User login
VIDEO: Troponin acts as atherosclerotic biomarker in patients with lupus
MADRID – Measuring levels of troponin, a well-known cardiac biomarker, could help identify patients with systemic lupus erythematosus (SLE) at particularly high risk for cardiovascular (CV) events, according to the results of a cross-sectional study presented at the European Congress of Rheumatology.
Karim Sacré, MD, presented the findings of the study that looked for possible biomarkers of atherosclerosis in patients with SLE and provide preliminary evidence that high-sensitivity troponin T (HS-cTnT) was predictive regardless of whether or not patients already had visible atherosclerotic plaques on vascular ultrasound.
“Patients with SLE have been known to be at risk for cardiovascular disease for at least a decade,” Dr. Sacré of Bichat Hospital, University of Paris-Diderot, France, said in an interview at the meeting. Today, SLE is considered an independent risk factor for CV events, much like diabetes, he added.
However, determining which patients with lupus will and which will not develop cardiac problems is still tricky in routine practice. This is because the traditional ways of assessing CV events do not fully account for the increased risk seen in lupus patients. Indeed, the Framingham risk score, which is based on several risk factors such as tobacco use, hypertension, and dyslipidemia, has been shown to underestimate the cardiovascular risk of lupus patients, he observed.
“So, we need something that will help clinicians to better define the real risk of cardiovascular disease in such populations,” he said at an earlier press conference.
Thus, the objective of the study he presented was to try to find a biomarker in the blood that might aid clinicians in identifying which patients who had SLE and no obvious cardiac symptoms might be at risk for future CV events.
The study involved 63 patients with SLE who were consecutively recruited and 18 individuals without SLE who were used as controls. None had any symptoms of cardiovascular disease at recruitment, and all were assessed prospectively by vascular ultrasound for the presence of atherosclerotic plaques in the carotid artery.
The concentration of HS-cTnT was measured in the serum by using an electrochemiluminescence method, which could detect a concentration level greater than 3 ng/L .
At recruitment, the Framingham risk score was low (2.1) in both patients and controls, none of whom showed any signs of already having cardiovascular disease. The results of the carotid ultrasound, however, showed a different story for the SLE patients, with 23 (36.5%) identified as having carotid plaques, compared with just 2 (11.1%) of the control group.
Serum HS-cTNT could be detected in more SLE patients than controls (58.7% vs. 33.3%; P = .057), and the SLE patients who had detectable levels were nine times more likely than controls to have a carotid plaque, Dr. Sacré reported, although the 95% confidence interval was wide (1.55 to 90.07; P = .033).
Interestingly, a higher percentage of SLE patients with carotid plaques than those without had detectable HS-cTNT (87% vs. 42.5%; P less than .001). Conversely, more patients with detectable HS-cTnT than without had a carotid plaque (54.5% vs. 11.5%; P less than .001).
In multivariate analyses, only SLE status and age were significantly associated with having carotid plaques, and body mass index and HS-cTnT (P = .033) were statistically associated with the presence of carotid plaques in SLE patients.
The research is, of course, preliminary, Dr. Sacré emphasized, and further investigation is needed. The study looked at subclinical disease rather than actual CV events, and that is something to look at next in a larger cohort of patients with a longer follow-up period, he said.
Dr. Sacré disclosed that he had received support for travel to the EULAR Congress from Roche Diagnostics France.
*This story was updated 6/20/2017.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Measuring levels of troponin, a well-known cardiac biomarker, could help identify patients with systemic lupus erythematosus (SLE) at particularly high risk for cardiovascular (CV) events, according to the results of a cross-sectional study presented at the European Congress of Rheumatology.
Karim Sacré, MD, presented the findings of the study that looked for possible biomarkers of atherosclerosis in patients with SLE and provide preliminary evidence that high-sensitivity troponin T (HS-cTnT) was predictive regardless of whether or not patients already had visible atherosclerotic plaques on vascular ultrasound.
“Patients with SLE have been known to be at risk for cardiovascular disease for at least a decade,” Dr. Sacré of Bichat Hospital, University of Paris-Diderot, France, said in an interview at the meeting. Today, SLE is considered an independent risk factor for CV events, much like diabetes, he added.
However, determining which patients with lupus will and which will not develop cardiac problems is still tricky in routine practice. This is because the traditional ways of assessing CV events do not fully account for the increased risk seen in lupus patients. Indeed, the Framingham risk score, which is based on several risk factors such as tobacco use, hypertension, and dyslipidemia, has been shown to underestimate the cardiovascular risk of lupus patients, he observed.
“So, we need something that will help clinicians to better define the real risk of cardiovascular disease in such populations,” he said at an earlier press conference.
Thus, the objective of the study he presented was to try to find a biomarker in the blood that might aid clinicians in identifying which patients who had SLE and no obvious cardiac symptoms might be at risk for future CV events.
The study involved 63 patients with SLE who were consecutively recruited and 18 individuals without SLE who were used as controls. None had any symptoms of cardiovascular disease at recruitment, and all were assessed prospectively by vascular ultrasound for the presence of atherosclerotic plaques in the carotid artery.
The concentration of HS-cTnT was measured in the serum by using an electrochemiluminescence method, which could detect a concentration level greater than 3 ng/L .
At recruitment, the Framingham risk score was low (2.1) in both patients and controls, none of whom showed any signs of already having cardiovascular disease. The results of the carotid ultrasound, however, showed a different story for the SLE patients, with 23 (36.5%) identified as having carotid plaques, compared with just 2 (11.1%) of the control group.
Serum HS-cTNT could be detected in more SLE patients than controls (58.7% vs. 33.3%; P = .057), and the SLE patients who had detectable levels were nine times more likely than controls to have a carotid plaque, Dr. Sacré reported, although the 95% confidence interval was wide (1.55 to 90.07; P = .033).
Interestingly, a higher percentage of SLE patients with carotid plaques than those without had detectable HS-cTNT (87% vs. 42.5%; P less than .001). Conversely, more patients with detectable HS-cTnT than without had a carotid plaque (54.5% vs. 11.5%; P less than .001).
In multivariate analyses, only SLE status and age were significantly associated with having carotid plaques, and body mass index and HS-cTnT (P = .033) were statistically associated with the presence of carotid plaques in SLE patients.
The research is, of course, preliminary, Dr. Sacré emphasized, and further investigation is needed. The study looked at subclinical disease rather than actual CV events, and that is something to look at next in a larger cohort of patients with a longer follow-up period, he said.
Dr. Sacré disclosed that he had received support for travel to the EULAR Congress from Roche Diagnostics France.
*This story was updated 6/20/2017.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Measuring levels of troponin, a well-known cardiac biomarker, could help identify patients with systemic lupus erythematosus (SLE) at particularly high risk for cardiovascular (CV) events, according to the results of a cross-sectional study presented at the European Congress of Rheumatology.
Karim Sacré, MD, presented the findings of the study that looked for possible biomarkers of atherosclerosis in patients with SLE and provide preliminary evidence that high-sensitivity troponin T (HS-cTnT) was predictive regardless of whether or not patients already had visible atherosclerotic plaques on vascular ultrasound.
“Patients with SLE have been known to be at risk for cardiovascular disease for at least a decade,” Dr. Sacré of Bichat Hospital, University of Paris-Diderot, France, said in an interview at the meeting. Today, SLE is considered an independent risk factor for CV events, much like diabetes, he added.
However, determining which patients with lupus will and which will not develop cardiac problems is still tricky in routine practice. This is because the traditional ways of assessing CV events do not fully account for the increased risk seen in lupus patients. Indeed, the Framingham risk score, which is based on several risk factors such as tobacco use, hypertension, and dyslipidemia, has been shown to underestimate the cardiovascular risk of lupus patients, he observed.
“So, we need something that will help clinicians to better define the real risk of cardiovascular disease in such populations,” he said at an earlier press conference.
Thus, the objective of the study he presented was to try to find a biomarker in the blood that might aid clinicians in identifying which patients who had SLE and no obvious cardiac symptoms might be at risk for future CV events.
The study involved 63 patients with SLE who were consecutively recruited and 18 individuals without SLE who were used as controls. None had any symptoms of cardiovascular disease at recruitment, and all were assessed prospectively by vascular ultrasound for the presence of atherosclerotic plaques in the carotid artery.
The concentration of HS-cTnT was measured in the serum by using an electrochemiluminescence method, which could detect a concentration level greater than 3 ng/L .
At recruitment, the Framingham risk score was low (2.1) in both patients and controls, none of whom showed any signs of already having cardiovascular disease. The results of the carotid ultrasound, however, showed a different story for the SLE patients, with 23 (36.5%) identified as having carotid plaques, compared with just 2 (11.1%) of the control group.
Serum HS-cTNT could be detected in more SLE patients than controls (58.7% vs. 33.3%; P = .057), and the SLE patients who had detectable levels were nine times more likely than controls to have a carotid plaque, Dr. Sacré reported, although the 95% confidence interval was wide (1.55 to 90.07; P = .033).
