User login
Biologics for psoriasis may also reduce coronary plaque
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
FROM CIRCULATION: CARDIOVASCULAR IMAGING
Many providers don’t follow hypertension guidelines
Many health care professionals are not following current, evidence-based guidelines to screen for and diagnose hypertension, and appear to have substantial gaps in knowledge, beliefs, and use of recommended practices, results from a large survey suggest.
“One surprising finding was that there was so much trust in the stethoscope, because the automated monitors are a better way to take blood pressure,” lead author Beverly Green, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The results of the survey were presented Sept. 10 at the virtual joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The U.S. Preventive Services Task Force (USPSTF) and the American Heart Association/American College of Cardiology recommend out-of-office blood pressure measurements – via ambulatory blood pressure monitoring (ABPM) or home BP monitoring – before making a new diagnosis of hypertension.
To gauge provider knowledge, beliefs, and practices related to BP diagnostic tests, the researchers surveyed 282 providers: 102 medical assistants (MA), 28 licensed practical nurses (LPNs), 33 registered nurses (RNs), 86 primary care physicians, and 33 advanced practitioners (APs).
More than three-quarters of providers (79%) felt that BP measured manually with a stethoscope and ABPM were “very or highly” accurate ways to measure BP when making a new diagnosis of hypertension.
Most did not think that automated clinic BPs, home BP, or kiosk BP measurements were very or highly accurate.
Nearly all providers surveyed (96%) reported that they “always or almost always” rely on clinic BP measurements when diagnosing hypertension, but the majority of physicians/APs would prefer using ABPM (61%) if available.
The problem with ABPM, said Dr. Green, is “it’s just not very available or convenient for patients, and a lot of providers think that patients won’t tolerate it.” Yet, without it, there is a risk for misclassification, she said.
Karen A. Griffin, MD, who chairs the AHA Council on Hypertension, said it became “customary to use clinic BP since ABPM was not previously reimbursed for the routine diagnosis of hypertension.
“Now that the payment for ABPM has been expanded, the number of machines at most institutions is not adequate for the need. Consequently, it will take some time to catch up with the current guidelines for diagnosing hypertension,” she said in an interview.
The provider survey by Dr. Green and colleagues also shows slow uptake of updated thresholds for high blood pressure.
Eighty-four percent of physicians/APs and 68% of MA/LPN/RNs said they used a clinic BP threshold of at least 140/90 mm Hg for making a new diagnosis of hypertension.
Only 3.5% and 9.0%, respectively, reported using the updated threshold of at least 130/80 mm Hg put forth in 2017.
Dr. Griffin said part of this stems from the fact that the survey began before the updated guidelines were released in 2017, “not to mention the fact that some societies have opposed the new threshold of 130/80 mm Hg.”
“I think, with time, the data on morbidity and mortality associated with the goal of 130/80 mm Hg will hopefully convince those who have not yet implemented these new guidelines that it is a safe and effective BP goal,” Dr. Griffin said.
This research had no specific funding. Dr. Green and Dr. Griffin have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Many health care professionals are not following current, evidence-based guidelines to screen for and diagnose hypertension, and appear to have substantial gaps in knowledge, beliefs, and use of recommended practices, results from a large survey suggest.
“One surprising finding was that there was so much trust in the stethoscope, because the automated monitors are a better way to take blood pressure,” lead author Beverly Green, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The results of the survey were presented Sept. 10 at the virtual joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The U.S. Preventive Services Task Force (USPSTF) and the American Heart Association/American College of Cardiology recommend out-of-office blood pressure measurements – via ambulatory blood pressure monitoring (ABPM) or home BP monitoring – before making a new diagnosis of hypertension.
To gauge provider knowledge, beliefs, and practices related to BP diagnostic tests, the researchers surveyed 282 providers: 102 medical assistants (MA), 28 licensed practical nurses (LPNs), 33 registered nurses (RNs), 86 primary care physicians, and 33 advanced practitioners (APs).
More than three-quarters of providers (79%) felt that BP measured manually with a stethoscope and ABPM were “very or highly” accurate ways to measure BP when making a new diagnosis of hypertension.
Most did not think that automated clinic BPs, home BP, or kiosk BP measurements were very or highly accurate.
Nearly all providers surveyed (96%) reported that they “always or almost always” rely on clinic BP measurements when diagnosing hypertension, but the majority of physicians/APs would prefer using ABPM (61%) if available.
The problem with ABPM, said Dr. Green, is “it’s just not very available or convenient for patients, and a lot of providers think that patients won’t tolerate it.” Yet, without it, there is a risk for misclassification, she said.
Karen A. Griffin, MD, who chairs the AHA Council on Hypertension, said it became “customary to use clinic BP since ABPM was not previously reimbursed for the routine diagnosis of hypertension.
“Now that the payment for ABPM has been expanded, the number of machines at most institutions is not adequate for the need. Consequently, it will take some time to catch up with the current guidelines for diagnosing hypertension,” she said in an interview.
The provider survey by Dr. Green and colleagues also shows slow uptake of updated thresholds for high blood pressure.
Eighty-four percent of physicians/APs and 68% of MA/LPN/RNs said they used a clinic BP threshold of at least 140/90 mm Hg for making a new diagnosis of hypertension.
Only 3.5% and 9.0%, respectively, reported using the updated threshold of at least 130/80 mm Hg put forth in 2017.
Dr. Griffin said part of this stems from the fact that the survey began before the updated guidelines were released in 2017, “not to mention the fact that some societies have opposed the new threshold of 130/80 mm Hg.”
“I think, with time, the data on morbidity and mortality associated with the goal of 130/80 mm Hg will hopefully convince those who have not yet implemented these new guidelines that it is a safe and effective BP goal,” Dr. Griffin said.
