant women, older individuals with refractory hypertension, and those with an abdominal bruit.13
Psychological issues. The disfigurement caused by neurofibromas and the uncertainty of an unpredictable disease course can cause psychological manifestations for patients with NF1. Anxiety and depression are common. Not surprisingly, patients with more severe disease report more adverse psychological effects.
Orthopedic deformities. Spinal deformities are the most common skeletal manifestation of NF1, with an incidence estimated from 10% to 25% in various studies. Bone mineral density, as measured by age- and gender-adjusted Z-scores, is significantly lower in NF1 patients than in the general population.14 Children may develop bowing of the long bones, particularly the tibia, and pseudarthrosis, a false joint in a long bone. Children with NF1 need yearly assessment of the spine. Patients with clinical evidence of scoliosis should be referred to Orthopedics for further evaluation.
Eye issues. A majority of adult patients develop neurofibroma-like nodules in the iris known as Lisch nodules. The nodules are not thought to cause any ophthalmologic complications. Patients may also develop palpebral neurofibroma, which may become large and sporadically show malignant transformation. Optic nerve glioma may cause strabismus and proptosis, and a large number of patients will also develop glaucoma and globe enlargement.15
Gastrointestinal lesions and cancer. Neurofibromas can grow in the stomach, liver, mesentery, retroperitoneum, and bowel. Adenocarcinoma developed in 23% of patients.16 Gastrointestinal tract bleeding, pseudo-obstruction, and protein-losing enteropathy also may occur.17
Continue to: Central nervous system manifestations
Central nervous system manifestations. Neurological manifestations have been observed in 55% of patients with NF1.18 These include headache, hydrocephalus, epilepsy, lacunar stroke, white matter disease, intraspinal neurofibroma, facial palsy, radiculopathy, and polyneuropathy. Tumors include optic pathway tumors, meningioma, and cerebral glioma. Glioma is the predominant tumor type in NF1 and occurs in all parts of the nervous system, with a predilection for the optic pathways, brainstem, and cerebellum.18
Malignant peripheral nerve sheath tumors. There is an 8% to 13% lifetime risk for malignant peripheral nerve sheath tumors (MPNST), predominantly in individuals between the ages of 20 and 35.19,20 Any change in neurofibroma from soft to hard, or a rapid increase in the size, is suspicious for MPNST. Other symptoms include persistent pain lasting for longer than a month, pain that disturbs sleep, and new neurological deficits. These cancers can be hard to detect, leading to poor prognosis secondary to metastasis.19,20 The greatest risk factors for MPNST are pain associated with a mass and the presence of cutaneous and subcutaneous neurofibromas.21
Treatment is symptom based, but there is a new option
Treatment is individualized to the patient’s symptoms. Neurofibromas that are disfiguring, disruptive, or malignant may be surgically removed.
In April 2020, the US Food and Drug Administration (FDA) approved selumetinib (Koselugo) for the treatment of pediatric patients (ages ≥ 2 years) with NF1 who have symptomatic, inoperable plexiform neurofibromas (PNs).22 In a clinical trial, patients received selumetinib 25 mg/m2 orally twice a day until they demonstrated disease progression or experienced “unacceptable” adverse events.22,23 The overall response rate was 66%, defined as “the percentage of patients with a complete response and those who experienced more than a 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3 to 6 months.”22
Of note, all patients had a partial, not complete, response. Common adverse effects included vomiting, rash, abdominal pain, diarrhea, and nausea.23 Selumetinib may also cause more serious adverse effects, including cardiomyopathy and ocular toxicity. Prior to treatment initiation and at regular intervals during treatment, patients should undergo cardiac and ophthalmic evaluation.22,23 Selumetinib was granted priority review and orphan drug status by the FDA.22
Continue to: You play a key role in ongoing monitoring
You play a key role in ongoing monitoring
In light of the condition’s heterogeneity, the goals of care include early recognition and treatment of complications, especially neoplasms; optimization of quality of life; and identification and treatment of comorbidities. Family physicians are well positioned to monitor patients with NF1 for age-specific disease manifestations and potential complications.9 All patients require:
- an annual physical examination by a physician who is familiar with the individual and with the disease
- annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- regular blood pressure monitoring
- other studies (eg, MRI) only as indicated on the basis of clinically apparent signs or symptoms
- monitoring by an appropriate specialist if there are abnormalities of the central nervous, skeletal, or cardiovascular systems
- referral to a neurologist for any unexplained neurological signs and symptoms. Referral should be urgent if there are acute symptoms of progressive sensory disturbance, motor deficit and incoordination, or sphincter disturbances since these might indicate an intracranial lesion or spinal cord compression. Headaches on waking, morning vomiting, and altered consciousness are suggestive of raised intracranial pressure.
Children with NF1 benefit from coordinated care between the FP and a pediatrician or other specialist familiar with the disease. In addition to providing usual well care, perform regular assessment of development and school performance. Pay careful attention to the cardiovascular system (particularly blood pressure) and evaluate for scoliosis.
Young adults should be continually monitored for all complications, especially hypertension. This population requires continued education about NF1 and its possible complications and may benefit from counseling about disease inheritance. Screen for anxiety and depression; offer psychological support.
Adults require monitoring based on patient preference and disease severity. For this population, blood pressure should be measured annually, or more frequently if the patient’s values indicate borderline hypertension. Provide education about complications, especially MPNSTs and spinal cord compression. Patients who have abnormalities of the central nervous, skeletal, or cardiovascular systems should be monitored by an appropriate specialist. If desired, the patient may be referred to a geneticist, especially if he or she expresses concern about inheritance. Cutaneous neurofibromas can be removed if they cause discomfort, although removal occasionally results in neurological deficit.
CORRESPONDENCE
T. Grant Phillips, MD, Associate Director, UPMC Altoona Family Physicians Residency, 501 Howard Avenue, Altoona, PA 16601-4899; phillipstg2@upmc.edu
1. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019;103:1035-1054.
2. Miller DT, Freedenberg D, Schorry E, et al; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143:e20190660.
3. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 200l;61:1-14.
4. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-844.
5. Ben-Shachar S, Dubov T, Toledano-Alhadef H, et al. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J Am Acad Dermatol. 2017;76:1077-1083.e3.
6. Friedman JM. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. www.ncbi.nlm.nih.gov/books/NBK1109. Accessed Septemeber 28, 2020.
7. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A:1624-1633.
8. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD, July 13-15, 1987. Neurofibromatosis. 1988;1:172-178. https://consensus.nih.gov/1987/1987Neurofibramatosis064html.htm. Accessed Septemeber 28, 2020.
9. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
10. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6:185-194.
11. North KN, Riccardi VM, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121-1127.
12. Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
13. Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: a report of the NF1 Cardiovascular Task Force. Genet Med. 2003;4:105-111.
14. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
15. Abdolrahimzadeh B, Piraino DC, Albanese G, et al. Neurofibromatosis: an update of ophthalmic characteristics and applications of optical coherence tomography. Clin Ophthalmol. 2016;10:851-860.
16. Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105.
17. Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J Gastroenterol. 2008;14:80-82.
18. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(pt 3):473-481.
19. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res. 2002;62:1573-1577.
20. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311-314.
21. King AA, Debaun MR, Riccardi VM, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392.
22. US Food and Drug Administration. FDA approves first therapy for children with debilitating and disfiguring rare disease [news release]. April 10, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed September 28, 2020.
23. Koselugo (selumetinib) [product information]. Wilmington, DC: AstraZeneca Pharmaceuticals LP; April 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed September 24, 2020.
1. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am. 2019;103:1035-1054.
2. Miller DT, Freedenberg D, Schorry E, et al; Council on Genetics, American College of Medical Genetics and Genomics. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143:e20190660.
3. Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 200l;61:1-14.
4. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-844.
5. Ben-Shachar S, Dubov T, Toledano-Alhadef H, et al. Predicting neurofibromatosis type 1 risk among children with isolated café-au-lait macules. J Am Acad Dermatol. 2017;76:1077-1083.e3.
6. Friedman JM. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. www.ncbi.nlm.nih.gov/books/NBK1109. Accessed Septemeber 28, 2020.
7. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A. 2008;146A:1624-1633.
8. National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, MD, July 13-15, 1987. Neurofibromatosis. 1988;1:172-178. https://consensus.nih.gov/1987/1987Neurofibramatosis064html.htm. Accessed Septemeber 28, 2020.
9. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.
10. Koth CW, Cutting LE, Denckla MB. The association of neurofibromatosis type 1 and attention deficit hyperactivity disorder. Child Neuropsychol. 2000;6:185-194.
