User login
For MD-IQ use only
Concern grows over ‘medical assistance in dying for mental illness’ law
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Canada already has the largest number of deaths by MAID of any nation, with 10,064 in 2021, a 32% increase from 2020. With the addition of serious mental illness (SMI) as an eligible category, the country is on track to have the most liberal assisted-death policy in the world.
Concerns about the additional number of patients who could become eligible for MAID, and a lack of evidence-backed standards from disability rights groups, mental health advocates, First Nations leaders, psychiatrists, and other mental health providers, seems to have led the Canadian government to give the proposed law some sober second thought.
“Listening to experts and Canadians, we believe this date needs to be temporarily delayed,” said David Lametti, Canada’s minister of Justice and attorney general of Canada; Jean-Yves Duclos, minister of Health; and Carolyn Bennett, minister of Mental Health and Addictions, in a Dec. 15, 2022, joint statement.
Canada’s Parliament – which approved the expansion – will now have to vote on whether to okay a pause on the legislation.
However, the Canadian Psychiatric Association has not been calling for a delay in the proposed legislation. In a November 2021 statement, the CPA said it “does not take a position on the legality or morality of MAID,” but added that to deny MAID to people with mental illness was discriminatory, and that, as it was the law, it must be followed.
“CPA has not taken a position about MAID,” the association’s president Gary Chaimowitz, MBChB, told this news organization. “We know this is coming and our organization is trying to get its members ready for what will be most likely the ability of people with mental conditions to be able to request MAID,” said Dr. Chaimowitz, who is also head of forensic psychiatry at St. Joseph’s Healthcare and a professor of psychiatry at McMaster University, both in Hamilton, Ont.
Dr. Chaimowitz acknowledges that “a majority of psychiatrists do not want to be involved in anything to do with MAID.”
“The idea, certainly in psychiatry, is to get people well and we’ve been taught that people dying from a major mental disorder is something that we’re trained to prevent,” he added.
A ‘clinical option’
Assisted medical death is especially fraught in psychiatry, said Rebecca Brendel, MD, president of the American Psychiatric Association. She noted a 25-year life expectancy gap between people with SMI and those who do not have such conditions.
“As a profession we have very serious obligations to advance treatment so that a person with serious mental illness can live [a] full, productive, and healthy [life],” Dr. Brendel, associate director of the Center for Bioethics at Harvard Medical School in Boston, said in an interview.
Under the Canadian proposal, psychiatrists would be allowed to suggest MAID as a “clinical option.”
Harold Braswell, PhD, a fellow with The Hastings Center, a bioethics research institute, calls that problematic.
“It’s not neutral to suggest to someone that it would be theoretically reasonable to end their lives,” Dr. Braswell, associate professor at the Albert Gnaegi Center for Health Care Ethics at Saint Louis University, told this news organization.
It also creates a double standard in the treatment of suicidal ideation, in which suicide prevention is absolute for some, but encouraging it as a possibility for others, he added.
“To have that come from an authority figure is something that’s very harsh and, in my opinion, very potentially destructive,” especially for vulnerable groups, like First Nations people, who already have elevated rates of suicide, said Dr. Braswell.
Fierce debate
Since 2016, Canada has allowed MAID for medical conditions and diseases that will not improve and in cases where the evidence shows that medical providers can accurately predict the condition will not improve.
However, in 2019, a Quebec court ruled that the law unconstitutionally barred euthanasia in people who were not terminally ill. In March 2021, Canada’s criminal code was amended to allow MAID for people whose natural death was not “reasonably foreseeable,” but it excluded SMI for a period of 2 years, ending in March 2023.
The 2-year stay was intended to allow for study and to give mental health providers and MAID assessors time to develop standards.
The federal government charged a 12-member expert panel with determining how to safely allow MAID for SMI. In its final report released in May 2022 it recommended that standards be developed.
The panel acknowledged that for many conditions it may be impossible to make predictions about whether an individual might improve. However, it did not mention SMI.
In those cases, when MAID is requested, “establishing incurability and irreversibility on the basis of the evolution and response to past interventions is necessary,” the panel noted, adding that these are the criteria used by psychiatrists assessing euthanasia requests in the Netherlands and Belgium.
But the notion that mental illness can be irremediable has been fiercely debated.
Soon after the expert report was released, the Center for Addiction and Mental Health in Toronto noted on its website that there are currently “no agreed upon standards for psychiatrists or other health care practitioners to use to determine if a person’s mental illness is ‘grievous and irremediable’ for the purposes of MAID.”
