User login
Erythema extent predicts death in cutaneous GVHD
“There is value in collecting erythema serially over time as a continuous variable on a scale of 0%-100%” to identify high-risk patients for prophylactic and preemptive treatment, say investigators led by dermatologist Emily Baumrin, MD, director of the GVHD clinic at the University of Pennsylvania, Philadelphia.
They report a study of more than 300 patients with ccGVHD, which found that the extent of skin erythema strongly predicted the risk for death from GVHD.
Of the 267 patients with cutaneous GVHD at baseline, 103 patients died, the majority without a relapse of their blood cancer.
With additional research, erythema body surface area (BSA) should be “introduced as an outcome measure in clinical practice and trials,” they conclude.
At the moment, the NIH Skin Score is commonly used for risk assessment in cutaneous GVHD, but the researchers found that erythema BSA out-predicts this score.
The investigators explain that the NIH Skin Score does incorporate erythema surface area, but it does so as a categorical variable, not a continuous variable. Among other additional factors, it also includes assessments of skin sclerosis, which the investigators found was not associated with GVHD mortality.
Overall, the composite score waters down the weight given to erythema BSA because the score is “driven by stable sclerotic features, and erythema changes are missed,” they explain.
The study was published online in JAMA Dermatology.
Study details
The study included 469 patients with chronic GVHD (cGVHD), of whom 267 (57%) had cutaneous cGVHD at enrollment and 89 (19%) developed skin involvement subsequently.
All of the patients were on systemic immunosuppression for GVHD after allogeneic stem cell transplants for various blood cancers.
They were enrolled from 2007 through 2012 at nine U.S. medical centers – all members of the Chronic Graft Versus Host Disease Consortium – and they were followed until 2018.
Erythema BSA and NIH Skin Score were assessed at baseline and then every 3-6 months. Erythema was the first manifestation of skin involvement in the majority of patients, with a median surface area involvement of 11% at baseline.
The study team found that the extent of erythema at first follow-up visit was associated with both nonrelapse mortality (hazard ratio, 1.33 per 10% BSA increase; P < .001) and overall survival (HR, 1.28 per 10% BSA increase; P < .001), whereas extent of sclerotic skin involvement was not associated with either.
Participants in the study were predominantly White. The investigators note that “BSA assessments of erythema may be less reliable in patients with darker skin.”
The work was funded by the Department of Veterans Affairs and the National Institutes of Health. Dr. Baumrin had no disclosures; one coauthor is an employee of CorEvitas, and two others reported grants/adviser fees from several companies, including Janssen, Mallinckrodt, and Pfizer.
A version of this article first appeared on Medscape.com.
“There is value in collecting erythema serially over time as a continuous variable on a scale of 0%-100%” to identify high-risk patients for prophylactic and preemptive treatment, say investigators led by dermatologist Emily Baumrin, MD, director of the GVHD clinic at the University of Pennsylvania, Philadelphia.
They report a study of more than 300 patients with ccGVHD, which found that the extent of skin erythema strongly predicted the risk for death from GVHD.
Of the 267 patients with cutaneous GVHD at baseline, 103 patients died, the majority without a relapse of their blood cancer.
With additional research, erythema body surface area (BSA) should be “introduced as an outcome measure in clinical practice and trials,” they conclude.
At the moment, the NIH Skin Score is commonly used for risk assessment in cutaneous GVHD, but the researchers found that erythema BSA out-predicts this score.
The investigators explain that the NIH Skin Score does incorporate erythema surface area, but it does so as a categorical variable, not a continuous variable. Among other additional factors, it also includes assessments of skin sclerosis, which the investigators found was not associated with GVHD mortality.
Overall, the composite score waters down the weight given to erythema BSA because the score is “driven by stable sclerotic features, and erythema changes are missed,” they explain.
The study was published online in JAMA Dermatology.
Study details
The study included 469 patients with chronic GVHD (cGVHD), of whom 267 (57%) had cutaneous cGVHD at enrollment and 89 (19%) developed skin involvement subsequently.
All of the patients were on systemic immunosuppression for GVHD after allogeneic stem cell transplants for various blood cancers.
They were enrolled from 2007 through 2012 at nine U.S. medical centers – all members of the Chronic Graft Versus Host Disease Consortium – and they were followed until 2018.
Erythema BSA and NIH Skin Score were assessed at baseline and then every 3-6 months. Erythema was the first manifestation of skin involvement in the majority of patients, with a median surface area involvement of 11% at baseline.
The study team found that the extent of erythema at first follow-up visit was associated with both nonrelapse mortality (hazard ratio, 1.33 per 10% BSA increase; P < .001) and overall survival (HR, 1.28 per 10% BSA increase; P < .001), whereas extent of sclerotic skin involvement was not associated with either.
Participants in the study were predominantly White. The investigators note that “BSA assessments of erythema may be less reliable in patients with darker skin.”
The work was funded by the Department of Veterans Affairs and the National Institutes of Health. Dr. Baumrin had no disclosures; one coauthor is an employee of CorEvitas, and two others reported grants/adviser fees from several companies, including Janssen, Mallinckrodt, and Pfizer.
A version of this article first appeared on Medscape.com.
“There is value in collecting erythema serially over time as a continuous variable on a scale of 0%-100%” to identify high-risk patients for prophylactic and preemptive treatment, say investigators led by dermatologist Emily Baumrin, MD, director of the GVHD clinic at the University of Pennsylvania, Philadelphia.
They report a study of more than 300 patients with ccGVHD, which found that the extent of skin erythema strongly predicted the risk for death from GVHD.
Of the 267 patients with cutaneous GVHD at baseline, 103 patients died, the majority without a relapse of their blood cancer.
With additional research, erythema body surface area (BSA) should be “introduced as an outcome measure in clinical practice and trials,” they conclude.
At the moment, the NIH Skin Score is commonly used for risk assessment in cutaneous GVHD, but the researchers found that erythema BSA out-predicts this score.
The investigators explain that the NIH Skin Score does incorporate erythema surface area, but it does so as a categorical variable, not a continuous variable. Among other additional factors, it also includes assessments of skin sclerosis, which the investigators found was not associated with GVHD mortality.
Overall, the composite score waters down the weight given to erythema BSA because the score is “driven by stable sclerotic features, and erythema changes are missed,” they explain.
The study was published online in JAMA Dermatology.
Study details
The study included 469 patients with chronic GVHD (cGVHD), of whom 267 (57%) had cutaneous cGVHD at enrollment and 89 (19%) developed skin involvement subsequently.
All of the patients were on systemic immunosuppression for GVHD after allogeneic stem cell transplants for various blood cancers.
They were enrolled from 2007 through 2012 at nine U.S. medical centers – all members of the Chronic Graft Versus Host Disease Consortium – and they were followed until 2018.
Erythema BSA and NIH Skin Score were assessed at baseline and then every 3-6 months. Erythema was the first manifestation of skin involvement in the majority of patients, with a median surface area involvement of 11% at baseline.
The study team found that the extent of erythema at first follow-up visit was associated with both nonrelapse mortality (hazard ratio, 1.33 per 10% BSA increase; P < .001) and overall survival (HR, 1.28 per 10% BSA increase; P < .001), whereas extent of sclerotic skin involvement was not associated with either.
Participants in the study were predominantly White. The investigators note that “BSA assessments of erythema may be less reliable in patients with darker skin.”
The work was funded by the Department of Veterans Affairs and the National Institutes of Health. Dr. Baumrin had no disclosures; one coauthor is an employee of CorEvitas, and two others reported grants/adviser fees from several companies, including Janssen, Mallinckrodt, and Pfizer.
A version of this article first appeared on Medscape.com.
DEA proposals on telehealth for controlled substances draw fire
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The proposed rules – one for Schedule III-V substances, and the other for buprenorphine – are due to go into effect on May 11, when the COVID-19 public health emergency (PHE), and temporary flexibilities, end.
Essentially, both proposals would allow providers to prescribe a 30-day supply of a controlled substance or buprenorphine, but then require a face-to-face meeting for patients to receive additional prescriptions.
The DEA says that the rules are aimed at preventing abuse and diversion of the substances, but clinicians claim they are creating unnecessary hurdles that will probably lead to some patients dropping out of treatment.
“We were happy to see that there is ongoing flexibility to be able to initiate buprenorphine through telehealth, but we were disappointed to see that the DEA set an arbitrary time frame, in this case, a 30-day time frame after which the patient would have to be seen in person before ongoing care with buprenorphine for opioid use disorder could be provided,” Brian Hurley, MD, MBA, the president-elect of the American Society of Addiction Medicine told this news organization.
Dr. Hurley agreed that it is best practice to see patients in person for ongoing care, but he noted they have many reasons why they might not be able to make it into an office every month.
“What this rule would do if instituted as written is prevent me from continuing care for patients unless I can get them in in person,” he said. “And while I’d make every effort as a clinician, it’s not always feasible to do so.”
The addiction specialist noted that only about 20% of Americans with opioid use disorder have access to medications for the disorder. “I would posit that untreated opioid use disorder is a bigger threat to public safety currently than the risk of diversion,” he said.
The DEA is also proposing to allow state laws to supersede its regulations, which concerns Dr. Hurley and other clinicians because some states are more restrictive. “Our position is that state laws that restrict access to medications for opioid use disorder through telehealth means are inconsistent with our policy recommendation. I certainly hope that the DEA hears our concerns and amends the proposal,” said Dr. Hurley.
A potential ‘telehealth cliff’
Shabana Khan, MD, chair of the American Psychiatric Association’s telepsychiatry committee, said that “because of potential overlap with state rules that may be more stringent than these new regulations, APA is concerned that the proposed rules will create a telehealth cliff for those in most need of critical psychiatric and opioid use disorder treatment, particularly in communities where this specialty care is limited or nonexistent.”
Dr. Khan noted that “clarification is necessary on how patients who started treatment during the PHE can continue treatment with a prescribing provider, if at all, through an in-person evaluation with a DEA-registered provider referral.”
Telehealth companies were also disappointed in the DEA proposals.
“The continuity of care for countless Americans will be severed, potentially leaving these patients to fall through the cracks of our health care system without access to needed medications,” said Kyle Zebley, the American Telemedicine Association’s senior vice president of public policy, in a statement.