11. North KN, Riccardi VM, Samango‐Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48:1121-1127.
12. Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
13. Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: a report of the NF1 Cardiovascular Task Force. Genet Med. 2003;4:105-111.
14. Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161-1166.
15. Abdolrahimzadeh B, Piraino DC, Albanese G, et al. Neurofibromatosis: an update of ophthalmic characteristics and applications of optical coherence tomography. Clin Ophthalmol. 2016;10:851-860.
16. Bakker JR, Haber MM, Garcia FU. Gastrointestinal neurofibromatosis: an unusual cause of gastric outlet obstruction. Am Surg. 2005;71:100-105.
17. Rastogi R. Intra-abdominal manifestations of von Recklinghausen’s neurofibromatosis. Saudi J Gastroenterol. 2008;14:80-82.
18. Créange A, Zeller J, Rostaing-Rigattieri S, et al. Neurological complications of neurofibromatosis type 1 in adulthood. Brain. 1999;122(pt 3):473-481.
19. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Cancer Res. 2002;62:1573-1577.
20. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311-314.
21. King AA, Debaun MR, Riccardi VM, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet. 2000;93:388-392.
22. US Food and Drug Administration. FDA approves first therapy for children with debilitating and disfiguring rare disease [news release]. April 10, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-therapy-children-debilitating-and-disfiguring-rare-disease. Accessed September 28, 2020.
23. Koselugo (selumetinib) [product information]. Wilmington, DC: AstraZeneca Pharmaceuticals LP; April 2020. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed September 24, 2020.
An unplanned ‘vacation’
Looking back at the calendar, I realized that the insane year of 2020 will be the first in memory that I never took a vacation. Not a single trip outside the Phoenix metropolitan area. For that matter, there were only a handful of times I even ventured beyond the borders of Scottsdale.
The vacation is such an ingrained part of western culture that it’s hard to believe I haven’t gone anywhere since a cruise in November, 2019, and I have no vacation plans in the foreseeable future.
Do I feel horribly stressed from the lack of time off? Mmmm … Not really.
I suspect a big part of that is because I have had a lot of time off, albeit unintentionally. Looking back at my schedule, the last completely full day of patients was March 12, 2020. Since then I’ve averaged days that are only one-quarter to one-third full.
One of my idols, Dr. Arlan Cohn, once wrote “When holes appear in your appointment schedule, celebrate.” So, as he suggested, I use the extra time with the patients I do have and organize my drug samples. But there’s only so much time you can spend with a patient before you both get bored, and at this point my sample cabinet is about as organized – and devoid of expired drugs – as it can be.
In the modern age a lot can be handled by email, so if I’m done at the office I’ll often head home and nap, then answer patient queries for the rest of the day.
From a practical viewpoint, you could argue that, since mid-March, 2020 has been a strange, slow-motion vacation. Realistically, I’ve probably had more time off this year than I ever have, even if I haven’t gone too far. My kids have been home from college, giving me more time with them than I thought I’d have, and that’s been an enjoyable plus.
Of course, there are limits to any trip. At some time you reach the point where you’re sick of the whole thing and want your normal life back. I’m there now. There’s only so much nonwork you can do before you start climbing the walls, and obviously the financial worries take over, too. Seeing patients is how I earn a living.
At this point,
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Looking back at the calendar, I realized that the insane year of 2020 will be the first in memory that I never took a vacation. Not a single trip outside the Phoenix metropolitan area. For that matter, there were only a handful of times I even ventured beyond the borders of Scottsdale.
The vacation is such an ingrained part of western culture that it’s hard to believe I haven’t gone anywhere since a cruise in November, 2019, and I have no vacation plans in the foreseeable future.
Do I feel horribly stressed from the lack of time off? Mmmm … Not really.
I suspect a big part of that is because I have had a lot of time off, albeit unintentionally. Looking back at my schedule, the last completely full day of patients was March 12, 2020. Since then I’ve averaged days that are only one-quarter to one-third full.
One of my idols, Dr. Arlan Cohn, once wrote “When holes appear in your appointment schedule, celebrate.” So, as he suggested, I use the extra time with the patients I do have and organize my drug samples. But there’s only so much time you can spend with a patient before you both get bored, and at this point my sample cabinet is about as organized – and devoid of expired drugs – as it can be.
In the modern age a lot can be handled by email, so if I’m done at the office I’ll often head home and nap, then answer patient queries for the rest of the day.
From a practical viewpoint, you could argue that, since mid-March, 2020 has been a strange, slow-motion vacation. Realistically, I’ve probably had more time off this year than I ever have, even if I haven’t gone too far. My kids have been home from college, giving me more time with them than I thought I’d have, and that’s been an enjoyable plus.
Of course, there are limits to any trip. At some time you reach the point where you’re sick of the whole thing and want your normal life back. I’m there now. There’s only so much nonwork you can do before you start climbing the walls, and obviously the financial worries take over, too. Seeing patients is how I earn a living.
At this point,
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Looking back at the calendar, I realized that the insane year of 2020 will be the first in memory that I never took a vacation. Not a single trip outside the Phoenix metropolitan area. For that matter, there were only a handful of times I even ventured beyond the borders of Scottsdale.
The vacation is such an ingrained part of western culture that it’s hard to believe I haven’t gone anywhere since a cruise in November, 2019, and I have no vacation plans in the foreseeable future.
Do I feel horribly stressed from the lack of time off? Mmmm … Not really.
I suspect a big part of that is because I have had a lot of time off, albeit unintentionally. Looking back at my schedule, the last completely full day of patients was March 12, 2020. Since then I’ve averaged days that are only one-quarter to one-third full.
One of my idols, Dr. Arlan Cohn, once wrote “When holes appear in your appointment schedule, celebrate.” So, as he suggested, I use the extra time with the patients I do have and organize my drug samples. But there’s only so much time you can spend with a patient before you both get bored, and at this point my sample cabinet is about as organized – and devoid of expired drugs – as it can be.
In the modern age a lot can be handled by email, so if I’m done at the office I’ll often head home and nap, then answer patient queries for the rest of the day.
From a practical viewpoint, you could argue that, since mid-March, 2020 has been a strange, slow-motion vacation. Realistically, I’ve probably had more time off this year than I ever have, even if I haven’t gone too far. My kids have been home from college, giving me more time with them than I thought I’d have, and that’s been an enjoyable plus.
Of course, there are limits to any trip. At some time you reach the point where you’re sick of the whole thing and want your normal life back. I’m there now. There’s only so much nonwork you can do before you start climbing the walls, and obviously the financial worries take over, too. Seeing patients is how I earn a living.
At this point,
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
T2D treatments create tension between glycemic and cardiovascular goals
It was no surprise that updated guidelines recently published by the European Society of Cardiology for managing cardiovascular disease in patients with diabetes highlighted optimized treatment from a cardiovascular disease perspective, while a nearly concurrent update from two major diabetes societies saw the same issue from a more glycemic point of view.
This difference led to divergent approaches to managing hyperglycemia in patients with type 2 diabetes (T2D). The two diabetes societies that wrote one set of recommendations, the American Diabetes Association and the European Association for the Study of Diabetes, put metformin at the pinnacle of their drug hierarchy. Patients with T2D and established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure should all receive metformin first unless contraindicated or not tolerated, their updated consensus report said.
Once metformin is on board, a clinician can then add a second diabetes agent from among the two drug classes recently proven to also reduce cardiovascular and renal events, either the SGLT2 (sodium-glucose transporter 2) inhibitors, or GLP-1 (glucagonlike peptide–1) receptor agonists, they advised.
Cardiovascular disease focus represents a ‘major paradigm shift’
In contrast, the ESC guidelines called for upfront, systematic assessment of CVD risk in patients with T2D before treatment starts, and for patients in high- or very high–risk strata, the guidelines recommended starting the patient first on an SGLT2 inhibitor or a GLP-1 receptor agonist, and only adding metformin in patients who need additional glycemic control.
The guidelines also recommended starting treatment-naive patients with moderate CVD risk on metformin. For patients already on metformin, the new ESC guidelines called for adding an agent from at least one of these two drug classes with proven CVD benefits for those at high or very high CVD risk. The guidelines also note that the CVD benefits of the two newer drug classes differ and hence require further individualization depending on the risks faced by each patient, such as the risk for heart failure hospitalizations.