This research had no specific funding. Dr. Green and Dr. Griffin have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Many health care professionals are not following current, evidence-based guidelines to screen for and diagnose hypertension, and appear to have substantial gaps in knowledge, beliefs, and use of recommended practices, results from a large survey suggest.
“One surprising finding was that there was so much trust in the stethoscope, because the automated monitors are a better way to take blood pressure,” lead author Beverly Green, MD, of Kaiser Permanente Washington Health Research Institute, Seattle, said in an interview.
The results of the survey were presented Sept. 10 at the virtual joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The U.S. Preventive Services Task Force (USPSTF) and the American Heart Association/American College of Cardiology recommend out-of-office blood pressure measurements – via ambulatory blood pressure monitoring (ABPM) or home BP monitoring – before making a new diagnosis of hypertension.
To gauge provider knowledge, beliefs, and practices related to BP diagnostic tests, the researchers surveyed 282 providers: 102 medical assistants (MA), 28 licensed practical nurses (LPNs), 33 registered nurses (RNs), 86 primary care physicians, and 33 advanced practitioners (APs).
More than three-quarters of providers (79%) felt that BP measured manually with a stethoscope and ABPM were “very or highly” accurate ways to measure BP when making a new diagnosis of hypertension.
Most did not think that automated clinic BPs, home BP, or kiosk BP measurements were very or highly accurate.
Nearly all providers surveyed (96%) reported that they “always or almost always” rely on clinic BP measurements when diagnosing hypertension, but the majority of physicians/APs would prefer using ABPM (61%) if available.
The problem with ABPM, said Dr. Green, is “it’s just not very available or convenient for patients, and a lot of providers think that patients won’t tolerate it.” Yet, without it, there is a risk for misclassification, she said.
Karen A. Griffin, MD, who chairs the AHA Council on Hypertension, said it became “customary to use clinic BP since ABPM was not previously reimbursed for the routine diagnosis of hypertension.
“Now that the payment for ABPM has been expanded, the number of machines at most institutions is not adequate for the need. Consequently, it will take some time to catch up with the current guidelines for diagnosing hypertension,” she said in an interview.
The provider survey by Dr. Green and colleagues also shows slow uptake of updated thresholds for high blood pressure.
Eighty-four percent of physicians/APs and 68% of MA/LPN/RNs said they used a clinic BP threshold of at least 140/90 mm Hg for making a new diagnosis of hypertension.
Only 3.5% and 9.0%, respectively, reported using the updated threshold of at least 130/80 mm Hg put forth in 2017.
Dr. Griffin said part of this stems from the fact that the survey began before the updated guidelines were released in 2017, “not to mention the fact that some societies have opposed the new threshold of 130/80 mm Hg.”
“I think, with time, the data on morbidity and mortality associated with the goal of 130/80 mm Hg will hopefully convince those who have not yet implemented these new guidelines that it is a safe and effective BP goal,” Dr. Griffin said.
This research had no specific funding. Dr. Green and Dr. Griffin have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
COVID-19 and the psychological side effects of PPE
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at cpnews@mdedge.com.
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at cpnews@mdedge.com.
A few months ago, I published a short thought piece on the use of “sitters” with patients who were COVID-19 positive, or patients under investigation. In it, I recommended the use of telesitters for those who normally would warrant a human sitter, to decrease the discomfort of sitting in full personal protective equipment (PPE) (gown, mask, gloves, etc.) while monitoring a suicidal patient.
I received several queries, which I want to address here. In addition, I want to draw from my Army days in terms of the claustrophobia often experienced with PPE.
The first of the questions was about evidence-based practices. The second was about the discomfort of having sitters sit for many hours in the full gear.
I do not know of any evidence-based practices, but I hope we will develop them.
I agree that spending many hours in full PPE can be discomforting, which is why I wrote the essay.
As far as lessons learned from the Army time, I briefly learned how to wear a “gas mask” or Mission-Oriented Protective Posture (MOPP gear) while at Fort Bragg. We were run through the “gas chamber,” where sergeants released tear gas while we had the mask on. We were then asked to lift it up, and then tearing and sputtering, we could leave the small wooden building.
We wore the mask as part of our Army gear, usually on the right leg. After that, I mainly used the protective mask in its bag as a pillow when I was in the field.
Fast forward to August 1990. I arrived at Camp Casey, near the Korean demilitarized zone. Four days later, Saddam Hussein invaded Kuwait. The gas mask moved from a pillow to something we had to wear while doing 12-mile road marches in “full ruck.” In full ruck, you have your uniform on, with TA-50, knapsack, and weapon. No, I do not remember any more what TA-50 stands for, but essentially it is the webbing that holds your bullets and bandages.
Many could not tolerate it. They developed claustrophobia – sweating, air hunger, and panic. If stationed in the Gulf for Operation Desert Storm, they were evacuated home.
I wrote a couple of short articles on treatment of gas mask phobia.1,2 I basically advised desensitization. Start by watching TV in it for 5 minutes. Graduate to ironing your uniform in the mask. Go then to shorter runs. Work up to the 12-mile road march.
In my second tour in Korea, we had exercises where we simulated being hit by nerve agents and had to operate the hospital for days at a time in partial or full PPE. It was tough but we did it, and felt more confident about surviving attacks from North Korea.
So back to the pandemic present. I have gotten more used to my constant wearing of a surgical mask. I get anxious when I see others with masks below their noses.
The pandemic is not going away anytime soon, in my opinion. Furthermore, there are other viruses that are worse, such as Ebola. It is only a matter of time.
So, let us train with our PPE. If health care workers cannot tolerate them, use desensitization- and anxiety-reducing techniques to help them.