“Requiring every patient who has initiated treatment via telemedicine during the pandemic to now visit a provider in person clearly falls on the side of being overly restrictive,” Mr. Zebley added.
The DEA is proposing to allow patients who have been receiving telehealth over the past 3 years to continue to do so for 180 days after the PHE ends.
But the American Telemedicine Association and others said that they still want to see a change in the proposal as written. “Our hope is that the DEA works with us to avoid unnecessary and inappropriate restrictions on the prescription of essential medications for these vulnerable and underserved populations,” Mr. Zebley said in the statement.
DEA Administrator Anne Milgram said in a statement that the agency believes that “the telemedicine regulations would continue to expand access to buprenorphine for patients with opioid use disorder,” and that the DEA “is committed to the expansion of telemedicine with guardrails that prevent the online overprescribing of controlled medications that can cause harm.”
Rahul Gupta, MD, director of the White House Office of National Drug Control Policy, said in a statement that “This proposed rule builds on President Biden’s historic move to eliminate the X-waiver that prevented many prescribers from treating patients with buprenorphine.” He added, “Thanks to these changes, millions of Americans will be able to access the lifesaving care they need.”
The DEA estimated that there were 15.7 million prescriptions for buprenorphine in 2021 and that about 67,000 were for initial prescriptions.
Ketamine confusion
The rule on controlled substances has also caused some consternation, especially given that it does not differentiate between racemic ketamine and esketamine, said Lisa Marie Harding, MD, vice president of the board of the American Society of Ketamine Physicians, Psychotherapists & Practitioners.
Esketamine (Spravato) is approved by the Food and Drug Administration and, under a Risk Evaluation and Mitigation Strategy, can only be administered in FDA-monitored treatment facilities. Racemic ketamine is being prescribed – often for home use – with almost no regulatory oversight.
Dr. Harding, who is an approved Spravato provider and also administers intravenous ketamine in her practice, does not believe that ketamine should be used at home without supervision.
“I had a patient who had a very powerful dissociative experience in my office earlier this week,” Dr. Harding said in an interview. One of her staff asked what would happen if the patient had experienced that at home. “We don’t know. Nor do we want this to happen,” said Dr. Harding.
However, the DEA proposal would continue to allow for home use, at least initially. “If it’s open to interpretation, those people that prescribe ketamine for home use can use that leeway to then continue to do it,” she said. “That is not safe.”
Dr. Harding approves of the proposed DEA requirement for face-to-face visits. “It’s good patient care,” she said. But she wants the administration to adjust the rules to make it harder to offer home ketamine therapy.
“Lots of people are using racemic ketamine off-label for treating depression with success but doing it in treatment settings that are appropriate,” said Dr. Harding.
Dr. Hurley and Dr. Harding report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can SGLT2 inhibitors limit acute kidney injury in type 2 diabetes?
Adults with type 2 diabetes treated with an SGLT2 inhibitor had roughly a third fewer episodes of acute kidney injury (AKI) compared with matched people with type 2 diabetes treated with a DPP4 inhibitor, in an analysis of health insurance data from more than 100,000 Taiwan residents during 2016-2018.
The findings add to, and expand on, prior evidence that treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class cuts the incidence of AKI, say the authors of the report, which was recently published in JAMA Network Open.
The long-term risk for AKI among people with type 2 diabetes treated with an SGLT2 inhibitor “appears to be quite low” compared with adults who received an agent from the dipeptidyl peptidase 4 (DPP4) inhibitor class.
Treatment with an SGLT2 inhibitor – such as canagliflozin (Invokana), dapagliflozin (Farxiga), or empagliflozin (Jardiance) – causes a transient drop in kidney function that manifests as a temporary dip in estimated glomerular filtration rate, which caused concerns about AKI when the drugs were first introduced.
Indeed, canagliflozin and dapagliflozin had warnings strengthened 7 years ago by the Food and Drug Administration in a Drug Safety Communication for accumulating reports of AKI linked to their use.
More recent experience has calmed AKI concerns, however.
Commenting on the new study, F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn., said: “It’s a nice piece of data to demonstrate that the long-term risk from SGLT2 inhibitor treatment is low.” Dr. Wilson was not involved with the new study.
The Taiwan study found a cumulative incidence of AKI events during about 2.5 years of follow-up of 5.55 events/1,000 patient-years among adults with type 2 diabetes receiving an SGLT2 inhibitor and 7.88 events/1,000 patient-years among those taking a DPP4 inhibitor such as sitagliptin (Januvia).
Main barrier to SGLT2 inhibitor use is unfamiliarity, not AKI risk
“My impression is that the main barrier to wider use of the SGLT2 inhibitor class is not a perceived risk for causing AKI, but rather ongoing unfamiliarity with the class,” Dr. Wilson said in an interview.
Although he sees “relatively broad comfort with and enthusiasm for the class among nephrologists and cardiologists,” routine prescribing does not seem to have caught on nearly as much among primary care physicians, he said.
Clinicians in primary care “still perceive the SGLT2 inhibitor class as something of a ‘specialty drug,’ and they defer initiating it on that basis,” Dr. Wilson observed. “That’s probably not a good thing,” as many people with type 2 diabetes do not have access to a specialized clinician who might be more amenable to prescribing an SGLT2 inhibitor.
One example of the lag in SGLT2 inhibitor uptake for people with type 2 diabetes in practice was a recent report from the Centers for Disease Control and Prevention published in Annals of Internal Medicine. Researchers identified a representative U.S. sample of 1,330 adults with type 2 diabetes studied in depth during 2017-2020, of whom 82% fulfilled criteria published in 2022 for receiving treatment with an SGLT2 inhibitor. Despite this high prevalence of medical appropriateness, a scant 5.3% of those with a recommended indication actually received an agent from this class.
Early AKI concern has diminished
Results from more recent studies, such as a 2019 meta-analysis of more than 100 randomized studies and four large observational studies that together included about 180,000 people receiving SGLT2 inhibitor treatment, showed the opposite of SGLT2 inhibitor treatment triggering AKI.
In the trials, people taking an SGLT2 inhibitor had a relative 25% lower rate of AKI events, while in the observational studies, SGLT2 inhibitor treatment was linked with a 60% relative reduction in AKI. The study also found that SGLT2 inhibitor use in the trials was linked with a significant 20% relative increase in the incidence of low fluid volume.
Despite accumulated evidence exonerating AKI risk, U.S. labels for canagliflozin, dapagliflozin, and empagliflozin continue to cite AKI as a potential adverse reaction, especially in patients who undergo volume depletion while on SGLT2 inhibitor treatment.
The new Taiwan study used data from the country’s National Health Insurance Research Database. Out of more than 250,000 adults with type 2 diabetes in the system from May 2016 to December 2018, the researchers identified 52,231 propensity-score matched pairs of people where one was on treatment with an SGLT2 inhibitor and the other with a DPP4 inhibitor.
During follow-up, 856 of these people (0.8%) had an AKI event, including 102 people with AKI that required dialysis.
A logistic regression analysis that adjusted for 16 potential confounders showed that SGLT2 inhibitor treatment linked with a significant 34% reduction in AKI events compared with DPP4 inhibitor treatment, as well as with a significant 44% relative risk reduction in the incidence of AKI events requiring dialysis, reported the authors from several medical institutions in Taiwan.
The study’s main limitation was its reliance on “quite insensitive” administrative coding data to identify AKI cases, said Dr. Wilson.
He noted that although concern about AKI events secondary to SGLT2 inhibitor treatment is uncommon among U.S. clinicians they do worry about the potential risk for fungal infections, urinary tract infection, or gangrene in people with diabetes who receive an agent from this class.
The study received no commercial funding, and none of the authors had disclosures. Dr. Wilson has reported receiving research funding from AstraZeneca, Boehringer Ingelheim, Vifor, and Whoop.
A version of this article originally appeared on Medscape.com.
Adults with type 2 diabetes treated with an SGLT2 inhibitor had roughly a third fewer episodes of acute kidney injury (AKI) compared with matched people with type 2 diabetes treated with a DPP4 inhibitor, in an analysis of health insurance data from more than 100,000 Taiwan residents during 2016-2018.
The findings add to, and expand on, prior evidence that treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class cuts the incidence of AKI, say the authors of the report, which was recently published in JAMA Network Open.
The long-term risk for AKI among people with type 2 diabetes treated with an SGLT2 inhibitor “appears to be quite low” compared with adults who received an agent from the dipeptidyl peptidase 4 (DPP4) inhibitor class.
Treatment with an SGLT2 inhibitor – such as canagliflozin (Invokana), dapagliflozin (Farxiga), or empagliflozin (Jardiance) – causes a transient drop in kidney function that manifests as a temporary dip in estimated glomerular filtration rate, which caused concerns about AKI when the drugs were first introduced.
Indeed, canagliflozin and dapagliflozin had warnings strengthened 7 years ago by the Food and Drug Administration in a Drug Safety Communication for accumulating reports of AKI linked to their use.
More recent experience has calmed AKI concerns, however.
Commenting on the new study, F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn., said: “It’s a nice piece of data to demonstrate that the long-term risk from SGLT2 inhibitor treatment is low.” Dr. Wilson was not involved with the new study.
The Taiwan study found a cumulative incidence of AKI events during about 2.5 years of follow-up of 5.55 events/1,000 patient-years among adults with type 2 diabetes receiving an SGLT2 inhibitor and 7.88 events/1,000 patient-years among those taking a DPP4 inhibitor such as sitagliptin (Januvia).
Main barrier to SGLT2 inhibitor use is unfamiliarity, not AKI risk
“My impression is that the main barrier to wider use of the SGLT2 inhibitor class is not a perceived risk for causing AKI, but rather ongoing unfamiliarity with the class,” Dr. Wilson said in an interview.
Although he sees “relatively broad comfort with and enthusiasm for the class among nephrologists and cardiologists,” routine prescribing does not seem to have caught on nearly as much among primary care physicians, he said.
