User login
Three or more nonadvanced adenomas no longer spell increased CRC risk
SAN ANTONIO – , Carol Rouphael, MD, reported at the annual meeting of the American College of Gastroenterology.
She presented a retrospective study of 3,377 patients who had their first colonoscopies at age 50 or older in 2006 or later, when high-definition colonscopes took over.
The clinical implications of the study are clear: “Our findings suggest the colonoscopy interval for individuals with three or more nonadvanced adenomas should be similar to low-risk adenoma patients; that is, 5-10 years,” said Dr. Rouphael, a gastroenterology fellow at the Cleveland Clinic.
Studies conducted in the early 2000s using standard-definition colonoscopes showed that the risk of metachronous advanced neoplasia (MAN) – that is, colorectal cancer or a pathologically advanced adenoma – was twice as great in patients with three or more small tubular adenomas compared with patients with just one or two of them. Thus, guidelines called for such patients to undergo repeat colonoscopy at a shorter time interval post polypectomy, typically 3 years. But with contemporary colonoscopy using high-definition optics, gastroenterologists are detecting a lot more small adenomas. Dr. Rouphael and coworkers wondered if the definition of high risk established more than a decade ago, prior to the use of high-definition colonoscopes, still held true. They concluded that the answer is no.
Eleven percent of patients in their study had features indicative of high-risk adenoma on the initial colonoscopy. Twenty-four percent of these patients had an adenoma with advanced pathology, meaning villous features or high-grade dysplasia; 51% had an adenoma 10 mm or more in size without advanced pathology; and the remaining 25% of patients were classified as having high-risk adenoma on the basis of having three or more small tubular adenomas.
Follow-up colonoscopy was performed a median of 42 months later in the high-risk adenoma group, 54 months later in the low-risk adenoma patients with one or two small tubular adenomas, and at 61 months in those with no adenomas. At follow-up, MAN was discovered in 3.8% of patients with no adenomas at baseline, 4.6% of the low-risk adenoma group, and 9.3% of the overall high-risk adenoma group. However, within the high-risk adenoma group the risk of MAN varied widely: 6.3% in patients with three or more nonadvanced adenomas, 6.1% in those with three or four nonadvanced adenomas, 7.7% in patients with five or more nonadvanced adenomas, 8.3% in those with a 10-mm or larger adenoma without advanced pathology, and 14.6% in patients with an adenoma with advanced pathology at baseline.
In a multivariate analysis adjusted for age, sex, ethnicity, and time between first and follow-up colonoscopy, the risk of MAN did not differ significantly between patients with three or four nonadvanced adenomas and those with one or two, nor between patients with five or more versus one or two. In addition, there was no significant difference in MAN risk between patients with no adenomas at baseline and those with one or two low-risk, nonadvanced adenomas. In contrast, patients with a 10-mm or larger adenoma without advanced pathology at baseline were 1.9-fold more likely to have MAN at follow-up colonoscopy than were patients with one or two small tubular adenomas. And patients having an adenoma with advanced pathology at baseline were at 3.7-fold greater risk of developing MAN than were those with baseline low-risk adenoma, according to Dr. Rouphael.
She reported having no financial conflicts regarding her study, which won the Fellows-in-Training Award at the annual meeting.
SOURCE: Rouphael C. ACG 2019 Abstract 9.
SAN ANTONIO – , Carol Rouphael, MD, reported at the annual meeting of the American College of Gastroenterology.
She presented a retrospective study of 3,377 patients who had their first colonoscopies at age 50 or older in 2006 or later, when high-definition colonscopes took over.
The clinical implications of the study are clear: “Our findings suggest the colonoscopy interval for individuals with three or more nonadvanced adenomas should be similar to low-risk adenoma patients; that is, 5-10 years,” said Dr. Rouphael, a gastroenterology fellow at the Cleveland Clinic.
Studies conducted in the early 2000s using standard-definition colonoscopes showed that the risk of metachronous advanced neoplasia (MAN) – that is, colorectal cancer or a pathologically advanced adenoma – was twice as great in patients with three or more small tubular adenomas compared with patients with just one or two of them. Thus, guidelines called for such patients to undergo repeat colonoscopy at a shorter time interval post polypectomy, typically 3 years. But with contemporary colonoscopy using high-definition optics, gastroenterologists are detecting a lot more small adenomas. Dr. Rouphael and coworkers wondered if the definition of high risk established more than a decade ago, prior to the use of high-definition colonoscopes, still held true. They concluded that the answer is no.
Eleven percent of patients in their study had features indicative of high-risk adenoma on the initial colonoscopy. Twenty-four percent of these patients had an adenoma with advanced pathology, meaning villous features or high-grade dysplasia; 51% had an adenoma 10 mm or more in size without advanced pathology; and the remaining 25% of patients were classified as having high-risk adenoma on the basis of having three or more small tubular adenomas.
Follow-up colonoscopy was performed a median of 42 months later in the high-risk adenoma group, 54 months later in the low-risk adenoma patients with one or two small tubular adenomas, and at 61 months in those with no adenomas. At follow-up, MAN was discovered in 3.8% of patients with no adenomas at baseline, 4.6% of the low-risk adenoma group, and 9.3% of the overall high-risk adenoma group. However, within the high-risk adenoma group the risk of MAN varied widely: 6.3% in patients with three or more nonadvanced adenomas, 6.1% in those with three or four nonadvanced adenomas, 7.7% in patients with five or more nonadvanced adenomas, 8.3% in those with a 10-mm or larger adenoma without advanced pathology, and 14.6% in patients with an adenoma with advanced pathology at baseline.
In a multivariate analysis adjusted for age, sex, ethnicity, and time between first and follow-up colonoscopy, the risk of MAN did not differ significantly between patients with three or four nonadvanced adenomas and those with one or two, nor between patients with five or more versus one or two. In addition, there was no significant difference in MAN risk between patients with no adenomas at baseline and those with one or two low-risk, nonadvanced adenomas. In contrast, patients with a 10-mm or larger adenoma without advanced pathology at baseline were 1.9-fold more likely to have MAN at follow-up colonoscopy than were patients with one or two small tubular adenomas. And patients having an adenoma with advanced pathology at baseline were at 3.7-fold greater risk of developing MAN than were those with baseline low-risk adenoma, according to Dr. Rouphael.
She reported having no financial conflicts regarding her study, which won the Fellows-in-Training Award at the annual meeting.
SOURCE: Rouphael C. ACG 2019 Abstract 9.
SAN ANTONIO – , Carol Rouphael, MD, reported at the annual meeting of the American College of Gastroenterology.
She presented a retrospective study of 3,377 patients who had their first colonoscopies at age 50 or older in 2006 or later, when high-definition colonscopes took over.
The clinical implications of the study are clear: “Our findings suggest the colonoscopy interval for individuals with three or more nonadvanced adenomas should be similar to low-risk adenoma patients; that is, 5-10 years,” said Dr. Rouphael, a gastroenterology fellow at the Cleveland Clinic.
Studies conducted in the early 2000s using standard-definition colonoscopes showed that the risk of metachronous advanced neoplasia (MAN) – that is, colorectal cancer or a pathologically advanced adenoma – was twice as great in patients with three or more small tubular adenomas compared with patients with just one or two of them. Thus, guidelines called for such patients to undergo repeat colonoscopy at a shorter time interval post polypectomy, typically 3 years. But with contemporary colonoscopy using high-definition optics, gastroenterologists are detecting a lot more small adenomas. Dr. Rouphael and coworkers wondered if the definition of high risk established more than a decade ago, prior to the use of high-definition colonoscopes, still held true. They concluded that the answer is no.
Eleven percent of patients in their study had features indicative of high-risk adenoma on the initial colonoscopy. Twenty-four percent of these patients had an adenoma with advanced pathology, meaning villous features or high-grade dysplasia; 51% had an adenoma 10 mm or more in size without advanced pathology; and the remaining 25% of patients were classified as having high-risk adenoma on the basis of having three or more small tubular adenomas.
Follow-up colonoscopy was performed a median of 42 months later in the high-risk adenoma group, 54 months later in the low-risk adenoma patients with one or two small tubular adenomas, and at 61 months in those with no adenomas. At follow-up, MAN was discovered in 3.8% of patients with no adenomas at baseline, 4.6% of the low-risk adenoma group, and 9.3% of the overall high-risk adenoma group. However, within the high-risk adenoma group the risk of MAN varied widely: 6.3% in patients with three or more nonadvanced adenomas, 6.1% in those with three or four nonadvanced adenomas, 7.7% in patients with five or more nonadvanced adenomas, 8.3% in those with a 10-mm or larger adenoma without advanced pathology, and 14.6% in patients with an adenoma with advanced pathology at baseline.
In a multivariate analysis adjusted for age, sex, ethnicity, and time between first and follow-up colonoscopy, the risk of MAN did not differ significantly between patients with three or four nonadvanced adenomas and those with one or two, nor between patients with five or more versus one or two. In addition, there was no significant difference in MAN risk between patients with no adenomas at baseline and those with one or two low-risk, nonadvanced adenomas. In contrast, patients with a 10-mm or larger adenoma without advanced pathology at baseline were 1.9-fold more likely to have MAN at follow-up colonoscopy than were patients with one or two small tubular adenomas. And patients having an adenoma with advanced pathology at baseline were at 3.7-fold greater risk of developing MAN than were those with baseline low-risk adenoma, according to Dr. Rouphael.
She reported having no financial conflicts regarding her study, which won the Fellows-in-Training Award at the annual meeting.
SOURCE: Rouphael C. ACG 2019 Abstract 9.