There are no easy answers here, in the time of the COVID pandemic. However, we owe it to ourselves, our patients, and society to do the best we can.
References
1. Ritchie EC. Milit Med. 1992 Feb;157(2):104-6.
2. Ritchie EC. Milit Med. 2001 Dec;166. Suppl. 2(1)83-4.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures and can be reached at cpnews@mdedge.com.
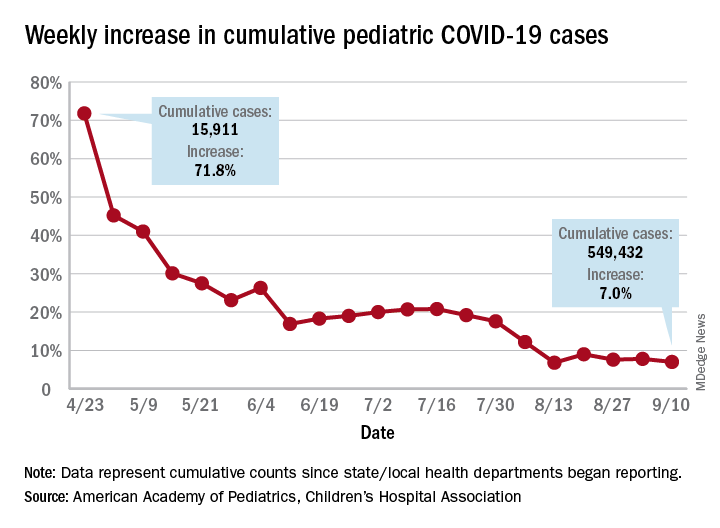
Children and COVID-19: New cases may be leveling off
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Growth in new pediatric COVID-19 cases has evened out in recent weeks, but children now represent 10% of all COVID-19 cases in the United States, and that measurement has been rising throughout the pandemic, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in the report, based on data from 49 states (New York City is included but not New York state), the District of Columbia, Puerto Rico, and Guam.
The weekly percentage of increase in the number of new cases has not reached double digits since early August and has been no higher than 7.8% over the last 3 weeks. The number of child COVID-19 cases, however, has finally reached 10% of the total for Americans of all ages, which stands at 5.49 million in the jurisdictions included in the report, the AHA and CHA reported.
Measures, however, continue to show low levels of severe illness in children, they noted, including the following:
- Child cases as a proportion of all COVID-19 hospitalizations: 1.7%.
- Hospitalization rate for children: 1.8%.
- Child deaths as a proportion of all deaths: 0.07%.
- Percent of child cases resulting in death: 0.01%.
The number of cumulative cases per 100,000 children is now up to 728.5 nationally, with a range by state that goes from 154.0 in Vermont to 1,670.3 in Tennessee, which is one of only two states reporting cases in those aged 0-20 years as children (the other is South Carolina). The age range for children is 0-17 or 0-19 for most other states, although Florida uses a range of 0-14, the report notes.
Other than Tennessee, there are 10 states with overall rates higher than 1,000 COVID-19 cases per 100,000 children, and there are nine states with cumulative totals over 15,000 cases (California is the highest with just over 75,000), according to the report.
Conspiracy theories
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
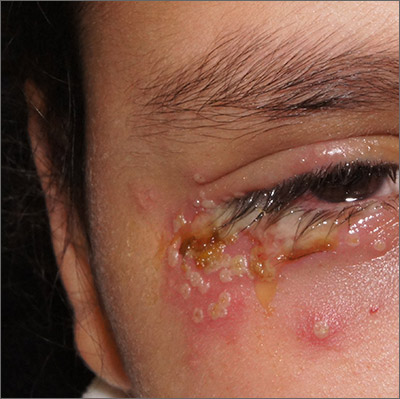
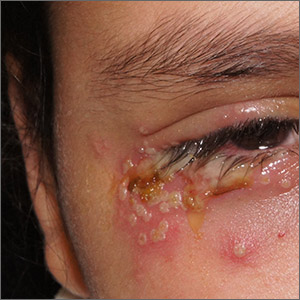
Painful periocular rash
This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
This patient was given a diagnosis of primary herpes simplex virus (HSV) based on the appearance of her eyelid. Swabs were performed for bacterial culture, and polymerase chain reaction (PCR) testing was done for HSV and varicella, but results were pending prior to her transfer to the Emergency Department (ED).
The patient was given a single dose of 800 mg oral acyclovir (200 mg/5mL) and 500 mg of oral cephalexin (250 mg/5mL) and referred to the ED for a more detailed eye exam and to exclude orbital erosions.
HSV classically causes clustered vesicles on an erythematous base. Superinfection with skin flora can cause pustules instead of vesicles. Severe complications of HSV can include widespread skin involvement, eczema herpeticum, local destruction, central nervous system involvement, throat infections (affecting airway and oral intake), and dissemination in immunocompromised hosts. Ocular or periorbital infections increase the risk of keratitis, corneal ulcers, and loss of sight. Viral involvement of the cornea is best seen with fluorescein staining.
In cases like this one, PCR is the preferred method of testing over viral cultures or serology, given its speed, accuracy, and temporal relevance. Ophthalmology referral is warranted, although it should not delay treatment. Topical and oral antivirals are both effective when treating corneal disease; patient preference should be considered.
Most cases of HSV may resolve without treatment; however, treatment started while vesicles are present and within 72 hours of infection may shorten the time of viral replication and prevent progression to stromal involvement.
After a 12-hour wait in the ED, this patient was seen by an ophthalmology resident who did not observe orbital erosions but did note umbilication and misdiagnosed molluscum contagiosum. Umbilication is not pathognomonic for molluscum; few experienced in diagnosing molluscum contagiosum would make this error.