Clinicians in primary care “still perceive the SGLT2 inhibitor class as something of a ‘specialty drug,’ and they defer initiating it on that basis,” Dr. Wilson observed. “That’s probably not a good thing,” as many people with type 2 diabetes do not have access to a specialized clinician who might be more amenable to prescribing an SGLT2 inhibitor.
One example of the lag in SGLT2 inhibitor uptake for people with type 2 diabetes in practice was a recent report from the Centers for Disease Control and Prevention published in Annals of Internal Medicine. Researchers identified a representative U.S. sample of 1,330 adults with type 2 diabetes studied in depth during 2017-2020, of whom 82% fulfilled criteria published in 2022 for receiving treatment with an SGLT2 inhibitor. Despite this high prevalence of medical appropriateness, a scant 5.3% of those with a recommended indication actually received an agent from this class.
Early AKI concern has diminished
Results from more recent studies, such as a 2019 meta-analysis of more than 100 randomized studies and four large observational studies that together included about 180,000 people receiving SGLT2 inhibitor treatment, showed the opposite of SGLT2 inhibitor treatment triggering AKI.
In the trials, people taking an SGLT2 inhibitor had a relative 25% lower rate of AKI events, while in the observational studies, SGLT2 inhibitor treatment was linked with a 60% relative reduction in AKI. The study also found that SGLT2 inhibitor use in the trials was linked with a significant 20% relative increase in the incidence of low fluid volume.
Despite accumulated evidence exonerating AKI risk, U.S. labels for canagliflozin, dapagliflozin, and empagliflozin continue to cite AKI as a potential adverse reaction, especially in patients who undergo volume depletion while on SGLT2 inhibitor treatment.
The new Taiwan study used data from the country’s National Health Insurance Research Database. Out of more than 250,000 adults with type 2 diabetes in the system from May 2016 to December 2018, the researchers identified 52,231 propensity-score matched pairs of people where one was on treatment with an SGLT2 inhibitor and the other with a DPP4 inhibitor.
During follow-up, 856 of these people (0.8%) had an AKI event, including 102 people with AKI that required dialysis.
A logistic regression analysis that adjusted for 16 potential confounders showed that SGLT2 inhibitor treatment linked with a significant 34% reduction in AKI events compared with DPP4 inhibitor treatment, as well as with a significant 44% relative risk reduction in the incidence of AKI events requiring dialysis, reported the authors from several medical institutions in Taiwan.
The study’s main limitation was its reliance on “quite insensitive” administrative coding data to identify AKI cases, said Dr. Wilson.
He noted that although concern about AKI events secondary to SGLT2 inhibitor treatment is uncommon among U.S. clinicians they do worry about the potential risk for fungal infections, urinary tract infection, or gangrene in people with diabetes who receive an agent from this class.
The study received no commercial funding, and none of the authors had disclosures. Dr. Wilson has reported receiving research funding from AstraZeneca, Boehringer Ingelheim, Vifor, and Whoop.
A version of this article originally appeared on Medscape.com.
Adults with type 2 diabetes treated with an SGLT2 inhibitor had roughly a third fewer episodes of acute kidney injury (AKI) compared with matched people with type 2 diabetes treated with a DPP4 inhibitor, in an analysis of health insurance data from more than 100,000 Taiwan residents during 2016-2018.
The findings add to, and expand on, prior evidence that treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class cuts the incidence of AKI, say the authors of the report, which was recently published in JAMA Network Open.
The long-term risk for AKI among people with type 2 diabetes treated with an SGLT2 inhibitor “appears to be quite low” compared with adults who received an agent from the dipeptidyl peptidase 4 (DPP4) inhibitor class.
Treatment with an SGLT2 inhibitor – such as canagliflozin (Invokana), dapagliflozin (Farxiga), or empagliflozin (Jardiance) – causes a transient drop in kidney function that manifests as a temporary dip in estimated glomerular filtration rate, which caused concerns about AKI when the drugs were first introduced.
Indeed, canagliflozin and dapagliflozin had warnings strengthened 7 years ago by the Food and Drug Administration in a Drug Safety Communication for accumulating reports of AKI linked to their use.
More recent experience has calmed AKI concerns, however.
Commenting on the new study, F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn., said: “It’s a nice piece of data to demonstrate that the long-term risk from SGLT2 inhibitor treatment is low.” Dr. Wilson was not involved with the new study.
The Taiwan study found a cumulative incidence of AKI events during about 2.5 years of follow-up of 5.55 events/1,000 patient-years among adults with type 2 diabetes receiving an SGLT2 inhibitor and 7.88 events/1,000 patient-years among those taking a DPP4 inhibitor such as sitagliptin (Januvia).
Main barrier to SGLT2 inhibitor use is unfamiliarity, not AKI risk
“My impression is that the main barrier to wider use of the SGLT2 inhibitor class is not a perceived risk for causing AKI, but rather ongoing unfamiliarity with the class,” Dr. Wilson said in an interview.
Although he sees “relatively broad comfort with and enthusiasm for the class among nephrologists and cardiologists,” routine prescribing does not seem to have caught on nearly as much among primary care physicians, he said.
Clinicians in primary care “still perceive the SGLT2 inhibitor class as something of a ‘specialty drug,’ and they defer initiating it on that basis,” Dr. Wilson observed. “That’s probably not a good thing,” as many people with type 2 diabetes do not have access to a specialized clinician who might be more amenable to prescribing an SGLT2 inhibitor.
One example of the lag in SGLT2 inhibitor uptake for people with type 2 diabetes in practice was a recent report from the Centers for Disease Control and Prevention published in Annals of Internal Medicine. Researchers identified a representative U.S. sample of 1,330 adults with type 2 diabetes studied in depth during 2017-2020, of whom 82% fulfilled criteria published in 2022 for receiving treatment with an SGLT2 inhibitor. Despite this high prevalence of medical appropriateness, a scant 5.3% of those with a recommended indication actually received an agent from this class.
Early AKI concern has diminished
Results from more recent studies, such as a 2019 meta-analysis of more than 100 randomized studies and four large observational studies that together included about 180,000 people receiving SGLT2 inhibitor treatment, showed the opposite of SGLT2 inhibitor treatment triggering AKI.
In the trials, people taking an SGLT2 inhibitor had a relative 25% lower rate of AKI events, while in the observational studies, SGLT2 inhibitor treatment was linked with a 60% relative reduction in AKI. The study also found that SGLT2 inhibitor use in the trials was linked with a significant 20% relative increase in the incidence of low fluid volume.
Despite accumulated evidence exonerating AKI risk, U.S. labels for canagliflozin, dapagliflozin, and empagliflozin continue to cite AKI as a potential adverse reaction, especially in patients who undergo volume depletion while on SGLT2 inhibitor treatment.
The new Taiwan study used data from the country’s National Health Insurance Research Database. Out of more than 250,000 adults with type 2 diabetes in the system from May 2016 to December 2018, the researchers identified 52,231 propensity-score matched pairs of people where one was on treatment with an SGLT2 inhibitor and the other with a DPP4 inhibitor.
During follow-up, 856 of these people (0.8%) had an AKI event, including 102 people with AKI that required dialysis.
A logistic regression analysis that adjusted for 16 potential confounders showed that SGLT2 inhibitor treatment linked with a significant 34% reduction in AKI events compared with DPP4 inhibitor treatment, as well as with a significant 44% relative risk reduction in the incidence of AKI events requiring dialysis, reported the authors from several medical institutions in Taiwan.
The study’s main limitation was its reliance on “quite insensitive” administrative coding data to identify AKI cases, said Dr. Wilson.
He noted that although concern about AKI events secondary to SGLT2 inhibitor treatment is uncommon among U.S. clinicians they do worry about the potential risk for fungal infections, urinary tract infection, or gangrene in people with diabetes who receive an agent from this class.
The study received no commercial funding, and none of the authors had disclosures. Dr. Wilson has reported receiving research funding from AstraZeneca, Boehringer Ingelheim, Vifor, and Whoop.
A version of this article originally appeared on Medscape.com.
Tobramycin inhaled solution and quality of life in patients with bronchiectasis
Airway Disorders Network
Bronchiectasis Section
Bronchiectasis is a condition of dilated, inflamed airways and mucous production caused by a myriad of diseases. Bronchiectasis entails chronic productive cough and an increased risk of infections leading to exacerbations. Chronic bacterial infections are often a hallmark of severe disease, especially with Pseudomonas aeruginosa (O’Donnell AE. N Engl J Med. 2022;387[6]:533). Prophylactic inhaled antibiotics have been used as off-label therapies with mixed evidence, particularly in non-cystic fibrosis bronchiectasis (Rubin BK, et al. Respiration. 2014;88[3]:177).
In a recent publication, Guan and colleagues evaluated the efficacy and safety of tobramycin inhaled solution (TIS) for bronchiectasis with chronic P. aeruginosa in a phase 3, 16-week, multicenter, double-blind randomized, controlled trial (Guan W-J, et al. Chest. 2023;163[1]:64). A regimen of twice-daily TIS, compared with nebulized normal saline, demonstrated a more significant reduction in P. aeruginosa sputum density after two cycles of 28 days on-treatment and 28 days off-treatment (adjusted mean difference, 1.74 log10 colony-forming units/g; 95% CI, 1.12-2.35; (P < .001), and more patients became culture-negative for P. aeruginosa in the TIS group than in the placebo group on day 29 (29.3% vs 10.6%). Adverse events were similar in both groups. Importantly, there was an improvement in quality-of-life bronchiectasis respiratory symptom score by 7.91 points at day 29 and 6.72 points at day 85; all three were statistically significant but just below the minimal clinically important difference of 8 points.
Dr. Conroy Wong and Dr. Miguel Angel Martinez-Garcia (Chest. 2023 Jan;163[1]:3) highlighted in their accompanying editorial that use of health-related quality of life score was a “distinguishing feature” of the trial as “most studies have used the change in microbial density as the primary outcome measure alone.”
Future studies evaluating cyclical vs continuous antibiotic administration, treatment duration, and impact on exacerbations continue to be needed.