Dr. Chaimowitz acknowledged that “there’s no agreed-upon definition of incurability” in mental illness. Some psychiatrists “will argue that there’s always another treatment that can be attempted,” he said, adding that there has been a lack of consensus on irremediability among CPA members.
Protecting vulnerable populations
Matt Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora, said the question of irremediability is crucial. “Most people with mental illness do get better, especially if they’re in treatment,” Dr. Wynia said.
For MAID assessors it may be difficult to know when someone has tried all possible treatments, especially given the wide array of options, including psychedelics, said Dr. Wynia.
Dr. Braswell said there is not enough evidence that mental illness is incurable. With SMI, “there’s a lot more potential for the causes of the individual’s suffering to be ameliorated. By offering MAID, you’re going to kill people who might have been able to get out of this through other nonlethal means.”
Currently, MAID is provided for an irremediable medical condition, “in other words, a condition that will not improve and that we can predict will not improve,” said Karandeep S. Gaind, MD, chief of psychiatry at Toronto’s Humber River Hospital and physician chair of the hospital’s MAID team.
“If that’s the premise, then I think we cannot provide MAID for sole mental illness,” Dr. Gaind said. “Because we can’t honestly make those predictions” with mental illness, he added.
Dr. Gaind does not support MAID for mental illness and believes that it will put the vulnerable – including those living in poverty – at particular risk.
With the proposed expansion, MAID is “now becoming something which is being sought as a way to escape a painful life rather than to avoid a painful death,” said Dr. Gaind, who is also a past president of the CPA.
One member of the federal government’s expert panel – Ellen Cohen, who had a psychiatric condition – wrote in The Globe and Mail that she quit early on when it became apparent that the panel was not seriously considering her own experiences or the possibility that poverty and lack of access to care or social supports could strongly influence a request for MAID.
Social determinants of suffering
People with mental illness often are without homes, have substance use disorders, have been stigmatized and discriminated against, and have poor social supports, said Dr. Wynia. “You worry that it’s all of those things that are making them want to end their lives,” he said.
The Daily Mail ran a story in December 2022 about a 65-year-old Canadian who said he’d applied for MAID solely because of fears that his disability benefits for various chronic health conditions were being cut off and that he didn’t want to live in poverty.
A 51-year-old Ontario woman with multiple chemical sensitivities was granted MAID after she said she could not find housing that could keep her safe, according to an August report by CTV News.
Tarek Rajji, MD, chief of the Adult Neurodevelopment and Geriatric Psychiatry Division at CAMH, said social determinants of health need to be considered in standards created to guide MAID for mental illness.
“We’re very mindful of the fact that the suffering, that is, the grievousness that the person is living with, in the context of mental illness, many times is due to the social determinants of their illness and the social determinants of their suffering,” Dr. Rajji said.
Many are also concerned that it will be difficult to separate out suicidality from sheer hopelessness.
The CPA has advised a group that’s working on developing guidelines for MAID in SMI and is also developing a curriculum for mental health providers, Dr. Chaimowitz said. As part of that, there will be a process to ensure that someone who is actively suicidal is not granted MAID.
“I do not believe that it’s contemplated that MAID is going to accelerate or facilitate suicidal ideation,” he said. Someone who is suicidal will be referred to treatment, said Dr. Chaimowitz.
“People with depression often feel hopeless,” and may refuse treatments that have worked in the past, countered Dr. Gaind. Some of his patients “are absolutely convinced that nothing will help,” he said.
Troublesome cases
The expert panel said in its final report that “it is not possible to provide fixed rules for how many attempts at interventions, how many types of interventions, and over how much time,” are necessary to establish “irreversibility” of mental illness.
Dr. Chaimowitz said MAID will not be offered to anyone “refusing treatment for their condition without any good reason.” They will be “unlikely to meet criteria for incurable,” as they will have needed to avail themselves of the array of treatments available, he said.
That would be similar to rules in Belgium and the Netherlands, which allow euthanasia for psychiatric conditions.
An estimated 100-300 psychiatric patients receive euthanasia each year in those countries, according to a 2021 commentary in Psychiatric Times (Jun 7;38[6]) by Mark S. Komrad, MD, a Towson, Maryland-based psychiatrist.
There are still troublesome cases.
As previously reported by this news organization, many in Belgium were distressed recently at the news that a 23-year-old woman who had survived a terrorist attack, Shanti De Corte, requested and was granted euthanasia.
As the deadline for implementation of MAID grew closer, calls for delay grew louder, especially given the lack of concrete standards for providers.