REPORTING FROM ACG 2019
February 2020
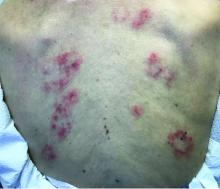
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
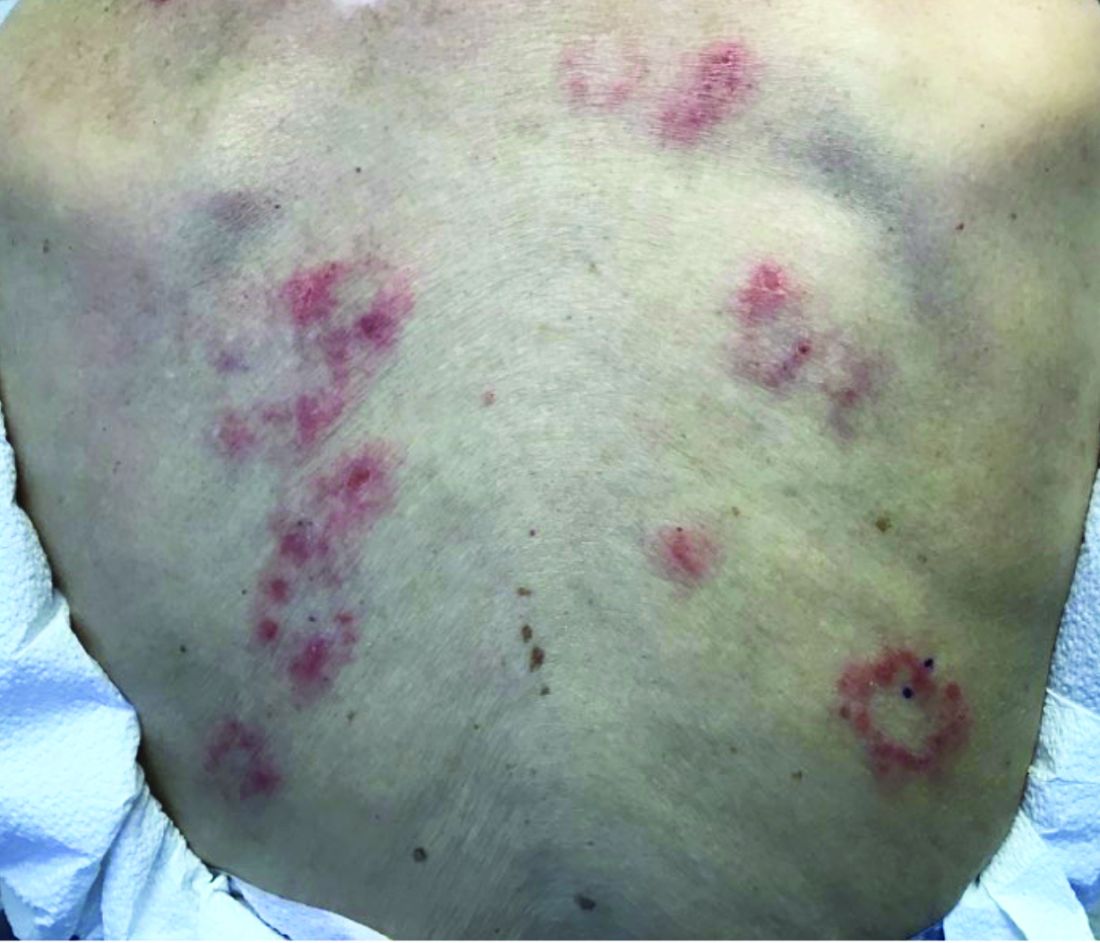
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
New year, old you
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
HCV a risk in HIV-negative MSM who use PrEP
Hepatitis C virus (HCV) is known to be a common sexually transmitted infection (STI) among HIV-positive men who have sex with men (MSM). To examine this relationship in HIV-negative MSM, researchers in the Amsterdam PrEP Project team in the HIV Transmission Elimination AMsterdam (H-TEAM) Initiative evaluated HCV-incidence and its risk-factors in this population, who were using pre-exposure prophylaxis (PrEP).
Participants in the Amsterdam PrEP project were tested for HCV antibodies or HCV-RNA every 6 months. During the period, participants used daily or event-driven PrEP and could switch regimens during follow-up, according to the report by published in the Journal of Hepatology.
HIV-negative MSM on PrEP are at risk for incident HCV-infection, while identified risk-factors are similar to those in HIV-positive MSM.
Among 350 participants, they detected 15 HCV infections in 14 participants, finding 8 primary infections and 7 reinfections. The researchers found that the factors associated with incident HCV-infection were higher number of receptive condomless anal sex acts with casual partners, anal STI, injecting drug use, and sharing straws when snorting drugs. These are similar risk-factors to those found among in HIV-positive MSM.
They concluded that, because HIV-negative MSM on PrEP are at risk for incident HCV-infection, regular HCV-testing was needed, especially for those with a previous HCV-infection.
Hepatitis C virus (HCV) is known to be a common sexually transmitted infection (STI) among HIV-positive men who have sex with men (MSM). To examine this relationship in HIV-negative MSM, researchers in the Amsterdam PrEP Project team in the HIV Transmission Elimination AMsterdam (H-TEAM) Initiative evaluated HCV-incidence and its risk-factors in this population, who were using pre-exposure prophylaxis (PrEP).
Participants in the Amsterdam PrEP project were tested for HCV antibodies or HCV-RNA every 6 months. During the period, participants used daily or event-driven PrEP and could switch regimens during follow-up, according to the report by published in the Journal of Hepatology.
HIV-negative MSM on PrEP are at risk for incident HCV-infection, while identified risk-factors are similar to those in HIV-positive MSM.
Among 350 participants, they detected 15 HCV infections in 14 participants, finding 8 primary infections and 7 reinfections. The researchers found that the factors associated with incident HCV-infection were higher number of receptive condomless anal sex acts with casual partners, anal STI, injecting drug use, and sharing straws when snorting drugs. These are similar risk-factors to those found among in HIV-positive MSM.
They concluded that, because HIV-negative MSM on PrEP are at risk for incident HCV-infection, regular HCV-testing was needed, especially for those with a previous HCV-infection.
Hepatitis C virus (HCV) is known to be a common sexually transmitted infection (STI) among HIV-positive men who have sex with men (MSM). To examine this relationship in HIV-negative MSM, researchers in the Amsterdam PrEP Project team in the HIV Transmission Elimination AMsterdam (H-TEAM) Initiative evaluated HCV-incidence and its risk-factors in this population, who were using pre-exposure prophylaxis (PrEP).
Participants in the Amsterdam PrEP project were tested for HCV antibodies or HCV-RNA every 6 months. During the period, participants used daily or event-driven PrEP and could switch regimens during follow-up, according to the report by published in the Journal of Hepatology.
HIV-negative MSM on PrEP are at risk for incident HCV-infection, while identified risk-factors are similar to those in HIV-positive MSM.
Among 350 participants, they detected 15 HCV infections in 14 participants, finding 8 primary infections and 7 reinfections. The researchers found that the factors associated with incident HCV-infection were higher number of receptive condomless anal sex acts with casual partners, anal STI, injecting drug use, and sharing straws when snorting drugs. These are similar risk-factors to those found among in HIV-positive MSM.
They concluded that, because HIV-negative MSM on PrEP are at risk for incident HCV-infection, regular HCV-testing was needed, especially for those with a previous HCV-infection.
FROM THE JOURNAL OF HEPATOLOGY
TNFi treatment shows hint of slowing axial spondyloarthritis radiographic progression
according to an analysis of studies with low risk of bias, but no evidence exists for slowed disease progression at the sacroiliac joint, according to a systematic review and meta-analysis of 24 studies.
The review, conducted by Paras Karmacharya, MBBS, and colleagues at the Mayo Clinic in Rochester, Minn., did not find a significant protective effect overall for tumor necrosis factor inhibitor (TNFi) treatment on radiographic progression of ankylosing spondylitis at the spine at 2 and 4 years. But when the researchers restricted the analysis to six studies of TNFi with low risk of bias, the results were significant for slowing radiographic progression at 4 years or more (modified Stoke Ankylosing Spondylitis Spine Score [mSASSS] difference, –2.17).
NSAIDs did not show any benefit in slowing progression at either the spine or sacroiliac joint over a shorter 2-year time span for which results were available. The single study of secukinumab (Cosentyx) that was included in the analysis did not show a significant difference in radiographic progression over 2 years (mean mSASSS difference, –0.34).
For the few studies that included data on radiographic progression in patients with nonradiographic axial spondyloarthritis, there was no effect seen on the spine with either high or low NSAID use at 2 years and no evidence for an effect of TNFi on progression at the sacroiliac joint.
“Although our study showed a significant effect of TNFi on long-term radiographic progression (in sensitivity analysis), none of the included studies provide prospective, long-term, controlled comparison. Most included studies were judged to have a low risk of bias; however predominance of observational and open-label extensions of randomized, controlled trials limits overall level of evidence,” the authors wrote in Arthritis & Rheumatology.
Any benefits of early treatment on slowing the natural progression of disease might support introducing the treatment early with a treat-to-target strategy, similar to RA, the researchers noted. However, “the current guidelines recommend against this due to lack of evidence.”
The analysis involved 18 studies with TNFi, 8 with NSAIDs, and 1 with secukinumab (3 studies contained data for both NSAIDs and TNFi). The investigators used a change of 2 mSASSS units in 2 years or one new syndesmophyte formation in 2 years as the primary endpoint for radiographic progression.
“Further studies should explore the effect of NSAIDs and biologics alone and in combination in patients with early axial spondyloarthritis; their use in the group with high risk of progression should be evaluated with a follow-up [longer than] 4 years to see if effects are more pronounced over time. Newer measures with higher sensitivity to detect structural changes, such as those based on quantitative low-dose CT should be compared to mSASSS for use in clinical trials,” the researchers concluded.
The work was funded by various grants from the National Institutes of Health. The authors reported no relevant disclosures.
SOURCE: Karmacharya P et al. Arthritis Rheumatol. 2020 Jan 20. doi: 10.1002/art.41206.
according to an analysis of studies with low risk of bias, but no evidence exists for slowed disease progression at the sacroiliac joint, according to a systematic review and meta-analysis of 24 studies.