Interestingly, a higher percentage of SLE patients with carotid plaques than those without had detectable HS-cTNT (87% vs. 42.5%; P less than .001). Conversely, more patients with detectable HS-cTnT than without had a carotid plaque (54.5% vs. 11.5%; P less than .001).
In multivariate analyses, only SLE status and age were significantly associated with having carotid plaques, and body mass index and HS-cTnT (P = .033) were statistically associated with the presence of carotid plaques in SLE patients.
The research is, of course, preliminary, Dr. Sacré emphasized, and further investigation is needed. The study looked at subclinical disease rather than actual CV events, and that is something to look at next in a larger cohort of patients with a longer follow-up period, he said.
Dr. Sacré disclosed that he had received support for travel to the EULAR Congress from Roche Diagnostics France.
*This story was updated 6/20/2017.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2017 CONGRESS
Measles immunization is a major challenge in HIV-infected children
MADRID – Impaired response to measles immunization is common in HIV-infected children, and revaccination – while an important strategy – helps only some of them, Ruth del Valle, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
The key predictor of an impaired response to measles immunization appears to be a CD4/CD8 cell ratio of less than 1.0, added Dr. del Valle of Infant Sofia University Hospital in Madrid.
Of note, 48% of the children had a CD4/CD8 ratio of less than 1.0 at baseline. This ratio is gaining credence as a predictor of HIV-positive patients’ immunologic response to a variety of vaccines: most recently, in Dr. del Valle’s study, to measles vaccine and, in two earlier studies published in 2016, to yellow fever vaccine (PLoS Negl Trop Dis. 2016 Dec 12;10[12]:e0005219) and hepatitis B vaccine (Vaccine. 2016 Apr 7;34[16]:1889-95).
In Dr. del Valle’s study, only 52% of HIV-positive children developed protective antibody levels following completion of the standard two-dose MMR schedule. In accord with current Paediatric European Network for Treatment of AIDS (PENTA) guidelines, 41 unresponsive patients then received a booster MMR dose (HIV Med. 2012 Jul;13[6]:333-6). Of the 41, 10 (24%) did not respond to the booster dose, either. Thus, the final measles protection rate in this study was only 77%.
These study findings highlight the importance of routinely testing measles vaccine response in HIV-infected children in clinical practice, she noted.
The CD4/CD8 ratio was 1.3 in good responders to measles immunization and 0.9 in nonresponders. In a multivariate analysis, a CD4/CD8 ratio of less than 1.0 conferred a 3.2 times increased risk of impaired response to the measles vaccine.
Dr. del Valle and her coinvestigators in CORISPES (the Spanish Cohort of HIV-infected Children) plan further studies to determine the duration of protection against measles in those patients who responded to the booster dose.
Session cochair Nigel Klein, MD, commented that Dr. del Valle’s study is “one of a number of reports that HIV-infected children, as they get older, are predisposed to getting infections that we should be able to prevent.”
“I think we don’t know best how to manage this population of patients. You can give more vaccinations, but, as Dr. del Valle has shown, not all of them will respond. And I think this study is further evidence that the better we can maintain our children’s immune systems by treating early, the better our chances of getting good responses when we vaccinate,” said Dr. Klein, professor of pediatric infectious diseases and immunology at Great Ormond Street Children’s Hospital and University College London.
Dr. del Valle reported having no relevant financial disclosures.
MADRID – Impaired response to measles immunization is common in HIV-infected children, and revaccination – while an important strategy – helps only some of them, Ruth del Valle, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
The key predictor of an impaired response to measles immunization appears to be a CD4/CD8 cell ratio of less than 1.0, added Dr. del Valle of Infant Sofia University Hospital in Madrid.
Of note, 48% of the children had a CD4/CD8 ratio of less than 1.0 at baseline. This ratio is gaining credence as a predictor of HIV-positive patients’ immunologic response to a variety of vaccines: most recently, in Dr. del Valle’s study, to measles vaccine and, in two earlier studies published in 2016, to yellow fever vaccine (PLoS Negl Trop Dis. 2016 Dec 12;10[12]:e0005219) and hepatitis B vaccine (Vaccine. 2016 Apr 7;34[16]:1889-95).
In Dr. del Valle’s study, only 52% of HIV-positive children developed protective antibody levels following completion of the standard two-dose MMR schedule. In accord with current Paediatric European Network for Treatment of AIDS (PENTA) guidelines, 41 unresponsive patients then received a booster MMR dose (HIV Med. 2012 Jul;13[6]:333-6). Of the 41, 10 (24%) did not respond to the booster dose, either. Thus, the final measles protection rate in this study was only 77%.
These study findings highlight the importance of routinely testing measles vaccine response in HIV-infected children in clinical practice, she noted.
The CD4/CD8 ratio was 1.3 in good responders to measles immunization and 0.9 in nonresponders. In a multivariate analysis, a CD4/CD8 ratio of less than 1.0 conferred a 3.2 times increased risk of impaired response to the measles vaccine.
Dr. del Valle and her coinvestigators in CORISPES (the Spanish Cohort of HIV-infected Children) plan further studies to determine the duration of protection against measles in those patients who responded to the booster dose.
Session cochair Nigel Klein, MD, commented that Dr. del Valle’s study is “one of a number of reports that HIV-infected children, as they get older, are predisposed to getting infections that we should be able to prevent.”
“I think we don’t know best how to manage this population of patients. You can give more vaccinations, but, as Dr. del Valle has shown, not all of them will respond. And I think this study is further evidence that the better we can maintain our children’s immune systems by treating early, the better our chances of getting good responses when we vaccinate,” said Dr. Klein, professor of pediatric infectious diseases and immunology at Great Ormond Street Children’s Hospital and University College London.
Dr. del Valle reported having no relevant financial disclosures.
MADRID – Impaired response to measles immunization is common in HIV-infected children, and revaccination – while an important strategy – helps only some of them, Ruth del Valle, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
The key predictor of an impaired response to measles immunization appears to be a CD4/CD8 cell ratio of less than 1.0, added Dr. del Valle of Infant Sofia University Hospital in Madrid.
Of note, 48% of the children had a CD4/CD8 ratio of less than 1.0 at baseline. This ratio is gaining credence as a predictor of HIV-positive patients’ immunologic response to a variety of vaccines: most recently, in Dr. del Valle’s study, to measles vaccine and, in two earlier studies published in 2016, to yellow fever vaccine (PLoS Negl Trop Dis. 2016 Dec 12;10[12]:e0005219) and hepatitis B vaccine (Vaccine. 2016 Apr 7;34[16]:1889-95).
In Dr. del Valle’s study, only 52% of HIV-positive children developed protective antibody levels following completion of the standard two-dose MMR schedule. In accord with current Paediatric European Network for Treatment of AIDS (PENTA) guidelines, 41 unresponsive patients then received a booster MMR dose (HIV Med. 2012 Jul;13[6]:333-6). Of the 41, 10 (24%) did not respond to the booster dose, either. Thus, the final measles protection rate in this study was only 77%.
These study findings highlight the importance of routinely testing measles vaccine response in HIV-infected children in clinical practice, she noted.
The CD4/CD8 ratio was 1.3 in good responders to measles immunization and 0.9 in nonresponders. In a multivariate analysis, a CD4/CD8 ratio of less than 1.0 conferred a 3.2 times increased risk of impaired response to the measles vaccine.
Dr. del Valle and her coinvestigators in CORISPES (the Spanish Cohort of HIV-infected Children) plan further studies to determine the duration of protection against measles in those patients who responded to the booster dose.
Session cochair Nigel Klein, MD, commented that Dr. del Valle’s study is “one of a number of reports that HIV-infected children, as they get older, are predisposed to getting infections that we should be able to prevent.”
“I think we don’t know best how to manage this population of patients. You can give more vaccinations, but, as Dr. del Valle has shown, not all of them will respond. And I think this study is further evidence that the better we can maintain our children’s immune systems by treating early, the better our chances of getting good responses when we vaccinate,” said Dr. Klein, professor of pediatric infectious diseases and immunology at Great Ormond Street Children’s Hospital and University College London.
Dr. del Valle reported having no relevant financial disclosures.
AT ESPID 2017
Key clinical point:
Major finding: After two doses of MMR and, if needed, a third booster dose, 23% of a group of HIV-infected children remained unprotected against measles.
Data source: This multicenter study included 120 HIV-infected Spanish children who received two doses of the MMR vaccine and, if still unprotected against measles, a booster dose.
Disclosures: Dr. del Valle reported having no relevant financial disclosures.
Cancer, heart disease increase MRSA mortality
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Key clinical point:
Major finding: Cancer is associated with a more than twofold increase in 30-day mortality from MRSA.
Data source: A 9-year retrospective study of 1,168 patients with MRSA infection.
Disclosures: Two authors declared research grants from the pharmaceutical industry.
Want to take action on health care legislation? Here’s how
The U.S. House of Representatives recently passed a revised version of the American Health Care Act (AHCA), which the nonpartisan Congressional Budget Office concluded would result in 14 million people losing coverage by 2018 and 23 million people losing coverage by 2026.
The American Congress of Obstetricians and Gynecologists is opposed to this bill and has stated that it will leave Americans “worse off than they are today” by cutting Medicaid, eliminating Medicaid expansion, allowing states to opt out of covering essential benefits like maternity care, and weakening protections for people with preexisting conditions.