During the waning months of 2022, Dr. Gaind – who said he was suspended from CPA for “unprofessional interactions” and allegedly misrepresenting CPA’s processes and governance matters – announced the launch of a new organization, the Society of Canadian Psychiatry, in November calling for a delay in MAID of at least 1 year so that evidence-based safeguards could be implemented. The petition has been signed by more than 200 psychiatrists, along with several dozen physicians, MAID assessors, and individuals with mental illness and family members.
The Association of Chairs of Psychiatry in Canada, the Canadian Association for Suicide Prevention, the Council of Canadians with Disabilities, a group of indigenous leaders, and the Ontario Association for ACT and FACT, psychiatrists who provide care to individuals with severe mental illness, among other groups, joined the call for a delay.
In its December announcement, the Canadian federal ministers said a factor in seeking a delay was that standards guiding clinicians would not be delivered until at least February – too close to when applications would be opened.
Upon hearing about the federal government’s intentions, the chair of the expert panel, Mona Gupta, MD, told The Canadian Press that she did not think it was necessary to put off implementation because necessary safeguards were already in place.
Dr. Chaimowitz awaits the standards but is optimistic that for mental illness, “the process will be tightly controlled, closely monitored, and open to scrutiny,” he said.
Dr. Braswell is not convinced. The concern is that adding people with mental illness is “going to overload the capacity of the government to monitor this practice,” he said.
Is the United States next?
Although Canada and the United States share a border, it’s unlikely that U.S. states will allow aid in dying for nonterminal illness, much less for psychiatric conditions any time soon, said Dr. Braswell and others.
Ten states – California, Colorado, Hawaii, Maine, Montana, New Jersey, New Mexico, Oregon, Vermont, and Washington – have laws allowing assistance in dying, but for terminal illness only.
In 2016, the APA adopted the American Medical Association policy on medical euthanasia, stating, “that a psychiatrist should not prescribe or administer any intervention to a nonterminally ill person for the purpose of causing death.”
Dr. Brendel said the field is acutely aware that people with mental illness do suffer, but that more work needs to be done – and is being done – on “distinguishing wishes to hasten death or end one’s life from these historical or traditional notions that any premature death is a suicide.”
There is also increasing discussion within the medical community, not just psychiatry, about a physician’s duty to relieve suffering, said Dr. Wynia. “There’s debate basically about whether we stand for preserving life essentially at all costs and never being involved in the taking of life, or whether we stand for reduction of suffering and being the advocate for the patients that we serve,” he said.
“Those are both legitimate,” said Dr. Wynia, adding, “there are good reasons to want both of those to be true.”
“I suspect that 20 years from now we will still be having conversations about how physicians, how psychiatrists ought to participate in preserving life and in shepherding death,” said Dr. Brendel.
But to Dr. Gaind, the debate is not just esoteric, it’s a soon-to-be reality in Canada. “When we’re providing death to people who aren’t dying, to me that’s like providing what amounts to a wrongful death,” he said.
A version of this article originally appeared on Medscape.com.
Artificial intelligence applications in colonoscopy
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4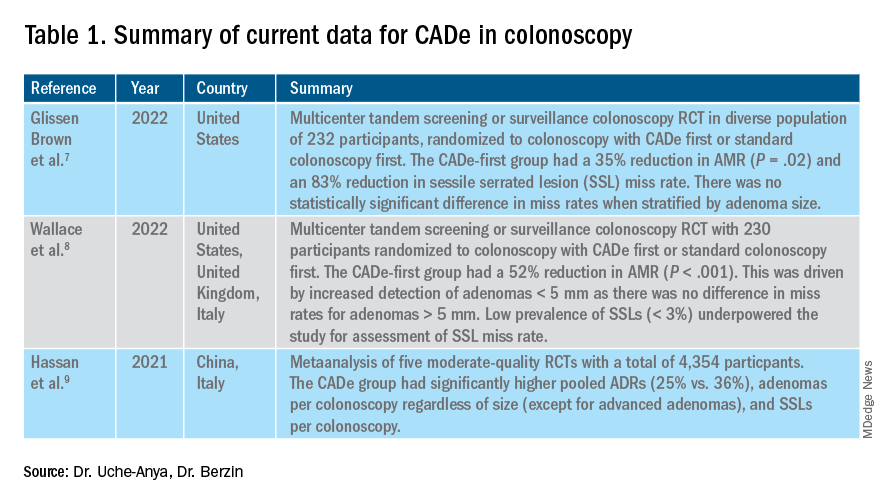
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.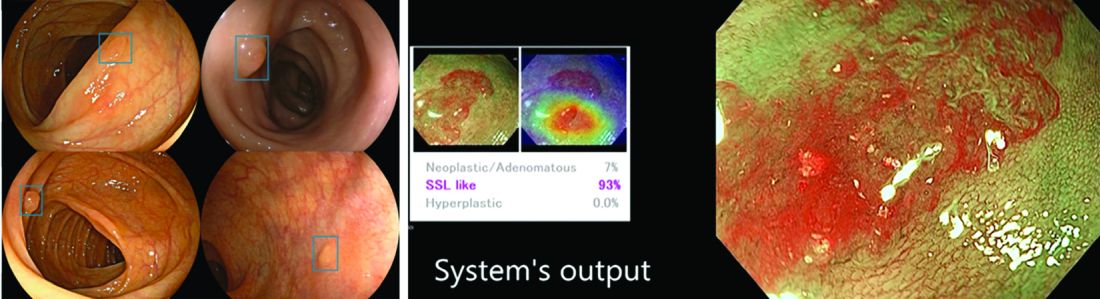