The review, conducted by Paras Karmacharya, MBBS, and colleagues at the Mayo Clinic in Rochester, Minn., did not find a significant protective effect overall for tumor necrosis factor inhibitor (TNFi) treatment on radiographic progression of ankylosing spondylitis at the spine at 2 and 4 years. But when the researchers restricted the analysis to six studies of TNFi with low risk of bias, the results were significant for slowing radiographic progression at 4 years or more (modified Stoke Ankylosing Spondylitis Spine Score [mSASSS] difference, –2.17).
NSAIDs did not show any benefit in slowing progression at either the spine or sacroiliac joint over a shorter 2-year time span for which results were available. The single study of secukinumab (Cosentyx) that was included in the analysis did not show a significant difference in radiographic progression over 2 years (mean mSASSS difference, –0.34).
For the few studies that included data on radiographic progression in patients with nonradiographic axial spondyloarthritis, there was no effect seen on the spine with either high or low NSAID use at 2 years and no evidence for an effect of TNFi on progression at the sacroiliac joint.
“Although our study showed a significant effect of TNFi on long-term radiographic progression (in sensitivity analysis), none of the included studies provide prospective, long-term, controlled comparison. Most included studies were judged to have a low risk of bias; however predominance of observational and open-label extensions of randomized, controlled trials limits overall level of evidence,” the authors wrote in Arthritis & Rheumatology.
Any benefits of early treatment on slowing the natural progression of disease might support introducing the treatment early with a treat-to-target strategy, similar to RA, the researchers noted. However, “the current guidelines recommend against this due to lack of evidence.”
The analysis involved 18 studies with TNFi, 8 with NSAIDs, and 1 with secukinumab (3 studies contained data for both NSAIDs and TNFi). The investigators used a change of 2 mSASSS units in 2 years or one new syndesmophyte formation in 2 years as the primary endpoint for radiographic progression.
“Further studies should explore the effect of NSAIDs and biologics alone and in combination in patients with early axial spondyloarthritis; their use in the group with high risk of progression should be evaluated with a follow-up [longer than] 4 years to see if effects are more pronounced over time. Newer measures with higher sensitivity to detect structural changes, such as those based on quantitative low-dose CT should be compared to mSASSS for use in clinical trials,” the researchers concluded.
The work was funded by various grants from the National Institutes of Health. The authors reported no relevant disclosures.
SOURCE: Karmacharya P et al. Arthritis Rheumatol. 2020 Jan 20. doi: 10.1002/art.41206.
according to an analysis of studies with low risk of bias, but no evidence exists for slowed disease progression at the sacroiliac joint, according to a systematic review and meta-analysis of 24 studies.
The review, conducted by Paras Karmacharya, MBBS, and colleagues at the Mayo Clinic in Rochester, Minn., did not find a significant protective effect overall for tumor necrosis factor inhibitor (TNFi) treatment on radiographic progression of ankylosing spondylitis at the spine at 2 and 4 years. But when the researchers restricted the analysis to six studies of TNFi with low risk of bias, the results were significant for slowing radiographic progression at 4 years or more (modified Stoke Ankylosing Spondylitis Spine Score [mSASSS] difference, –2.17).
NSAIDs did not show any benefit in slowing progression at either the spine or sacroiliac joint over a shorter 2-year time span for which results were available. The single study of secukinumab (Cosentyx) that was included in the analysis did not show a significant difference in radiographic progression over 2 years (mean mSASSS difference, –0.34).
For the few studies that included data on radiographic progression in patients with nonradiographic axial spondyloarthritis, there was no effect seen on the spine with either high or low NSAID use at 2 years and no evidence for an effect of TNFi on progression at the sacroiliac joint.
“Although our study showed a significant effect of TNFi on long-term radiographic progression (in sensitivity analysis), none of the included studies provide prospective, long-term, controlled comparison. Most included studies were judged to have a low risk of bias; however predominance of observational and open-label extensions of randomized, controlled trials limits overall level of evidence,” the authors wrote in Arthritis & Rheumatology.
Any benefits of early treatment on slowing the natural progression of disease might support introducing the treatment early with a treat-to-target strategy, similar to RA, the researchers noted. However, “the current guidelines recommend against this due to lack of evidence.”
The analysis involved 18 studies with TNFi, 8 with NSAIDs, and 1 with secukinumab (3 studies contained data for both NSAIDs and TNFi). The investigators used a change of 2 mSASSS units in 2 years or one new syndesmophyte formation in 2 years as the primary endpoint for radiographic progression.
“Further studies should explore the effect of NSAIDs and biologics alone and in combination in patients with early axial spondyloarthritis; their use in the group with high risk of progression should be evaluated with a follow-up [longer than] 4 years to see if effects are more pronounced over time. Newer measures with higher sensitivity to detect structural changes, such as those based on quantitative low-dose CT should be compared to mSASSS for use in clinical trials,” the researchers concluded.
The work was funded by various grants from the National Institutes of Health. The authors reported no relevant disclosures.
SOURCE: Karmacharya P et al. Arthritis Rheumatol. 2020 Jan 20. doi: 10.1002/art.41206.
FROM ARTHRITIS & RHEUMATOLOGY
Carbs, fat, and mortality: Types matter more than levels
The health consequences of diet don’t largely depend on whether a person eats a high or low level of carbohydrates or a diet high or low in fat. What’s much more important is where the carbs and fats come from, according to an analysis that related diet and mortality rates in more than 37,000 American adults.
“Unhealthy low carbohydrate diet [LCD] and low-fat diet [LFD] scores were associated with higher total mortality, whereas healthy LCD and LFD scores were associated with lower total mortality,” Zhilei Shan, MD, and associates wrote in an article (JAMA Intern Med. 2020 Jan 21; doi: 10.1001/jamainternmed.2019.6980). The findings “suggest that the association of LCDs and LFDs with mortality may depend on the quality of food sources of macronutrients,” said the researchers, based at the Harvard T.H. Chan School of Public Health in Boston.
The analysis included follow-up of almost 300,000 person-years. It showed that, for every 20-percentile increase in a person’s unhealthy LCD score, their relative rate of total mortality increased by a statistically significant 7%; and for every 20-percentile rise in unhealthy LFD score, the relative, total mortality rate rose by a statistically significant 6%, after adjustment for several demographic and clinical measures and family and personal histories of diabetes, cancer, and heart disease. In contrast, for each 20-percentile increase in healthy LCD score relative, total mortality fell by 9%, and similar increases in healthy LFD score linked with an 11% relative drop in total mortality, also statistically significant associations in these confounder-adjusted analyses.
The findings “extend the previous evidence” for these links, and the data suggest that “the health benefits of an LCD or LFD may depend not only on the types of protein and fat or carbohydrate but also on the quality of carbohydrate or fat remaining in the diet,” the researchers wrote. They cited the documented health problems caused by eating significant amounts of low-quality carbohydrates such as refined grains and added sugars, which provide limited nutrition and introduce a high glycemic load, and can produce high levels of postprandial glucose and insulin, inflammation, insulin resistance, and dyslipidemia.
The foods people ate that produced healthy diet scores and linked with better survival were diets high in plant protein and unsaturated fat, and low in carbohydrates from refined grains, added sugar, starchy vegetables, and similar sources as part of a low carbohydrate diet. The foods that formed a healthy LFD included whole grains, whole fruit, legumes, and nonstarchy vegetables, along with higher intake of plant protein and low levels of saturated fat.
The study used data from 24-hour diet-recall surveys completed by 37,233 American adults collected by the National Health and Nutrition Examination Survey (NHANES) during 1999-2014, and linked the diet scores calculated for these people with U.S. national death records collected by the National Death Index through the end of 2015. The people included averaged about 50 years of age at the time of their dietary interview, and 53% were women. During 297,768 person-years of follow-up, 4,866 total deaths occurred, including 849 from heart disease and 1,068 from cancer. The analyses found no statistically significant links between overall LCD or LFD scores and mortality; the significant links only existed when the researchers further classified the diet scores into healthy and unhealthy subtypes.
The results also showed statistically significant links or strong trends between high or low levels of healthy or unhealthy LCD and LFD scores and cancer deaths. A 20-percentile increase in unhealthy LCD score linked with an 11% relative increase in cancer deaths, while a 20-percentile increase in the healthy LCD score linked with a 10% decrease in cancer deaths. A 20-percentile increase in the healthy LFD score linked with a 15% relative decrease in cancer mortality.
The study received no commercial fundings, and the authors had no commercial disclosures.
SOURCE: Shan Z et al. JAMA Intern Med. 2020 Jan 21; doi: 10.1001/jamainternmed.2019.6980.
This is an important study because the findings reinforce the already established concept that it’s the quality of the fat and carbohydrate a person eats that matters for health, rather than the relative levels of these nutrients. Eating unsaturated fats and unprocessed carbohydrates like whole grains, fruits, and legumes produces the greatest health and survival, while higher levels of saturated fats and processed carbs in the diet produce health problems. That’s much more important than whether a diet is low fat or low carb. This means sticking with the food principles advanced by the AHA diet, the DASH diet, and a Mediterranean diet.
High-fat and low-carb diets are popular because people who follow them lose weight over the short term, but those weight losses are hard to sustain longer term and create an opportunity for unhealthy effects if people eat the wrong fats, carbohydrates, and proteins. Strategies that focus on healthier food choices like the Mediterranean or AHA diets can minimize disease and produce more sustainable weight control.
Robert A. Vogel, MD , is a cardiologist in Denver affiliated with the University of Colorado School of Medicine and the VA Medical Center in Denver. He has been a consultant to the Pritikin Longevity Institute in Doral, Fla. He made these comments in an interview.
This is an important study because the findings reinforce the already established concept that it’s the quality of the fat and carbohydrate a person eats that matters for health, rather than the relative levels of these nutrients. Eating unsaturated fats and unprocessed carbohydrates like whole grains, fruits, and legumes produces the greatest health and survival, while higher levels of saturated fats and processed carbs in the diet produce health problems. That’s much more important than whether a diet is low fat or low carb. This means sticking with the food principles advanced by the AHA diet, the DASH diet, and a Mediterranean diet.