The patient was instructed to stop the acyclovir. Two days later when the PCR came back positive for HSV-1 and the bacterial culture confirmed growth of superimposed Staphylococcus aureus, the patient had been lost to follow-up. A better approach would have been for the ophthalmology resident to continue the acyclovir until PCR excluded herpetic disease.
Text courtesy of Tristan Reynolds, DO, Maine Dartmouth Family Medicine Residency, and Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
Barker NH. Ocular herpes simplex. BMJ Clin Evid. 2008;2008:0707.
The importance of character
Early autumn is typically a quiet time for outpatient pediatricians. The school physicals are finished. The last-minute school physicals are finished. The “I forgot to get my child’s physical” physicals are finished. Respiratory syncytial virus and influenza seasons haven’t started. There is time for some self-reflection and sharpening the saw.
My reflective period each year tends to start with the unresolved “What do I want to be when I grow up?” Mind you, just because I’ve grown old doesn’t mean I’ve grown up. I never wanted to be a “grande personne” who, per Antoine de Saint-Exupéry in “Le Petit Prince,” will never understand why a minor item (Did the lamb eat the flower?) makes all the difference in the universe to a child. Awe and wonderment should remain a part of life. I enjoy reading that short story in the original French because, as my high school French vocabulary and conjugation have faded, any word I don’t recognize means exactly what my journey of a lifetime tells me it means, neither more nor less, just as Humpty Dumpty explained to Alice in Lewis Carroll’s “Through the Looking Glass.”
Along with my perennial favorites like “Le Petit Prince” and the Gettysburg Address, in this year’s folder for reflection are two essays I’ve collected this year. The first is a letter addressed from medical ethicist Ira Bedzow, PhD, to this year’s incoming class of medical students.
The essay gives advice to first-year medical students entering the profession of medicine. It talks about finding “something to say that you communicate with the whole and essence of your being.” There is lots of great counsel in the letter. It claims, “Only in a professional does one’s voice sing in harmony with one’s being. Want that for yourselves, for only a life undivided is a life of full integrity.”
I agree with the harmony part. I hesitate with the undivided part. A professional singer could be dedicated to opera but still sing in a barbershop quartet and a church choir, motivated by fun and fellowship. It is important to emphasize integrity and dedication to medical students. The letter does that well, but students must also develop a work-life balance. The ascetic life is not for everyone.
Life needs balance and moderation. I am pretty sure that Aristotle said that, but I never did spend much time studying the Classics. I use my periods of self-reflection to chart my life’s vector. I choose new skills to learn and challenges to meet. But as I grow older, I spend more time pruning those roles that no longer give me joy. Delayed gratification is an important character trait for success, but its value lessens as it becomes clear there are more days behind me than ahead.
The second essay reflects the views of Canon Brodar, a third-year medical student and divinity school graduate.
He attests to the willingness of medical trainees to accept their duties and personal risk during the crisis of the COVID-19 pandemic. He correctly points out the contributions his fellow students could make, but underestimates the negatives. During March 2020 when decisions were made to send third-year medical students home, the administrative focus was on the cost of their participation (consumption of scarce personal protective equipment) and the potential negative consequences (an additional person who might transmit the virus among patients.) Four months later, most medical students were back on the job.
Mr. Brodar’s eloquent description of duty and responsibility complement, and perhaps have evolved from, the integrity and dedication that Dr. Bedzow emphasized to incoming medical students. These are all character traits. These traits are not knowledge of anatomy or skill with a scalpel. With experience come two more key character traits – the moderation of a work-life balance and the judgment to weigh benefits, risks, and costs.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
Early autumn is typically a quiet time for outpatient pediatricians. The school physicals are finished. The last-minute school physicals are finished. The “I forgot to get my child’s physical” physicals are finished. Respiratory syncytial virus and influenza seasons haven’t started. There is time for some self-reflection and sharpening the saw.
My reflective period each year tends to start with the unresolved “What do I want to be when I grow up?” Mind you, just because I’ve grown old doesn’t mean I’ve grown up. I never wanted to be a “grande personne” who, per Antoine de Saint-Exupéry in “Le Petit Prince,” will never understand why a minor item (Did the lamb eat the flower?) makes all the difference in the universe to a child. Awe and wonderment should remain a part of life. I enjoy reading that short story in the original French because, as my high school French vocabulary and conjugation have faded, any word I don’t recognize means exactly what my journey of a lifetime tells me it means, neither more nor less, just as Humpty Dumpty explained to Alice in Lewis Carroll’s “Through the Looking Glass.”
Along with my perennial favorites like “Le Petit Prince” and the Gettysburg Address, in this year’s folder for reflection are two essays I’ve collected this year. The first is a letter addressed from medical ethicist Ira Bedzow, PhD, to this year’s incoming class of medical students.
The essay gives advice to first-year medical students entering the profession of medicine. It talks about finding “something to say that you communicate with the whole and essence of your being.” There is lots of great counsel in the letter. It claims, “Only in a professional does one’s voice sing in harmony with one’s being. Want that for yourselves, for only a life undivided is a life of full integrity.”
I agree with the harmony part. I hesitate with the undivided part. A professional singer could be dedicated to opera but still sing in a barbershop quartet and a church choir, motivated by fun and fellowship. It is important to emphasize integrity and dedication to medical students. The letter does that well, but students must also develop a work-life balance. The ascetic life is not for everyone.
Life needs balance and moderation. I am pretty sure that Aristotle said that, but I never did spend much time studying the Classics. I use my periods of self-reflection to chart my life’s vector. I choose new skills to learn and challenges to meet. But as I grow older, I spend more time pruning those roles that no longer give me joy. Delayed gratification is an important character trait for success, but its value lessens as it becomes clear there are more days behind me than ahead.
The second essay reflects the views of Canon Brodar, a third-year medical student and divinity school graduate.