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya eucheanya@mgh.harvard.edu Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya eucheanya@mgh.harvard.edu Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya eucheanya@mgh.harvard.edu Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Mastocytosis: Rare, underdiagnosed, potentially fatal
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Nationwide, approximately 1,000 adults are diagnosed with systemic mastocytosis annually. This rare disease is a myeloid neoplasm with a highly variable phenotypic expression, in which abnormal mast cells proliferate and infiltrate organs and tissues. It swings widely from a nonadvanced form, composed of indolent or smoldering disease, to advanced disease that progresses to leukemia in 6% of cases.
More than 80% of systemic mastocytosis is driven by the KIT D816V mutation. Along with a host of other rare KIT mutations, KIT D816V activates KIT-receptor tyrosine kinase to trigger mast cell proliferation.
Dr. Gotlib could not be contacted for an interview. However, there are many good reasons to identify patients with systemic mastocytosis, according to Attilio Orazi, MD, professor and chair of the department of pathology at Texas Tech University, El Paso. The chief reason is that the patient may be in grave peril.
“The degree of heterogeneity is amazing. ... There’s very indolent [disease], which is really not a big deal. And then you have a disease in which you’re dead in 3 months,” Dr. Orazi said. “So you run the gamut between an indolent, no-problem cutaneous disease to a very nasty systemic, aggressive leukemia-like neoplasm.”
Since 2001, the diagnosis of mastocytosis has been guided by the World Health Organization Classification of Tumours, or “Blue Book.” In 2022, Dr. Orazi along with 137 other senior experts, most of whom were involved in past editions of the Blue Book, published their own version: The International Consensus Classification of Myeloid Neoplasms and Acute Leukemias (the ICC 2022).
In September 2021, this group of specialists held a virtual/in-person advisory committee meeting at the University of Chicago to create the document. One factor in their decision to go it alone, Dr. Orazi said, was that WHO decided to proceed with the fifth edition of the Blue Book using its own internal editorial group without convening an advisory committee, despite repeated requests to do so.
ICC 2022 divides advanced systemic mastocytosis into three subtypes: aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Median survival is 3.5 years for patients with ASM, 2 years for those with SM-AHN and as low as 2 months for MCL.
The second key reason to increase awareness of mastocytosis among physicians, said Dr. Orazi, is that patients falling through the net are likely to be ambulatory, and their presentation can be “a little confusing.”
Patients with indolent disease are relatively straightforward to recognize, explained Dr. Orazi. Similarly, very sick patients with SM-AHN or MCL are easily recognized by hem-oncs.
“But if you see a patient in an ambulatory setting, in your clinic or whatever, and you’re suspicious, then you need to decide [how] you’re going to investigate that patient further,” he said, Dr. Orazi noted the next step is not always obvious, especially for primary-practice or internal medicine physicians likely to be unfamiliar with such a rare disease.
A practice survey published in 2022 by other researchers backed up Dr. Orazi’s remarks. The study found that community/solo-practice physicians were less likely to have tested systemic mastocytosis patients for KIT816V mutation than academic/specialty physicians (58% vs. 80%; P = .004; n = 111). Clinicians treating these patients estimated that it took an average of 8.5 months for a “typical” patient to receive the diagnosis from the time of symptom onset.
The research was headed by Ruben Mesa, MD, director of University of Texas Health, San Antonio, and funded by Blueprint Medicines, the manufacturer of avapritinib (Ayvakit), a new drug for the disease.