It’s an approach “driven by data from the cardiovascular outcome trials,” that showed several drugs from both the SGLT2 inhibitor and GLP-1 receptor agonist classes have substantial benefit for preventing cardiovascular events, renal events, hospitalizations for heart failure, and in some studies all-cause mortality, said Francesco Cosentino, MD, during a discussion of the guideline differences at the virtual annual meeting of the European Association for the Study of Diabetes.
The ESC approach also represents “a major paradigm shift,” a “change from a glucose-centric approach to an approach driven by cardiovascular disease events,” summed up Dr. Cosentino, professor of cardiology at the Karolinska Institute in Stockholm and chair of the task force that wrote the ESC’s 2019 updated guidelines. The ESC approach advocates initiating drugs for treating patients with T2D “based on cardiovascular disease risk classification,” he highlighted. Results from some SGLT2 inhibitor cardiovascular outcome trials showed that the CVD benefit was similar regardless of whether or not patients also received metformin.
ADA, EASD call for ‘a different emphasis’
“There is a different emphasis” in the statement issued by the diabetologists of the ADA and EASD, admitted Peter J. Grant, MD, a professor of diabetes and endocrinology at the University of Leeds (England) and cochair of the ESC guidelines task force. Dr. Grant represented the EASD on the task force, and the Association collaborated with the ESC in producing its guidelines.
“The ADA and EASD recommendations “look primarily at glucose control, with cardiovascular disease management as secondary.” In contrast, the ESC guidelines “are primarily cardiovascular disease risk guidelines, with a glucose interest,” Dr. Grant declared.
Despite his involvement in writing the ESC guidelines, Dr. Grant tilted toward the ADA/EASD statement as more globally relevant.
“There is much more to vasculopathy in diabetes than just macrovascular disease. Many patients with type 2 diabetes without macrovascular complications have microvascular disease,” including the potential for retinopathy, nephropathy, and neuropathy, he said. These complications can also have a strong impact on psychological well being and treatment satisfaction.
“It’s important that we’re not glucocentric any more, but it’s equally important that we treat glucose because it has such a benefit for microvascular disease.” Dr. Grant also cited metformin’s long history of safety and good tolerance, clinician comfort prescribing it, and its low price. Heavier reliance on SGLT2 inhibitors and GLP-1 receptor agonists will be expensive for the short term while the cost of these drugs remains high, which places a higher burden on “knowing we’re doing it right,” said Dr. Grant.
Dr. Cosentino pointed out that the higher cost of the drugs in the two classes shown to exert important cardiovascular and renal effects needs to be considered in a cost-effectiveness context, not just by cost alone.
‘Clinical inertia’ could be a danger
Dr. Cosentino played down a major disagreement between the two guidelines, suggesting that “focusing on the differences leads to clinical inertia” by the practicing community when they are unsure how to reconcile the two positions.
Dr. Grant agreed that adding a second drug to metformin right away made sense in at least selected patients. “Look at each patient and decide whether they need glycemic control. If so, and if they also have cardiovascular disease, use both drugs,” metformin, plus one agent from one of the two newer classes.
Something both experts agreed on is that it’s time to generally steer clear of sulfonylurea drugs. “We have evidence for harmful effects from sulfonylureas,” Dr. Cosentino said.
“I’d dump sulfonylureas,” was Dr. Grant’s assessment, but he added that they still have a role for patients who need additional glycemic control but can’t afford the newer drugs.
Dr. Cosentino has had financial relationships with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Mundipharma, Novo Nordisk, and Pfizer, Dr. Grant has lectured on behalf of AstraZeneca, GlaxoSmithKline, Merck, Novo Nordisk, the Medicines Company, and Takeda, and he has been an adviser to Amgen, AstraZeneca, Novartis, Novo Nordisk, and Synexus.
It was no surprise that updated guidelines recently published by the European Society of Cardiology for managing cardiovascular disease in patients with diabetes highlighted optimized treatment from a cardiovascular disease perspective, while a nearly concurrent update from two major diabetes societies saw the same issue from a more glycemic point of view.
This difference led to divergent approaches to managing hyperglycemia in patients with type 2 diabetes (T2D). The two diabetes societies that wrote one set of recommendations, the American Diabetes Association and the European Association for the Study of Diabetes, put metformin at the pinnacle of their drug hierarchy. Patients with T2D and established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure should all receive metformin first unless contraindicated or not tolerated, their updated consensus report said.
Once metformin is on board, a clinician can then add a second diabetes agent from among the two drug classes recently proven to also reduce cardiovascular and renal events, either the SGLT2 (sodium-glucose transporter 2) inhibitors, or GLP-1 (glucagonlike peptide–1) receptor agonists, they advised.
Cardiovascular disease focus represents a ‘major paradigm shift’
In contrast, the ESC guidelines called for upfront, systematic assessment of CVD risk in patients with T2D before treatment starts, and for patients in high- or very high–risk strata, the guidelines recommended starting the patient first on an SGLT2 inhibitor or a GLP-1 receptor agonist, and only adding metformin in patients who need additional glycemic control.
The guidelines also recommended starting treatment-naive patients with moderate CVD risk on metformin. For patients already on metformin, the new ESC guidelines called for adding an agent from at least one of these two drug classes with proven CVD benefits for those at high or very high CVD risk. The guidelines also note that the CVD benefits of the two newer drug classes differ and hence require further individualization depending on the risks faced by each patient, such as the risk for heart failure hospitalizations.
It’s an approach “driven by data from the cardiovascular outcome trials,” that showed several drugs from both the SGLT2 inhibitor and GLP-1 receptor agonist classes have substantial benefit for preventing cardiovascular events, renal events, hospitalizations for heart failure, and in some studies all-cause mortality, said Francesco Cosentino, MD, during a discussion of the guideline differences at the virtual annual meeting of the European Association for the Study of Diabetes.
The ESC approach also represents “a major paradigm shift,” a “change from a glucose-centric approach to an approach driven by cardiovascular disease events,” summed up Dr. Cosentino, professor of cardiology at the Karolinska Institute in Stockholm and chair of the task force that wrote the ESC’s 2019 updated guidelines. The ESC approach advocates initiating drugs for treating patients with T2D “based on cardiovascular disease risk classification,” he highlighted. Results from some SGLT2 inhibitor cardiovascular outcome trials showed that the CVD benefit was similar regardless of whether or not patients also received metformin.
ADA, EASD call for ‘a different emphasis’
“There is a different emphasis” in the statement issued by the diabetologists of the ADA and EASD, admitted Peter J. Grant, MD, a professor of diabetes and endocrinology at the University of Leeds (England) and cochair of the ESC guidelines task force. Dr. Grant represented the EASD on the task force, and the Association collaborated with the ESC in producing its guidelines.
“The ADA and EASD recommendations “look primarily at glucose control, with cardiovascular disease management as secondary.” In contrast, the ESC guidelines “are primarily cardiovascular disease risk guidelines, with a glucose interest,” Dr. Grant declared.
Despite his involvement in writing the ESC guidelines, Dr. Grant tilted toward the ADA/EASD statement as more globally relevant.
“There is much more to vasculopathy in diabetes than just macrovascular disease. Many patients with type 2 diabetes without macrovascular complications have microvascular disease,” including the potential for retinopathy, nephropathy, and neuropathy, he said. These complications can also have a strong impact on psychological well being and treatment satisfaction.
“It’s important that we’re not glucocentric any more, but it’s equally important that we treat glucose because it has such a benefit for microvascular disease.” Dr. Grant also cited metformin’s long history of safety and good tolerance, clinician comfort prescribing it, and its low price. Heavier reliance on SGLT2 inhibitors and GLP-1 receptor agonists will be expensive for the short term while the cost of these drugs remains high, which places a higher burden on “knowing we’re doing it right,” said Dr. Grant.
Dr. Cosentino pointed out that the higher cost of the drugs in the two classes shown to exert important cardiovascular and renal effects needs to be considered in a cost-effectiveness context, not just by cost alone.
‘Clinical inertia’ could be a danger
Dr. Cosentino played down a major disagreement between the two guidelines, suggesting that “focusing on the differences leads to clinical inertia” by the practicing community when they are unsure how to reconcile the two positions.
Dr. Grant agreed that adding a second drug to metformin right away made sense in at least selected patients. “Look at each patient and decide whether they need glycemic control. If so, and if they also have cardiovascular disease, use both drugs,” metformin, plus one agent from one of the two newer classes.
Something both experts agreed on is that it’s time to generally steer clear of sulfonylurea drugs. “We have evidence for harmful effects from sulfonylureas,” Dr. Cosentino said.