He attests to the willingness of medical trainees to accept their duties and personal risk during the crisis of the COVID-19 pandemic. He correctly points out the contributions his fellow students could make, but underestimates the negatives. During March 2020 when decisions were made to send third-year medical students home, the administrative focus was on the cost of their participation (consumption of scarce personal protective equipment) and the potential negative consequences (an additional person who might transmit the virus among patients.) Four months later, most medical students were back on the job.
Mr. Brodar’s eloquent description of duty and responsibility complement, and perhaps have evolved from, the integrity and dedication that Dr. Bedzow emphasized to incoming medical students. These are all character traits. These traits are not knowledge of anatomy or skill with a scalpel. With experience come two more key character traits – the moderation of a work-life balance and the judgment to weigh benefits, risks, and costs.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
Early autumn is typically a quiet time for outpatient pediatricians. The school physicals are finished. The last-minute school physicals are finished. The “I forgot to get my child’s physical” physicals are finished. Respiratory syncytial virus and influenza seasons haven’t started. There is time for some self-reflection and sharpening the saw.
My reflective period each year tends to start with the unresolved “What do I want to be when I grow up?” Mind you, just because I’ve grown old doesn’t mean I’ve grown up. I never wanted to be a “grande personne” who, per Antoine de Saint-Exupéry in “Le Petit Prince,” will never understand why a minor item (Did the lamb eat the flower?) makes all the difference in the universe to a child. Awe and wonderment should remain a part of life. I enjoy reading that short story in the original French because, as my high school French vocabulary and conjugation have faded, any word I don’t recognize means exactly what my journey of a lifetime tells me it means, neither more nor less, just as Humpty Dumpty explained to Alice in Lewis Carroll’s “Through the Looking Glass.”
Along with my perennial favorites like “Le Petit Prince” and the Gettysburg Address, in this year’s folder for reflection are two essays I’ve collected this year. The first is a letter addressed from medical ethicist Ira Bedzow, PhD, to this year’s incoming class of medical students.
The essay gives advice to first-year medical students entering the profession of medicine. It talks about finding “something to say that you communicate with the whole and essence of your being.” There is lots of great counsel in the letter. It claims, “Only in a professional does one’s voice sing in harmony with one’s being. Want that for yourselves, for only a life undivided is a life of full integrity.”
I agree with the harmony part. I hesitate with the undivided part. A professional singer could be dedicated to opera but still sing in a barbershop quartet and a church choir, motivated by fun and fellowship. It is important to emphasize integrity and dedication to medical students. The letter does that well, but students must also develop a work-life balance. The ascetic life is not for everyone.
Life needs balance and moderation. I am pretty sure that Aristotle said that, but I never did spend much time studying the Classics. I use my periods of self-reflection to chart my life’s vector. I choose new skills to learn and challenges to meet. But as I grow older, I spend more time pruning those roles that no longer give me joy. Delayed gratification is an important character trait for success, but its value lessens as it becomes clear there are more days behind me than ahead.
The second essay reflects the views of Canon Brodar, a third-year medical student and divinity school graduate.
He attests to the willingness of medical trainees to accept their duties and personal risk during the crisis of the COVID-19 pandemic. He correctly points out the contributions his fellow students could make, but underestimates the negatives. During March 2020 when decisions were made to send third-year medical students home, the administrative focus was on the cost of their participation (consumption of scarce personal protective equipment) and the potential negative consequences (an additional person who might transmit the virus among patients.) Four months later, most medical students were back on the job.
Mr. Brodar’s eloquent description of duty and responsibility complement, and perhaps have evolved from, the integrity and dedication that Dr. Bedzow emphasized to incoming medical students. These are all character traits. These traits are not knowledge of anatomy or skill with a scalpel. With experience come two more key character traits – the moderation of a work-life balance and the judgment to weigh benefits, risks, and costs.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
COVID-19 prompts ‘democratization’ of cancer trials
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
The pandemic has taught researchers how to decentralize trials, which should not only improve patient satisfaction but increase trial accrual by providing access to typically underserved populations, Patricia M. LoRusso, DO, of Yale University, New Haven, Conn., said at the meeting.
Dr. LoRusso was one of six panelists who participated in a forum about changes to cancer trials that were prompted by the pandemic. The forum was moderated by Keith T. Flaherty, MD, of Massachusetts General Hospital in Boston.
Dr. Flaherty asked the panelists to explain adjustments their organizations have made in response to the pandemic, discuss accomplishments, and speculate on future challenges and priorities.
Trial, administrative, and patient-care modifications
COVID-19 put some cancer trials on hold. For others, the pandemic forced sponsors and study chairs to reduce trial complexity and identify nonessential aspects of the studies, according to panelist José Baselga, MD, PhD, of AstraZeneca.
Specifically, exploratory objectives were subjugated to patient safety and a focus on the primary endpoints of each trial.
Once the critical data were identified, study chairs were asked to determine whether data could be obtained through technologies that could substitute for face-to-face contact between patients and staff – for example, patient-reported outcome tools and at-home digital monitoring.
Modifications prompted by the pandemic include the following:
- On-site auditing was suspended.
- Oral investigational agents were shipped directly to patients.
- “Remote” informed consent (telephone or video consenting) was permitted.
- Local providers could perform study-related services, with oversight by the research site.
- Minor deviations from the written protocols were allowed, provided the deviations did not affect patient care or data integrity.
“Obviously, the pandemic has been horrible, but what it has allowed us to do, as investigators in the clinical research landscape, … is to change our focus somewhat and realize, first and foremost, the patient is at the center of this,” Dr. LoRusso said.