Dr. Orazi urged clinicians to have a high degree of suspicion for mastocytosis in a patient who walks into the clinic with any combination of the following: urticarial-type skin manifestations, especially if persistent into adulthood; history of undue reaction to an insect sting; a big spleen in a patient with a history of cutaneous flushing or rash; chronic diarrhea, especially if a biopsy has shown “too many mast cells” in the lamina propria of the small bowel; and positivity for KIT816V mutation.
Dr. Orazi stressed that the majority of patients will have indolent disease, but for the few patients for whom immediate treatment is essential, “the distinction between indolent and aggressive [disease] is really very, very important.”
Patients with advanced systemic mastocytosis can now be effectively treated, following the arrival of midostaurin (Rydapt, Tauritmo) and avapritinib.
Midostaurin, a multikinase/KIT inhibitor, was approved by the Food and Drug Administration in 2017 for the treatment of advanced systemic mastocytosis (ASM, SM-AHN, and MCL). Avapritinib, a selective kinase inhibitor of KIT816V and platelet-derived growth factor receptor alpha as well as multiple KIT exon 11, 11/17 and 17 mutants, gained the same indication in June 2021.
As with all rare diseases, it is challenging to obtain accurate numbers on how many patients are affected by systemic mastocytosis. The first population-based study of the disorder, presented at the 2018 annual meeting of the American Society of Hematology, used the Surveillance, Epidemiology, and End Results database from 2000 to 2014 to estimate incidence at 0.046 per 10,000, which translates to 1,050 new adult cases per year. The study data have never been published in full.
How many of these cases are advanced disease? There are no U.S. data but extrapolating from a Danish registry study that found 82% of systemic mastocytosis cases to be indolent disease, the incidence of advanced systemic mastocytosis in the United States could be as low as 200 adults a year.
This information, in turn, suggests that identifying more patients with advanced disease would not only benefit those patients but would also benefit clinical trial investigators who are seeking the proverbial needle in the haystack.
Nationwide, five clinical trials are recruiting individuals with advanced systemic mastocytosis, collectively looking for 352 patients in the United States. Two of the studies focus on mast-cell activation (NCT0544944) and cutaneous mastocytoses (NCT04846348). Two trials in a range of hematological malignancies are testing bispecific antibodies flotetuzumab and MGD024 (both from Macrogenics; NCT04681105, NCT05362773).
Apex, a phase 2 study of tyrosine-kinase inhibitor bezuclastinib (a Cogent hopeful), is specifically focusing on advanced disease. Dr. Gotlib and coinvestigators are aiming for 140 participants.
As a pathologist, Dr. Orazi said he find mastocytosis fascinating because he believes he has “a truly useful role,” contrasting with some other hematological diseases in which the molecular profile rules.
“Pathology plays a major role here,” he explained, “because you have to correlate what you see at the microscope with the full clinical picture, selected laboratory tests such as CBC and serum tryptase, and molecular results. You often need integration through a pathologist to put all the pieces together.
“It’s easier to treat once you know exactly what disease you’re dealing with and whether it is an aggressive or indolent subtype,” Dr. Orazi concluded.
Dr. Orazi disclosed no conflicts of interest. Dr. Gotlib has disclosed ties with Blueprint Medicines, Deciphera, Incyte, and Kartos Therapeutics, and has led committees for Blueprint Medicine’s EXPLORER and PATHFINDER studies, Deciphera’s Study Steering Committee for ripretinib in AdvSM, and the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM.
Guidelines recommend CBT alone for mild acute depression, more options for more severe cases
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
The guidelines also state that patients with mild depression should start with CBT alone, and if a patient with moderate to severe depression prefers, they can use a combination of both CBT and an SGA.
These nuanced recommendations contrast sharply with the 2016 ACP guidelines for depression, which lumped all stages and severity levels together, and came with just one recommendation: Clinicians should choose between CBT and an SGA.
More data have come to light over the years, requiring the present update, reported lead author Amir Qaseem, MD, PhD, vice president of Clinical Policy and the Center for Evidence Reviews at the ACP, and adjunct faculty at Thomas Jefferson University, Philadelphia, and colleagues.
In addition to the focus on acute depression, Dr. Qaseem and colleagues highlighted the new guidelines' “consideration of patient values and preferences, and costs,” as well as responses to therapy.
Recommendations were derived from a network meta-analysis that included studies evaluating nonpharmacologic and pharmacologic therapies, the authors wrote in Annals of Internal Medicine. They compared effectiveness across a range of SGAs, “including selective serotonin reuptake inhibitors; serotonin-norepinephrine reuptake inhibitors; and others such as bupropion, mirtazapine, nefazodone, trazodone, vilazodone, and vortioxetine.”