“I’d dump sulfonylureas,” was Dr. Grant’s assessment, but he added that they still have a role for patients who need additional glycemic control but can’t afford the newer drugs.
Dr. Cosentino has had financial relationships with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Mundipharma, Novo Nordisk, and Pfizer, Dr. Grant has lectured on behalf of AstraZeneca, GlaxoSmithKline, Merck, Novo Nordisk, the Medicines Company, and Takeda, and he has been an adviser to Amgen, AstraZeneca, Novartis, Novo Nordisk, and Synexus.
It was no surprise that updated guidelines recently published by the European Society of Cardiology for managing cardiovascular disease in patients with diabetes highlighted optimized treatment from a cardiovascular disease perspective, while a nearly concurrent update from two major diabetes societies saw the same issue from a more glycemic point of view.
This difference led to divergent approaches to managing hyperglycemia in patients with type 2 diabetes (T2D). The two diabetes societies that wrote one set of recommendations, the American Diabetes Association and the European Association for the Study of Diabetes, put metformin at the pinnacle of their drug hierarchy. Patients with T2D and established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure should all receive metformin first unless contraindicated or not tolerated, their updated consensus report said.
Once metformin is on board, a clinician can then add a second diabetes agent from among the two drug classes recently proven to also reduce cardiovascular and renal events, either the SGLT2 (sodium-glucose transporter 2) inhibitors, or GLP-1 (glucagonlike peptide–1) receptor agonists, they advised.
Cardiovascular disease focus represents a ‘major paradigm shift’
In contrast, the ESC guidelines called for upfront, systematic assessment of CVD risk in patients with T2D before treatment starts, and for patients in high- or very high–risk strata, the guidelines recommended starting the patient first on an SGLT2 inhibitor or a GLP-1 receptor agonist, and only adding metformin in patients who need additional glycemic control.
The guidelines also recommended starting treatment-naive patients with moderate CVD risk on metformin. For patients already on metformin, the new ESC guidelines called for adding an agent from at least one of these two drug classes with proven CVD benefits for those at high or very high CVD risk. The guidelines also note that the CVD benefits of the two newer drug classes differ and hence require further individualization depending on the risks faced by each patient, such as the risk for heart failure hospitalizations.
It’s an approach “driven by data from the cardiovascular outcome trials,” that showed several drugs from both the SGLT2 inhibitor and GLP-1 receptor agonist classes have substantial benefit for preventing cardiovascular events, renal events, hospitalizations for heart failure, and in some studies all-cause mortality, said Francesco Cosentino, MD, during a discussion of the guideline differences at the virtual annual meeting of the European Association for the Study of Diabetes.
The ESC approach also represents “a major paradigm shift,” a “change from a glucose-centric approach to an approach driven by cardiovascular disease events,” summed up Dr. Cosentino, professor of cardiology at the Karolinska Institute in Stockholm and chair of the task force that wrote the ESC’s 2019 updated guidelines. The ESC approach advocates initiating drugs for treating patients with T2D “based on cardiovascular disease risk classification,” he highlighted. Results from some SGLT2 inhibitor cardiovascular outcome trials showed that the CVD benefit was similar regardless of whether or not patients also received metformin.
ADA, EASD call for ‘a different emphasis’
“There is a different emphasis” in the statement issued by the diabetologists of the ADA and EASD, admitted Peter J. Grant, MD, a professor of diabetes and endocrinology at the University of Leeds (England) and cochair of the ESC guidelines task force. Dr. Grant represented the EASD on the task force, and the Association collaborated with the ESC in producing its guidelines.
“The ADA and EASD recommendations “look primarily at glucose control, with cardiovascular disease management as secondary.” In contrast, the ESC guidelines “are primarily cardiovascular disease risk guidelines, with a glucose interest,” Dr. Grant declared.
Despite his involvement in writing the ESC guidelines, Dr. Grant tilted toward the ADA/EASD statement as more globally relevant.
“There is much more to vasculopathy in diabetes than just macrovascular disease. Many patients with type 2 diabetes without macrovascular complications have microvascular disease,” including the potential for retinopathy, nephropathy, and neuropathy, he said. These complications can also have a strong impact on psychological well being and treatment satisfaction.
“It’s important that we’re not glucocentric any more, but it’s equally important that we treat glucose because it has such a benefit for microvascular disease.” Dr. Grant also cited metformin’s long history of safety and good tolerance, clinician comfort prescribing it, and its low price. Heavier reliance on SGLT2 inhibitors and GLP-1 receptor agonists will be expensive for the short term while the cost of these drugs remains high, which places a higher burden on “knowing we’re doing it right,” said Dr. Grant.
Dr. Cosentino pointed out that the higher cost of the drugs in the two classes shown to exert important cardiovascular and renal effects needs to be considered in a cost-effectiveness context, not just by cost alone.
‘Clinical inertia’ could be a danger
Dr. Cosentino played down a major disagreement between the two guidelines, suggesting that “focusing on the differences leads to clinical inertia” by the practicing community when they are unsure how to reconcile the two positions.
Dr. Grant agreed that adding a second drug to metformin right away made sense in at least selected patients. “Look at each patient and decide whether they need glycemic control. If so, and if they also have cardiovascular disease, use both drugs,” metformin, plus one agent from one of the two newer classes.
Something both experts agreed on is that it’s time to generally steer clear of sulfonylurea drugs. “We have evidence for harmful effects from sulfonylureas,” Dr. Cosentino said.
“I’d dump sulfonylureas,” was Dr. Grant’s assessment, but he added that they still have a role for patients who need additional glycemic control but can’t afford the newer drugs.
Dr. Cosentino has had financial relationships with Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck, Mundipharma, Novo Nordisk, and Pfizer, Dr. Grant has lectured on behalf of AstraZeneca, GlaxoSmithKline, Merck, Novo Nordisk, the Medicines Company, and Takeda, and he has been an adviser to Amgen, AstraZeneca, Novartis, Novo Nordisk, and Synexus.
FROM EASD 2020
Primary prevention of VTE spans a spectrum
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment
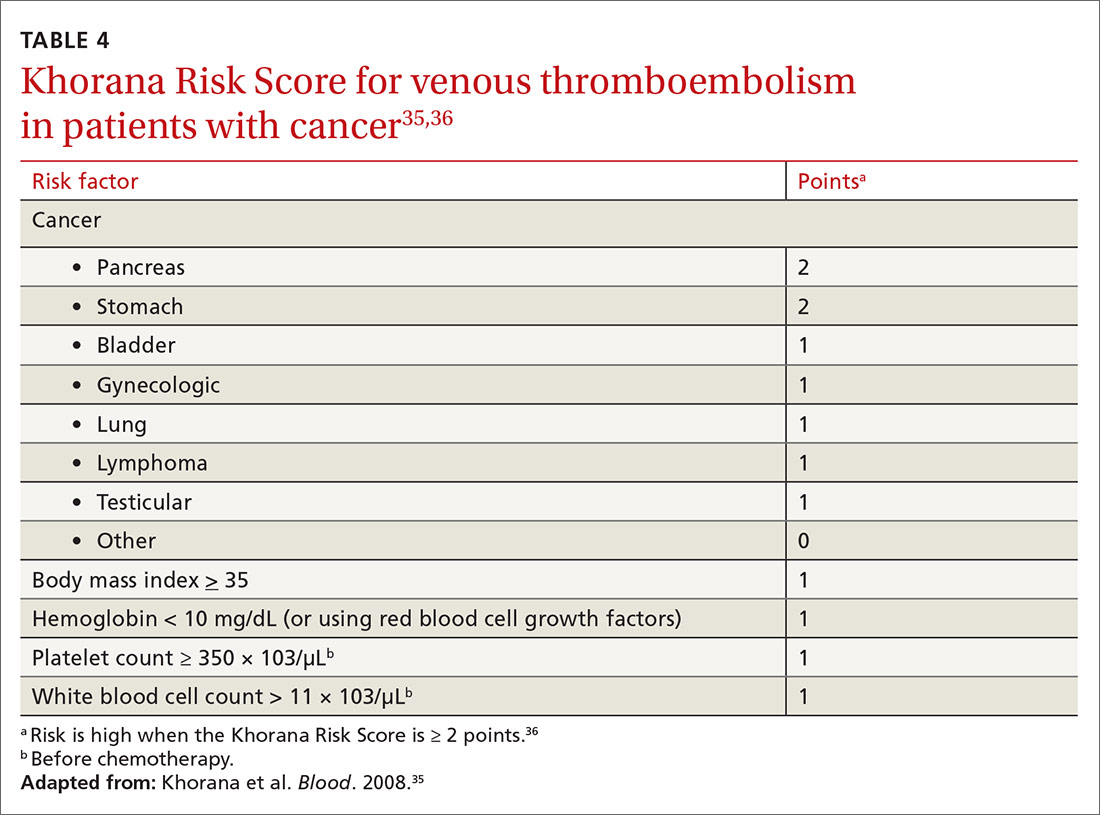
Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; michael.arnold@usuhs.edu.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; michael.arnold@usuhs.edu.