Alicia Mirza, MD
Section Member-at-Large
Airway Disorders Network
Bronchiectasis Section
Bronchiectasis is a condition of dilated, inflamed airways and mucous production caused by a myriad of diseases. Bronchiectasis entails chronic productive cough and an increased risk of infections leading to exacerbations. Chronic bacterial infections are often a hallmark of severe disease, especially with Pseudomonas aeruginosa (O’Donnell AE. N Engl J Med. 2022;387[6]:533). Prophylactic inhaled antibiotics have been used as off-label therapies with mixed evidence, particularly in non-cystic fibrosis bronchiectasis (Rubin BK, et al. Respiration. 2014;88[3]:177).
In a recent publication, Guan and colleagues evaluated the efficacy and safety of tobramycin inhaled solution (TIS) for bronchiectasis with chronic P. aeruginosa in a phase 3, 16-week, multicenter, double-blind randomized, controlled trial (Guan W-J, et al. Chest. 2023;163[1]:64). A regimen of twice-daily TIS, compared with nebulized normal saline, demonstrated a more significant reduction in P. aeruginosa sputum density after two cycles of 28 days on-treatment and 28 days off-treatment (adjusted mean difference, 1.74 log10 colony-forming units/g; 95% CI, 1.12-2.35; (P < .001), and more patients became culture-negative for P. aeruginosa in the TIS group than in the placebo group on day 29 (29.3% vs 10.6%). Adverse events were similar in both groups. Importantly, there was an improvement in quality-of-life bronchiectasis respiratory symptom score by 7.91 points at day 29 and 6.72 points at day 85; all three were statistically significant but just below the minimal clinically important difference of 8 points.
Dr. Conroy Wong and Dr. Miguel Angel Martinez-Garcia (Chest. 2023 Jan;163[1]:3) highlighted in their accompanying editorial that use of health-related quality of life score was a “distinguishing feature” of the trial as “most studies have used the change in microbial density as the primary outcome measure alone.”
Future studies evaluating cyclical vs continuous antibiotic administration, treatment duration, and impact on exacerbations continue to be needed.
Alicia Mirza, MD
Section Member-at-Large
Airway Disorders Network
Bronchiectasis Section
Bronchiectasis is a condition of dilated, inflamed airways and mucous production caused by a myriad of diseases. Bronchiectasis entails chronic productive cough and an increased risk of infections leading to exacerbations. Chronic bacterial infections are often a hallmark of severe disease, especially with Pseudomonas aeruginosa (O’Donnell AE. N Engl J Med. 2022;387[6]:533). Prophylactic inhaled antibiotics have been used as off-label therapies with mixed evidence, particularly in non-cystic fibrosis bronchiectasis (Rubin BK, et al. Respiration. 2014;88[3]:177).
In a recent publication, Guan and colleagues evaluated the efficacy and safety of tobramycin inhaled solution (TIS) for bronchiectasis with chronic P. aeruginosa in a phase 3, 16-week, multicenter, double-blind randomized, controlled trial (Guan W-J, et al. Chest. 2023;163[1]:64). A regimen of twice-daily TIS, compared with nebulized normal saline, demonstrated a more significant reduction in P. aeruginosa sputum density after two cycles of 28 days on-treatment and 28 days off-treatment (adjusted mean difference, 1.74 log10 colony-forming units/g; 95% CI, 1.12-2.35; (P < .001), and more patients became culture-negative for P. aeruginosa in the TIS group than in the placebo group on day 29 (29.3% vs 10.6%). Adverse events were similar in both groups. Importantly, there was an improvement in quality-of-life bronchiectasis respiratory symptom score by 7.91 points at day 29 and 6.72 points at day 85; all three were statistically significant but just below the minimal clinically important difference of 8 points.
Dr. Conroy Wong and Dr. Miguel Angel Martinez-Garcia (Chest. 2023 Jan;163[1]:3) highlighted in their accompanying editorial that use of health-related quality of life score was a “distinguishing feature” of the trial as “most studies have used the change in microbial density as the primary outcome measure alone.”
Future studies evaluating cyclical vs continuous antibiotic administration, treatment duration, and impact on exacerbations continue to be needed.
Alicia Mirza, MD
Section Member-at-Large
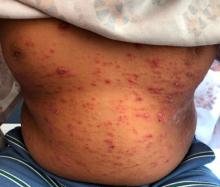
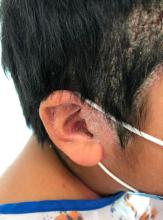
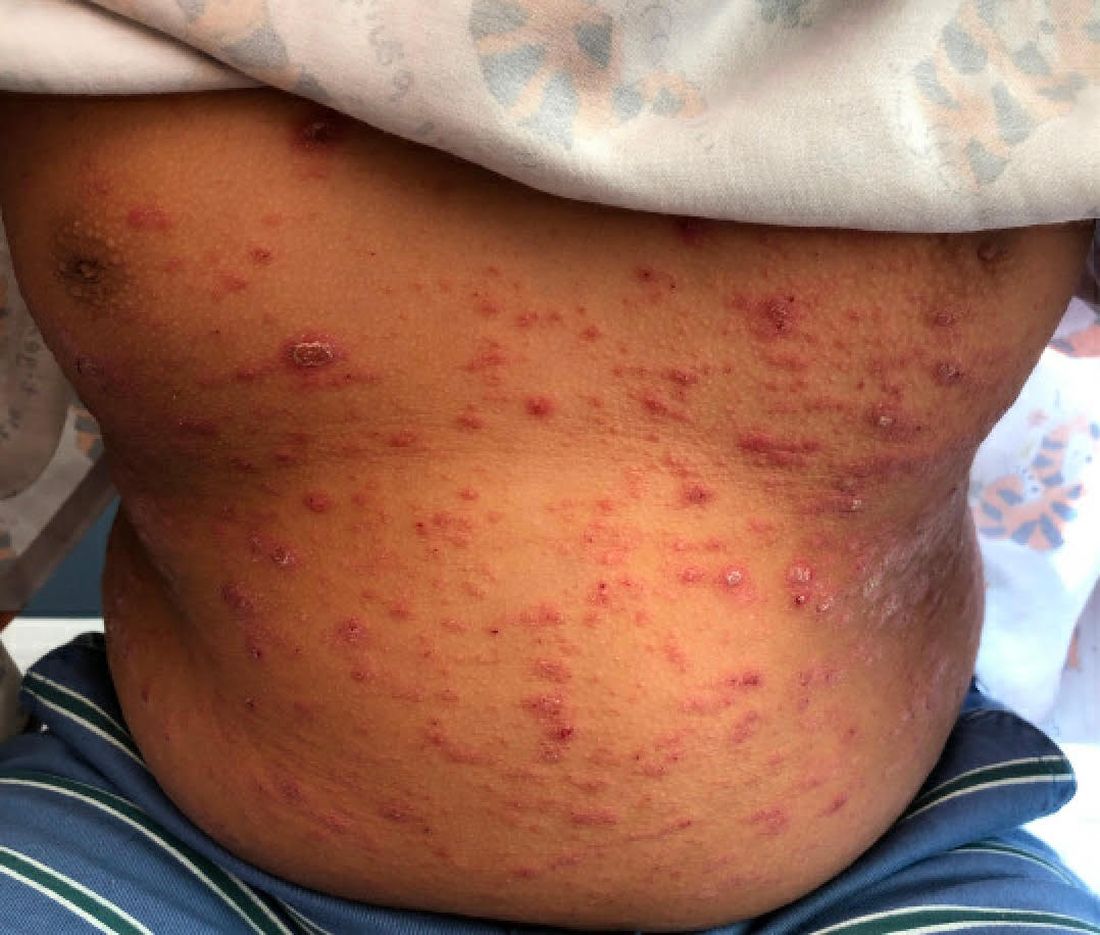
A 9-year-old male presents with multiple thick scaly plaques on scalp, ears, and trunk
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
A 9-year-old male is seen in the clinic with a 1-year history of multiple thick scaly plaques on scalp, ears, and trunk. He has been treated with hydrocortisone 1% ointment with no change in the lesions. He had upper respiratory tract symptoms 3 weeks prior to the visit.
Examination reveals erythematous, well-demarcated plaques of the anterior scalp with thick overlying micaceous scale with some extension onto the forehead and temples. Additionally, erythematous scaly patches on the ear, axilla, and umbilicus were noted. There was no palmar or plantar involvement. He denied joint swelling, stiffness, or pain in the morning.
Wearable fluid sensor lowers risk of HF rehospitalizations: BMAD
NEW ORLEANS – A wearable device that monitors thoracic fluid and can signal elevated levels can improve outcomes after heart failure hospitalization, according to a comparative but nonrandomized trial.
In this study, management adjustments made in response to a threshold alert from the device led to several improvements in outcome at 90 days, including a significant 38% reduction in the primary outcome of rehospitalization, relative to controls (P = .02), reported John P. Boehmer, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The same relative risk reduction at 90 days was observed for a composite outcome of time to first hospitalization, visit to an emergency room, or death (hazard ratio, 0.62; P = .03).
Quality of life, as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), improved steadily in both the experimental and control arm over the 90-day study, but the curves separated at about 30 days, Dr. Boehmer reported. By the end of the study, the mean KCCQ difference was 12 points favoring the experimental arm on a scale in which 5 points is considered clinically meaningful.
70% report improved quality of life
“Responder analysis revealed that nearly 70% of patients in the arm managed with the monitor reported a clinically meaningful improvement in quality of life, compared to 50% of patients in the control arm,” said Dr. Boehmer, professor of medicine and surgery at Penn State Health, Hershey.
Fluid overload is an indication of worsening disease and a frequent cause of heart failure hospitalization. The Zoll Heart Failure Monitoring System (HFMS) that was tested in this study already has regulatory approval. It is equipped to monitor several biomarkers, including heart rate and respiration rate, but its ability to measure lung fluid through low electromagnetic radiofrequency pulses was the function of interest for this study.
In this nonrandomized study, called Benefits of Microcor in Ambulatory
Decompensated Heart Failure (BMAD), a control arm was enrolled first. By monitoring the initial patients enrolled in the control arm, the investigators established a threshold of thoracic fluid that would be used to trigger an alert in the intervention arm. This ultimately was defined as 3 standard deviations from the population mean.