High-fat and low-carb diets are popular because people who follow them lose weight over the short term, but those weight losses are hard to sustain longer term and create an opportunity for unhealthy effects if people eat the wrong fats, carbohydrates, and proteins. Strategies that focus on healthier food choices like the Mediterranean or AHA diets can minimize disease and produce more sustainable weight control.
Robert A. Vogel, MD , is a cardiologist in Denver affiliated with the University of Colorado School of Medicine and the VA Medical Center in Denver. He has been a consultant to the Pritikin Longevity Institute in Doral, Fla. He made these comments in an interview.
This is an important study because the findings reinforce the already established concept that it’s the quality of the fat and carbohydrate a person eats that matters for health, rather than the relative levels of these nutrients. Eating unsaturated fats and unprocessed carbohydrates like whole grains, fruits, and legumes produces the greatest health and survival, while higher levels of saturated fats and processed carbs in the diet produce health problems. That’s much more important than whether a diet is low fat or low carb. This means sticking with the food principles advanced by the AHA diet, the DASH diet, and a Mediterranean diet.
High-fat and low-carb diets are popular because people who follow them lose weight over the short term, but those weight losses are hard to sustain longer term and create an opportunity for unhealthy effects if people eat the wrong fats, carbohydrates, and proteins. Strategies that focus on healthier food choices like the Mediterranean or AHA diets can minimize disease and produce more sustainable weight control.
Robert A. Vogel, MD , is a cardiologist in Denver affiliated with the University of Colorado School of Medicine and the VA Medical Center in Denver. He has been a consultant to the Pritikin Longevity Institute in Doral, Fla. He made these comments in an interview.
The health consequences of diet don’t largely depend on whether a person eats a high or low level of carbohydrates or a diet high or low in fat. What’s much more important is where the carbs and fats come from, according to an analysis that related diet and mortality rates in more than 37,000 American adults.
“Unhealthy low carbohydrate diet [LCD] and low-fat diet [LFD] scores were associated with higher total mortality, whereas healthy LCD and LFD scores were associated with lower total mortality,” Zhilei Shan, MD, and associates wrote in an article (JAMA Intern Med. 2020 Jan 21; doi: 10.1001/jamainternmed.2019.6980). The findings “suggest that the association of LCDs and LFDs with mortality may depend on the quality of food sources of macronutrients,” said the researchers, based at the Harvard T.H. Chan School of Public Health in Boston.
The analysis included follow-up of almost 300,000 person-years. It showed that, for every 20-percentile increase in a person’s unhealthy LCD score, their relative rate of total mortality increased by a statistically significant 7%; and for every 20-percentile rise in unhealthy LFD score, the relative, total mortality rate rose by a statistically significant 6%, after adjustment for several demographic and clinical measures and family and personal histories of diabetes, cancer, and heart disease. In contrast, for each 20-percentile increase in healthy LCD score relative, total mortality fell by 9%, and similar increases in healthy LFD score linked with an 11% relative drop in total mortality, also statistically significant associations in these confounder-adjusted analyses.
The findings “extend the previous evidence” for these links, and the data suggest that “the health benefits of an LCD or LFD may depend not only on the types of protein and fat or carbohydrate but also on the quality of carbohydrate or fat remaining in the diet,” the researchers wrote. They cited the documented health problems caused by eating significant amounts of low-quality carbohydrates such as refined grains and added sugars, which provide limited nutrition and introduce a high glycemic load, and can produce high levels of postprandial glucose and insulin, inflammation, insulin resistance, and dyslipidemia.
The foods people ate that produced healthy diet scores and linked with better survival were diets high in plant protein and unsaturated fat, and low in carbohydrates from refined grains, added sugar, starchy vegetables, and similar sources as part of a low carbohydrate diet. The foods that formed a healthy LFD included whole grains, whole fruit, legumes, and nonstarchy vegetables, along with higher intake of plant protein and low levels of saturated fat.
The study used data from 24-hour diet-recall surveys completed by 37,233 American adults collected by the National Health and Nutrition Examination Survey (NHANES) during 1999-2014, and linked the diet scores calculated for these people with U.S. national death records collected by the National Death Index through the end of 2015. The people included averaged about 50 years of age at the time of their dietary interview, and 53% were women. During 297,768 person-years of follow-up, 4,866 total deaths occurred, including 849 from heart disease and 1,068 from cancer. The analyses found no statistically significant links between overall LCD or LFD scores and mortality; the significant links only existed when the researchers further classified the diet scores into healthy and unhealthy subtypes.
The results also showed statistically significant links or strong trends between high or low levels of healthy or unhealthy LCD and LFD scores and cancer deaths. A 20-percentile increase in unhealthy LCD score linked with an 11% relative increase in cancer deaths, while a 20-percentile increase in the healthy LCD score linked with a 10% decrease in cancer deaths. A 20-percentile increase in the healthy LFD score linked with a 15% relative decrease in cancer mortality.
The study received no commercial fundings, and the authors had no commercial disclosures.
SOURCE: Shan Z et al. JAMA Intern Med. 2020 Jan 21; doi: 10.1001/jamainternmed.2019.6980.
The health consequences of diet don’t largely depend on whether a person eats a high or low level of carbohydrates or a diet high or low in fat. What’s much more important is where the carbs and fats come from, according to an analysis that related diet and mortality rates in more than 37,000 American adults.
“Unhealthy low carbohydrate diet [LCD] and low-fat diet [LFD] scores were associated with higher total mortality, whereas healthy LCD and LFD scores were associated with lower total mortality,” Zhilei Shan, MD, and associates wrote in an article (JAMA Intern Med. 2020 Jan 21; doi: 10.1001/jamainternmed.2019.6980). The findings “suggest that the association of LCDs and LFDs with mortality may depend on the quality of food sources of macronutrients,” said the researchers, based at the Harvard T.H. Chan School of Public Health in Boston.
The analysis included follow-up of almost 300,000 person-years. It showed that, for every 20-percentile increase in a person’s unhealthy LCD score, their relative rate of total mortality increased by a statistically significant 7%; and for every 20-percentile rise in unhealthy LFD score, the relative, total mortality rate rose by a statistically significant 6%, after adjustment for several demographic and clinical measures and family and personal histories of diabetes, cancer, and heart disease. In contrast, for each 20-percentile increase in healthy LCD score relative, total mortality fell by 9%, and similar increases in healthy LFD score linked with an 11% relative drop in total mortality, also statistically significant associations in these confounder-adjusted analyses.
The findings “extend the previous evidence” for these links, and the data suggest that “the health benefits of an LCD or LFD may depend not only on the types of protein and fat or carbohydrate but also on the quality of carbohydrate or fat remaining in the diet,” the researchers wrote. They cited the documented health problems caused by eating significant amounts of low-quality carbohydrates such as refined grains and added sugars, which provide limited nutrition and introduce a high glycemic load, and can produce high levels of postprandial glucose and insulin, inflammation, insulin resistance, and dyslipidemia.
The foods people ate that produced healthy diet scores and linked with better survival were diets high in plant protein and unsaturated fat, and low in carbohydrates from refined grains, added sugar, starchy vegetables, and similar sources as part of a low carbohydrate diet. The foods that formed a healthy LFD included whole grains, whole fruit, legumes, and nonstarchy vegetables, along with higher intake of plant protein and low levels of saturated fat.
The study used data from 24-hour diet-recall surveys completed by 37,233 American adults collected by the National Health and Nutrition Examination Survey (NHANES) during 1999-2014, and linked the diet scores calculated for these people with U.S. national death records collected by the National Death Index through the end of 2015. The people included averaged about 50 years of age at the time of their dietary interview, and 53% were women. During 297,768 person-years of follow-up, 4,866 total deaths occurred, including 849 from heart disease and 1,068 from cancer. The analyses found no statistically significant links between overall LCD or LFD scores and mortality; the significant links only existed when the researchers further classified the diet scores into healthy and unhealthy subtypes.
The results also showed statistically significant links or strong trends between high or low levels of healthy or unhealthy LCD and LFD scores and cancer deaths. A 20-percentile increase in unhealthy LCD score linked with an 11% relative increase in cancer deaths, while a 20-percentile increase in the healthy LCD score linked with a 10% decrease in cancer deaths. A 20-percentile increase in the healthy LFD score linked with a 15% relative decrease in cancer mortality.
The study received no commercial fundings, and the authors had no commercial disclosures.
SOURCE: Shan Z et al. JAMA Intern Med. 2020 Jan 21; doi: 10.1001/jamainternmed.2019.6980.
REPORTING FROM JAMA INTERNAL MEDICINE
Celebrating 50 years of Dermatology News
The first issue of Skin & Allergy News, now Dermatology News, was published in January 1970. One front-page story highlighted the "continued improvement and more widespread use of steroids" as the most important development of the 1960s in dermatology. Another covered the launch of a national program for dermatology "to design a pattern for its future instead of simply drifting and letting its fate be determined by others."
Throughout 2020, look for articles and features marking the publication's golden anniversary. And read the first ever issue in the PDF above.
The first issue of Skin & Allergy News, now Dermatology News, was published in January 1970. One front-page story highlighted the "continued improvement and more widespread use of steroids" as the most important development of the 1960s in dermatology. Another covered the launch of a national program for dermatology "to design a pattern for its future instead of simply drifting and letting its fate be determined by others."
Throughout 2020, look for articles and features marking the publication's golden anniversary. And read the first ever issue in the PDF above.
The first issue of Skin & Allergy News, now Dermatology News, was published in January 1970. One front-page story highlighted the "continued improvement and more widespread use of steroids" as the most important development of the 1960s in dermatology. Another covered the launch of a national program for dermatology "to design a pattern for its future instead of simply drifting and letting its fate be determined by others."
Throughout 2020, look for articles and features marking the publication's golden anniversary. And read the first ever issue in the PDF above.
Redo PCI or CABG, left main patients pay a price: EXCEL
Repeat revascularization was more frequent after left main percutaneous coronary intervention than after coronary artery bypass surgery, but raised the mortality risk after both procedures in a secondary EXCEL analysis.