This analysis yielded three pieces of clinical advice.
First, patients in the acute phase of mild depression should receive CBT alone as their initial treatment.
Dr. Qaseem and colleagues noted that many depression studies for pharmacologic therapies excluded these patients in favor of those with moderate to severe depression, leaving an evidence gap.
“Furthermore, the Clinical Guidelines Committee had concerns about adverse effects of SGAs in these patients and suggests that the use of SGAs as initial treatment of these patients should be based on additional considerations, such as limited access to or cost of CBT, history of moderate or severe major depressive disorder, or patient preferences,” they added.
The committee’s next recommendation, based on moderate-certainty evidence, suggested that CBT alone or an SGA alone should be considered for patients in the acute phase of moderate to severe depression. This call for monotherapy is balanced by a conditional recommendation based on low-certainty evidence that the same group may benefit from initial combination therapy with both CBT and an SGA.
“The informed decision on the options of monotherapy with CBT versus SGAs, or combination therapy, should be personalized and based on discussion of potential treatment benefits, harms, adverse effect profiles, cost, feasibility, patients’ specific symptoms (such as insomnia, hypersomnia, or fluctuation in appetite), comorbidities, concomitant medication use, and patient preferences,” the guidelines state.
The third and final recommendation offers an algorithm for patients who do not respond to initial therapy with an SGA. Multiple pathways are provided: Switch to CBT or augment with CBT; or switch to a different SGA or augment with a second pharmacologic therapy, such as mirtazapine, bupropion, or buspirone.
“These second-line treatment strategies show similar efficacy when compared with each other,” the guidelines committee noted.
Again, the guidelines suggest that second-line choices should be personalized based on the various factors previously discussed.
A timely update
“The new guideline is very different from the last guideline,” said Ryan Mire, MD, president of the ACP and practicing internal medicine physician in Nashville, Tenn. in a written comment. “ACP decided to update the depression guidelines with a focus on acute depression because approximately 70% of patients with major depressive disorder do not achieve remission and remain in the acute phase after the initial pharmacologic treatment attempt. In addition, there is new evidence on second-line treatments since the 2016 ACP guideline was published.”
Neil S. Skolnik, MD, of Thomas Jefferson University, Philadelphia, agreed that the guidelines offer a necessary and fresh perspective on caring for patients with depression.
“These guidelines are a helpful update, assuring us that we are using the latest, evidence-based therapies, and [they] are written in a practical, easy-to-implement manner,” Dr. Skolnik said in a written comment.
“First, the guidelines reaffirm that CBT is an effective first-line option, with or without the concurrent use of an SGA,” Dr. Skolnik said, noting that CBT alone may reduce likelihood of recurrence, compared with an SGA alone. “Many patients do not like the idea of medication, or the potential side effects of medications, and CBT is an evidenced-based approach that can be very helpful for patients.”
Dr. Skolnik also applauded the guidelines authors for offering a clear path forward for patients who do not have full remission after treatment – a common clinical scenario.
He went on to offer some more detailed steps forward.
“If someone chooses to be treated with an SGA alone and has not had much response at all to an initial SGA, usually a selective serotonin reuptake inhibitor, I’ll usually switch to a different SSRI or serotonin and norepinephrine reuptake inhibitor (SNRI) and/or add CBT,” Dr. Skolnik said. “If they have had a partial response, I’ll often encourage CBT and consider the addition of augmentation with an additional medication as discussed in the guidelines.”
Valuable despite the gaps
Other experts expressed mixed impressions of the update, noting both highs and lows.
“Although [this guideline] has some gaps, it is more valuable in several ways than other widely consulted practice guidelines for depression,” wrote Miriam Shuchman, MD and Elia Abi-Jaoude, MSc, MD, PhD, of the University of Toronto, in an accompanying editorial.
Specifically, they praised the publication’s focus on shared decision-making in the treatment planning process.
“This effort to respond to patient preferences is crucial and may even increase the chance that patients will improve with treatment,” they wrote.
They also applauded the ACP’s efforts to recuse any committee members who may have had conflicts of interest “that could affect their judgment about treatments for depression.”
After highlighting these attributes, Dr. Shuchman and Dr. Abi-Jaoude noted that the guidelines still contain “significant gaps.”
Foremost, they pointed out the guidelines' emphasis on CBT to the exclusion of other nonpharmacologic options.
“The guideline does patients a disservice by leaving out several nonmedication treatment options that clinicians can offer as first- or second-line therapies,” they wrote.
This oversight may increase risk that patients simply hop from one SGA to another, which is a common, and often ineffective, strategy, according to Dr. Shuchman and Dr. Abi-Jaoude.