[polldaddy:9770191]
State level
At the state level, the best person to contact is your ACOG section chairman, who can then direct you to your state’s legislative chairman. The legislative chairman will provide you with information on legislative actions that have been taken by the section. If you are interested in women’s health legislation, there may be an advocacy list to join that will provide legislative alerts. You may even choose to tweet about the alerts.
Often the state legislative chairman will send out information about an upcoming bill and ask for ACOG members to provide testimony. If there is a bill of particular interest, you can offer to testify either in person or submit written testimony. Often talking points are made available to help in the preparation of testimony.
Many states also have a Lobby Day, a day when members of the ob.gyn. community meet at the state house to advocate for or oppose legislation. This action can be easier than providing testimony because the time and date are predictable, while legislative hearings may not be.
There is a great deal that can be done at the state level in support of women’s health. Perhaps your state does not have a maternal mortality committee, perhaps there needs to be funding for vaccinations, or perhaps you want to support legislation that continues to provide health care for women even if the AHCA becomes law.
National action
Legislative action at the national level mirrors that at the state level, but it is coordinated by the ACOG Government Affairs Department. Contact this department via acog.org. One option is to become an advocate and receive legislative alerts. This alert system informs ACOG members about congressional actions and gives you the option to directly contact your members of Congress through email with a specific message. Some physicians may prefer to send an email different than the one provided by ACOG, while others may tweet using hashtags like #obgynaction or #docs4coverage.
You may prefer to contact your member of Congress by phone. The U.S. Capitol Switchboard number is 202-224-3121. Phone calls typically are taken by staff members. It is reasonable to ask for a staff member who handles the issue you wish to discuss. This person may be referred to as the L.A. (legislative assistant). Inform the staff member that you are a constituent and you would like to leave a brief message for the Senator or Representative. Your comments may be as brief as stating that you support or oppose a particular piece of legislation. As with the letter, you should state the reasons for your opinion and may include a short personal story. If you do not already know it, you should ask for the lawmaker’s position on the bill.
An ideal method of interacting with members of Congress is through town hall meetings. These meetings typically are posted on the member’s website and hearing from constituents in person can have a tremendous impact on legislators. Letters to the Editor, interviews with journalists, and advocating for candidates are also options to consider. In such cases, consider contacting the ACOG Government Affairs Department. They can provide helpful dos and don’ts before an interview is scheduled or an article is written.
Health care policymaking that is not based on scientific or medical evidence is dangerous for our patients. We, as their physicians, need to advocate on their behalf. Stay or get involved to help ensure that our patients can get the health care they need when they need it.
Dr. Bohon is an ob.gyn. in private practice in Washington, and an ACOG state legislative chair from the District of Columbia. She is a member of the Ob.Gyn. News Editorial Advisory Board. Dr. Bohon reported having no relevant financial disclosures.
The U.S. House of Representatives recently passed a revised version of the American Health Care Act (AHCA), which the nonpartisan Congressional Budget Office concluded would result in 14 million people losing coverage by 2018 and 23 million people losing coverage by 2026.
The American Congress of Obstetricians and Gynecologists is opposed to this bill and has stated that it will leave Americans “worse off than they are today” by cutting Medicaid, eliminating Medicaid expansion, allowing states to opt out of covering essential benefits like maternity care, and weakening protections for people with preexisting conditions.
[polldaddy:9770191]
State level
At the state level, the best person to contact is your ACOG section chairman, who can then direct you to your state’s legislative chairman. The legislative chairman will provide you with information on legislative actions that have been taken by the section. If you are interested in women’s health legislation, there may be an advocacy list to join that will provide legislative alerts. You may even choose to tweet about the alerts.
Often the state legislative chairman will send out information about an upcoming bill and ask for ACOG members to provide testimony. If there is a bill of particular interest, you can offer to testify either in person or submit written testimony. Often talking points are made available to help in the preparation of testimony.
Many states also have a Lobby Day, a day when members of the ob.gyn. community meet at the state house to advocate for or oppose legislation. This action can be easier than providing testimony because the time and date are predictable, while legislative hearings may not be.
There is a great deal that can be done at the state level in support of women’s health. Perhaps your state does not have a maternal mortality committee, perhaps there needs to be funding for vaccinations, or perhaps you want to support legislation that continues to provide health care for women even if the AHCA becomes law.
National action
Legislative action at the national level mirrors that at the state level, but it is coordinated by the ACOG Government Affairs Department. Contact this department via acog.org. One option is to become an advocate and receive legislative alerts. This alert system informs ACOG members about congressional actions and gives you the option to directly contact your members of Congress through email with a specific message. Some physicians may prefer to send an email different than the one provided by ACOG, while others may tweet using hashtags like #obgynaction or #docs4coverage.
You may prefer to contact your member of Congress by phone. The U.S. Capitol Switchboard number is 202-224-3121. Phone calls typically are taken by staff members. It is reasonable to ask for a staff member who handles the issue you wish to discuss. This person may be referred to as the L.A. (legislative assistant). Inform the staff member that you are a constituent and you would like to leave a brief message for the Senator or Representative. Your comments may be as brief as stating that you support or oppose a particular piece of legislation. As with the letter, you should state the reasons for your opinion and may include a short personal story. If you do not already know it, you should ask for the lawmaker’s position on the bill.
An ideal method of interacting with members of Congress is through town hall meetings. These meetings typically are posted on the member’s website and hearing from constituents in person can have a tremendous impact on legislators. Letters to the Editor, interviews with journalists, and advocating for candidates are also options to consider. In such cases, consider contacting the ACOG Government Affairs Department. They can provide helpful dos and don’ts before an interview is scheduled or an article is written.
Health care policymaking that is not based on scientific or medical evidence is dangerous for our patients. We, as their physicians, need to advocate on their behalf. Stay or get involved to help ensure that our patients can get the health care they need when they need it.
Dr. Bohon is an ob.gyn. in private practice in Washington, and an ACOG state legislative chair from the District of Columbia. She is a member of the Ob.Gyn. News Editorial Advisory Board. Dr. Bohon reported having no relevant financial disclosures.
The U.S. House of Representatives recently passed a revised version of the American Health Care Act (AHCA), which the nonpartisan Congressional Budget Office concluded would result in 14 million people losing coverage by 2018 and 23 million people losing coverage by 2026.
The American Congress of Obstetricians and Gynecologists is opposed to this bill and has stated that it will leave Americans “worse off than they are today” by cutting Medicaid, eliminating Medicaid expansion, allowing states to opt out of covering essential benefits like maternity care, and weakening protections for people with preexisting conditions.
[polldaddy:9770191]
State level
At the state level, the best person to contact is your ACOG section chairman, who can then direct you to your state’s legislative chairman. The legislative chairman will provide you with information on legislative actions that have been taken by the section. If you are interested in women’s health legislation, there may be an advocacy list to join that will provide legislative alerts. You may even choose to tweet about the alerts.
Often the state legislative chairman will send out information about an upcoming bill and ask for ACOG members to provide testimony. If there is a bill of particular interest, you can offer to testify either in person or submit written testimony. Often talking points are made available to help in the preparation of testimony.
Many states also have a Lobby Day, a day when members of the ob.gyn. community meet at the state house to advocate for or oppose legislation. This action can be easier than providing testimony because the time and date are predictable, while legislative hearings may not be.
There is a great deal that can be done at the state level in support of women’s health. Perhaps your state does not have a maternal mortality committee, perhaps there needs to be funding for vaccinations, or perhaps you want to support legislation that continues to provide health care for women even if the AHCA becomes law.
National action
Legislative action at the national level mirrors that at the state level, but it is coordinated by the ACOG Government Affairs Department. Contact this department via acog.org. One option is to become an advocate and receive legislative alerts. This alert system informs ACOG members about congressional actions and gives you the option to directly contact your members of Congress through email with a specific message. Some physicians may prefer to send an email different than the one provided by ACOG, while others may tweet using hashtags like #obgynaction or #docs4coverage.
You may prefer to contact your member of Congress by phone. The U.S. Capitol Switchboard number is 202-224-3121. Phone calls typically are taken by staff members. It is reasonable to ask for a staff member who handles the issue you wish to discuss. This person may be referred to as the L.A. (legislative assistant). Inform the staff member that you are a constituent and you would like to leave a brief message for the Senator or Representative. Your comments may be as brief as stating that you support or oppose a particular piece of legislation. As with the letter, you should state the reasons for your opinion and may include a short personal story. If you do not already know it, you should ask for the lawmaker’s position on the bill.
An ideal method of interacting with members of Congress is through town hall meetings. These meetings typically are posted on the member’s website and hearing from constituents in person can have a tremendous impact on legislators. Letters to the Editor, interviews with journalists, and advocating for candidates are also options to consider. In such cases, consider contacting the ACOG Government Affairs Department. They can provide helpful dos and don’ts before an interview is scheduled or an article is written.
Health care policymaking that is not based on scientific or medical evidence is dangerous for our patients. We, as their physicians, need to advocate on their behalf. Stay or get involved to help ensure that our patients can get the health care they need when they need it.