The 3-year rate of any repeat revascularization was 12.9% after PCI and 7.6% after CABG (hazard ratio, 1.73; 95% confidence interval, 1.28-2.33).
“It’s a real difference and shouldn’t be minimized. About 1 in 20 patients will need an additional repeat revascularization after PCI, compared with surgery,” study author Gregg Stone, MD, Icahn School of Medicine at Mount Sinai in New York, said in an interview. “Surgery is a more durable procedure in that regard, and patients need to be informed of that by heart team discussions.”
That said, Dr. Stone highlighted other differences between the two strategies, including more bleeding and atrial fibrillation after surgery and better early quality of life after PCI. There’s also an early myocardial infarction (MI) benefit with PCI but a late MI benefit with surgery, which “is probably a more important difference between the two, as opposed to the difference in repeat revascularization,” he added.
Although the increased need to perform repeat vascularization after PCI is not unexpected, the analysis of 346 repeat revascularizations in 185 patients provides more details on the timing and prognosis of these procedures in left main disease.
The need for repeat revascularization was independently associated with 3-year all-cause mortality (adjusted HR, 2.05; 95% CI, 1.13-3.70) and cardiovascular mortality (adjusted HR, 4.22; 95% CI, 2.10-8.48) for both PCI and CABG (P for interaction = .85 for both outcomes).
The increase in mortality risk, however, was smaller than that for MI (adjusted HR, 4.03; 95% CI, 2.43-6.67) or stroke (adjusted HR, 16.62; 95% CI, 9.97-27.69).
The risk for death peaked in the 30 days after redo revascularization and then declined during follow-up. Most of the deaths were cardiovascular (74/128).
The incidence of repeat left main PCI was only 17.5%, whereas the left main was the most common site for redo revascularization in the CABG group.
Repeat revascularization of the index target vessel and target lesion – but not of other lesions – were both strongly associated with increased all-cause and cardiovascular mortality, the authors reported January 15 in JACC: Cardiovascular Interventions.
“It just continues to show that, no matter what intervention we use, we haven’t achieved perfection yet and the opportunities for improvement and decision making between a PCI and a CABG is still up in the air,” Richard J. Shemin, MD, chief of cardiac surgery, UCLA Medical Center, Los Angeles, said in an interview. “And there’s some evidence to suggest coronary bypass might be better in terms of mortality and the need for repeat revascularization.”
Enhancing durability
“Measures to reduce the need for repeat revascularization including improved stent platforms and implantation technique, use of pan-arterial bypass grafting, and aggressive risk factor control with guideline-directed medical therapy may improve prognosis after both PCI and CABG,” the authors concluded.
In a linked editorial, David O. Williams, MD, and Pinak B. Shah, MD, both with Brigham and Women’s Hospital and Harvard Medical School, Boston, say intravascular imaging should be “mandatory for all complex PCI,” but that intravascular ultrasound was used in only 77.2% of cases in EXCEL.
“There are also data suggesting careful image guidance during complex PCI is associated with a mortality benefit,” they wrote. “In a similar fashion, arterial revascularization (especially with a mammary artery graft to the [left anterior descending]) and complete revascularization during CABG needs to be achieved.”
“Surgeons need to be intellectually challenged to not take the easy way out and just do a saphenous vein graft,” Dr. Shemin agreed. “And because we are dealing with an underlying progressive disease, continued medical and preventive measures to prevent atherosclerosis are key.”
Higher body mass index, insulin-treated diabetes, and hemodynamic support during the procedure were associated with a higher risk for repeat revascularization after PCI, whereas statin use at discharge was protective.
Younger age, female sex, and peripheral vascular disease were independent predictors of repeat revascularization after CABG.
Most redo procedures were performed by PCI in both groups. However, repeat revascularization by CABG was more common during follow-up in patients randomized to initial PCI vs. CABG (3.3% vs .0.8%; P = .0002) and was significantly associated with increased all-cause mortality.
“This observation suggests that CABG should be reserved for repeat revascularization procedures that are not amenable to repeat PCI, irrespective of the initial revascularization approach,” the authors wrote.
The editorialists point out that more than half of EXCEL patients with one repeat revascularization went on to have another. Overall, 55.1% of patients underwent one repeat revascularization, 22.2% underwent two redos, and 22.7% underwent more than two redos.
Although enhancing the durability of the initial revascularization is an important goal, “one might also conclude that a safer and potentially more durable treatment specifically developed for recurrent lesions is as equally an important objective,” they opined.
5-year kerfuffle
As previously reported, the EXCEL trial’s 5-year analysis showed no significant difference between PCI and CABG for the primary endpoint of all-cause death, MI, or stroke.
However, recent allegations that key MI data were withheld have called into question the final conclusion of relative parity and led the European Association for Cardio-Thoracic Surgery (EACTS) to withdraw support for the left main portion of the 2018 EACTS-European Society of Cardiology (ESC) clinical guidelines based on 3-year EXCEL outcomes.
On January 14, the Society of Thoracic Surgeons (STS) joined EACTS and the American Association for Thoracic Surgery in calling for independent reanalysis of the EXCEL data.
“Any final conclusions drawn from the EXCEL trial will not only affect the actions of physicians, surgeons, regulatory agencies, and third-party payers but, more importantly, they will seriously impact the health and wellbeing of our patients and their families for years to come,” the statement says.
“Given such potentially profound consequences, the Society believes that the final interpretation regarding the outcomes of the EXCEL study should wait until an independent analysis of all aspects of the EXCEL study has been performed.”
EXCEL was sponsored by Abbott Vascular. Dr. Stone reported speaker honoraria from Terumo and Amaranth and serving as a consultant to Reva. Coauthor conflict of interest disclosures are listed in the paper. Dr. Shemin reported no relevant conflicts of interest.
This article first appeared on Medscape.com.
Repeat revascularization was more frequent after left main percutaneous coronary intervention than after coronary artery bypass surgery, but raised the mortality risk after both procedures in a secondary EXCEL analysis.
The 3-year rate of any repeat revascularization was 12.9% after PCI and 7.6% after CABG (hazard ratio, 1.73; 95% confidence interval, 1.28-2.33).
“It’s a real difference and shouldn’t be minimized. About 1 in 20 patients will need an additional repeat revascularization after PCI, compared with surgery,” study author Gregg Stone, MD, Icahn School of Medicine at Mount Sinai in New York, said in an interview. “Surgery is a more durable procedure in that regard, and patients need to be informed of that by heart team discussions.”
That said, Dr. Stone highlighted other differences between the two strategies, including more bleeding and atrial fibrillation after surgery and better early quality of life after PCI. There’s also an early myocardial infarction (MI) benefit with PCI but a late MI benefit with surgery, which “is probably a more important difference between the two, as opposed to the difference in repeat revascularization,” he added.
Although the increased need to perform repeat vascularization after PCI is not unexpected, the analysis of 346 repeat revascularizations in 185 patients provides more details on the timing and prognosis of these procedures in left main disease.
The need for repeat revascularization was independently associated with 3-year all-cause mortality (adjusted HR, 2.05; 95% CI, 1.13-3.70) and cardiovascular mortality (adjusted HR, 4.22; 95% CI, 2.10-8.48) for both PCI and CABG (P for interaction = .85 for both outcomes).
The increase in mortality risk, however, was smaller than that for MI (adjusted HR, 4.03; 95% CI, 2.43-6.67) or stroke (adjusted HR, 16.62; 95% CI, 9.97-27.69).
The risk for death peaked in the 30 days after redo revascularization and then declined during follow-up. Most of the deaths were cardiovascular (74/128).
The incidence of repeat left main PCI was only 17.5%, whereas the left main was the most common site for redo revascularization in the CABG group.
Repeat revascularization of the index target vessel and target lesion – but not of other lesions – were both strongly associated with increased all-cause and cardiovascular mortality, the authors reported January 15 in JACC: Cardiovascular Interventions.
“It just continues to show that, no matter what intervention we use, we haven’t achieved perfection yet and the opportunities for improvement and decision making between a PCI and a CABG is still up in the air,” Richard J. Shemin, MD, chief of cardiac surgery, UCLA Medical Center, Los Angeles, said in an interview. “And there’s some evidence to suggest coronary bypass might be better in terms of mortality and the need for repeat revascularization.”
Enhancing durability
“Measures to reduce the need for repeat revascularization including improved stent platforms and implantation technique, use of pan-arterial bypass grafting, and aggressive risk factor control with guideline-directed medical therapy may improve prognosis after both PCI and CABG,” the authors concluded.
In a linked editorial, David O. Williams, MD, and Pinak B. Shah, MD, both with Brigham and Women’s Hospital and Harvard Medical School, Boston, say intravascular imaging should be “mandatory for all complex PCI,” but that intravascular ultrasound was used in only 77.2% of cases in EXCEL.
“There are also data suggesting careful image guidance during complex PCI is associated with a mortality benefit,” they wrote. “In a similar fashion, arterial revascularization (especially with a mammary artery graft to the [left anterior descending]) and complete revascularization during CABG needs to be achieved.”
“Surgeons need to be intellectually challenged to not take the easy way out and just do a saphenous vein graft,” Dr. Shemin agreed. “And because we are dealing with an underlying progressive disease, continued medical and preventive measures to prevent atherosclerosis are key.”
Higher body mass index, insulin-treated diabetes, and hemodynamic support during the procedure were associated with a higher risk for repeat revascularization after PCI, whereas statin use at discharge was protective.
Younger age, female sex, and peripheral vascular disease were independent predictors of repeat revascularization after CABG.
Most redo procedures were performed by PCI in both groups. However, repeat revascularization by CABG was more common during follow-up in patients randomized to initial PCI vs. CABG (3.3% vs .0.8%; P = .0002) and was significantly associated with increased all-cause mortality.
“This observation suggests that CABG should be reserved for repeat revascularization procedures that are not amenable to repeat PCI, irrespective of the initial revascularization approach,” the authors wrote.