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; michael.arnold@usuhs.edu.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
PRACTICE RECOMMENDATIONS
› Consider the mild reduction in the risk of venous thromboembolism (VTE) provided by statins when contemplating their use for cardiovascular disease prevention. B
› Avoid testing for thrombophilia to determine the risk of VTE, except in pregnant patients who meet criteria for antiphospholipid syndrome or have a family history of VTE. B
› Recommend an intrauterine device or progestin-only pill for contraception if the patient’s risk of VTE is high. B
› Stratify hospitalized medical and nonorthopedic surgical patients by risk score to determine the need for VTE prophylaxis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A 31-year-old with a 3-week history of a waxing and waning, mildly pruritic eruption on his neck, chest, and back
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Prurigo pigmentosa is an inflammatory disorder of uncertain etiology characterized by the eruption of erythematous, markedly pruritic, urticaria-like papules and vesicles on the posterior neck, mid- to upper back, and chest. Crops of papules appear rapidly and then involute within days, leaving behind postinflammatory hyperpigmentation in a netlike configuration. New papules may appear prior to resolution of hyperpigmented macules, resulting in a mixed presentation of erythematous papules overlying reticulated hyperpigmentation.1
The condition was initially described in Japanese individuals, and to date, most cases have occurred in this population.2 However, the incidence of prurigo pigmentosa is increasing worldwide, including in the United States, which has led to the identification of several metabolic risk factors including diabetes mellitus, fasting, and dieting, with the common etiologic endpoint of ketosis.3With the increasing popularity of diets with strict carbohydrate limits, often with the goal of ketosis, dermatologists should be aware of the clinical appearance and common history of this rash to facilitate prompt diagnosis and treatment.
Clinical exam with appropriate history is usually sufficient for diagnosis. However, biopsy with histopathologic analysis can be utilized to confirm atypical cases. Histopathologic findings depend on the stage of the lesion biopsied. The earliest finding is a shallow perivascular neutrophilic infiltrate, neutrophil exocytosis, and epidermal and superficial dermal edema. As lesions progress, the prominent findings include epidermal vesiculation with necrotic keratinocytes and a lichenoid infiltrate dominated by lymphocytes and eosinophils. In the final stages, lesions demonstrate variable parakeratosis and acanthosis, as well as prominent dermal melanophagia.1
Treatment of prurigo pigmentosa includes modification of the patient’s underlying health issues to avoid ketosis, and in the case of diet-induced ketosis, reinstitution of a more balanced diet with sufficient carbohydrates. In the case of the patient presented here, rash resolved 1 week following instruction to include more carbohydrates in his diet. For recalcitrant cases or those without a clear precipitating factor, the addition of oral antibiotics is often helpful. Tetracyclines or dapsone are typically employed, usually in courses of 1-2 months.3,4
Dr. Johnson is a PGY-4 dermatology resident at Carilion Clinic in Roanoke, Va. He provided the case and photos. Donna Bilu Martin, MD, is the editor of the column.
References
1. Boer A et al. Am J Dermatopathol. 2003 Apr;25(2):117-292.
2. Satter E et al. J Cutan Pathol. 2016 Oct;43(10):809-14.
3. Alshaya M et al. JAAD Case Rep. 2019 Jun 8;5(6):504-7.
4. Hartman M et al. Cutis. 2019 Mar;103(3):E10-3.
Is your patient’s cannabis use problematic?
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.
Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).
CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27
Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).
Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; mhsu7@partners.org.
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.
Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).
CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27
Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).
Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; mhsu7@partners.org.
CASE
Jessica F is a new 23-year-old patient at your clinic who is seeing you to discuss her severe anxiety. She also has asthma and reports during your exploration of her family history that her father has been diagnosed with schizophrenia. She has been using 3 cartridges of cannabis vape daily to help “calm her mind” but has never tried other psychotropic medications and has never been referred to a psychiatrist.
How would you proceed with this patient?
Despite emerging evidence of the harmful effects of cannabis consumption, public perception of harm has steadily declined over the past 10 years.1,2 More adults are using cannabis than before and using it more frequently. Among primary care patients who consume cannabis recreationally, about half report less than monthly consumption; 15% use it weekly, and 20% daily.3 The potency of cannabis products has also increased. In the past 2 decades, the average tetrahydrocannabinol (THC) content of recreational cannabis rose from 3% to 19%, and high-THC content delivery modalities such as vaporizer pens (“vapes”) were introduced.4,5
Health hazards of cannabis use include gastrointestinal dysfunction (eg, cannabinoid hyperemesis syndrome), acute psychosis or exacerbation of an existing mood, anxiety, or psychotic disorder, and cardiovascular sequelae such as myocardial infarction or dysrhythmia.6 Potential long-term effects include neurocognitive impairment among adolescents who use cannabis,7-9 worse outcomes in anxiety and mood disorders,10 schizophrenia,11 cardiovascular sequelae,12 chronic bronchitis,13 negative impact on reproductive function,14 and poor birth outcomes.15-17
Hidden in plain sight. Many patients who use cannabis report that their primary care physicians are unaware of their cannabis consumption.18 Inadequate screening for cannabis can be attributed to time constraints, inconsistent definitions for problematic or risky cannabis use, and lack of guidance.19,20 This article offers a more inclusive definition of “problematic cannabis use,” presents an up-to-date framework for evaluating it in the outpatient setting, and outlines potential interventions.
Your patient doesn’t meetthe DSM criteria, but …
Although it is important to identify cannabis use disorder (CUD) as defined in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; TABLE 121,22), consider also the immediate and long-term consequences of cannabis use for individuals who do not meet criteria for CUD. “Problematic cannabis use,” as we define it, may also involve (a) high-risk behaviors or (b) contraindicating medical or psychiatric comorbidities (TABLE 26-9).
CASE
The patient in our case exhibited
Continue to: Guidelines for screening and evaluation
Guidelines for screening and evaluation
All primary care patients should be screened for problematic cannabis use, but especially teenagers, young adults, pregnant women, and patients with a mental health or substance use history. A variation of the single question used to screen for alcohol use disorder can be applied to cannabis use.23 We recommend asking the initial question, “Over the past month, how many days a week on average have you used cannabis and products that contain THC?” Although some guidelines emphasize frequency of cannabis use when identifying problematic consumption,24,25 duration of behavior and content of THC are also important indicators.19 Inquire about cannabis consumption over 1 month to differentiate sporadic use from longstanding persistent use.
Explore what types of cannabis the patient is ingesting and whether the patient uses cannabis heavily (4 or more times a week on average). Also determine the method of ingestion (eg, eating, vaping, smoking), THC-content (%, if known), and estimated weight of daily cannabis use in grams (TABLE 326). Although patients may not always be able to provide accurate answers, you can gain a sense of the quantity and forms of cannabis a patient is ingesting to inform future conversations on risk and harm reduction.27
Assess a patient’s risk for harm
Cannabis use has the potential to cause immediate harm (linked to a single event of problematic cannabis use) and long-term harm (linked to a recurring pattern of problematic consumption). Cannabis can be especially harmful for patients with the following medical comorbidities or psychosocial factors, and should be avoided.
Cardiovascular disease. Cannabis is associated with an elevated risk for acute coronary syndrome and cardiovascular disease.28 Long-term cannabis use is linked to increased frequency of anginal events, development of cardiac arrhythmias, peripheral arteritis, coronary vasospasms, and problems with platelet aggregation.29,30 Strongly caution against cannabis use with patients who have a history of cardiovascular disease, orthostatic hypotension, tachyarrhythmia, or hypertension.