Dr. Bohon is an ob.gyn. in private practice in Washington, and an ACOG state legislative chair from the District of Columbia. She is a member of the Ob.Gyn. News Editorial Advisory Board. Dr. Bohon reported having no relevant financial disclosures.
Average cost of Healthcare.gov policy up 105% since 2013
The average monthly premium for individuals purchasing a plan from Healthcare.gov increased by 105% from 2013 to 2017, according to the Department of Health & Human Services.
In the 39 states that use Healthcare.gov, the average monthly exchange plan premium went from $232 in 2013 to $476 in 2017, an increase of $244 (105%), the HHS Office of the Assistant Secretary for Planning and Evaluation (ASPE) reported.
All 39 states experienced an increase in the cost of an average premium, but there was considerable variation in the size. Alabama had the largest percent increase at 223%, but Alaska had the largest absolute increase – $697 – to go with the second-largest percent increase – 203%. Oklahoma, where the average premium jumped 201%, was third, the ASPE said.
The state with the smallest change, both in terms of dollars and percents, was New Jersey, which had an increase of $51 (12%) over the 4-year period. The only other states with less than a 50% increase were New Hampshire at 32% and North Dakota at 44%, the report showed.
“States with benefit mandates similar to those required in the [Affordable Care Act] in effect before 2014 had smaller premium increases between 2013 and 2017,” the ASPE noted.
One limitation to the analysis is the change among those enrolling from 2013 to 2017. “Older and less healthy people are a larger share of the individual market risk pool now than in 2013. The changing mix of enrollees and adverse selection pressure has likely been a significant cause of the large average premium increases,” the ASPE said.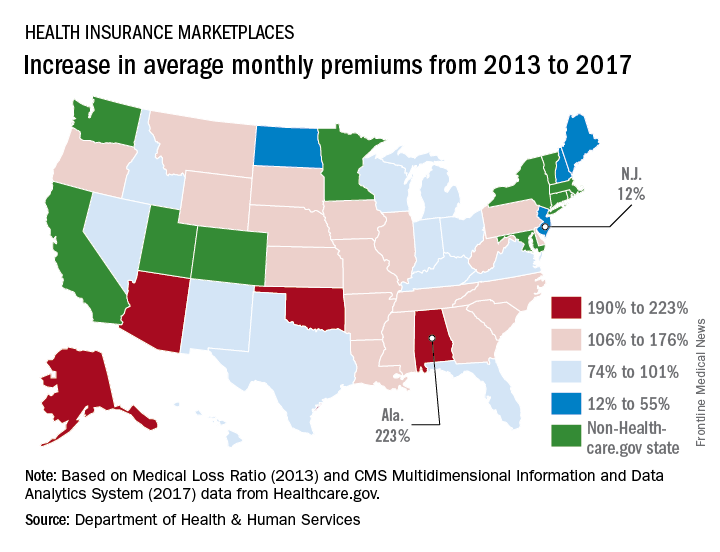
The average monthly premium for individuals purchasing a plan from Healthcare.gov increased by 105% from 2013 to 2017, according to the Department of Health & Human Services.
In the 39 states that use Healthcare.gov, the average monthly exchange plan premium went from $232 in 2013 to $476 in 2017, an increase of $244 (105%), the HHS Office of the Assistant Secretary for Planning and Evaluation (ASPE) reported.
All 39 states experienced an increase in the cost of an average premium, but there was considerable variation in the size. Alabama had the largest percent increase at 223%, but Alaska had the largest absolute increase – $697 – to go with the second-largest percent increase – 203%. Oklahoma, where the average premium jumped 201%, was third, the ASPE said.
The state with the smallest change, both in terms of dollars and percents, was New Jersey, which had an increase of $51 (12%) over the 4-year period. The only other states with less than a 50% increase were New Hampshire at 32% and North Dakota at 44%, the report showed.
“States with benefit mandates similar to those required in the [Affordable Care Act] in effect before 2014 had smaller premium increases between 2013 and 2017,” the ASPE noted.
One limitation to the analysis is the change among those enrolling from 2013 to 2017. “Older and less healthy people are a larger share of the individual market risk pool now than in 2013. The changing mix of enrollees and adverse selection pressure has likely been a significant cause of the large average premium increases,” the ASPE said.
The average monthly premium for individuals purchasing a plan from Healthcare.gov increased by 105% from 2013 to 2017, according to the Department of Health & Human Services.
In the 39 states that use Healthcare.gov, the average monthly exchange plan premium went from $232 in 2013 to $476 in 2017, an increase of $244 (105%), the HHS Office of the Assistant Secretary for Planning and Evaluation (ASPE) reported.
All 39 states experienced an increase in the cost of an average premium, but there was considerable variation in the size. Alabama had the largest percent increase at 223%, but Alaska had the largest absolute increase – $697 – to go with the second-largest percent increase – 203%. Oklahoma, where the average premium jumped 201%, was third, the ASPE said.
The state with the smallest change, both in terms of dollars and percents, was New Jersey, which had an increase of $51 (12%) over the 4-year period. The only other states with less than a 50% increase were New Hampshire at 32% and North Dakota at 44%, the report showed.
“States with benefit mandates similar to those required in the [Affordable Care Act] in effect before 2014 had smaller premium increases between 2013 and 2017,” the ASPE noted.
One limitation to the analysis is the change among those enrolling from 2013 to 2017. “Older and less healthy people are a larger share of the individual market risk pool now than in 2013. The changing mix of enrollees and adverse selection pressure has likely been a significant cause of the large average premium increases,” the ASPE said.
Daytime sleepiness linked to subsequent brain amyloid
BOSTON – Daytime sleepiness in cognitively intact older adults was significantly associated with subsequent neuroimaging evidence of brain amyloid deposition, according to a data analysis presented at the annual meeting of the Associated Professional Sleep Societies.
Self-reported regular nappers were also more likely to have brain amyloid on subsequent imaging, compared with non-nappers, but this difference just missed statistical significance.
Complementing these findings, Adam P. Spira, PhD, and his colleagues from John’s Hopkins University, Baltimore, previously published a study showing that self-reported sleep duration and poorer sleep quality were associated with greater beta-amyloid deposition in cognitively normal adults. (Spira et al. JAMA Neurology, 2013).
This new study by Dr. Spira and his colleagues sought to look at the link between excessive daytime sleepiness (EDS)/napping in older adults and beta-amyloid burden 15 years later as determined by positron emission tomography (PET) scanning. EDS, defined as a level of sleepiness during the day sufficient to interfere with daily activities, is a common manifestation of sleep disorders, in particular sleep disordered breathing, and is tied to cognitive impairment and accidents.
In unadjusted analyses, participants with EDS were beyond three times more likely to be beta-amyloid+ (odds ratio, 3.37), compared with those who did not meet criteria for EDS. This finding remained significant after adjustment for age, body mass index, and education level (OR, 2.59).
Nappers had an almost twofold greater odds of being beta-amyloid+ than non-nappers after adjustment, but this difference was not statistically meaningful (multivariate adjusted odds ratio, 1.82).
The researchers analyzed data on 124 participants drawn from the National Institute of Aging’s Baltimore Longitudinal Study of Aging (BLSA), the longest-running study of human aging in the U.S. The mean age of these patients at baseline was 60.1 years, and 50.8% of the sample was female. All participants were determined to be cognitively normal at baseline.
At the beginning of the study, participants were asked about their daytime sleepiness and napping habits. Those who reported often being drowsy or falling asleep during the daytime when they preferred to be awake were considered to having EDS. Those who napped once or twice a week or more were considered nappers.
About one-quarter of participants (24.4%) reported EDS, and 28.5% identified themselves as regular nappers. An average of 15.7 years later, participants completed Pittsburgh Compound B PET imaging, at which time 34.7% were deemed beta-amyloid+.
“We were kind of shocked that a single question with a yes/no response was robustly associated with [beta-amyloid] status that many years later,” reported Dr. Spira.
Still unknown, he added, is whether there is utility in quantifying or screening for preclinical Alzheimer’s disease risk using sleep variables. Also unclear is how EDS itself might drive beta-amyloid deposition. “What is likely to be happening here is something like sleep disordered breathing – which has been linked to cognitive impairment and dementia and has been linked to [Alzheimer’s disease] biomarkers – is driving this association,” he speculated.
“We have to keep in mind that even the people at baseline, even though they were cognitively normal, may have had some beta-amyloid deposition, which might have contributed to their EDS,” said Dr. Spira.
Dr. Spira intends to conduct a prospective study using laboratory-based sleep testing (polysomnography) and repeated measures of brain amyloid to clarify whether sleep disordered breathing or other factors were driving the association he found.
Dr. Spira reported having no financial disclosures. This study was supported by grants from the National Institute on Aging.
BOSTON – Daytime sleepiness in cognitively intact older adults was significantly associated with subsequent neuroimaging evidence of brain amyloid deposition, according to a data analysis presented at the annual meeting of the Associated Professional Sleep Societies.
Self-reported regular nappers were also more likely to have brain amyloid on subsequent imaging, compared with non-nappers, but this difference just missed statistical significance.