Patients were eligible for this study if they were discharged from a hospital with heart failure in the previous 10 days. Of exclusion criteria, a short life expectancy (< 1 year) and a wearable cardiac defibrillator were notable. Left ventricular ejection fraction (LVEF) was not considered for inclusion or exclusion.
All subjects participated in weekly phone calls and monthly office visits. However, both investigators and patients were blinded to the device data in the control arm. Conversely, subjects and investigators in the intervention arm were able to access data generated by the device through a secure website.
Of the 245 eligible patients in the control arm, 168 were available for evaluation at 90 days. Among the 249 eligible patients in the intervention arm, 176 were included in the 90-day evaluation. Of those who were not available, the most common reason was study withdrawal. About 20% died before the 90-day evaluation.
The majority of patients in both arms were in class III or IV heart failure. About half had LVEF less than 40%, and more than 40% of patients in each group had chronic kidney disease (CKD). Roughly 55% of patients were at least 65 years of age.
At 90 days, the absolute risk reduction in rehospitalization was 7%, producing a number to treat with the device of 14.3 to prevent one rehospitalization. In a subgroup stratification, the benefit was similar by age, sex, presence or absence of CKD, LVEF greater or lower than 40%, Black or non-Black race, and ischemic or nonischemic etiology.
Patient access to data considered a plus
If lack of randomization is a weakness of this study, the decision to unblind the data for both investigators and patients might not be, according to Lynne Stevenson, MD, director of the cardiomyopathy program, Vanderbilt University Medical Center, Nashville, Tenn.
“You might be criticized for this [allowing patients to monitor their data], but I actually think this is a strength of the study,” said Dr. Stevenson, who believes the growing trend to involve heart failure patients in self-management has been a positive direction in clinical care.
She indicated that, despite the potential bias derived from being aware of fluid fluctuations, this information might also be contributing to patient motivation for adherence and appropriate lifestyle modifications.
Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, made a similar point but for a different reason. She expressed concern about the work that monitoring the wearable device creates for clinicians. Despite the positive data generated by this study, Dr. Bozkurt said the device as used in the study demanded “a lot of clinical time and effort” when these are both in short supply.
While she called for a larger and randomized study to corroborate the results of this investigation, she also thinks that it would make sense to compare the clinical value of this device against alternative methods for monitoring heart failure, including other wearable devices. Dr. Bozkurt asserted that some of the most helpful devices from a clinical perspective might be those that patients monitor themselves.
“Hopefully in the future, we will be offering tools that provide patients information they can use without the immediate need of a clinician,” she said.
Dr. Boehmer reports financial relationships with Abbott, Boston Scientific, Medtronic, and Zoll Medical Corporation, which provided the funding for this study. Dr. Stevenson reports no potential conflicts of interest. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
NEW ORLEANS – A wearable device that monitors thoracic fluid and can signal elevated levels can improve outcomes after heart failure hospitalization, according to a comparative but nonrandomized trial.
In this study, management adjustments made in response to a threshold alert from the device led to several improvements in outcome at 90 days, including a significant 38% reduction in the primary outcome of rehospitalization, relative to controls (P = .02), reported John P. Boehmer, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The same relative risk reduction at 90 days was observed for a composite outcome of time to first hospitalization, visit to an emergency room, or death (hazard ratio, 0.62; P = .03).
Quality of life, as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), improved steadily in both the experimental and control arm over the 90-day study, but the curves separated at about 30 days, Dr. Boehmer reported. By the end of the study, the mean KCCQ difference was 12 points favoring the experimental arm on a scale in which 5 points is considered clinically meaningful.
70% report improved quality of life
“Responder analysis revealed that nearly 70% of patients in the arm managed with the monitor reported a clinically meaningful improvement in quality of life, compared to 50% of patients in the control arm,” said Dr. Boehmer, professor of medicine and surgery at Penn State Health, Hershey.
Fluid overload is an indication of worsening disease and a frequent cause of heart failure hospitalization. The Zoll Heart Failure Monitoring System (HFMS) that was tested in this study already has regulatory approval. It is equipped to monitor several biomarkers, including heart rate and respiration rate, but its ability to measure lung fluid through low electromagnetic radiofrequency pulses was the function of interest for this study.
In this nonrandomized study, called Benefits of Microcor in Ambulatory
Decompensated Heart Failure (BMAD), a control arm was enrolled first. By monitoring the initial patients enrolled in the control arm, the investigators established a threshold of thoracic fluid that would be used to trigger an alert in the intervention arm. This ultimately was defined as 3 standard deviations from the population mean.
Patients were eligible for this study if they were discharged from a hospital with heart failure in the previous 10 days. Of exclusion criteria, a short life expectancy (< 1 year) and a wearable cardiac defibrillator were notable. Left ventricular ejection fraction (LVEF) was not considered for inclusion or exclusion.
All subjects participated in weekly phone calls and monthly office visits. However, both investigators and patients were blinded to the device data in the control arm. Conversely, subjects and investigators in the intervention arm were able to access data generated by the device through a secure website.
Of the 245 eligible patients in the control arm, 168 were available for evaluation at 90 days. Among the 249 eligible patients in the intervention arm, 176 were included in the 90-day evaluation. Of those who were not available, the most common reason was study withdrawal. About 20% died before the 90-day evaluation.
The majority of patients in both arms were in class III or IV heart failure. About half had LVEF less than 40%, and more than 40% of patients in each group had chronic kidney disease (CKD). Roughly 55% of patients were at least 65 years of age.
At 90 days, the absolute risk reduction in rehospitalization was 7%, producing a number to treat with the device of 14.3 to prevent one rehospitalization. In a subgroup stratification, the benefit was similar by age, sex, presence or absence of CKD, LVEF greater or lower than 40%, Black or non-Black race, and ischemic or nonischemic etiology.
Patient access to data considered a plus
If lack of randomization is a weakness of this study, the decision to unblind the data for both investigators and patients might not be, according to Lynne Stevenson, MD, director of the cardiomyopathy program, Vanderbilt University Medical Center, Nashville, Tenn.
“You might be criticized for this [allowing patients to monitor their data], but I actually think this is a strength of the study,” said Dr. Stevenson, who believes the growing trend to involve heart failure patients in self-management has been a positive direction in clinical care.
She indicated that, despite the potential bias derived from being aware of fluid fluctuations, this information might also be contributing to patient motivation for adherence and appropriate lifestyle modifications.
Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, made a similar point but for a different reason. She expressed concern about the work that monitoring the wearable device creates for clinicians. Despite the positive data generated by this study, Dr. Bozkurt said the device as used in the study demanded “a lot of clinical time and effort” when these are both in short supply.
While she called for a larger and randomized study to corroborate the results of this investigation, she also thinks that it would make sense to compare the clinical value of this device against alternative methods for monitoring heart failure, including other wearable devices. Dr. Bozkurt asserted that some of the most helpful devices from a clinical perspective might be those that patients monitor themselves.
“Hopefully in the future, we will be offering tools that provide patients information they can use without the immediate need of a clinician,” she said.
Dr. Boehmer reports financial relationships with Abbott, Boston Scientific, Medtronic, and Zoll Medical Corporation, which provided the funding for this study. Dr. Stevenson reports no potential conflicts of interest. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
NEW ORLEANS – A wearable device that monitors thoracic fluid and can signal elevated levels can improve outcomes after heart failure hospitalization, according to a comparative but nonrandomized trial.
In this study, management adjustments made in response to a threshold alert from the device led to several improvements in outcome at 90 days, including a significant 38% reduction in the primary outcome of rehospitalization, relative to controls (P = .02), reported John P. Boehmer, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The same relative risk reduction at 90 days was observed for a composite outcome of time to first hospitalization, visit to an emergency room, or death (hazard ratio, 0.62; P = .03).
Quality of life, as measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), improved steadily in both the experimental and control arm over the 90-day study, but the curves separated at about 30 days, Dr. Boehmer reported. By the end of the study, the mean KCCQ difference was 12 points favoring the experimental arm on a scale in which 5 points is considered clinically meaningful.
70% report improved quality of life
“Responder analysis revealed that nearly 70% of patients in the arm managed with the monitor reported a clinically meaningful improvement in quality of life, compared to 50% of patients in the control arm,” said Dr. Boehmer, professor of medicine and surgery at Penn State Health, Hershey.
Fluid overload is an indication of worsening disease and a frequent cause of heart failure hospitalization. The Zoll Heart Failure Monitoring System (HFMS) that was tested in this study already has regulatory approval. It is equipped to monitor several biomarkers, including heart rate and respiration rate, but its ability to measure lung fluid through low electromagnetic radiofrequency pulses was the function of interest for this study.
In this nonrandomized study, called Benefits of Microcor in Ambulatory
Decompensated Heart Failure (BMAD), a control arm was enrolled first. By monitoring the initial patients enrolled in the control arm, the investigators established a threshold of thoracic fluid that would be used to trigger an alert in the intervention arm. This ultimately was defined as 3 standard deviations from the population mean.
Patients were eligible for this study if they were discharged from a hospital with heart failure in the previous 10 days. Of exclusion criteria, a short life expectancy (< 1 year) and a wearable cardiac defibrillator were notable. Left ventricular ejection fraction (LVEF) was not considered for inclusion or exclusion.
All subjects participated in weekly phone calls and monthly office visits. However, both investigators and patients were blinded to the device data in the control arm. Conversely, subjects and investigators in the intervention arm were able to access data generated by the device through a secure website.
Of the 245 eligible patients in the control arm, 168 were available for evaluation at 90 days. Among the 249 eligible patients in the intervention arm, 176 were included in the 90-day evaluation. Of those who were not available, the most common reason was study withdrawal. About 20% died before the 90-day evaluation.
The majority of patients in both arms were in class III or IV heart failure. About half had LVEF less than 40%, and more than 40% of patients in each group had chronic kidney disease (CKD). Roughly 55% of patients were at least 65 years of age.
At 90 days, the absolute risk reduction in rehospitalization was 7%, producing a number to treat with the device of 14.3 to prevent one rehospitalization. In a subgroup stratification, the benefit was similar by age, sex, presence or absence of CKD, LVEF greater or lower than 40%, Black or non-Black race, and ischemic or nonischemic etiology.