Pulmonary disease. Patients with pulmonary disease such as asthma may find cannabis helpful as a short-term bronchodilator.31 However, for patients with underlying pulmonary disease who also smoke cigarettes, strongly discourage the smoking of cannabis or hashish, as that may worsen asthma symptoms,32 increase risk of chronic bronchitis,33 and increase cough, sputum production, and wheezing.31 There is currently insufficient evidence to suggest a positive association between cannabis use and the development of chronic obstructive pulmonary disease.34
Continue to: Family history of psychotic disorders
Family history of psychotic disorders. Cannabis is associated with a dose-dependent risk of schizophrenia, which is especially pronounced in patients with a family history of schizophrenia.35 Among patients with a history of psychosis, heavy cannabis use has been associated with increased hospitalizations, increased positive symptoms, and more frequent relapses.36-38
Pregnancy, current or planned. Some women turn to cannabis during pregnancy due to its antiemetic properties. However, perinatal exposure to cannabis is associated with significant risk to the offspring. Maternal cannabis use during the first and second trimesters of pregnancy is associated with decreased performance of the child on measures of function at 3 years of age.39 In addition, cannabis consumption during pregnancy is linked to increased frequency of childhood behavioral issues, inattention, hyperactivity, and impulsivity.40 Peripartum cannabis exposure can affect birth outcomes and is correlated with lower birth weight, incidence of preterm labor, and neonatal intensive care unit admission.15-17,41 Of note, the THC concentration in breast milk peaks at 1 hour after the nursing mother inhales cannabis and typically dissipates after 4 hours.42
Age < 25 years. Chronic heavy use of cannabis in those younger than 25 is associated with higher likelihood of developing CUD, lower IQ,9 lower level of educational attainment, lower income,43 and decreased executive function.8
Substance use disorder history. Recreational cannabis use can hinder recovery from other substance use disorders.44
Consider these 5 interventions
Physicians can address problematic cannabis use with a 5-pronged approach: (1) harm reduction, (2) motivational interviewing, (3) addressing underlying conditions, (4) mitigating withdrawal symptoms, and (5) referring to an addiction specialist (FIGURE).
Continue to: Harm reduction
Harm reduction
Harm reduction applies to all individuals who use cannabis but especially to problematic cannabis users. Ask users to abstain from cannabis for limited periods of time to see how such abstinence affects other areas of their life. While abstinence is a goal, be prepared to perform non-abstinence-based interventions. The goal of harm reduction is to encourage behaviors that minimize health risks to which cannabis users are exposed. Encourage patients to:
Abstain from driving while intoxicated. Cannabis use while driving slows reaction time,45 impairs road tracking (driving with correct road position),46 increases weaving,47 and causes a loss of anticipatory reactions learned in driving practice.48 Risk of crashing is significantly increased with elevated levels of THC, and driving within 1 hour of cannabis ingestion nearly doubles the risk of a crash.49-51
Abstain from vaping THC-containing products. The Centers for Disease Control and Prevention recommends that patients minimize the use of THC-containing e-cigarette or vaping products in light of the thousands of reports in the United States of product-associated lung injury, which in some cases have led to death.52
Clarify serving sizes and recognize delayed effects. Inexperienced cannabis users often are confused by recommended serving sizes for edible cannabis products. A typical cannabis-infused brownie may contain 100 mg of THC when the recommended serving size typically is 10 mg. THC content is included on the label of cannabis edibles purchased in state-regulated stores; these products are tested regularly in laboratories designated by the state.
Due to the delayed onset of THC’s effect, there have been numerous cases of patients taking a higher-than-intended dose of edible cannabis that caused acute intoxication and psychomedical sequelae leading to emergency hospital visits and, in some cases, death.6,53 Individuals should start at a low dose and gradually work up to a higher dose as tolerated. Patients naïve to cannabis should be especially cautious when ingesting edible products.
Continue to: Abstain from cannabis with high THC content
Abstain from cannabis with high THC content. High-potency cannabis (> 10% THC) is associated with earlier onset of first-episode psychosis.54,55
Motivational interviewing
Motivational interviewing (MI) is a psychosocial approach that emphasizes a patient’s self-efficacy and an interviewer’s positive feedback to collaboratively address substance use.56 MI can be performed in short, discrete sessions. Such interventions can reduce the average number of days of cannabis use. One large-scale Cochrane review found that cognitive behavioral therapy (CBT), motivational enhancement therapy, or the 2 therapies combined most consistently reduced the frequency of cannabis use reported by patients at early follow-up.57
Address underlying conditions
Some patients use cannabis to self-medicate for pain, insomnia, nausea, and anxiety. Identify these conditions and address them with first-line pharmacologic or psychotherapeutic interventions when possible. This is especially important for conditions in which long-term cannabis use may adversely impact outcomes, such as in posttraumatic stress disorder, anxiety, and mood disorders.58-60 Little evidence exists for the use of cannabis as treatment of any primary psychiatric disorder.61,62 Family physicians who are uncomfortable treating a specific underlying condition can consult specialists in pain management, sleep medicine, psychiatry, and neurology.
Mitigate withdrawal symptoms
Discontinuation of cannabis use may lead to withdrawal symptoms such as waxing and waning irritability, restlessness, sweating, aggression, anxiety, depressed mood, sleep disturbance, or changes in appetite.63,64 These symptoms typically emerge within the first couple days of abstinence and can last up to 28 days.63,64 Although the US Food and Drug Administration has not approved any medications for CUD treatment, and there are no established protocols for detoxification, there is evidence that CBT or medications such as gabapentin or zolpidem can reduce the intensity of withdrawal symptoms.65,66
Refer to an addiction specialist
Consider referring patients with problematic cannabis use to an addiction specialist with expertise in psychopharmacologic and psychotherapeutic approaches to managing substance use.
Continue to: CASE
CASE
You renew Ms. F’s asthma medications, discuss her cannabis use, start her on a selective serotonin reuptake inhibitor, and refer her to an outpatient psychiatrist. Over the next few weeks, you and the outpatient psychiatrist employ brief motivational interviewing around cannabis use, and you provide psychoeducation around potential harms of use when driving and in light of the patient’s asthma.
The patient’s anxiety symptoms decrease with up-titration of the SSRI by the outpatient psychiatrist and with enrollment in individual CBT. She is slowly able to taper off cannabis vaping with continued motivational interviewing and encouragement, despite withdrawal-induced anxiety and sleep disturbance.
CORRESPONDENCE
Michael Hsu, MD, Brigham & Women’s Hospital, 75 Francis Street, Boston, MA 02215; mhsu7@partners.org.
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
1. Sarvet AL, Wall MM, Keyes KM, et al. Recent rapid decrease in adolescents’ perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. 2018;186:68-74.
2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002-14: analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016;3:954-964.
3. Lapham GT, Lee AK, Caldeiro RM, et al. Frequency of cannabis use among primary care patients in Washington state. J Am Board Fam Med. 2017;30:795‐805.
4. Chandra S, Radwan MM, Majumdar CG, et al. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. 2019;269:5-15.
5. Sevigny EL, Pacula RL, Heaton P. The effects of medical marijuana laws on potency. Int J Drug Policy. 2014;25:308-319.
6. Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med. 2019;170:531-537.
7. Scott JC, Slomiak ST, Jones JD, et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:585-595.
8. Gruber SA, Sagar KA, Dahlgren MK, et al. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26:496-506.
9. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657-E2664.
10. Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79:17r11839.
11. Gage SH, Hickman M, Zammit S. Association between cannabis and psychosis: epidemiologic evidence. Biol Psychiatry. 2016;79:549-556.
12. Singh A, Saluja S, Kumar A, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7:45-59.
13. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219-2227.
14. Bari M, Battista N, Pirazzi V, et al. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed). 2011;16:498-516.
15. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215-219.
16. Saurel-Cubizolles M-J, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971-977.
17. Prunet C, Delnord M, Saurel-Cubizolles M-J, et al. Risk factors of preterm birth in France in 2010 and changes since 1995: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46:19-28.
18. Kondrad EC, Reed AJ, Simpson MJ, et al. Lack of communication about medical marijuana use between doctors and their patients. J Am Board Fam Med. 2018;31:805-808.
19. Casajuana C, López-Pelayo H, Balcells MM, et al. Definitions of risky and problematic cannabis use: a systematic review. Subst Use Misuse. 2016;51:1760-1770.
20. Norberg MM, Gates P, Dillon P, et al. Screening and managing cannabis use: comparing GP’s and nurses’ knowledge, beliefs, and behavior. Subst Abuse Treat Prev Policy. 2012;7:31.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: APA Publishing; 2013:509-516.
22. Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry. 2015;72:1235-1242.
23. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
24. Fischer B, Jones W, Shuper P, et al. 12-month follow-up of an exploratory ‘brief intervention’ for high-frequency cannabis users among Canadian university students. Subst Abuse Treat Prev Policy. 2012;7:15.
25. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Physician. 2014;60:801-808.