Complementing these findings, Adam P. Spira, PhD, and his colleagues from John’s Hopkins University, Baltimore, previously published a study showing that self-reported sleep duration and poorer sleep quality were associated with greater beta-amyloid deposition in cognitively normal adults. (Spira et al. JAMA Neurology, 2013).
This new study by Dr. Spira and his colleagues sought to look at the link between excessive daytime sleepiness (EDS)/napping in older adults and beta-amyloid burden 15 years later as determined by positron emission tomography (PET) scanning. EDS, defined as a level of sleepiness during the day sufficient to interfere with daily activities, is a common manifestation of sleep disorders, in particular sleep disordered breathing, and is tied to cognitive impairment and accidents.
In unadjusted analyses, participants with EDS were beyond three times more likely to be beta-amyloid+ (odds ratio, 3.37), compared with those who did not meet criteria for EDS. This finding remained significant after adjustment for age, body mass index, and education level (OR, 2.59).
Nappers had an almost twofold greater odds of being beta-amyloid+ than non-nappers after adjustment, but this difference was not statistically meaningful (multivariate adjusted odds ratio, 1.82).
The researchers analyzed data on 124 participants drawn from the National Institute of Aging’s Baltimore Longitudinal Study of Aging (BLSA), the longest-running study of human aging in the U.S. The mean age of these patients at baseline was 60.1 years, and 50.8% of the sample was female. All participants were determined to be cognitively normal at baseline.
At the beginning of the study, participants were asked about their daytime sleepiness and napping habits. Those who reported often being drowsy or falling asleep during the daytime when they preferred to be awake were considered to having EDS. Those who napped once or twice a week or more were considered nappers.
About one-quarter of participants (24.4%) reported EDS, and 28.5% identified themselves as regular nappers. An average of 15.7 years later, participants completed Pittsburgh Compound B PET imaging, at which time 34.7% were deemed beta-amyloid+.
“We were kind of shocked that a single question with a yes/no response was robustly associated with [beta-amyloid] status that many years later,” reported Dr. Spira.
Still unknown, he added, is whether there is utility in quantifying or screening for preclinical Alzheimer’s disease risk using sleep variables. Also unclear is how EDS itself might drive beta-amyloid deposition. “What is likely to be happening here is something like sleep disordered breathing – which has been linked to cognitive impairment and dementia and has been linked to [Alzheimer’s disease] biomarkers – is driving this association,” he speculated.
“We have to keep in mind that even the people at baseline, even though they were cognitively normal, may have had some beta-amyloid deposition, which might have contributed to their EDS,” said Dr. Spira.
Dr. Spira intends to conduct a prospective study using laboratory-based sleep testing (polysomnography) and repeated measures of brain amyloid to clarify whether sleep disordered breathing or other factors were driving the association he found.
Dr. Spira reported having no financial disclosures. This study was supported by grants from the National Institute on Aging.
BOSTON – Daytime sleepiness in cognitively intact older adults was significantly associated with subsequent neuroimaging evidence of brain amyloid deposition, according to a data analysis presented at the annual meeting of the Associated Professional Sleep Societies.
Self-reported regular nappers were also more likely to have brain amyloid on subsequent imaging, compared with non-nappers, but this difference just missed statistical significance.
Complementing these findings, Adam P. Spira, PhD, and his colleagues from John’s Hopkins University, Baltimore, previously published a study showing that self-reported sleep duration and poorer sleep quality were associated with greater beta-amyloid deposition in cognitively normal adults. (Spira et al. JAMA Neurology, 2013).
This new study by Dr. Spira and his colleagues sought to look at the link between excessive daytime sleepiness (EDS)/napping in older adults and beta-amyloid burden 15 years later as determined by positron emission tomography (PET) scanning. EDS, defined as a level of sleepiness during the day sufficient to interfere with daily activities, is a common manifestation of sleep disorders, in particular sleep disordered breathing, and is tied to cognitive impairment and accidents.
In unadjusted analyses, participants with EDS were beyond three times more likely to be beta-amyloid+ (odds ratio, 3.37), compared with those who did not meet criteria for EDS. This finding remained significant after adjustment for age, body mass index, and education level (OR, 2.59).
Nappers had an almost twofold greater odds of being beta-amyloid+ than non-nappers after adjustment, but this difference was not statistically meaningful (multivariate adjusted odds ratio, 1.82).
The researchers analyzed data on 124 participants drawn from the National Institute of Aging’s Baltimore Longitudinal Study of Aging (BLSA), the longest-running study of human aging in the U.S. The mean age of these patients at baseline was 60.1 years, and 50.8% of the sample was female. All participants were determined to be cognitively normal at baseline.
At the beginning of the study, participants were asked about their daytime sleepiness and napping habits. Those who reported often being drowsy or falling asleep during the daytime when they preferred to be awake were considered to having EDS. Those who napped once or twice a week or more were considered nappers.
About one-quarter of participants (24.4%) reported EDS, and 28.5% identified themselves as regular nappers. An average of 15.7 years later, participants completed Pittsburgh Compound B PET imaging, at which time 34.7% were deemed beta-amyloid+.
“We were kind of shocked that a single question with a yes/no response was robustly associated with [beta-amyloid] status that many years later,” reported Dr. Spira.
Still unknown, he added, is whether there is utility in quantifying or screening for preclinical Alzheimer’s disease risk using sleep variables. Also unclear is how EDS itself might drive beta-amyloid deposition. “What is likely to be happening here is something like sleep disordered breathing – which has been linked to cognitive impairment and dementia and has been linked to [Alzheimer’s disease] biomarkers – is driving this association,” he speculated.
“We have to keep in mind that even the people at baseline, even though they were cognitively normal, may have had some beta-amyloid deposition, which might have contributed to their EDS,” said Dr. Spira.
Dr. Spira intends to conduct a prospective study using laboratory-based sleep testing (polysomnography) and repeated measures of brain amyloid to clarify whether sleep disordered breathing or other factors were driving the association he found.
Dr. Spira reported having no financial disclosures. This study was supported by grants from the National Institute on Aging.
AT SLEEP 2017
Key clinical point: Cognitively normal older adults who reported excessive sleepiness or napping were more likely, at follow-up 15 years later, to have brain amyloid deposition, one of the hallmarks of Alzheimer’s disease.
Major finding: Compared with those who did not report excessive daytime sleepiness, those who did were more than three times more likely to have beta-amyloid deposition on PET imaging more than 15 years later.
Data source: The data analysis included 124 participants in the Baltimore Longitudinal Study of Aging who were queried on their sleep habits and then underwent PET imaging more than 15 years later.
Disclosures: Dr. Spira reported having no financial disclosures. This study was supported by grants from the National Institute on Aging.
Monoclonal antibody holds promise for S. aureus pneumonia
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
AT ASM MICROBE 2017
Key clinical point: A monoclonal antibody that neutralizes the alpha-toxin secreted by S. aureus appeared safe and effective in an early trial.
Major finding: AR-301 appeared safe, with 8 of 343 adverse events, or 2.3%, considered treatment related.
Data source: Randomized, double-blind, placebo-controlled, international study in 48 patients from 13 ICUs.
Disclosures: Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
‘Pink tax’ found on OTC minoxidil
Minoxidil 5% foam costs a mean of 40% more when it’s marketed to women instead of men, according to a review of 24 pharmacies in four northeastern states.
The statistically significant difference – $11.27/30 mL versus $8.05/30 mL – “could be a result of differential pricing by gender or could reflect production costs that are not related to medication strength,” reported the investigators from the University of Pennsylvania, Philadelphia. Also, the formulation for women, approved in 2014, is still on-patent and available only as Rogaine, whereas generics are available for the men’s foam (JAMA Dermatol. 2017 Jun 7. doi: 10.1001/jamadermatol.2017.1394).
The team also found that women’s 2% minoxidil solution costs about the same as men’s 5% minoxidil solution, despite the difference in strength ($7.61/30 mL versus $7.63/30 mL).
“These are products with the exact same ingredients. They come in different amounts and packaging based on gender, so, for the most part, women probably do not even realize they are paying more,” lead author Jules Lipoff, MD, of the department of dermatology, said in a University of Pennsylvania press release. “We recommend that our female patients buy the male version of the product because it doesn’t seem right to ask a woman to pay more when the products are, for all intents and purposes, identical,” he said.
Gender-based price differences – the “pink tax” – have been documented in consumer products before. A 2015 report found that women pay about 13% more than men for equivalent personal products in New York City. “However, to our knowledge, gender-based price differences for medications have not been previously studied,” the investigators said.
Pricing data came from CVS, Kroger, Rite Aid, Target, Walgreens, and Walmart pharmacies in Pennsylvania, New York, Ohio, and Indiana. The analysis included 14 OTC minoxidil preparations marketed to women and 27 marketed to men. Products were matched based on concentration and inactive ingredients to arrive at overall prices. When prices varied for the same product at different stores in the same chain, the mean price was used.
Dr. Lipoff reported receiving a grant from Pfizer. No other disclosures were reported.
Minoxidil 5% foam costs a mean of 40% more when it’s marketed to women instead of men, according to a review of 24 pharmacies in four northeastern states.