The editorialists point out that more than half of EXCEL patients with one repeat revascularization went on to have another. Overall, 55.1% of patients underwent one repeat revascularization, 22.2% underwent two redos, and 22.7% underwent more than two redos.
Although enhancing the durability of the initial revascularization is an important goal, “one might also conclude that a safer and potentially more durable treatment specifically developed for recurrent lesions is as equally an important objective,” they opined.
5-year kerfuffle
As previously reported, the EXCEL trial’s 5-year analysis showed no significant difference between PCI and CABG for the primary endpoint of all-cause death, MI, or stroke.
However, recent allegations that key MI data were withheld have called into question the final conclusion of relative parity and led the European Association for Cardio-Thoracic Surgery (EACTS) to withdraw support for the left main portion of the 2018 EACTS-European Society of Cardiology (ESC) clinical guidelines based on 3-year EXCEL outcomes.
On January 14, the Society of Thoracic Surgeons (STS) joined EACTS and the American Association for Thoracic Surgery in calling for independent reanalysis of the EXCEL data.
“Any final conclusions drawn from the EXCEL trial will not only affect the actions of physicians, surgeons, regulatory agencies, and third-party payers but, more importantly, they will seriously impact the health and wellbeing of our patients and their families for years to come,” the statement says.
“Given such potentially profound consequences, the Society believes that the final interpretation regarding the outcomes of the EXCEL study should wait until an independent analysis of all aspects of the EXCEL study has been performed.”
EXCEL was sponsored by Abbott Vascular. Dr. Stone reported speaker honoraria from Terumo and Amaranth and serving as a consultant to Reva. Coauthor conflict of interest disclosures are listed in the paper. Dr. Shemin reported no relevant conflicts of interest.
This article first appeared on Medscape.com.
Repeat revascularization was more frequent after left main percutaneous coronary intervention than after coronary artery bypass surgery, but raised the mortality risk after both procedures in a secondary EXCEL analysis.
The 3-year rate of any repeat revascularization was 12.9% after PCI and 7.6% after CABG (hazard ratio, 1.73; 95% confidence interval, 1.28-2.33).
“It’s a real difference and shouldn’t be minimized. About 1 in 20 patients will need an additional repeat revascularization after PCI, compared with surgery,” study author Gregg Stone, MD, Icahn School of Medicine at Mount Sinai in New York, said in an interview. “Surgery is a more durable procedure in that regard, and patients need to be informed of that by heart team discussions.”
That said, Dr. Stone highlighted other differences between the two strategies, including more bleeding and atrial fibrillation after surgery and better early quality of life after PCI. There’s also an early myocardial infarction (MI) benefit with PCI but a late MI benefit with surgery, which “is probably a more important difference between the two, as opposed to the difference in repeat revascularization,” he added.
Although the increased need to perform repeat vascularization after PCI is not unexpected, the analysis of 346 repeat revascularizations in 185 patients provides more details on the timing and prognosis of these procedures in left main disease.
The need for repeat revascularization was independently associated with 3-year all-cause mortality (adjusted HR, 2.05; 95% CI, 1.13-3.70) and cardiovascular mortality (adjusted HR, 4.22; 95% CI, 2.10-8.48) for both PCI and CABG (P for interaction = .85 for both outcomes).
The increase in mortality risk, however, was smaller than that for MI (adjusted HR, 4.03; 95% CI, 2.43-6.67) or stroke (adjusted HR, 16.62; 95% CI, 9.97-27.69).
The risk for death peaked in the 30 days after redo revascularization and then declined during follow-up. Most of the deaths were cardiovascular (74/128).
The incidence of repeat left main PCI was only 17.5%, whereas the left main was the most common site for redo revascularization in the CABG group.
Repeat revascularization of the index target vessel and target lesion – but not of other lesions – were both strongly associated with increased all-cause and cardiovascular mortality, the authors reported January 15 in JACC: Cardiovascular Interventions.
“It just continues to show that, no matter what intervention we use, we haven’t achieved perfection yet and the opportunities for improvement and decision making between a PCI and a CABG is still up in the air,” Richard J. Shemin, MD, chief of cardiac surgery, UCLA Medical Center, Los Angeles, said in an interview. “And there’s some evidence to suggest coronary bypass might be better in terms of mortality and the need for repeat revascularization.”
Enhancing durability
“Measures to reduce the need for repeat revascularization including improved stent platforms and implantation technique, use of pan-arterial bypass grafting, and aggressive risk factor control with guideline-directed medical therapy may improve prognosis after both PCI and CABG,” the authors concluded.
In a linked editorial, David O. Williams, MD, and Pinak B. Shah, MD, both with Brigham and Women’s Hospital and Harvard Medical School, Boston, say intravascular imaging should be “mandatory for all complex PCI,” but that intravascular ultrasound was used in only 77.2% of cases in EXCEL.
“There are also data suggesting careful image guidance during complex PCI is associated with a mortality benefit,” they wrote. “In a similar fashion, arterial revascularization (especially with a mammary artery graft to the [left anterior descending]) and complete revascularization during CABG needs to be achieved.”
“Surgeons need to be intellectually challenged to not take the easy way out and just do a saphenous vein graft,” Dr. Shemin agreed. “And because we are dealing with an underlying progressive disease, continued medical and preventive measures to prevent atherosclerosis are key.”
Higher body mass index, insulin-treated diabetes, and hemodynamic support during the procedure were associated with a higher risk for repeat revascularization after PCI, whereas statin use at discharge was protective.
Younger age, female sex, and peripheral vascular disease were independent predictors of repeat revascularization after CABG.
Most redo procedures were performed by PCI in both groups. However, repeat revascularization by CABG was more common during follow-up in patients randomized to initial PCI vs. CABG (3.3% vs .0.8%; P = .0002) and was significantly associated with increased all-cause mortality.
“This observation suggests that CABG should be reserved for repeat revascularization procedures that are not amenable to repeat PCI, irrespective of the initial revascularization approach,” the authors wrote.
The editorialists point out that more than half of EXCEL patients with one repeat revascularization went on to have another. Overall, 55.1% of patients underwent one repeat revascularization, 22.2% underwent two redos, and 22.7% underwent more than two redos.
Although enhancing the durability of the initial revascularization is an important goal, “one might also conclude that a safer and potentially more durable treatment specifically developed for recurrent lesions is as equally an important objective,” they opined.
5-year kerfuffle
As previously reported, the EXCEL trial’s 5-year analysis showed no significant difference between PCI and CABG for the primary endpoint of all-cause death, MI, or stroke.
However, recent allegations that key MI data were withheld have called into question the final conclusion of relative parity and led the European Association for Cardio-Thoracic Surgery (EACTS) to withdraw support for the left main portion of the 2018 EACTS-European Society of Cardiology (ESC) clinical guidelines based on 3-year EXCEL outcomes.
On January 14, the Society of Thoracic Surgeons (STS) joined EACTS and the American Association for Thoracic Surgery in calling for independent reanalysis of the EXCEL data.
“Any final conclusions drawn from the EXCEL trial will not only affect the actions of physicians, surgeons, regulatory agencies, and third-party payers but, more importantly, they will seriously impact the health and wellbeing of our patients and their families for years to come,” the statement says.
“Given such potentially profound consequences, the Society believes that the final interpretation regarding the outcomes of the EXCEL study should wait until an independent analysis of all aspects of the EXCEL study has been performed.”
EXCEL was sponsored by Abbott Vascular. Dr. Stone reported speaker honoraria from Terumo and Amaranth and serving as a consultant to Reva. Coauthor conflict of interest disclosures are listed in the paper. Dr. Shemin reported no relevant conflicts of interest.
This article first appeared on Medscape.com.
USPSTF recommendations on screening for abdominal aortic aneurysm
The prevalence of abdominal aortic aneurysms (AAAs) is decreasing, thought to be caused by a decrease in smoking. But the risk of death if one ruptures is as high as 81%. So, screening is still an important part of preventive medicine.
When the abdominal aorta enlarges to greater than 3.0 cm, it is considered an aneurysm. Risk factors that can lead to an enlarged aorta include older age, male sex, smoking, history of AAA in a first-degree relative, hypertension, history of other aneurysms, coronary artery disease, cerebrovascular disease, atherosclerosis, and hypercholesterolemia.
History of AAA in a first-degree relative puts patients at double the risk of developing an abdominal aortic aneurysm. Interestingly, diabetes has been associated with a reduced risk of AAA. People of African American, Asian, and Hispanic descent have a reduced risk of AAA.
Screening
Screening is performed using abdominal duplex ultrasound. It has high sensitivity (94%-100%) and specificity (98%-100%), is low cost, and has low risk to the patient. The U.S. Preventive Services Task Force breaks its screening recommendations into four categories:
1. Men aged 65-75 years who have ever smoked (at least 100 cigarettes in their lifetime): One-time screening (grade B, moderate net benefit).
2. Men aged 65-75 years who have never smoked: Selectively offer screening (grade C, small net benefit). “To determine whether this service is appropriate, patients and clinicians should consider the patient’s medical history, family history, other risk factors, and personal values.”
3. Women without a smoking history or family history of AAA: Do not perform screening (grade D, recommendation against the service).
4. Women aged 65-75 years who have a smoking history or family history of AAA: There is insufficient evidence on whether or not to screen for AAA (grade I, insufficient evidence).
To assess screening and treatment of AAAs, the USPSTF looked at four randomized, controlled trials largely focused on men older than 65 years. With the combined data, they found 246 men would need to be screened to prevent 1 AAA rupture, and 305 men would need to be screened to prevent 1 death from AAA.
The USPSTF does note that, while the risk of death is lower for elective AAA repair than ruptured AAA, there is still increased risk with elective surgery. In addition, increased screening and detection increases the rate of elective surgery. Overdiagnosis and overtreatment could represent a harm.
Treatment
Surgical repair of AAA in men depends on the size of the aneurysm and rate of growth.