26. Smart R, Caulkins JP, Kilmer B, et al. Variation in cannabis potency & prices in a newly-legal market: evidence from 30 million cannabis sales in Washington State. Addiction. 2017;112:2167-2177.
27. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708-1709.
28. Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin Toxicol (Phila). 2019;57:831-841.
29. Subramaniam VN, Menezes AR, DeSchutter A, et al. The cardiovascular effects of marijuana: are the potential adverse effects worth the high? Mo Med. 2019;116:146-153.
30. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S-63S.
31. Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221-228.
32. Bramness JG, von Soest T. A longitudinal study of cannabis use increasing the use of asthma medication in young Norwegian adults. BMC Pulm Med. 2019;19:52.
33. Moore BA, Augustson EM, Moser RP, et al. Respiratory effects of marijuana and tobacco use in a U.S. sample. J Gen Intern Med. 2005;20:33-37.
34. Tashkin DP. Does marijuana pose risks for chronic airflow obstruction? Ann Am Thorac Soc. 2015;12:235-236.
35. McGuire PK, Jones P, Harvey I, et al. Morbid risk of schizophrenia for relatives of patients with cannabis-associated psychosis. Schizophr Res. 1995;15:277-281.
36. Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68-71.
37. Gerlach J, Koret B, Gereš N, et al. Clinical challenges in patients with first episode psychosis and cannabis use: mini-review and a case study. Psychiatr Danub. 2019;31(suppl 2):162-170.
38. Patel R, Wilson R, Jackson R, et al. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888.
39. Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169-175.
40. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
41. Corsi DJ, Walsh L, Weiss D, et al. Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA. 2019;322:145-152.
42. Baker T, Datta P, Rewers-Felkins K, et al. Transfer of inhaled cannabis into human breast milk. Obstet Gynecol. 2018;131:783-788.
43. Thompson K, Leadbeater B, Ames M, et al. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. 2019;20:257-269.
44. Yuan M, Kanellopoulos T, Kotbi N. Cannabis use and psychiatric illness in the context of medical marijuana legalization: a clinical perspective. Gen Hosp Psychiatry. 2019;61:82-83.
45. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40:926-934.
46. Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol Clin Exp. 1998;13(suppl 2):S70-S78.
47. Lenné MG, Dietze PM, Triggs TJ, et al. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859-866.
48. Anderson BM, Rizzo M, Block RI, et al. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19-30.
49. Laumon B, Gadegbeku B, Martin J-L, Biecheler M-B. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371.
50. Asbridge M, Poulin C, Donato A. Motor vehicle collision risk and driving under the influence of cannabis: evidence from adolescents in Atlantic Canada. Accid Anal Prev. 2005;37:1025-1034.
51. Mann RE, Adlaf E, Zhao J, et al. Cannabis use and self-reported collisions in a representative sample of adult drivers. J Safety Res. 2007;38:669-674.
52. Taylor J, Wiens T, Peterson J, et al. Characteristics of e-cigarette, or vaping, products used by patients with associated lung injury and products seized by law enforcement—Minnesota, 2018 and 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1096-1100.
53. Hancock-Allen JB, Barker L, VanDyke M, et al. Notes from the field: death following ingestion of an edible marijuana product—Colorado, March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:771-772.
54. Murray RM, Quigley H, Quattrone D, et al. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry. 2016;15:195-204.
55. Di Forti MD, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509-1517.
56. Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842.
57. Gates PJ, Sabioni P, Copeland J, et al. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;(5):CD005336.
58. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76:1174-1180.
59. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25:554-558.
60. Johnson MJ, Pierce JD, Mavandadi S, et al. Mental health symptom severity in cannabis using and non-using veterans with probable PTSD. J Affect Disord. 2016;190:439-442.
61. Wilkinson ST, Radhakrishnan R, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77:1050-1064.
62. Black N, Stockings E, Campbell G, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:995-1010.
63. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9-37.
64. Vandrey R, Smith MT, McCann UD, et al. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38-44.
65. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689-1698.
66. Weinstein A, Miller H, Tal E, et al. Treatment of cannabis withdrawal syndrome using cognitive-behavioral therapy and relapse prevention for cannabis dependence. J Groups Addict Recover. 2010;5:240-263.
PRACTICE RECOMMENDATIONS
› Address underlying conditions for which patients use recreational cannabis to manage symptoms. B
› Consider discrete, in-office sessions of motivational interviewing and referral for cognitive behavioral therapy for patients with problematic cannabis use. B
› Provide counseling around harm reduction for all patients—especially those with problematic cannabis use. C
› Consider referral to an addiction specialist for patients with cannabis use disorder or other problematic cannabis use. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Durable efficacy with MK-6482 in VHL-associated RCC
according to a presentation at the European Society for Medical Oncology Virtual Congress 2020.
MK-6482 is an oral inhibitor of hypoxia inducible factor-(HIF) 2-alpha. The drug previously showed favorable safety and antitumor activity in advanced RCC, Ramaprasad Srinivasan, MD, PhD, of the National Cancer Institute, Bethesda, Md., said when presenting data from the phase 2 trial.
Dr. Srinivasan noted that, in VHL disease, RCC occurs in 25%-60% of individuals and is a key cause of morbidity and shortened life expectancy despite aggressive treatment. HIF-2-alpha accumulation activates genes that drive tumor growth in VHL-associated RCC.
The primary objective of Dr. Srinivasan’s phase 2 study was to evaluate the efficacy of the HIF-2-alpha inhibitor MK-6482 (at 120 mg daily) for the treatment of VHL-associated RCC.
The study included 61 treatment-naive patients with VHL diagnoses based on germline mutations. All subjects had RCC and additional non-RCC lesions, including pancreatic (100%), central nervous system (CNS) hemangioblastoma (70.5%), and retinal lesions (26.2%).
The patients’ median age at baseline was 41 years (range, 19-66), and 52.5% were men. Most (82%) had an European Cooperative Oncology Group performance status of 0.
Efficacy and safety
At a median follow-up of 68.7 weeks, 56 patients were receiving ongoing treatment.
By independent central review, the overall response rate in target RCC lesions was 36.1% (all partial responses), with unconfirmed partial responses in 11.5% and stable disease in 62.3%. There was no progression in target lesions. Decreases in target lesion size were observed in 91.8% of patients.
The median time to response was 31.1 weeks (range, 11.9-62.3 weeks), and the median duration of response was not reached (range, 11.9-62.3 weeks). The 1-year progression-free survival rate was 98.3%.
“Promising clinical activity was observed with MK-6482 in treatment-naive patients with VHL-associated RCC,” Dr. Srinivasan said. He added that efficacy was durable in both RCC and non-renal lesions.
Complete responses were observed in 6.6% (4/61) of pancreatic lesions and 11.6% (5/43) of CNS hemangioblastomas. Partial response and stable disease rates in pancreatic lesions were 57.4% and 34.4%, respectively. Partial response and stable disease rates in CNS hemangioblastomas were 18.6% and 65.1%, respectively.
In the 16 patients with retinal lesions, 68.8% saw an improvement and 25% had stable disease. No progression was reported.
“MK-6482 was well tolerated and has a favorable safety profile,” Dr. Srinivasan noted.
Most patients (98.4%) had treatment-related adverse events (AEs), with anemia being the most common. Grade 3 AEs included anemia (6.6%), fatigue (4.9%), dyspnea (1.6%), and hypoxia (1.6%). One patient (1.6%) discontinued treatment because of grade 1 dizziness. There was one grade 4 AE and one fatal AE, but both were considered unrelated to study treatment.
Remaining questions and next steps
The challenge in managing VHL-associated RCC tumors is finding a balance between the risk of cancer dissemination and renal morbidity, said study discussant Cristina Suárez, MD, PhD, of Hospital Universitari Vall d’Hebron in Barcelona.
“There is no standard of care systemic treatment, and recruitment for clinical trials is challenging,” Dr. Suárez added.
While response rates in RCC lesions with MK-6482 were generally in line with the experience reported for sunitinib and pazopanib, response rates were particularly favorable with MK-6482 in pancreatic lesions and CNS hemangioblastomas, Dr. Suárez said.
“These are the best response rates reported in non-RCC lesions,” she noted.
However, Dr. Suárez said, important questions remain. Specifically, how long should patients continue on treatment, and will lesion rebound occur after treatment discontinuation?
Larger multicenter trials are needed, Dr. Suárez said, pointing out that the current study is the largest to date of systemic therapy for patients with VHL disease.