The statistically significant difference – $11.27/30 mL versus $8.05/30 mL – “could be a result of differential pricing by gender or could reflect production costs that are not related to medication strength,” reported the investigators from the University of Pennsylvania, Philadelphia. Also, the formulation for women, approved in 2014, is still on-patent and available only as Rogaine, whereas generics are available for the men’s foam (JAMA Dermatol. 2017 Jun 7. doi: 10.1001/jamadermatol.2017.1394).
The team also found that women’s 2% minoxidil solution costs about the same as men’s 5% minoxidil solution, despite the difference in strength ($7.61/30 mL versus $7.63/30 mL).
“These are products with the exact same ingredients. They come in different amounts and packaging based on gender, so, for the most part, women probably do not even realize they are paying more,” lead author Jules Lipoff, MD, of the department of dermatology, said in a University of Pennsylvania press release. “We recommend that our female patients buy the male version of the product because it doesn’t seem right to ask a woman to pay more when the products are, for all intents and purposes, identical,” he said.
Gender-based price differences – the “pink tax” – have been documented in consumer products before. A 2015 report found that women pay about 13% more than men for equivalent personal products in New York City. “However, to our knowledge, gender-based price differences for medications have not been previously studied,” the investigators said.
Pricing data came from CVS, Kroger, Rite Aid, Target, Walgreens, and Walmart pharmacies in Pennsylvania, New York, Ohio, and Indiana. The analysis included 14 OTC minoxidil preparations marketed to women and 27 marketed to men. Products were matched based on concentration and inactive ingredients to arrive at overall prices. When prices varied for the same product at different stores in the same chain, the mean price was used.
Dr. Lipoff reported receiving a grant from Pfizer. No other disclosures were reported.
Minoxidil 5% foam costs a mean of 40% more when it’s marketed to women instead of men, according to a review of 24 pharmacies in four northeastern states.
The statistically significant difference – $11.27/30 mL versus $8.05/30 mL – “could be a result of differential pricing by gender or could reflect production costs that are not related to medication strength,” reported the investigators from the University of Pennsylvania, Philadelphia. Also, the formulation for women, approved in 2014, is still on-patent and available only as Rogaine, whereas generics are available for the men’s foam (JAMA Dermatol. 2017 Jun 7. doi: 10.1001/jamadermatol.2017.1394).
The team also found that women’s 2% minoxidil solution costs about the same as men’s 5% minoxidil solution, despite the difference in strength ($7.61/30 mL versus $7.63/30 mL).
“These are products with the exact same ingredients. They come in different amounts and packaging based on gender, so, for the most part, women probably do not even realize they are paying more,” lead author Jules Lipoff, MD, of the department of dermatology, said in a University of Pennsylvania press release. “We recommend that our female patients buy the male version of the product because it doesn’t seem right to ask a woman to pay more when the products are, for all intents and purposes, identical,” he said.
Gender-based price differences – the “pink tax” – have been documented in consumer products before. A 2015 report found that women pay about 13% more than men for equivalent personal products in New York City. “However, to our knowledge, gender-based price differences for medications have not been previously studied,” the investigators said.
Pricing data came from CVS, Kroger, Rite Aid, Target, Walgreens, and Walmart pharmacies in Pennsylvania, New York, Ohio, and Indiana. The analysis included 14 OTC minoxidil preparations marketed to women and 27 marketed to men. Products were matched based on concentration and inactive ingredients to arrive at overall prices. When prices varied for the same product at different stores in the same chain, the mean price was used.
Dr. Lipoff reported receiving a grant from Pfizer. No other disclosures were reported.
FROM JAMA DERMATOLOGY
Lead detected in 20% of baby food samples, surprising even researchers
Pediatricians and public health researchers know they have to be on the lookout for lead exposure from paint chips and contaminated drinking water. A new report suggests food – particularly baby food – could be a problem, too.
The Environmental Defense Fund, in an analysis of 11 years of federal data, found detectable levels of lead in 20% of 2,164 baby food samples. The toxic metal was most commonly found in fruit juices such as grape and apple, root vegetables such as sweet potatoes and carrots, and cookies such as teething biscuits.
The organization’s primary focus was on the baby foods because lead can be so detrimental to child development.
“Lead can have a number of effects on children, and it’s especially harmful during critical windows of development,” said Aparna Bole, MD, pediatrician at University Hospitals Rainbow Babies and Children’s Hospital in Cleveland, who was not involved with the report. “The largest burden that we often think about is neurocognitive that can occur even at low levels of lead exposure.”
Lead can cause problems with attention and behavior, cognitive development, the cardiovascular system, and immune system, Dr. Bole said.
The samples studied were not identified by brand, and the levels of lead are thought to be relatively low. Still, according to the Centers for Disease Control and Prevention, no safe blood lead level in children has been identified.
In a draft report released earlier this year, the Environmental Protection Agency estimated that over 5% of children consume more than 6 micrograms per day of lead – the maximum daily intake level set by the Food and Drug Administration in 1993 – in their diet.
This surprised Tom Neltner, JD, Environmental Defense Fund’s chemicals policy director, who has spent 20 years researching and working to reduce lead exposures. His further analysis of the EPA report was that food is the major source of lead exposure in two-thirds of toddlers.
This spurred the organization to examine data from the FDA’s Total Diet Study for specific sources of exposure for kids.
In the resulting report, releasedThursday, Mr. Neltner found that the baby food versions of apple juice, grape juice, and carrots had detectable lead more often than the regular versions. Researchers could determine how frequently contamination occurred, but not at what levels.
According to the FDA, lead makes its way into food through contaminated soil, but Mr. Neltner suspects that processing may also play a role.
“I can’t explain it other than I assume baby food is processed more,” Mr. Neltner said.
The Environmental Defense Fund report notes that more research on the sources of contamination is needed.
FDA has set guidance levels of 100 parts per billion (ppb) for candy and dried fruit and 50 ppb for fruit juices. The allowable level for lead in bottled water is 5 ppb.
Concern over fruit juices flared up in 2012 when Consumer Reports found that one in four samples of apple and grape juices had lead levels higher than the FDA’s bottled-water limit of 5 ppb.
“The FDA is continuing to work with industry to further limit the amount of lead in foods to the greatest extent feasible, especially in foods frequently consumed by children,” read an agency statement in response to the report. “The agency is in the process of reevaluating the analytical methods it uses for determining when it should take action with respect to measured levels of lead in particular foods, including those consumed by infants and toddlers.”
Mr. Neltner said he’s glad the FDA is working on the issue but wants them to “get it done. Move quicker.”
The Environmental Defense Fund isn’t recommending that parents avoid certain foods or brands for their children but does advise that they consult their pediatricians about all means of lead exposure.
“In many American communities, the most significant route of lead exposure is from paint and soil,” Dr. Bole said. “Avoiding all sources of exposure of lead poisoning is incredibly important … but the last thing I would want is for a parent to restrict their child’s diet or limit their intake of healthy food groups.”
She added that pediatricians recommend limiting or eliminating fruit juices from children’s diets, anyway, for nutritional reasons. “There are good reasons to limit juice other than this particular report,” Dr. Bole said.
But she said she wouldn’t want parents to avoid root vegetables altogether. “The benefits of those nutritious foods far outweigh any risk,” she said, especially in the context of where kids are most exposed to lead.
In response to a request for comment, Gerber said that samples of its baby foods and juices “consistently fall well within the available guidance levels and meet our own strict standards.” And samples of Gerber juices were all below the EPA standard for drinking water.
“We know parents may be concerned about a recent report on lead in foods and want to reassure them that Gerber foods and juices are safe,” the statement read.
The Environmental Defense Fund report was ultimately directed at the food industry and FDA in the hopes of getting limits and standards updated.
But lead in paint and drinking water shouldn’t fall by the wayside, Mr. Neltner said. “You’ve got to deal with this issue on multiple fronts.”
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Pediatricians and public health researchers know they have to be on the lookout for lead exposure from paint chips and contaminated drinking water. A new report suggests food – particularly baby food – could be a problem, too.
The Environmental Defense Fund, in an analysis of 11 years of federal data, found detectable levels of lead in 20% of 2,164 baby food samples. The toxic metal was most commonly found in fruit juices such as grape and apple, root vegetables such as sweet potatoes and carrots, and cookies such as teething biscuits.
The organization’s primary focus was on the baby foods because lead can be so detrimental to child development.
“Lead can have a number of effects on children, and it’s especially harmful during critical windows of development,” said Aparna Bole, MD, pediatrician at University Hospitals Rainbow Babies and Children’s Hospital in Cleveland, who was not involved with the report. “The largest burden that we often think about is neurocognitive that can occur even at low levels of lead exposure.”
Lead can cause problems with attention and behavior, cognitive development, the cardiovascular system, and immune system, Dr. Bole said.
The samples studied were not identified by brand, and the levels of lead are thought to be relatively low. Still, according to the Centers for Disease Control and Prevention, no safe blood lead level in children has been identified.