Patient access to data considered a plus
If lack of randomization is a weakness of this study, the decision to unblind the data for both investigators and patients might not be, according to Lynne Stevenson, MD, director of the cardiomyopathy program, Vanderbilt University Medical Center, Nashville, Tenn.
“You might be criticized for this [allowing patients to monitor their data], but I actually think this is a strength of the study,” said Dr. Stevenson, who believes the growing trend to involve heart failure patients in self-management has been a positive direction in clinical care.
She indicated that, despite the potential bias derived from being aware of fluid fluctuations, this information might also be contributing to patient motivation for adherence and appropriate lifestyle modifications.
Biykem Bozkurt, MD, PhD, chair of cardiology at Baylor College of Medicine, Houston, made a similar point but for a different reason. She expressed concern about the work that monitoring the wearable device creates for clinicians. Despite the positive data generated by this study, Dr. Bozkurt said the device as used in the study demanded “a lot of clinical time and effort” when these are both in short supply.
While she called for a larger and randomized study to corroborate the results of this investigation, she also thinks that it would make sense to compare the clinical value of this device against alternative methods for monitoring heart failure, including other wearable devices. Dr. Bozkurt asserted that some of the most helpful devices from a clinical perspective might be those that patients monitor themselves.
“Hopefully in the future, we will be offering tools that provide patients information they can use without the immediate need of a clinician,” she said.
Dr. Boehmer reports financial relationships with Abbott, Boston Scientific, Medtronic, and Zoll Medical Corporation, which provided the funding for this study. Dr. Stevenson reports no potential conflicts of interest. Dr. Bozkurt reports financial relationships with Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Cardurion, LivaNova, Relypsa, Renovacor, Sanofi-Aventis, and Vifor.
AT ACC 2023
FREEDOM COVID: Full-dose anticoagulation cut mortality but missed primary endpoint
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
Study conducted in noncritically ill
Study conducted in noncritically ill
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
NEW ORLEANS – In the international FREEDOM COVID trial that randomized non–critically ill hospitalized patients, a therapeutic dose of anticoagulation relative to a prophylactic dose significantly reduced death from COVID-19 at 30 days, even as a larger composite primary endpoint was missed.
The mortality reduction suggests therapeutic-dose anticoagulation “may improve outcomes in non–critically ill patients hospitalized with COVID-19 who are at increased risk for adverse events but do not yet require ICU-level of care,” reported Valentin Fuster, MD, PhD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
These data provide a suggestion rather than a demonstration of benefit because the primary composite endpoint of all-cause mortality, intubation requiring mechanical ventilation, systemic thromboembolism or ischemic stroke at 30 days was not met. Although this 30-day outcome was lower on the therapeutic dose (11.3% vs. 13.2%), the difference was only a trend (hazard ratio, 0.85; P = .11), said Dr. Fuster, physician-in-chief, Mount Sinai Hospital, New York.
Missed primary endpoint blamed on low events
The declining severity of more recent COVID-19 variants (the trial was conducted from August 2022 to September 2022) might be one explanation that the primary endpoint was not met, but the more likely explanation is the relatively good health status – and therefore a low risk of events – among patients randomized in India, 1 of 10 participating countries.
India accounted for roughly 40% of the total number of 3,398 patients in the intention-to-treat population. In India, the rates of events were 0.7 and 1.3 in the prophylactic and therapeutic anticoagulation arms, respectively. In contrast, they were 17.5 and 9.5, respectively in the United States. In combined data from the other eight countries, the rates were 22.78 and 20.4, respectively.
“These results emphasize that varying country-specific thresholds for hospitalization may affect patient prognosis and the potential utility of advanced therapies” Dr. Fuster said.
In fact, the therapeutic anticoagulation was linked to a nonsignificant twofold increase in the risk of the primary outcome in India (HR, 2.01; 95% confidence interval, 0.57-7.13) when outcomes were stratified by country. In the United States, where there was a much higher incidence of events, therapeutic anticoagulation was associated with a nearly 50% reduction (HR, 0.53; 95% CI, 0.31-0.91).
In the remaining countries, which included those in Latin America and Europe as well as the city of Hong Kong, the primary outcome was reduced numerically but not statistically by therapeutic relative to prophylactic anticoagulation (HR, 0.89; 95% CI, 0.71-1.11).
Enoxaparin and apixaban are studied
In FREEDOM COVID, patients were randomized to a therapeutic dose of the low-molecular-weight heparin (LMWH) enoxaparin (1 mg/kg every 12 hours), a prophylactic dose of enoxaparin (40 mg once daily), or a therapeutic dose of the direct factor Xa inhibitor apixaban (5 mg every 12 hours). Lower doses of enoxaparin and apixaban were used for those with renal impairment, and lower doses of apixaban were employed for elderly patients (≥ 80 years) and those with low body weight (≤ 60 kg).
The major inclusion criteria were confirmed COVID-19 infection with symptomatic systemic involvement. The major exclusion criteria were need for ICU level of care or active bleeding.
The therapeutic anticoagulation arms performed similarly and were combined for comparison to the prophylactic arm. Despite the failure to show a difference in the primary outcome, the rate of 30-day mortality was substantially lower in the therapeutic arm (4.9% vs. 7.0%), translating into a 30% risk reduction (HR, 0.70; P = .01).
Therapeutic anticoagulation was also associated with a lower rate of intubation/mechanical ventilation (6.4% vs. 8.4%) that reached statistical significance (HR, 0.75; P = .03). The risk reduction was also significant for a combination of these endpoints (HR, 0.77; P = .03).
The lower proportion of patients who eventually required ICU-level of care (9.9% vs. 11.7%) showed a trend in favor of therapeutic anticoagulation (HR, 0.84; P = .11).
Bleeding rates did not differ between arms
Bleeding Academic Research Consortium major bleeding types 3 and 5 were slightly numerically higher in the group randomized to therapeutic enoxaparin (0.5%) than prophylactic enoxaparin (0.1%) and therapeutic apixaban (0.3%), but the differences between any groups were not significant.
Numerous anticoagulation trials in patients with COVID-19 have been published previously. One 2021 trial published in the New England Journal of Medicine also suggested benefit from a therapeutic relative to prophylactic anticoagulation. In that trial, which compared heparin to usual-care thromboprophylaxis, benefits were derived from a Bayesian analysis. Significant differences were not shown for death or other major outcome assessed individually.
Even though this more recent trial missed its primary endpoint, Gregg Stone, MD, a coauthor of this study and a colleague of Dr. Fuster at the Mount Sinai School of Medicine, New York, reiterated that these results support routine anticoagulation in hospitalized COVID-19 patients.
“These are robust reductions in mortality and intubation rates, which are the most serious outcomes,” said Dr. Stone, who is first author of the paper, which was published in the Journal of the American College of Cardiology immediately after Dr. Fuster’s presentation.
COVID-19 has proven to be a very thrombogenic virus, but the literature has not been wholly consistent on which anticoagulation treatment provides the best balance of benefits and risks, according to Julia Grapsa, MD, PhD, attending cardiologist, Guys and St. Thomas Hospital, London. She said that this randomized trial, despite its failure to meet the primary endpoint, is useful.
“This demonstrates that a therapeutic dose of enoxaparin is likely to improve outcomes over a prophylactic dose with a low risk of bleeding,” Dr. Grapsa said. On the basis of the randomized study, “I feel more confident with this approach.”
Dr. Fuster reported no potential conflicts of interest. Dr. Stone has financial relationships with more than 30 companies that make pharmaceuticals and medical devices. Dr. Grapsa reported no potential conflicts of interest.
AT ACC 2023
Inspector General Finds Security Vulnerabilities and Risks at VA Medical Facilities
In 2022, the US Department of Veterans Affairs (VA) Office of Inspector General (OIG) received 36 separate serious incident reports involving 32 medical facilities—including a bomb threat. In response to those reports, OIG teams of auditors and criminal investigators visited 70 VA medical facilities in September 2022 to assess security and to issue a formal report.
Noting that VA policy includes an “extensive list of security safeguards” for medical facilities to implement, the OIG focused its review on those that “a person with a reasonable level of security knowledge could assess.” According to their report, released in February, they identified a variety of security vulnerabilities and deficiencies, ranging from shortages of police officers to radios with poor signal strength.
For one, the OIG says, the facilities were designed to provide a welcoming environment, which means the public can enter the grounds freely at many different points. But the open-campus design makes it more difficult to balance security with easy and prompt access for patients. Consequently, the OIG says, “threats may originate from many locations within the medical facility campus itself or in the nearby community.”
Walking around the perimeters of the facility buildings, the OIG teams assessed the security level of 2960 public and nonpublic access doors. They found that 87% of public doors did not have an active security presence, and of those, 23% also did not have a security camera. Moreover, 17% of nonpublic doors were unlocked; 97% of those did not have a security presence, and 43% did not have a security camera. Even more concerning: Some of those doors led to sensitive or restricted facility areas. For example, at one midwestern facility, an unlocked nonpublic access door led to the surgical intensive care unit.
The OIG teams also assessed training records for 170 police officers across the 70 facilities and found that nearly all officers were compliant with training requirements. Most respondents also reported they received adequate training to perform their job duties and provided numerous positive indicators. Well trained or not, though, a notable problem in maintaining security, the OIG teams found, was there simply weren’t enough officers.
The OIG has repeatedly issued reports on significant police officer staffing shortages since at least 2018. VA police officers are not only empowered to make arrests, carry firearms while on duty, and investigate criminal activity within VA’s jurisdiction, but also assist individuals on medical campuses “in myriad ways,” the OIG notes. Staffing shortages are likely to compromise overall facility security, morale, and staff retention and underscore the need for maintaining communication with local law enforcement agencies for assistance, the OIG report points out.
In the OIG surveys, security personnel often noted they were understaffed. Although VA guidance calls for at least 2 VA police officers on duty at all times, 21% of respondents said they were aware of a duty shift during which minimum police staffing requirements were not met. About 37% of respondents expressed concerns about the physical security at their facilities. Some pointed out that the lack of VA police on duty could make it difficult to respond to threats like an active shooter.