For men, surgical repair is standard when the AAA reaches 5.5 cm or if the AAA is growing faster than 1.0 cm per year and is larger than 4.0 cm. For women, surgical repair is often recommended between 5.0 cm and 5.4 cm in size.
Surgical repair is not recommended for AAAs that are less than 5.0 cm because the annual risk of rupture is 0%-1% below 5.0 cm. The risk increases to 11% for aneurysms that are 5.0-5.9 cm in size.
There are two methods of surgical repair: endovascular aneurysm repair and open repair. Recommendations for the surveillance of AAA between 3.0 cm and 5.5 cm is regular ultrasound surveillance, with the interval becoming shorter as the aneurysm size becomes larger. Exact intervals differ from one guideline group to another.
Screening and treatment in women
While it is true that AAAs in women are more likely to rupture at smaller sizes than AAAs in men, the AAAs that rupture in women are more likely to rupture at an older age than AAAs rupture in men.
The prevalence of AAAs in women is thought to be one-sixth of the prevalence of men. In addition, women had a higher 30-day mortality after surgical repair. They also had higher rates of complications for elective surgical repair of AAAs.
For these reasons, it is unclear that the benefits of AAA screening and treatment in women outweigh the risks, and the USPSTF cannot come to a conclusive recommendation for women who have ever smoked or women who have a family history of AAA.
The USPSTF is able to state definitively that they do not recommend screening in women with no smoking history or family history of AAA.
Bottom line
The USPSTF recommends screening men aged 65-75 years who have ever smoked and selectively screening men aged 65-75 years with no smoking history. The USPSTF recommends against screening women aged 65-75 years who have never smoked and have no family history of AAA. There is insufficient evidence to either recommend for or against screening women aged 65-75 years who have smoked or have a family history of AAA.
Reference
Owens DK et al. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2019 Dec 10;322(22):2211-18.
Dr. Sprogell is a second-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
The prevalence of abdominal aortic aneurysms (AAAs) is decreasing, thought to be caused by a decrease in smoking. But the risk of death if one ruptures is as high as 81%. So, screening is still an important part of preventive medicine.
When the abdominal aorta enlarges to greater than 3.0 cm, it is considered an aneurysm. Risk factors that can lead to an enlarged aorta include older age, male sex, smoking, history of AAA in a first-degree relative, hypertension, history of other aneurysms, coronary artery disease, cerebrovascular disease, atherosclerosis, and hypercholesterolemia.
History of AAA in a first-degree relative puts patients at double the risk of developing an abdominal aortic aneurysm. Interestingly, diabetes has been associated with a reduced risk of AAA. People of African American, Asian, and Hispanic descent have a reduced risk of AAA.
Screening
Screening is performed using abdominal duplex ultrasound. It has high sensitivity (94%-100%) and specificity (98%-100%), is low cost, and has low risk to the patient. The U.S. Preventive Services Task Force breaks its screening recommendations into four categories:
1. Men aged 65-75 years who have ever smoked (at least 100 cigarettes in their lifetime): One-time screening (grade B, moderate net benefit).
2. Men aged 65-75 years who have never smoked: Selectively offer screening (grade C, small net benefit). “To determine whether this service is appropriate, patients and clinicians should consider the patient’s medical history, family history, other risk factors, and personal values.”
3. Women without a smoking history or family history of AAA: Do not perform screening (grade D, recommendation against the service).
4. Women aged 65-75 years who have a smoking history or family history of AAA: There is insufficient evidence on whether or not to screen for AAA (grade I, insufficient evidence).
To assess screening and treatment of AAAs, the USPSTF looked at four randomized, controlled trials largely focused on men older than 65 years. With the combined data, they found 246 men would need to be screened to prevent 1 AAA rupture, and 305 men would need to be screened to prevent 1 death from AAA.
The USPSTF does note that, while the risk of death is lower for elective AAA repair than ruptured AAA, there is still increased risk with elective surgery. In addition, increased screening and detection increases the rate of elective surgery. Overdiagnosis and overtreatment could represent a harm.
Treatment
Surgical repair of AAA in men depends on the size of the aneurysm and rate of growth.
For men, surgical repair is standard when the AAA reaches 5.5 cm or if the AAA is growing faster than 1.0 cm per year and is larger than 4.0 cm. For women, surgical repair is often recommended between 5.0 cm and 5.4 cm in size.
Surgical repair is not recommended for AAAs that are less than 5.0 cm because the annual risk of rupture is 0%-1% below 5.0 cm. The risk increases to 11% for aneurysms that are 5.0-5.9 cm in size.
There are two methods of surgical repair: endovascular aneurysm repair and open repair. Recommendations for the surveillance of AAA between 3.0 cm and 5.5 cm is regular ultrasound surveillance, with the interval becoming shorter as the aneurysm size becomes larger. Exact intervals differ from one guideline group to another.
Screening and treatment in women
While it is true that AAAs in women are more likely to rupture at smaller sizes than AAAs in men, the AAAs that rupture in women are more likely to rupture at an older age than AAAs rupture in men.
The prevalence of AAAs in women is thought to be one-sixth of the prevalence of men. In addition, women had a higher 30-day mortality after surgical repair. They also had higher rates of complications for elective surgical repair of AAAs.
For these reasons, it is unclear that the benefits of AAA screening and treatment in women outweigh the risks, and the USPSTF cannot come to a conclusive recommendation for women who have ever smoked or women who have a family history of AAA.
The USPSTF is able to state definitively that they do not recommend screening in women with no smoking history or family history of AAA.
Bottom line
The USPSTF recommends screening men aged 65-75 years who have ever smoked and selectively screening men aged 65-75 years with no smoking history. The USPSTF recommends against screening women aged 65-75 years who have never smoked and have no family history of AAA. There is insufficient evidence to either recommend for or against screening women aged 65-75 years who have smoked or have a family history of AAA.
Reference
Owens DK et al. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2019 Dec 10;322(22):2211-18.
Dr. Sprogell is a second-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
The prevalence of abdominal aortic aneurysms (AAAs) is decreasing, thought to be caused by a decrease in smoking. But the risk of death if one ruptures is as high as 81%. So, screening is still an important part of preventive medicine.
When the abdominal aorta enlarges to greater than 3.0 cm, it is considered an aneurysm. Risk factors that can lead to an enlarged aorta include older age, male sex, smoking, history of AAA in a first-degree relative, hypertension, history of other aneurysms, coronary artery disease, cerebrovascular disease, atherosclerosis, and hypercholesterolemia.
History of AAA in a first-degree relative puts patients at double the risk of developing an abdominal aortic aneurysm. Interestingly, diabetes has been associated with a reduced risk of AAA. People of African American, Asian, and Hispanic descent have a reduced risk of AAA.
Screening
Screening is performed using abdominal duplex ultrasound. It has high sensitivity (94%-100%) and specificity (98%-100%), is low cost, and has low risk to the patient. The U.S. Preventive Services Task Force breaks its screening recommendations into four categories:
1. Men aged 65-75 years who have ever smoked (at least 100 cigarettes in their lifetime): One-time screening (grade B, moderate net benefit).
2. Men aged 65-75 years who have never smoked: Selectively offer screening (grade C, small net benefit). “To determine whether this service is appropriate, patients and clinicians should consider the patient’s medical history, family history, other risk factors, and personal values.”
3. Women without a smoking history or family history of AAA: Do not perform screening (grade D, recommendation against the service).
4. Women aged 65-75 years who have a smoking history or family history of AAA: There is insufficient evidence on whether or not to screen for AAA (grade I, insufficient evidence).
To assess screening and treatment of AAAs, the USPSTF looked at four randomized, controlled trials largely focused on men older than 65 years. With the combined data, they found 246 men would need to be screened to prevent 1 AAA rupture, and 305 men would need to be screened to prevent 1 death from AAA.
The USPSTF does note that, while the risk of death is lower for elective AAA repair than ruptured AAA, there is still increased risk with elective surgery. In addition, increased screening and detection increases the rate of elective surgery. Overdiagnosis and overtreatment could represent a harm.
Treatment
Surgical repair of AAA in men depends on the size of the aneurysm and rate of growth.
For men, surgical repair is standard when the AAA reaches 5.5 cm or if the AAA is growing faster than 1.0 cm per year and is larger than 4.0 cm. For women, surgical repair is often recommended between 5.0 cm and 5.4 cm in size.
Surgical repair is not recommended for AAAs that are less than 5.0 cm because the annual risk of rupture is 0%-1% below 5.0 cm. The risk increases to 11% for aneurysms that are 5.0-5.9 cm in size.
There are two methods of surgical repair: endovascular aneurysm repair and open repair. Recommendations for the surveillance of AAA between 3.0 cm and 5.5 cm is regular ultrasound surveillance, with the interval becoming shorter as the aneurysm size becomes larger. Exact intervals differ from one guideline group to another.
Screening and treatment in women
While it is true that AAAs in women are more likely to rupture at smaller sizes than AAAs in men, the AAAs that rupture in women are more likely to rupture at an older age than AAAs rupture in men.
The prevalence of AAAs in women is thought to be one-sixth of the prevalence of men. In addition, women had a higher 30-day mortality after surgical repair. They also had higher rates of complications for elective surgical repair of AAAs.
For these reasons, it is unclear that the benefits of AAA screening and treatment in women outweigh the risks, and the USPSTF cannot come to a conclusive recommendation for women who have ever smoked or women who have a family history of AAA.
The USPSTF is able to state definitively that they do not recommend screening in women with no smoking history or family history of AAA.
Bottom line
The USPSTF recommends screening men aged 65-75 years who have ever smoked and selectively screening men aged 65-75 years with no smoking history. The USPSTF recommends against screening women aged 65-75 years who have never smoked and have no family history of AAA. There is insufficient evidence to either recommend for or against screening women aged 65-75 years who have smoked or have a family history of AAA.
Reference
Owens DK et al. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2019 Dec 10;322(22):2211-18.
Dr. Sprogell is a second-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
Age, race affect preterm birth risk in women with obesity
according to new findings from an analysis that used a large, ethnically diverse population sample.