“Patients often go from one drug to the next in the hopes of landing on one that ‘works,’ ” the editorialists wrote. “This narrow clinical approach of pursuing medication-based treatments ignores the ways difficulties in a person’s work or relationships may contribute to their struggles with depression. At a time when the COVID-19 pandemic has underscored the importance of the social context of mental health, clinicians may need to consider other forms of support and tailor prescribing to what is most relevant and accessible for a particular patient.”
Dr. Shuchman and Dr. Abi-Jaoude went on to suggest several nonpharmacologic options beyond CBT, including interpersonal therapy, psychodynamic therapy, problem solving, behavioral activation, and guided self-help.
The other key gap they pointed out relates to withdrawal.
Although the guideline does advise physicians to taper antidepressants to reduce risk of withdrawal, the editorialists suggested that this recommendation lacked sufficient emphasis, as it can be a particularly difficult period in the treatment process.
“Tapering of an antidepressant may need to be done over months or years, not weeks, and a patient may need to visit a compounding pharmacy to obtain doses of a second-generation antidepressant not marketed by drug manufacturers so that prescriptions can be tapered even more slowly,” they suggested.
Financial costs remain unclear
Beyond the above medical considerations, one other piece of the depression puzzle remains unsolved: cost.
In a simultaneously published rapid review, Andreea Dobrescu, MD, PhD, of Cochrane Austria, and colleagues evaluated the relative cost-effectiveness of first- and second-step treatment strategies.
For most comparisons, evidence was insufficient to reach a conclusion, although they suggested that CBT may be more cost effective at the 5-year mark.
“For most pharmacologic and nonpharmacologic interventions for major depressive disorder, evidence was missing or was insufficient to draw conclusions about the cost-effectiveness of first- or second-step treatments for MDD,” Dr. Dobrescu and colleagues wrote. “The strongest evidence (albeit still low certainty of evidence) was for the cost-effectiveness of CBT compared with SGA as a first-step treatment over a 5-year time horizon from the societal and health care sector perspectives. However, this evidence should also be interpreted cautiously considering it is based on a single study.”
When asked about the financial findings, Dr. Mire agreed that more data are needed, especially because CBT and SGA costs range widely. He suggested that cost, for each patient, should be considered in the personalized approach now highlighted by the new guidelines.
The guidelines and the Cochrane cost-effectiveness study were supported by the ACP. The guidelines' authors and other individuals quoted in this article reported no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
A patient named ‘Settle’ decides to sue instead
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
On Nov. 1, 2020, Dallas Settle went to Plateau Medical Center, Oak Hill, W.Va., complaining of pain that was later described in court documents as being “in his right mid-abdomen migrating to his right lower abdomen.” Following a CT scan, Mr. Settle was diagnosed with diverticulitis resulting in pneumoperitoneum, which is the presence of air or other gas in the abdominal cavity. The patient, it was decided, required surgery to correct the problem, but Plateau Medical Center didn’t have the staff to perform the procedure.
Mr. Settle was then transferred to another West Virginia hospital, Charleston Area Medical Center (CAMC). Here, he was evaluated by doctors in the facility’s General Division, who initiated treatment with IV fluids and opiate analgesics. He was then placed under the care of a trauma surgeon, who initially decided to treat the patient nonoperatively. If that approach failed, the surgeon believed, Mr. Settle would probably require a laparotomy, bowel resection, and ostomy.
Another surgical team performed an exploratory laparotomy the following day. The team determined that Mr. Settle was suffering from a ruptured appendicitis and allegedly performed an appendectomy. But Mr. Settle’s condition continued to deteriorate the following day.
Another CT scan followed. It revealed various problems – multiple fluid collections, an ileus, distended loops of the patient’s small bowel, a left renal cyst, subcentimeter mesenteric, and retroperitoneal adenopathy. Additional CT scans conducted 4 days later indicated other problems, including fluid collections in the patient’s right- and left-lower quadrants.
Over the next few days, doctors performed further exploratory laparotomies. Finally, on Nov. 22, Mr. Settle was transferred out of the intensive care unit in preparation for his discharge the following day.
His pain continued to worsen, however, and he was readmitted to CAMC a day later. At this point, an examination revealed that his surgical incisions had become infected.
Worse news was on the horizon. On Nov. 28, the trauma surgeon who had first agreed to treat Mr. Settle informed him that, despite claims to the contrary, his appendix hadn’t been removed.
Eventually, Mr. Settle was referred to the Cleveland Clinic, where at press time he was still being treated.