Operational accomplishments and benefits
The pandemic caused a 40% decline in accrual to studies supported by the National Cancer Institute’s (NCI) Clinical Trials Network (NCTN) from mid-March to early April, according to James H. Doroshow, MD, of NCI.
However, after modifications to administrative and regulatory procedures, accrual to NCTN trials recovered to approximately 80% of prepandemic levels, Dr. Doroshow said.
The pandemic prompted investigators to leverage tools and technology they had not previously used frequently or at all, the panelists pointed out.
Investigators discovered perforce that telehealth could be used for almost all trial-related assessments. In lieu of physical examination, patients could send pictures of rashes and use electronic devices to monitor blood sugar values and vital signs.
Digital radiographic studies were performed at sites that were most convenient for patients, downloaded, and reinterpreted at the study institution. Visiting nurses and neighborhood laboratories enabled less-frequent in-person visits for assessments.
These adjustments have been particularly important for geographically and/or socioeconomically disadvantaged patients, the panelists said.
Overall, there was agreement among the panelists that shared values and trust among regulatory authorities, sponsors, investigators, and clinicians were impressive in their urgency, sincerity, and patient centricity.
“This pandemic … has forced us to think differently and be nimble and creative to our approach to maintaining our overriding goals while at the same time bringing these innovative therapies forward for patients with cancer and other serious and life-threatening diseases as quickly as possible,” said panelist Kristen M. Hege, MD, of Bristol-Myers Squibb.
In fact, Dr. Hege noted, some cancer-related therapies (e.g., BTK inhibitors, JAK inhibitors, and immunomodulatory agents) were “repurposed” rapidly and tested against COVID-related complications.
Streamlining trial regulatory processes
In addition to changing ongoing trials, the pandemic has affected how new research projects are launched.
One new study that came together quickly in response to the pandemic is the NCI COVID-19 in Cancer Patients Study (NCCAPS). NCCAPS is a natural history study with biospecimens and an imaging library. It was approved in just 5 weeks and is active in 650 sites, with “gangbusters” accrual, Dr. Doroshow said.
The rapidness of NCCAPS’ design and implementation should prompt the revision of previously accepted timelines for trial activation and lead to streamlined future processes.
Another project that was launched quickly in response to the pandemic is the COVID-19 evidence accelerator, according to Paul G. Kluetz, MD, of the Food and Drug Administration.
The COVID-19 evidence accelerator integrates real-world evidence into a database to provide investigators and health systems with the ability to gather information, design rapid turnaround queries, and share results. The evidence accelerator can provide study chairs with information that may have relevance to the safety of participants in clinical trials.
Future directions and challenges
The panelists agreed that pandemic-related modifications in processes will not only accelerate trial approval and activation but should facilitate higher study accrual, increase the diversity of protocol participants, and decrease the costs associated with clinical trial conduct.
With that in mind, the NCI is planning randomized clinical trials in which “process A” is compared with “process B,” Dr. Doroshow said. The goal is to determine which modifications are most likely to make trials available to patients without compromising data integrity or patient safety.
“How much less data do you need to have an outcome that will be similar?” Dr. Doroshow asked. “How many fewer visits, how many fewer tests, how much can you save? Physicians, clinical trialists, all of us respond to data, and if you get the same outcome at a third of the cost, then everybody benefits.”
Nonetheless, we will need to be vigilant for unintended vulnerabilities from well-intended efforts, according to Dr. Kluetz. Study chairs, sponsors, and regulatory agencies will need to be attentive to whether there are important differences in scan quality or interpretation, missing data that influence trial outcomes, and so on.
Dr. Hege pointed out that differences among data sources may be less important when treatments generate large effects but may be vitally important when the relative differences among treatments are small.
On a practical level, decentralizing clinical research may negatively impact the finances of tertiary care centers, which could threaten the required infrastructure for clinical trials, a few panelists noted.
The relative balance of NCI-, industry-, and investigator-initiated trials may require adjustment so that research income is adequate to maintain the costs associated with cancer clinical trials.
Shared goals and democratization
The pandemic has required all stakeholders in clinical research to rely on relationships of trust and shared goals, said Caroline Robert, MD, PhD, of Institut Gustave Roussy in Villejuif, France.
Dr. Kluetz summarized those goals as improving trial efficiencies, decreasing patient burden, decentralizing trials, and maintaining trial integrity.
A decentralized clinical trials operational model could lead to better generalizability of study outcomes, normalization of life for patients on studies, and lower costs of trial conduct. As such, decentralization would promote democratization.
Coupled with ongoing efforts to reduce eligibility criteria in cancer trials, the pandemic has brought operational solutions that should be perpetuated and has reminded us of the interlocking and mutually supportive relationships on which clinical research success depends.
Dr. Doroshow and Dr. Kluetz disclosed no conflicts of interest. All other panelists disclosed financial relationships, including employment, with a range of companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Flaherty KT et al. AACR: COVID-19 and Cancer, Regulatory and Operational Implications of Cancer Clinical Trial Changes During COVID-19.
FROM AACR: COVID-19 and Cancer
Baseline gene expression predicts TKI response in CML
Baseline gene expression in patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitor (TKI) therapy in the phase 3 ENESTnd trial differentiated those who achieved a good response from those with a poor response at 5 years in an exploratory analysis.
The investigators developed gene-expression models based on RNA sequencing of whole blood samples collected prior to treatment with nilotinib or imatinib in study participants who completed at least 5 years of therapy, including both good responders – those who achieved a major molecular response (MMR), defined as BCR-ABL1IS (a gene sequence found in an abnormal chromosome 22) less than 0.01% by 12 months and sustained deep molecular response (DMR) by 5 years, and poor responders – those without MMR by 12 months or with BCR-ABL1IS greater than 10% at 3 months.