Previous study findings have demonstrated that pregnant women with obesity have a higher risk of giving birth to preterm babies, but the effect of age and race on that risk was not clear until now.
In this latest study, Wei Bao, MD, and colleagues at the University of Iowa, Iowa City, looked at records from 7.14 million live births registered in the U.S. National Vital Statistics System for 2016 and 2017, of which about 7.4% were preterm. The researchers excluded from their sample women with preexisting diabetes or hypertension.
For the cohort overall, there was a significant association between prepregnancy body mass index and preterm birth, with mothers who were overweight (adjusted odds ratio, 1.02; 95% confidence interval, 1.01–1.03) or obese (aOR, 1.18; 95%CI, 1.18–1.19), having a significantly higher risk of preterm birth, compared with healthy weight mothers. Underweight women also had a greater risk of preterm birth, compared with the healthy weight references (aOR, 1.33; 95% CI, 1.31–1.35), the researchers reported, adding that the association between maternal underweight and preterm birth was consistent across the maternal age and race/ethnicity groups.
Dr. Bao and colleagues found that, among non-Hispanic white women (who made up about half the cohort), maternal obesity was inversely associated with preterm birth when mothers were younger than 20 years (aOR, 0.92; 95% CI, 0.88-0.97), but there was a crossover effect at age 20, when maternal obesity became positively associated with preterm birth until age 39 (aOR, 1.04 at ages 20-24, to 1.40 at ages 35-39). A similar pattern was seen in Hispanic women, for whom maternal obesity was not associated with preterm birth when they were younger than 20 (aOR, 0.98; 95% CI, 0.93-1.04), but was positively associated with preterm birth after age 20 until age 39 (aOR, 1.06 at ages 20-24, to 1.38 at ages 35-39).
However, the crossover effect occurred considerably later in black women with obesity, for whom maternal obesity remained inversely associated with preterm birth until age 30 (aOR, 0.76 before age 20; 0.83 at ages 20-24; 0.98 at ages 25-29), at which point the crossover effect kicked in, and maternal obesity became positively associated with preterm birth, increasing steadily with advancing age (aOR, 1.15 at ages 30-34; 1.26 at ages 35-39; 1.29 from age 40). “Our results, which are based on a large and diverse U.S. population, provide, for the first time, a comprehensive review of the association between maternal obesity and preterm birth for women [at a] range of ages,” Dr. Bao and colleagues wrote in their analysis, which was published in Lancet Diabetes & Endocrinology.
The researchers hypothesized that the inverse association between prepregnancy obesity and preterm birth in teenagers and younger women could be explained by the fact that “[healthy weight] teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition,” whereas pregnant teenagers with obesity “might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth.” The researchers stressed that more research was needed to understand the underlying mechanisms of the associations. The findings of a protective effect until age 30 in black women also require further study, Dr. Bao and colleagues said.
They stressed that the findings do not argue for weight gain as a preventive measure against preterm birth for normal weight young women, as “younger women, whether obese or not, have a higher risk of preterm birth than women aged 25-29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.”
The National Institutes of Health funded the study. The authors declared no conflicts of interest.
SOURCE: Bao et al. Lancet Diabetes Endocrinol. 2019;7:707-14.
according to new findings from an analysis that used a large, ethnically diverse population sample.
Previous study findings have demonstrated that pregnant women with obesity have a higher risk of giving birth to preterm babies, but the effect of age and race on that risk was not clear until now.
In this latest study, Wei Bao, MD, and colleagues at the University of Iowa, Iowa City, looked at records from 7.14 million live births registered in the U.S. National Vital Statistics System for 2016 and 2017, of which about 7.4% were preterm. The researchers excluded from their sample women with preexisting diabetes or hypertension.
For the cohort overall, there was a significant association between prepregnancy body mass index and preterm birth, with mothers who were overweight (adjusted odds ratio, 1.02; 95% confidence interval, 1.01–1.03) or obese (aOR, 1.18; 95%CI, 1.18–1.19), having a significantly higher risk of preterm birth, compared with healthy weight mothers. Underweight women also had a greater risk of preterm birth, compared with the healthy weight references (aOR, 1.33; 95% CI, 1.31–1.35), the researchers reported, adding that the association between maternal underweight and preterm birth was consistent across the maternal age and race/ethnicity groups.
Dr. Bao and colleagues found that, among non-Hispanic white women (who made up about half the cohort), maternal obesity was inversely associated with preterm birth when mothers were younger than 20 years (aOR, 0.92; 95% CI, 0.88-0.97), but there was a crossover effect at age 20, when maternal obesity became positively associated with preterm birth until age 39 (aOR, 1.04 at ages 20-24, to 1.40 at ages 35-39). A similar pattern was seen in Hispanic women, for whom maternal obesity was not associated with preterm birth when they were younger than 20 (aOR, 0.98; 95% CI, 0.93-1.04), but was positively associated with preterm birth after age 20 until age 39 (aOR, 1.06 at ages 20-24, to 1.38 at ages 35-39).
However, the crossover effect occurred considerably later in black women with obesity, for whom maternal obesity remained inversely associated with preterm birth until age 30 (aOR, 0.76 before age 20; 0.83 at ages 20-24; 0.98 at ages 25-29), at which point the crossover effect kicked in, and maternal obesity became positively associated with preterm birth, increasing steadily with advancing age (aOR, 1.15 at ages 30-34; 1.26 at ages 35-39; 1.29 from age 40). “Our results, which are based on a large and diverse U.S. population, provide, for the first time, a comprehensive review of the association between maternal obesity and preterm birth for women [at a] range of ages,” Dr. Bao and colleagues wrote in their analysis, which was published in Lancet Diabetes & Endocrinology.
The researchers hypothesized that the inverse association between prepregnancy obesity and preterm birth in teenagers and younger women could be explained by the fact that “[healthy weight] teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition,” whereas pregnant teenagers with obesity “might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth.” The researchers stressed that more research was needed to understand the underlying mechanisms of the associations. The findings of a protective effect until age 30 in black women also require further study, Dr. Bao and colleagues said.
They stressed that the findings do not argue for weight gain as a preventive measure against preterm birth for normal weight young women, as “younger women, whether obese or not, have a higher risk of preterm birth than women aged 25-29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.”
The National Institutes of Health funded the study. The authors declared no conflicts of interest.
SOURCE: Bao et al. Lancet Diabetes Endocrinol. 2019;7:707-14.
according to new findings from an analysis that used a large, ethnically diverse population sample.
Previous study findings have demonstrated that pregnant women with obesity have a higher risk of giving birth to preterm babies, but the effect of age and race on that risk was not clear until now.
In this latest study, Wei Bao, MD, and colleagues at the University of Iowa, Iowa City, looked at records from 7.14 million live births registered in the U.S. National Vital Statistics System for 2016 and 2017, of which about 7.4% were preterm. The researchers excluded from their sample women with preexisting diabetes or hypertension.
For the cohort overall, there was a significant association between prepregnancy body mass index and preterm birth, with mothers who were overweight (adjusted odds ratio, 1.02; 95% confidence interval, 1.01–1.03) or obese (aOR, 1.18; 95%CI, 1.18–1.19), having a significantly higher risk of preterm birth, compared with healthy weight mothers. Underweight women also had a greater risk of preterm birth, compared with the healthy weight references (aOR, 1.33; 95% CI, 1.31–1.35), the researchers reported, adding that the association between maternal underweight and preterm birth was consistent across the maternal age and race/ethnicity groups.
Dr. Bao and colleagues found that, among non-Hispanic white women (who made up about half the cohort), maternal obesity was inversely associated with preterm birth when mothers were younger than 20 years (aOR, 0.92; 95% CI, 0.88-0.97), but there was a crossover effect at age 20, when maternal obesity became positively associated with preterm birth until age 39 (aOR, 1.04 at ages 20-24, to 1.40 at ages 35-39). A similar pattern was seen in Hispanic women, for whom maternal obesity was not associated with preterm birth when they were younger than 20 (aOR, 0.98; 95% CI, 0.93-1.04), but was positively associated with preterm birth after age 20 until age 39 (aOR, 1.06 at ages 20-24, to 1.38 at ages 35-39).
However, the crossover effect occurred considerably later in black women with obesity, for whom maternal obesity remained inversely associated with preterm birth until age 30 (aOR, 0.76 before age 20; 0.83 at ages 20-24; 0.98 at ages 25-29), at which point the crossover effect kicked in, and maternal obesity became positively associated with preterm birth, increasing steadily with advancing age (aOR, 1.15 at ages 30-34; 1.26 at ages 35-39; 1.29 from age 40). “Our results, which are based on a large and diverse U.S. population, provide, for the first time, a comprehensive review of the association between maternal obesity and preterm birth for women [at a] range of ages,” Dr. Bao and colleagues wrote in their analysis, which was published in Lancet Diabetes & Endocrinology.
The researchers hypothesized that the inverse association between prepregnancy obesity and preterm birth in teenagers and younger women could be explained by the fact that “[healthy weight] teenagers, who are still growing and developing, might compete with the fetus for nutrients, which could subsequently affect physiological and metabolic systems involved with parturition,” whereas pregnant teenagers with obesity “might not need to compete (or compete to a lesser extent) for nutrients with their babies for their own growth.” The researchers stressed that more research was needed to understand the underlying mechanisms of the associations. The findings of a protective effect until age 30 in black women also require further study, Dr. Bao and colleagues said.
They stressed that the findings do not argue for weight gain as a preventive measure against preterm birth for normal weight young women, as “younger women, whether obese or not, have a higher risk of preterm birth than women aged 25-29 years do in Hispanic and in non-Hispanic white populations. Additionally, the adverse effects that maternal obesity has on other perinatal and neonatal outcomes should not be overlooked.”
The National Institutes of Health funded the study. The authors declared no conflicts of interest.
SOURCE: Bao et al. Lancet Diabetes Endocrinol. 2019;7:707-14.
FROM LANCET DIABETES & ENDOCRINOLOGY