In a draft report released earlier this year, the Environmental Protection Agency estimated that over 5% of children consume more than 6 micrograms per day of lead – the maximum daily intake level set by the Food and Drug Administration in 1993 – in their diet.
This surprised Tom Neltner, JD, Environmental Defense Fund’s chemicals policy director, who has spent 20 years researching and working to reduce lead exposures. His further analysis of the EPA report was that food is the major source of lead exposure in two-thirds of toddlers.
This spurred the organization to examine data from the FDA’s Total Diet Study for specific sources of exposure for kids.
In the resulting report, releasedThursday, Mr. Neltner found that the baby food versions of apple juice, grape juice, and carrots had detectable lead more often than the regular versions. Researchers could determine how frequently contamination occurred, but not at what levels.
According to the FDA, lead makes its way into food through contaminated soil, but Mr. Neltner suspects that processing may also play a role.
“I can’t explain it other than I assume baby food is processed more,” Mr. Neltner said.
The Environmental Defense Fund report notes that more research on the sources of contamination is needed.
FDA has set guidance levels of 100 parts per billion (ppb) for candy and dried fruit and 50 ppb for fruit juices. The allowable level for lead in bottled water is 5 ppb.
Concern over fruit juices flared up in 2012 when Consumer Reports found that one in four samples of apple and grape juices had lead levels higher than the FDA’s bottled-water limit of 5 ppb.
“The FDA is continuing to work with industry to further limit the amount of lead in foods to the greatest extent feasible, especially in foods frequently consumed by children,” read an agency statement in response to the report. “The agency is in the process of reevaluating the analytical methods it uses for determining when it should take action with respect to measured levels of lead in particular foods, including those consumed by infants and toddlers.”
Mr. Neltner said he’s glad the FDA is working on the issue but wants them to “get it done. Move quicker.”
The Environmental Defense Fund isn’t recommending that parents avoid certain foods or brands for their children but does advise that they consult their pediatricians about all means of lead exposure.
“In many American communities, the most significant route of lead exposure is from paint and soil,” Dr. Bole said. “Avoiding all sources of exposure of lead poisoning is incredibly important … but the last thing I would want is for a parent to restrict their child’s diet or limit their intake of healthy food groups.”
She added that pediatricians recommend limiting or eliminating fruit juices from children’s diets, anyway, for nutritional reasons. “There are good reasons to limit juice other than this particular report,” Dr. Bole said.
But she said she wouldn’t want parents to avoid root vegetables altogether. “The benefits of those nutritious foods far outweigh any risk,” she said, especially in the context of where kids are most exposed to lead.
In response to a request for comment, Gerber said that samples of its baby foods and juices “consistently fall well within the available guidance levels and meet our own strict standards.” And samples of Gerber juices were all below the EPA standard for drinking water.
“We know parents may be concerned about a recent report on lead in foods and want to reassure them that Gerber foods and juices are safe,” the statement read.
The Environmental Defense Fund report was ultimately directed at the food industry and FDA in the hopes of getting limits and standards updated.
But lead in paint and drinking water shouldn’t fall by the wayside, Mr. Neltner said. “You’ve got to deal with this issue on multiple fronts.”
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Pediatricians and public health researchers know they have to be on the lookout for lead exposure from paint chips and contaminated drinking water. A new report suggests food – particularly baby food – could be a problem, too.
The Environmental Defense Fund, in an analysis of 11 years of federal data, found detectable levels of lead in 20% of 2,164 baby food samples. The toxic metal was most commonly found in fruit juices such as grape and apple, root vegetables such as sweet potatoes and carrots, and cookies such as teething biscuits.
The organization’s primary focus was on the baby foods because lead can be so detrimental to child development.
“Lead can have a number of effects on children, and it’s especially harmful during critical windows of development,” said Aparna Bole, MD, pediatrician at University Hospitals Rainbow Babies and Children’s Hospital in Cleveland, who was not involved with the report. “The largest burden that we often think about is neurocognitive that can occur even at low levels of lead exposure.”
Lead can cause problems with attention and behavior, cognitive development, the cardiovascular system, and immune system, Dr. Bole said.
The samples studied were not identified by brand, and the levels of lead are thought to be relatively low. Still, according to the Centers for Disease Control and Prevention, no safe blood lead level in children has been identified.
In a draft report released earlier this year, the Environmental Protection Agency estimated that over 5% of children consume more than 6 micrograms per day of lead – the maximum daily intake level set by the Food and Drug Administration in 1993 – in their diet.
This surprised Tom Neltner, JD, Environmental Defense Fund’s chemicals policy director, who has spent 20 years researching and working to reduce lead exposures. His further analysis of the EPA report was that food is the major source of lead exposure in two-thirds of toddlers.
This spurred the organization to examine data from the FDA’s Total Diet Study for specific sources of exposure for kids.
In the resulting report, releasedThursday, Mr. Neltner found that the baby food versions of apple juice, grape juice, and carrots had detectable lead more often than the regular versions. Researchers could determine how frequently contamination occurred, but not at what levels.
According to the FDA, lead makes its way into food through contaminated soil, but Mr. Neltner suspects that processing may also play a role.
“I can’t explain it other than I assume baby food is processed more,” Mr. Neltner said.
The Environmental Defense Fund report notes that more research on the sources of contamination is needed.
FDA has set guidance levels of 100 parts per billion (ppb) for candy and dried fruit and 50 ppb for fruit juices. The allowable level for lead in bottled water is 5 ppb.
Concern over fruit juices flared up in 2012 when Consumer Reports found that one in four samples of apple and grape juices had lead levels higher than the FDA’s bottled-water limit of 5 ppb.
“The FDA is continuing to work with industry to further limit the amount of lead in foods to the greatest extent feasible, especially in foods frequently consumed by children,” read an agency statement in response to the report. “The agency is in the process of reevaluating the analytical methods it uses for determining when it should take action with respect to measured levels of lead in particular foods, including those consumed by infants and toddlers.”
Mr. Neltner said he’s glad the FDA is working on the issue but wants them to “get it done. Move quicker.”
The Environmental Defense Fund isn’t recommending that parents avoid certain foods or brands for their children but does advise that they consult their pediatricians about all means of lead exposure.
“In many American communities, the most significant route of lead exposure is from paint and soil,” Dr. Bole said. “Avoiding all sources of exposure of lead poisoning is incredibly important … but the last thing I would want is for a parent to restrict their child’s diet or limit their intake of healthy food groups.”
She added that pediatricians recommend limiting or eliminating fruit juices from children’s diets, anyway, for nutritional reasons. “There are good reasons to limit juice other than this particular report,” Dr. Bole said.
But she said she wouldn’t want parents to avoid root vegetables altogether. “The benefits of those nutritious foods far outweigh any risk,” she said, especially in the context of where kids are most exposed to lead.
In response to a request for comment, Gerber said that samples of its baby foods and juices “consistently fall well within the available guidance levels and meet our own strict standards.” And samples of Gerber juices were all below the EPA standard for drinking water.
“We know parents may be concerned about a recent report on lead in foods and want to reassure them that Gerber foods and juices are safe,” the statement read.
The Environmental Defense Fund report was ultimately directed at the food industry and FDA in the hopes of getting limits and standards updated.
But lead in paint and drinking water shouldn’t fall by the wayside, Mr. Neltner said. “You’ve got to deal with this issue on multiple fronts.”
This story was produced by Kaiser Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
VIDEO: Rheumatology biosimilars gain U.S. momentum
MADRID – With biosimilar infliximab on the U.S. market since November 2016 and producing an immediate, albeit modest, price drop for this tumor necrosis factor inhibitor (TNFi) and a second biosimilar infliximab now approved by the Food and Drug Administration and awaiting market entry, biosimilars are in a new phase of integration into U.S. practice.
“Physicians are willing to prescribe Inflectra,” the first biosimilar infliximab and the first TNFi to be sold in the United States last November, Jonathan Kay, MD, said in a video interview during the European Congress of Rheumatology. “Rheumatologists who were initially skeptical are now on the bandwagon and willing to prescribe biosimilars,” said Dr. Kay, a rheumatologist who has often consulted on biosimilar issues and has recently spoken to rheumatologists at various state society meetings to explain the U.S. biosimilar regulatory concepts and spread the message of the societal value of these agents.
“This is not a quick and casual drug evaluation” that produces “knockoff drugs,” but a “careful and extensive” FDA review that results in drugs that are equivalent in efficacy, safety, and immunogenicity to the reference drug and only compete on price, he explained.
When Pfizer began marketing Inflectra last Fall, it set the drug’s list price 15% lower than the list price at the time for Remicade, the reference-product infliximab. However, complex pricing and rebate strategies actually led to Remicade selling for a lower price than Inflectra, at least for some U.S. hospitals, including the University of Massachusetts in Worcester, where Dr. Kay is a professor of medicine.
“The effect of biosimilars is to reduce the cost to patients of an effective treatment. Whether that cost is for the reference drug or for the biosimilar drug doesn’t matter [from society’s perspective] as long as patients are able to receive an effective therapy at a [more] affordable cost, making the effective therapy available to more patients,” he said.