A model based on the comparison of gene signatures from 47 patients who achieved a molecular response of 4.5 (MR4.5) on the International Scale (BCR-ABL1S less than 0.00032%), compared with 23 patients with a poor response, best predicted 5-year responder status (area under the receiver operating characteristic curve, 0.76), Jerald P. Radich, MD, reported during the Society of Hematologic Oncology virtual meeting.
“For this kind of work, that’s really quite good,” said Dr. Radich of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle.
Notably, the differences in patient responses observed by 12 months in ENESTnd persisted for up to 10 years, he said.
The findings have potential implications for drug development and facilitation of DMR in patients on TKI therapy – a prerequisite for attempting treatment-free remission, he said.
Dr. Radich and colleagues assessed 24 clinical factors – such as Sokal risk score, TKI therapy type, age, and sex – according to responder status, and applied penalized regression to the clinical variables, to expression of 13,575 genes, and to a combination of the clinical variables and gene expression.
Clinical variables didn’t predict response in the trial, and including the clinical variables in the gene-expression model in the exploratory analysis did not improve it’s performance (AUC, 0.75). However, both the MR4.5 plus clinical variables model and the MR4.5-only model outperformed the clinical variables–only model (AUC, 0.59), he noted, adding: “So gene expression seems to be highly correlated with response.”
Of note, 458 genes were differentially expressed; those found in responders were most often associated with immune response, whereas those in poor responders were more likely to be associated with drug catabolism, WNT signaling, and cell cycle.
This suggests that good responders, compared with poor responders, have an activated immune system that is better able to engage after TKI therapy is administered to “cull through the heard, so to speak,” Dr. Radich said.
The findings were validated in an independent dataset of 19 good responders and 25 poor responders (AUC, 0.67 for the MR4.5 vs. poor-responder model).
A comparison of the expression of immune cell marker genes in good responders and poor responders further showed that T cells – particularly CD8 T cells, B cells, natural killer cells, and aggregate cytotoxic lymphocytes were expressed at significantly higher levels in good responders.
This was true in both the ENESTnd cohort and the validation dataset, he said.
The ENESTnd study is a randomized, open-label study comparing nilotinib and imatinib in adults with newly diagnosed Philadelphia chromosome–positive chronic-phase CML. A 5-year study update published in 2016 showed that 54% and 52% of patients in nilotinib 300- and 400-mg twice-daily arms, respectively, achieved MR4.5, compared with 31% of those in an imatinib 400-mg once-daily arm. In the current exploratory analysis, the gene expression model differentiated between good and poor responders regardless of the TKI used, Dr. Radich said.
The findings are of note because achieving sustained deep molecular response is necessary before CML patients can attempt treatment-free remission and because biomarkers for predicting DMR have been lacking, he explained.
“These findings could really be used, potentially, for a couple of things: One is to predict response, and that could drive patient goals, expectations, and maybe drug choice,” he said.
The findings could also be used to inform clinical trials to investigate how to best treat poor responders to improve their response, he added.
“I think there’s a lot of work to be done and a lot things to chew over, and we’re hoping that we’ll have more to talk to you about in the future,” he said.
The study was sponsored by Novartis. Dr. Radich is a paid consultant for Genentech, Cepheid, Bristol-Myers Squibb, Takeda, and Novartis.
SOURCE: Radich JP et al. SOHO 2020, Abstract CML-109.
Baseline gene expression in patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitor (TKI) therapy in the phase 3 ENESTnd trial differentiated those who achieved a good response from those with a poor response at 5 years in an exploratory analysis.
The investigators developed gene-expression models based on RNA sequencing of whole blood samples collected prior to treatment with nilotinib or imatinib in study participants who completed at least 5 years of therapy, including both good responders – those who achieved a major molecular response (MMR), defined as BCR-ABL1IS (a gene sequence found in an abnormal chromosome 22) less than 0.01% by 12 months and sustained deep molecular response (DMR) by 5 years, and poor responders – those without MMR by 12 months or with BCR-ABL1IS greater than 10% at 3 months.
A model based on the comparison of gene signatures from 47 patients who achieved a molecular response of 4.5 (MR4.5) on the International Scale (BCR-ABL1S less than 0.00032%), compared with 23 patients with a poor response, best predicted 5-year responder status (area under the receiver operating characteristic curve, 0.76), Jerald P. Radich, MD, reported during the Society of Hematologic Oncology virtual meeting.
“For this kind of work, that’s really quite good,” said Dr. Radich of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle.
Notably, the differences in patient responses observed by 12 months in ENESTnd persisted for up to 10 years, he said.
The findings have potential implications for drug development and facilitation of DMR in patients on TKI therapy – a prerequisite for attempting treatment-free remission, he said.
Dr. Radich and colleagues assessed 24 clinical factors – such as Sokal risk score, TKI therapy type, age, and sex – according to responder status, and applied penalized regression to the clinical variables, to expression of 13,575 genes, and to a combination of the clinical variables and gene expression.
Clinical variables didn’t predict response in the trial, and including the clinical variables in the gene-expression model in the exploratory analysis did not improve it’s performance (AUC, 0.75). However, both the MR4.5 plus clinical variables model and the MR4.5-only model outperformed the clinical variables–only model (AUC, 0.59), he noted, adding: “So gene expression seems to be highly correlated with response.”
Of note, 458 genes were differentially expressed; those found in responders were most often associated with immune response, whereas those in poor responders were more likely to be associated with drug catabolism, WNT signaling, and cell cycle.
This suggests that good responders, compared with poor responders, have an activated immune system that is better able to engage after TKI therapy is administered to “cull through the heard, so to speak,” Dr. Radich said.
The findings were validated in an independent dataset of 19 good responders and 25 poor responders (AUC, 0.67 for the MR4.5 vs. poor-responder model).