In May 2022, the VA issued a directive that established minimum police coverage at medical facilities, as well as a police officer staffing decision tool to help determine appropriate officer levels. It required facilities to have an active security presence in their emergency departments around-the-clock by May 2023. As of September 2022, 58% of the facilities’ emergency departments did not yet have a visible security presence.
The teams also found issues with security devices: 19% of all cameras were not functional; at 24 facilities, more than 20% of the cameras were not working. A few facilities had “highly functional” systems that allowed personnel to monitor the campus thoroughly and even search for specific individuals—but they weren’t always operable.
At one western facility, it came down to a problem that would be frustrating at any time, but alarming for security management: Security personnel could not access the monitoring system because the required security certificates had expired, and no one knew the administrative password. If the system went offline, the OIG team was told, no one could fix the problem without password access. Neither VA’s Office of Information and Technology nor the contractor for the facility’s cameras could override the administrative password.
On the bright side, the OIG teams found that camera video feeds were being actively surveilled by security personnel at 60 of 70 facilities. All but 1 site kept camera footage for an average of 2 weeks or more.
VA policy states that, in addition to at least 2 intermediate weapons (such as batons and pepper spray) uniformed officers must always be issued radios for use while on duty. Survey respondents generally indicated they received their equipment and that it was adequate, but 15% said theirs lacked functionality, such as battery life and signal strength.
Based on the teams’ findings, the OIG made 6 recommendations: (1) delegating a responsible official to monitor and report monthly on facilities’ security-related vacancies; (2) authorizing sufficient staff to inspect VA police forces per the OIG’s 2018 unimplemented recommendation; (3) ensuring medical facility directors appropriately assess VA police staffing needs, authorize associated positions, and leverage available mechanisms to fill vacancies; (4) committing sufficient resources to ensure that facility security measures are adequate, current, and operational; (5) directing Veterans Integrated Services Network police chiefs, in coordination with medical facility directors, facility police chiefs, and facility emergency management leaders, to present a plan to remedy identified security weaknesses; and (6) establishing policy that standardizes the review and retention requirements for facility security camera footage.
The VA concurred with all recommendations and submitted corrective action plans.
In 2022, the US Department of Veterans Affairs (VA) Office of Inspector General (OIG) received 36 separate serious incident reports involving 32 medical facilities—including a bomb threat. In response to those reports, OIG teams of auditors and criminal investigators visited 70 VA medical facilities in September 2022 to assess security and to issue a formal report.
Noting that VA policy includes an “extensive list of security safeguards” for medical facilities to implement, the OIG focused its review on those that “a person with a reasonable level of security knowledge could assess.” According to their report, released in February, they identified a variety of security vulnerabilities and deficiencies, ranging from shortages of police officers to radios with poor signal strength.
For one, the OIG says, the facilities were designed to provide a welcoming environment, which means the public can enter the grounds freely at many different points. But the open-campus design makes it more difficult to balance security with easy and prompt access for patients. Consequently, the OIG says, “threats may originate from many locations within the medical facility campus itself or in the nearby community.”
Walking around the perimeters of the facility buildings, the OIG teams assessed the security level of 2960 public and nonpublic access doors. They found that 87% of public doors did not have an active security presence, and of those, 23% also did not have a security camera. Moreover, 17% of nonpublic doors were unlocked; 97% of those did not have a security presence, and 43% did not have a security camera. Even more concerning: Some of those doors led to sensitive or restricted facility areas. For example, at one midwestern facility, an unlocked nonpublic access door led to the surgical intensive care unit.
The OIG teams also assessed training records for 170 police officers across the 70 facilities and found that nearly all officers were compliant with training requirements. Most respondents also reported they received adequate training to perform their job duties and provided numerous positive indicators. Well trained or not, though, a notable problem in maintaining security, the OIG teams found, was there simply weren’t enough officers.
The OIG has repeatedly issued reports on significant police officer staffing shortages since at least 2018. VA police officers are not only empowered to make arrests, carry firearms while on duty, and investigate criminal activity within VA’s jurisdiction, but also assist individuals on medical campuses “in myriad ways,” the OIG notes. Staffing shortages are likely to compromise overall facility security, morale, and staff retention and underscore the need for maintaining communication with local law enforcement agencies for assistance, the OIG report points out.
In the OIG surveys, security personnel often noted they were understaffed. Although VA guidance calls for at least 2 VA police officers on duty at all times, 21% of respondents said they were aware of a duty shift during which minimum police staffing requirements were not met. About 37% of respondents expressed concerns about the physical security at their facilities. Some pointed out that the lack of VA police on duty could make it difficult to respond to threats like an active shooter.
In May 2022, the VA issued a directive that established minimum police coverage at medical facilities, as well as a police officer staffing decision tool to help determine appropriate officer levels. It required facilities to have an active security presence in their emergency departments around-the-clock by May 2023. As of September 2022, 58% of the facilities’ emergency departments did not yet have a visible security presence.
The teams also found issues with security devices: 19% of all cameras were not functional; at 24 facilities, more than 20% of the cameras were not working. A few facilities had “highly functional” systems that allowed personnel to monitor the campus thoroughly and even search for specific individuals—but they weren’t always operable.
At one western facility, it came down to a problem that would be frustrating at any time, but alarming for security management: Security personnel could not access the monitoring system because the required security certificates had expired, and no one knew the administrative password. If the system went offline, the OIG team was told, no one could fix the problem without password access. Neither VA’s Office of Information and Technology nor the contractor for the facility’s cameras could override the administrative password.
On the bright side, the OIG teams found that camera video feeds were being actively surveilled by security personnel at 60 of 70 facilities. All but 1 site kept camera footage for an average of 2 weeks or more.
VA policy states that, in addition to at least 2 intermediate weapons (such as batons and pepper spray) uniformed officers must always be issued radios for use while on duty. Survey respondents generally indicated they received their equipment and that it was adequate, but 15% said theirs lacked functionality, such as battery life and signal strength.
Based on the teams’ findings, the OIG made 6 recommendations: (1) delegating a responsible official to monitor and report monthly on facilities’ security-related vacancies; (2) authorizing sufficient staff to inspect VA police forces per the OIG’s 2018 unimplemented recommendation; (3) ensuring medical facility directors appropriately assess VA police staffing needs, authorize associated positions, and leverage available mechanisms to fill vacancies; (4) committing sufficient resources to ensure that facility security measures are adequate, current, and operational; (5) directing Veterans Integrated Services Network police chiefs, in coordination with medical facility directors, facility police chiefs, and facility emergency management leaders, to present a plan to remedy identified security weaknesses; and (6) establishing policy that standardizes the review and retention requirements for facility security camera footage.
The VA concurred with all recommendations and submitted corrective action plans.
In 2022, the US Department of Veterans Affairs (VA) Office of Inspector General (OIG) received 36 separate serious incident reports involving 32 medical facilities—including a bomb threat. In response to those reports, OIG teams of auditors and criminal investigators visited 70 VA medical facilities in September 2022 to assess security and to issue a formal report.
Noting that VA policy includes an “extensive list of security safeguards” for medical facilities to implement, the OIG focused its review on those that “a person with a reasonable level of security knowledge could assess.” According to their report, released in February, they identified a variety of security vulnerabilities and deficiencies, ranging from shortages of police officers to radios with poor signal strength.
For one, the OIG says, the facilities were designed to provide a welcoming environment, which means the public can enter the grounds freely at many different points. But the open-campus design makes it more difficult to balance security with easy and prompt access for patients. Consequently, the OIG says, “threats may originate from many locations within the medical facility campus itself or in the nearby community.”
Walking around the perimeters of the facility buildings, the OIG teams assessed the security level of 2960 public and nonpublic access doors. They found that 87% of public doors did not have an active security presence, and of those, 23% also did not have a security camera. Moreover, 17% of nonpublic doors were unlocked; 97% of those did not have a security presence, and 43% did not have a security camera. Even more concerning: Some of those doors led to sensitive or restricted facility areas. For example, at one midwestern facility, an unlocked nonpublic access door led to the surgical intensive care unit.
The OIG teams also assessed training records for 170 police officers across the 70 facilities and found that nearly all officers were compliant with training requirements. Most respondents also reported they received adequate training to perform their job duties and provided numerous positive indicators. Well trained or not, though, a notable problem in maintaining security, the OIG teams found, was there simply weren’t enough officers.
The OIG has repeatedly issued reports on significant police officer staffing shortages since at least 2018. VA police officers are not only empowered to make arrests, carry firearms while on duty, and investigate criminal activity within VA’s jurisdiction, but also assist individuals on medical campuses “in myriad ways,” the OIG notes. Staffing shortages are likely to compromise overall facility security, morale, and staff retention and underscore the need for maintaining communication with local law enforcement agencies for assistance, the OIG report points out.
In the OIG surveys, security personnel often noted they were understaffed. Although VA guidance calls for at least 2 VA police officers on duty at all times, 21% of respondents said they were aware of a duty shift during which minimum police staffing requirements were not met. About 37% of respondents expressed concerns about the physical security at their facilities. Some pointed out that the lack of VA police on duty could make it difficult to respond to threats like an active shooter.
In May 2022, the VA issued a directive that established minimum police coverage at medical facilities, as well as a police officer staffing decision tool to help determine appropriate officer levels. It required facilities to have an active security presence in their emergency departments around-the-clock by May 2023. As of September 2022, 58% of the facilities’ emergency departments did not yet have a visible security presence.
The teams also found issues with security devices: 19% of all cameras were not functional; at 24 facilities, more than 20% of the cameras were not working. A few facilities had “highly functional” systems that allowed personnel to monitor the campus thoroughly and even search for specific individuals—but they weren’t always operable.
At one western facility, it came down to a problem that would be frustrating at any time, but alarming for security management: Security personnel could not access the monitoring system because the required security certificates had expired, and no one knew the administrative password. If the system went offline, the OIG team was told, no one could fix the problem without password access. Neither VA’s Office of Information and Technology nor the contractor for the facility’s cameras could override the administrative password.
On the bright side, the OIG teams found that camera video feeds were being actively surveilled by security personnel at 60 of 70 facilities. All but 1 site kept camera footage for an average of 2 weeks or more.