While Inflectra’s price impact my have been modest so far, the biosimilar effect on infliximab’s cost may soon intensify now that a second biosimilar of this TNFi, Renflexis – made by Samsung Bioepis and with U.S. marketing by Merck, received FDA approval on April 21, 2017. Until recently, U.S. pharmaceutical regulations had been understood to require a 180-day hiatus between FDA marketing approval for a biosimilar and the start of U.S. sales. But, on June 12, 2017, the U.S. Supreme Court, in a 9-0 decision, ruled that this 180-day wait was not required, making it possible for U.S. marketing of Renflexis to begin soon. (In mid-June, a statement on the Merck U.S. website for Renflexis says that the product is not currently available.)
Availability of a second biosimilar infliximab “is likely to drive the price down rapidly,” predicted Dr. Kay, citing what happened when multiple biosimilars for a reference drug came onto the European market.
Two other biosimilar TNFi have also received FDA marketing approvals but remain on hold as patent issues and litigation barriers play out. Erelzi – biosimilar etanercept – received FDA approval in August 2016, and Amjevita, biosimilar adalimumab, received FDA approval last September.
The efficacy and safety of Inflectra specifically, and by extension all biosimilars, received a recent boost with publication of findings from a randomized study with 482 patients that provided a real-world test of the core principle of biosimilar equivalence. After Inflectra came onto the Norwegian market, during July 2014 to August 2015, Norwegian researchers ran the NOR-SWTICH trial, which randomized patients who were on stable treatment with Remicade for a variety of indications (including 41% with a rheumatologic disease) to either stay on Remicade or to abruptly switch to treatment with Inflectra. During 1-year follow-up, the incidence of adverse effects and of episodes of disease worsening were virtually identical in the two treatment arms (Lancet. 2017 June 10;389[10086]:2304-16).
Dr. Kay has been a consultant to several companies that develop or market biosimilars, including Samsung Bioepis, Amgen, Pfizer, and Sandoz (Novartis), and to AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen, Roche, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – With biosimilar infliximab on the U.S. market since November 2016 and producing an immediate, albeit modest, price drop for this tumor necrosis factor inhibitor (TNFi) and a second biosimilar infliximab now approved by the Food and Drug Administration and awaiting market entry, biosimilars are in a new phase of integration into U.S. practice.
“Physicians are willing to prescribe Inflectra,” the first biosimilar infliximab and the first TNFi to be sold in the United States last November, Jonathan Kay, MD, said in a video interview during the European Congress of Rheumatology. “Rheumatologists who were initially skeptical are now on the bandwagon and willing to prescribe biosimilars,” said Dr. Kay, a rheumatologist who has often consulted on biosimilar issues and has recently spoken to rheumatologists at various state society meetings to explain the U.S. biosimilar regulatory concepts and spread the message of the societal value of these agents.
“This is not a quick and casual drug evaluation” that produces “knockoff drugs,” but a “careful and extensive” FDA review that results in drugs that are equivalent in efficacy, safety, and immunogenicity to the reference drug and only compete on price, he explained.
When Pfizer began marketing Inflectra last Fall, it set the drug’s list price 15% lower than the list price at the time for Remicade, the reference-product infliximab. However, complex pricing and rebate strategies actually led to Remicade selling for a lower price than Inflectra, at least for some U.S. hospitals, including the University of Massachusetts in Worcester, where Dr. Kay is a professor of medicine.
“The effect of biosimilars is to reduce the cost to patients of an effective treatment. Whether that cost is for the reference drug or for the biosimilar drug doesn’t matter [from society’s perspective] as long as patients are able to receive an effective therapy at a [more] affordable cost, making the effective therapy available to more patients,” he said.
While Inflectra’s price impact my have been modest so far, the biosimilar effect on infliximab’s cost may soon intensify now that a second biosimilar of this TNFi, Renflexis – made by Samsung Bioepis and with U.S. marketing by Merck, received FDA approval on April 21, 2017. Until recently, U.S. pharmaceutical regulations had been understood to require a 180-day hiatus between FDA marketing approval for a biosimilar and the start of U.S. sales. But, on June 12, 2017, the U.S. Supreme Court, in a 9-0 decision, ruled that this 180-day wait was not required, making it possible for U.S. marketing of Renflexis to begin soon. (In mid-June, a statement on the Merck U.S. website for Renflexis says that the product is not currently available.)
Availability of a second biosimilar infliximab “is likely to drive the price down rapidly,” predicted Dr. Kay, citing what happened when multiple biosimilars for a reference drug came onto the European market.
Two other biosimilar TNFi have also received FDA marketing approvals but remain on hold as patent issues and litigation barriers play out. Erelzi – biosimilar etanercept – received FDA approval in August 2016, and Amjevita, biosimilar adalimumab, received FDA approval last September.
The efficacy and safety of Inflectra specifically, and by extension all biosimilars, received a recent boost with publication of findings from a randomized study with 482 patients that provided a real-world test of the core principle of biosimilar equivalence. After Inflectra came onto the Norwegian market, during July 2014 to August 2015, Norwegian researchers ran the NOR-SWTICH trial, which randomized patients who were on stable treatment with Remicade for a variety of indications (including 41% with a rheumatologic disease) to either stay on Remicade or to abruptly switch to treatment with Inflectra. During 1-year follow-up, the incidence of adverse effects and of episodes of disease worsening were virtually identical in the two treatment arms (Lancet. 2017 June 10;389[10086]:2304-16).
Dr. Kay has been a consultant to several companies that develop or market biosimilars, including Samsung Bioepis, Amgen, Pfizer, and Sandoz (Novartis), and to AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen, Roche, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – With biosimilar infliximab on the U.S. market since November 2016 and producing an immediate, albeit modest, price drop for this tumor necrosis factor inhibitor (TNFi) and a second biosimilar infliximab now approved by the Food and Drug Administration and awaiting market entry, biosimilars are in a new phase of integration into U.S. practice.
“Physicians are willing to prescribe Inflectra,” the first biosimilar infliximab and the first TNFi to be sold in the United States last November, Jonathan Kay, MD, said in a video interview during the European Congress of Rheumatology. “Rheumatologists who were initially skeptical are now on the bandwagon and willing to prescribe biosimilars,” said Dr. Kay, a rheumatologist who has often consulted on biosimilar issues and has recently spoken to rheumatologists at various state society meetings to explain the U.S. biosimilar regulatory concepts and spread the message of the societal value of these agents.
“This is not a quick and casual drug evaluation” that produces “knockoff drugs,” but a “careful and extensive” FDA review that results in drugs that are equivalent in efficacy, safety, and immunogenicity to the reference drug and only compete on price, he explained.
When Pfizer began marketing Inflectra last Fall, it set the drug’s list price 15% lower than the list price at the time for Remicade, the reference-product infliximab. However, complex pricing and rebate strategies actually led to Remicade selling for a lower price than Inflectra, at least for some U.S. hospitals, including the University of Massachusetts in Worcester, where Dr. Kay is a professor of medicine.
“The effect of biosimilars is to reduce the cost to patients of an effective treatment. Whether that cost is for the reference drug or for the biosimilar drug doesn’t matter [from society’s perspective] as long as patients are able to receive an effective therapy at a [more] affordable cost, making the effective therapy available to more patients,” he said.
While Inflectra’s price impact my have been modest so far, the biosimilar effect on infliximab’s cost may soon intensify now that a second biosimilar of this TNFi, Renflexis – made by Samsung Bioepis and with U.S. marketing by Merck, received FDA approval on April 21, 2017. Until recently, U.S. pharmaceutical regulations had been understood to require a 180-day hiatus between FDA marketing approval for a biosimilar and the start of U.S. sales. But, on June 12, 2017, the U.S. Supreme Court, in a 9-0 decision, ruled that this 180-day wait was not required, making it possible for U.S. marketing of Renflexis to begin soon. (In mid-June, a statement on the Merck U.S. website for Renflexis says that the product is not currently available.)
Availability of a second biosimilar infliximab “is likely to drive the price down rapidly,” predicted Dr. Kay, citing what happened when multiple biosimilars for a reference drug came onto the European market.
Two other biosimilar TNFi have also received FDA marketing approvals but remain on hold as patent issues and litigation barriers play out. Erelzi – biosimilar etanercept – received FDA approval in August 2016, and Amjevita, biosimilar adalimumab, received FDA approval last September.
The efficacy and safety of Inflectra specifically, and by extension all biosimilars, received a recent boost with publication of findings from a randomized study with 482 patients that provided a real-world test of the core principle of biosimilar equivalence. After Inflectra came onto the Norwegian market, during July 2014 to August 2015, Norwegian researchers ran the NOR-SWTICH trial, which randomized patients who were on stable treatment with Remicade for a variety of indications (including 41% with a rheumatologic disease) to either stay on Remicade or to abruptly switch to treatment with Inflectra. During 1-year follow-up, the incidence of adverse effects and of episodes of disease worsening were virtually identical in the two treatment arms (Lancet. 2017 June 10;389[10086]:2304-16).
Dr. Kay has been a consultant to several companies that develop or market biosimilars, including Samsung Bioepis, Amgen, Pfizer, and Sandoz (Novartis), and to AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Janssen, Roche, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2017 CONGRESS