The study was funded by Merck Sharp & Dohme Corp. Dr. Srinivasan disclosed funding from Merck and Calithera Biosciences. Dr. Suárez disclosed relationships with Astellas, AstraZeneca, Bayer, and many other companies.
SOURCE: Srinivasan R et al. ESMO 2020. Abstract LBA26.
according to a presentation at the European Society for Medical Oncology Virtual Congress 2020.
MK-6482 is an oral inhibitor of hypoxia inducible factor-(HIF) 2-alpha. The drug previously showed favorable safety and antitumor activity in advanced RCC, Ramaprasad Srinivasan, MD, PhD, of the National Cancer Institute, Bethesda, Md., said when presenting data from the phase 2 trial.
Dr. Srinivasan noted that, in VHL disease, RCC occurs in 25%-60% of individuals and is a key cause of morbidity and shortened life expectancy despite aggressive treatment. HIF-2-alpha accumulation activates genes that drive tumor growth in VHL-associated RCC.
The primary objective of Dr. Srinivasan’s phase 2 study was to evaluate the efficacy of the HIF-2-alpha inhibitor MK-6482 (at 120 mg daily) for the treatment of VHL-associated RCC.
The study included 61 treatment-naive patients with VHL diagnoses based on germline mutations. All subjects had RCC and additional non-RCC lesions, including pancreatic (100%), central nervous system (CNS) hemangioblastoma (70.5%), and retinal lesions (26.2%).
The patients’ median age at baseline was 41 years (range, 19-66), and 52.5% were men. Most (82%) had an European Cooperative Oncology Group performance status of 0.
Efficacy and safety
At a median follow-up of 68.7 weeks, 56 patients were receiving ongoing treatment.
By independent central review, the overall response rate in target RCC lesions was 36.1% (all partial responses), with unconfirmed partial responses in 11.5% and stable disease in 62.3%. There was no progression in target lesions. Decreases in target lesion size were observed in 91.8% of patients.
The median time to response was 31.1 weeks (range, 11.9-62.3 weeks), and the median duration of response was not reached (range, 11.9-62.3 weeks). The 1-year progression-free survival rate was 98.3%.
“Promising clinical activity was observed with MK-6482 in treatment-naive patients with VHL-associated RCC,” Dr. Srinivasan said. He added that efficacy was durable in both RCC and non-renal lesions.
Complete responses were observed in 6.6% (4/61) of pancreatic lesions and 11.6% (5/43) of CNS hemangioblastomas. Partial response and stable disease rates in pancreatic lesions were 57.4% and 34.4%, respectively. Partial response and stable disease rates in CNS hemangioblastomas were 18.6% and 65.1%, respectively.
In the 16 patients with retinal lesions, 68.8% saw an improvement and 25% had stable disease. No progression was reported.
“MK-6482 was well tolerated and has a favorable safety profile,” Dr. Srinivasan noted.
Most patients (98.4%) had treatment-related adverse events (AEs), with anemia being the most common. Grade 3 AEs included anemia (6.6%), fatigue (4.9%), dyspnea (1.6%), and hypoxia (1.6%). One patient (1.6%) discontinued treatment because of grade 1 dizziness. There was one grade 4 AE and one fatal AE, but both were considered unrelated to study treatment.
Remaining questions and next steps
The challenge in managing VHL-associated RCC tumors is finding a balance between the risk of cancer dissemination and renal morbidity, said study discussant Cristina Suárez, MD, PhD, of Hospital Universitari Vall d’Hebron in Barcelona.
“There is no standard of care systemic treatment, and recruitment for clinical trials is challenging,” Dr. Suárez added.
While response rates in RCC lesions with MK-6482 were generally in line with the experience reported for sunitinib and pazopanib, response rates were particularly favorable with MK-6482 in pancreatic lesions and CNS hemangioblastomas, Dr. Suárez said.
“These are the best response rates reported in non-RCC lesions,” she noted.
However, Dr. Suárez said, important questions remain. Specifically, how long should patients continue on treatment, and will lesion rebound occur after treatment discontinuation?
Larger multicenter trials are needed, Dr. Suárez said, pointing out that the current study is the largest to date of systemic therapy for patients with VHL disease.
The study was funded by Merck Sharp & Dohme Corp. Dr. Srinivasan disclosed funding from Merck and Calithera Biosciences. Dr. Suárez disclosed relationships with Astellas, AstraZeneca, Bayer, and many other companies.
SOURCE: Srinivasan R et al. ESMO 2020. Abstract LBA26.
according to a presentation at the European Society for Medical Oncology Virtual Congress 2020.
MK-6482 is an oral inhibitor of hypoxia inducible factor-(HIF) 2-alpha. The drug previously showed favorable safety and antitumor activity in advanced RCC, Ramaprasad Srinivasan, MD, PhD, of the National Cancer Institute, Bethesda, Md., said when presenting data from the phase 2 trial.
Dr. Srinivasan noted that, in VHL disease, RCC occurs in 25%-60% of individuals and is a key cause of morbidity and shortened life expectancy despite aggressive treatment. HIF-2-alpha accumulation activates genes that drive tumor growth in VHL-associated RCC.
The primary objective of Dr. Srinivasan’s phase 2 study was to evaluate the efficacy of the HIF-2-alpha inhibitor MK-6482 (at 120 mg daily) for the treatment of VHL-associated RCC.
The study included 61 treatment-naive patients with VHL diagnoses based on germline mutations. All subjects had RCC and additional non-RCC lesions, including pancreatic (100%), central nervous system (CNS) hemangioblastoma (70.5%), and retinal lesions (26.2%).
The patients’ median age at baseline was 41 years (range, 19-66), and 52.5% were men. Most (82%) had an European Cooperative Oncology Group performance status of 0.
Efficacy and safety
At a median follow-up of 68.7 weeks, 56 patients were receiving ongoing treatment.
By independent central review, the overall response rate in target RCC lesions was 36.1% (all partial responses), with unconfirmed partial responses in 11.5% and stable disease in 62.3%. There was no progression in target lesions. Decreases in target lesion size were observed in 91.8% of patients.
The median time to response was 31.1 weeks (range, 11.9-62.3 weeks), and the median duration of response was not reached (range, 11.9-62.3 weeks). The 1-year progression-free survival rate was 98.3%.
“Promising clinical activity was observed with MK-6482 in treatment-naive patients with VHL-associated RCC,” Dr. Srinivasan said. He added that efficacy was durable in both RCC and non-renal lesions.
Complete responses were observed in 6.6% (4/61) of pancreatic lesions and 11.6% (5/43) of CNS hemangioblastomas. Partial response and stable disease rates in pancreatic lesions were 57.4% and 34.4%, respectively. Partial response and stable disease rates in CNS hemangioblastomas were 18.6% and 65.1%, respectively.
In the 16 patients with retinal lesions, 68.8% saw an improvement and 25% had stable disease. No progression was reported.
“MK-6482 was well tolerated and has a favorable safety profile,” Dr. Srinivasan noted.
Most patients (98.4%) had treatment-related adverse events (AEs), with anemia being the most common. Grade 3 AEs included anemia (6.6%), fatigue (4.9%), dyspnea (1.6%), and hypoxia (1.6%). One patient (1.6%) discontinued treatment because of grade 1 dizziness. There was one grade 4 AE and one fatal AE, but both were considered unrelated to study treatment.
Remaining questions and next steps
The challenge in managing VHL-associated RCC tumors is finding a balance between the risk of cancer dissemination and renal morbidity, said study discussant Cristina Suárez, MD, PhD, of Hospital Universitari Vall d’Hebron in Barcelona.
“There is no standard of care systemic treatment, and recruitment for clinical trials is challenging,” Dr. Suárez added.
While response rates in RCC lesions with MK-6482 were generally in line with the experience reported for sunitinib and pazopanib, response rates were particularly favorable with MK-6482 in pancreatic lesions and CNS hemangioblastomas, Dr. Suárez said.
“These are the best response rates reported in non-RCC lesions,” she noted.
However, Dr. Suárez said, important questions remain. Specifically, how long should patients continue on treatment, and will lesion rebound occur after treatment discontinuation?
Larger multicenter trials are needed, Dr. Suárez said, pointing out that the current study is the largest to date of systemic therapy for patients with VHL disease.
The study was funded by Merck Sharp & Dohme Corp. Dr. Srinivasan disclosed funding from Merck and Calithera Biosciences. Dr. Suárez disclosed relationships with Astellas, AstraZeneca, Bayer, and many other companies.
SOURCE: Srinivasan R et al. ESMO 2020. Abstract LBA26.
ESMO 2020