VA policy states that, in addition to at least 2 intermediate weapons (such as batons and pepper spray) uniformed officers must always be issued radios for use while on duty. Survey respondents generally indicated they received their equipment and that it was adequate, but 15% said theirs lacked functionality, such as battery life and signal strength.
Based on the teams’ findings, the OIG made 6 recommendations: (1) delegating a responsible official to monitor and report monthly on facilities’ security-related vacancies; (2) authorizing sufficient staff to inspect VA police forces per the OIG’s 2018 unimplemented recommendation; (3) ensuring medical facility directors appropriately assess VA police staffing needs, authorize associated positions, and leverage available mechanisms to fill vacancies; (4) committing sufficient resources to ensure that facility security measures are adequate, current, and operational; (5) directing Veterans Integrated Services Network police chiefs, in coordination with medical facility directors, facility police chiefs, and facility emergency management leaders, to present a plan to remedy identified security weaknesses; and (6) establishing policy that standardizes the review and retention requirements for facility security camera footage.
The VA concurred with all recommendations and submitted corrective action plans.
History of nonproductive cough
The history and findings in this case are suggestive of eosinophilic asthma.
Asthma is a common, chronic, and heterogeneous respiratory disease, most often characterized by chronic airway inflammation. Affected individuals experience respiratory symptoms (ie, wheezing, dyspnea, chest tightness, and cough) that may fluctuate over time and in intensity, as well as variable expiratory airflow limitation, which may become persistent. For many patients, asthma has a significant impact on quality of life. According to the World Health Organization, asthma affected an estimated 262 million people and caused 455,000 deaths. Currently, approximately 334 million people worldwide are believed to be affected by asthma.
Asthma frequently begins in childhood, but adult-onset asthma can occur and often presents as a nonatopic and eosinophilic condition. In fact, asthma is an umbrella diagnosis that encompasses several diseases with distinct mechanistic pathways (endotypes) and variable clinical presentations (phenotypes), all of which manifest with respiratory symptoms and are accompanied by variable airflow obstruction.
Broadly, asthma endotypes are categorized as type 2 (T2)-high or T2-low. Eosinophilic asthma falls under the T2-high endotype and comprises three phenotypes: atopic, late-onset, and aspirin-exacerbated respiratory disease. Late-onset T2-high asthma is characterized by prominent blood and sputum eosinophilia and is refractory to inhaled/oral corticosteroid treatment. Patients in this subgroup tend to be older and have more severe asthma with fixed airflow obstruction and more frequent exacerbations; patients may also have comorbid chronic rhinosinusitis with nasal polyps, which usually precedes asthma development. High FeNO levels and normal or elevated serum total IgE levels are also often seen in this subgroup.
The late-onset eosinophilic asthma phenotype accounts for 20%-40% of severe asthma cases and is associated with rapid decline of respiratory functions. Thus, earlier escalation of therapy may be indicated in patients with this phenotype.
According to a 2022 report from the Global Initiative for Asthma, the possibility of refractory T2 asthma should be considered when any of the following is found in patients taking high-dose ICS or daily oral corticosteroids:
• Blood eosinophils ≥ 150/μL, and/or
• FeNO ≥ 20 ppb, and/or
• Sputum eosinophils ≥ 2%, and/or
• Asthma is clinically allergen driven
Biologic T2-targeted therapies are available as add-on therapies for patients with T2 airway inflammation and severe asthma despite taking at least a high-dose ICS-LABA, and who have eosinophilic or allergic biomarkers or need maintenance oral corticosteroids. Available options for eosinophilic asthma include anti-interleukin (IL)-5/anti-IL-5R therapies (benralizumab, mepolizumab, reslizumab) and anti-IL-4R therapy (dupilumab).
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of eosinophilic asthma.
Asthma is a common, chronic, and heterogeneous respiratory disease, most often characterized by chronic airway inflammation. Affected individuals experience respiratory symptoms (ie, wheezing, dyspnea, chest tightness, and cough) that may fluctuate over time and in intensity, as well as variable expiratory airflow limitation, which may become persistent. For many patients, asthma has a significant impact on quality of life. According to the World Health Organization, asthma affected an estimated 262 million people and caused 455,000 deaths. Currently, approximately 334 million people worldwide are believed to be affected by asthma.
Asthma frequently begins in childhood, but adult-onset asthma can occur and often presents as a nonatopic and eosinophilic condition. In fact, asthma is an umbrella diagnosis that encompasses several diseases with distinct mechanistic pathways (endotypes) and variable clinical presentations (phenotypes), all of which manifest with respiratory symptoms and are accompanied by variable airflow obstruction.
Broadly, asthma endotypes are categorized as type 2 (T2)-high or T2-low. Eosinophilic asthma falls under the T2-high endotype and comprises three phenotypes: atopic, late-onset, and aspirin-exacerbated respiratory disease. Late-onset T2-high asthma is characterized by prominent blood and sputum eosinophilia and is refractory to inhaled/oral corticosteroid treatment. Patients in this subgroup tend to be older and have more severe asthma with fixed airflow obstruction and more frequent exacerbations; patients may also have comorbid chronic rhinosinusitis with nasal polyps, which usually precedes asthma development. High FeNO levels and normal or elevated serum total IgE levels are also often seen in this subgroup.
The late-onset eosinophilic asthma phenotype accounts for 20%-40% of severe asthma cases and is associated with rapid decline of respiratory functions. Thus, earlier escalation of therapy may be indicated in patients with this phenotype.
According to a 2022 report from the Global Initiative for Asthma, the possibility of refractory T2 asthma should be considered when any of the following is found in patients taking high-dose ICS or daily oral corticosteroids:
• Blood eosinophils ≥ 150/μL, and/or
• FeNO ≥ 20 ppb, and/or
• Sputum eosinophils ≥ 2%, and/or
• Asthma is clinically allergen driven
Biologic T2-targeted therapies are available as add-on therapies for patients with T2 airway inflammation and severe asthma despite taking at least a high-dose ICS-LABA, and who have eosinophilic or allergic biomarkers or need maintenance oral corticosteroids. Available options for eosinophilic asthma include anti-interleukin (IL)-5/anti-IL-5R therapies (benralizumab, mepolizumab, reslizumab) and anti-IL-4R therapy (dupilumab).
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of eosinophilic asthma.
Asthma is a common, chronic, and heterogeneous respiratory disease, most often characterized by chronic airway inflammation. Affected individuals experience respiratory symptoms (ie, wheezing, dyspnea, chest tightness, and cough) that may fluctuate over time and in intensity, as well as variable expiratory airflow limitation, which may become persistent. For many patients, asthma has a significant impact on quality of life. According to the World Health Organization, asthma affected an estimated 262 million people and caused 455,000 deaths. Currently, approximately 334 million people worldwide are believed to be affected by asthma.
Asthma frequently begins in childhood, but adult-onset asthma can occur and often presents as a nonatopic and eosinophilic condition. In fact, asthma is an umbrella diagnosis that encompasses several diseases with distinct mechanistic pathways (endotypes) and variable clinical presentations (phenotypes), all of which manifest with respiratory symptoms and are accompanied by variable airflow obstruction.
Broadly, asthma endotypes are categorized as type 2 (T2)-high or T2-low. Eosinophilic asthma falls under the T2-high endotype and comprises three phenotypes: atopic, late-onset, and aspirin-exacerbated respiratory disease. Late-onset T2-high asthma is characterized by prominent blood and sputum eosinophilia and is refractory to inhaled/oral corticosteroid treatment. Patients in this subgroup tend to be older and have more severe asthma with fixed airflow obstruction and more frequent exacerbations; patients may also have comorbid chronic rhinosinusitis with nasal polyps, which usually precedes asthma development. High FeNO levels and normal or elevated serum total IgE levels are also often seen in this subgroup.
The late-onset eosinophilic asthma phenotype accounts for 20%-40% of severe asthma cases and is associated with rapid decline of respiratory functions. Thus, earlier escalation of therapy may be indicated in patients with this phenotype.
According to a 2022 report from the Global Initiative for Asthma, the possibility of refractory T2 asthma should be considered when any of the following is found in patients taking high-dose ICS or daily oral corticosteroids:
• Blood eosinophils ≥ 150/μL, and/or
• FeNO ≥ 20 ppb, and/or
• Sputum eosinophils ≥ 2%, and/or
• Asthma is clinically allergen driven
Biologic T2-targeted therapies are available as add-on therapies for patients with T2 airway inflammation and severe asthma despite taking at least a high-dose ICS-LABA, and who have eosinophilic or allergic biomarkers or need maintenance oral corticosteroids. Available options for eosinophilic asthma include anti-interleukin (IL)-5/anti-IL-5R therapies (benralizumab, mepolizumab, reslizumab) and anti-IL-4R therapy (dupilumab).
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 42-year-old nonsmoking man presents with complaints of a 9-month history of wheezing, nonproductive cough, and exertional dyspnea. The patient reports nighttime awakenings from his symptoms three to five times per month. He was diagnosed with asthma by his primary care provider about 3 months after his symptoms began. On diagnosis, he was prescribed a short-acting beta-2 adrenergic agonist rescue inhaler and an inhaled corticosteroid (ICS), twice daily. Because the patient remained symptomatic, his primary care provider stepped up his daily therapy to a combined ICS and long-acting beta2-adrenergic agonist (LABA). At today's visit, the patient reports continued symptoms and use of his rescue inhaler at least twice per week. He has no other significant medical history aside from a history of mild atopic dermatitis. He is 5 ft 11 in and currently weighs 172 lb (BMI 24). He demonstrates proper inhaler technique and states that he is compliant with his therapy.
Physical examination reveals loud wheezing during inspiration and throughout expiration. The patient's heart rate is 110 beats/min; blood pressure is 130/70 mm Hg. Pulse oximetry is 93%. Spirometry reveals a forced expiratory volume in the first second (FEV1) of 78% predicted. Fractional exhaled nitric oxide (FeNO) is 56 ppb. Chest radiography is normal. High-resolution CT shows air trapping, mosaic lung attenuations, and bronchial wall thickening. IgE level is normal; sputum culture reveals 6% eosinophils.