A comparison of the expression of immune cell marker genes in good responders and poor responders further showed that T cells – particularly CD8 T cells, B cells, natural killer cells, and aggregate cytotoxic lymphocytes were expressed at significantly higher levels in good responders.
This was true in both the ENESTnd cohort and the validation dataset, he said.
The ENESTnd study is a randomized, open-label study comparing nilotinib and imatinib in adults with newly diagnosed Philadelphia chromosome–positive chronic-phase CML. A 5-year study update published in 2016 showed that 54% and 52% of patients in nilotinib 300- and 400-mg twice-daily arms, respectively, achieved MR4.5, compared with 31% of those in an imatinib 400-mg once-daily arm. In the current exploratory analysis, the gene expression model differentiated between good and poor responders regardless of the TKI used, Dr. Radich said.
The findings are of note because achieving sustained deep molecular response is necessary before CML patients can attempt treatment-free remission and because biomarkers for predicting DMR have been lacking, he explained.
“These findings could really be used, potentially, for a couple of things: One is to predict response, and that could drive patient goals, expectations, and maybe drug choice,” he said.
The findings could also be used to inform clinical trials to investigate how to best treat poor responders to improve their response, he added.
“I think there’s a lot of work to be done and a lot things to chew over, and we’re hoping that we’ll have more to talk to you about in the future,” he said.
The study was sponsored by Novartis. Dr. Radich is a paid consultant for Genentech, Cepheid, Bristol-Myers Squibb, Takeda, and Novartis.
SOURCE: Radich JP et al. SOHO 2020, Abstract CML-109.
Baseline gene expression in patients with chronic myeloid leukemia (CML) who received tyrosine kinase inhibitor (TKI) therapy in the phase 3 ENESTnd trial differentiated those who achieved a good response from those with a poor response at 5 years in an exploratory analysis.
The investigators developed gene-expression models based on RNA sequencing of whole blood samples collected prior to treatment with nilotinib or imatinib in study participants who completed at least 5 years of therapy, including both good responders – those who achieved a major molecular response (MMR), defined as BCR-ABL1IS (a gene sequence found in an abnormal chromosome 22) less than 0.01% by 12 months and sustained deep molecular response (DMR) by 5 years, and poor responders – those without MMR by 12 months or with BCR-ABL1IS greater than 10% at 3 months.
A model based on the comparison of gene signatures from 47 patients who achieved a molecular response of 4.5 (MR4.5) on the International Scale (BCR-ABL1S less than 0.00032%), compared with 23 patients with a poor response, best predicted 5-year responder status (area under the receiver operating characteristic curve, 0.76), Jerald P. Radich, MD, reported during the Society of Hematologic Oncology virtual meeting.
“For this kind of work, that’s really quite good,” said Dr. Radich of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle.
Notably, the differences in patient responses observed by 12 months in ENESTnd persisted for up to 10 years, he said.
The findings have potential implications for drug development and facilitation of DMR in patients on TKI therapy – a prerequisite for attempting treatment-free remission, he said.
Dr. Radich and colleagues assessed 24 clinical factors – such as Sokal risk score, TKI therapy type, age, and sex – according to responder status, and applied penalized regression to the clinical variables, to expression of 13,575 genes, and to a combination of the clinical variables and gene expression.
Clinical variables didn’t predict response in the trial, and including the clinical variables in the gene-expression model in the exploratory analysis did not improve it’s performance (AUC, 0.75). However, both the MR4.5 plus clinical variables model and the MR4.5-only model outperformed the clinical variables–only model (AUC, 0.59), he noted, adding: “So gene expression seems to be highly correlated with response.”
Of note, 458 genes were differentially expressed; those found in responders were most often associated with immune response, whereas those in poor responders were more likely to be associated with drug catabolism, WNT signaling, and cell cycle.
This suggests that good responders, compared with poor responders, have an activated immune system that is better able to engage after TKI therapy is administered to “cull through the heard, so to speak,” Dr. Radich said.
The findings were validated in an independent dataset of 19 good responders and 25 poor responders (AUC, 0.67 for the MR4.5 vs. poor-responder model).
A comparison of the expression of immune cell marker genes in good responders and poor responders further showed that T cells – particularly CD8 T cells, B cells, natural killer cells, and aggregate cytotoxic lymphocytes were expressed at significantly higher levels in good responders.
This was true in both the ENESTnd cohort and the validation dataset, he said.
The ENESTnd study is a randomized, open-label study comparing nilotinib and imatinib in adults with newly diagnosed Philadelphia chromosome–positive chronic-phase CML. A 5-year study update published in 2016 showed that 54% and 52% of patients in nilotinib 300- and 400-mg twice-daily arms, respectively, achieved MR4.5, compared with 31% of those in an imatinib 400-mg once-daily arm. In the current exploratory analysis, the gene expression model differentiated between good and poor responders regardless of the TKI used, Dr. Radich said.
The findings are of note because achieving sustained deep molecular response is necessary before CML patients can attempt treatment-free remission and because biomarkers for predicting DMR have been lacking, he explained.
“These findings could really be used, potentially, for a couple of things: One is to predict response, and that could drive patient goals, expectations, and maybe drug choice,” he said.
The findings could also be used to inform clinical trials to investigate how to best treat poor responders to improve their response, he added.
“I think there’s a lot of work to be done and a lot things to chew over, and we’re hoping that we’ll have more to talk to you about in the future,” he said.
The study was sponsored by Novartis. Dr. Radich is a paid consultant for Genentech, Cepheid, Bristol-Myers Squibb, Takeda, and Novartis.
SOURCE: Radich JP et al. SOHO 2020, Abstract CML-109.
FROM SOHO 2020
RAP device being investigated as a way to improve appearance of cellulite
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020