Mr. Settle has hired the firm Calwell Luce diTrapano to sue CAMC, accusing it of medical malpractice, medical negligence, and other lapses in the standard of care. In his complaint, he accused the hospital and its staff of breaching their duty of care “by negligently and improperly treating him” and by failing “to exercise the degree of care, skill, and learning required and expected of reasonable health care providers.”
His suit seeks not only compensatory damages and other relief but also punitive damages.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article originally appeared on Medscape.com.
How the Dobbs decision shapes the ObGyn workforce and training landscape

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
Characteristics of Matched vs Nonmatched Dermatology Applicants
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.
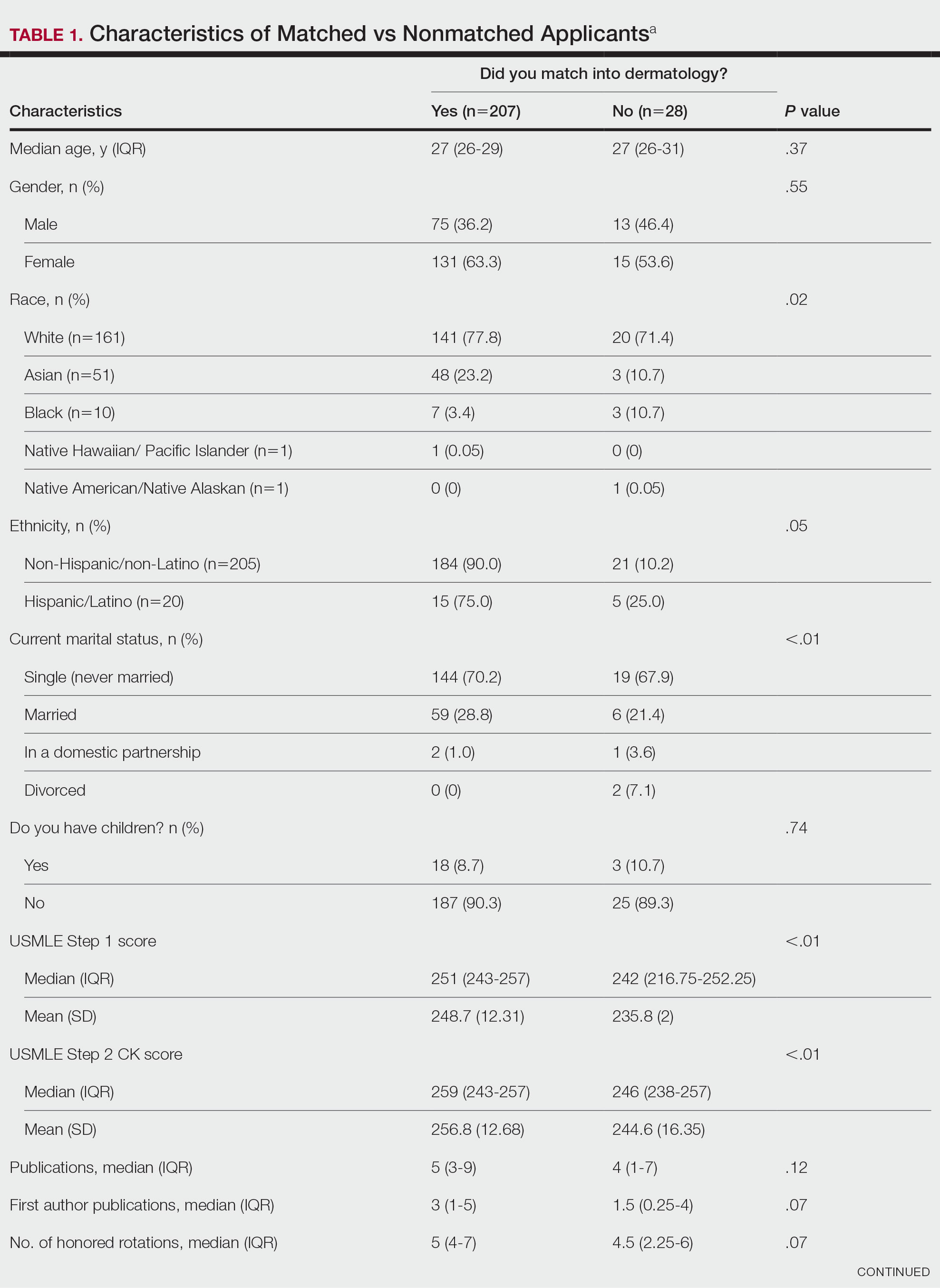
Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).
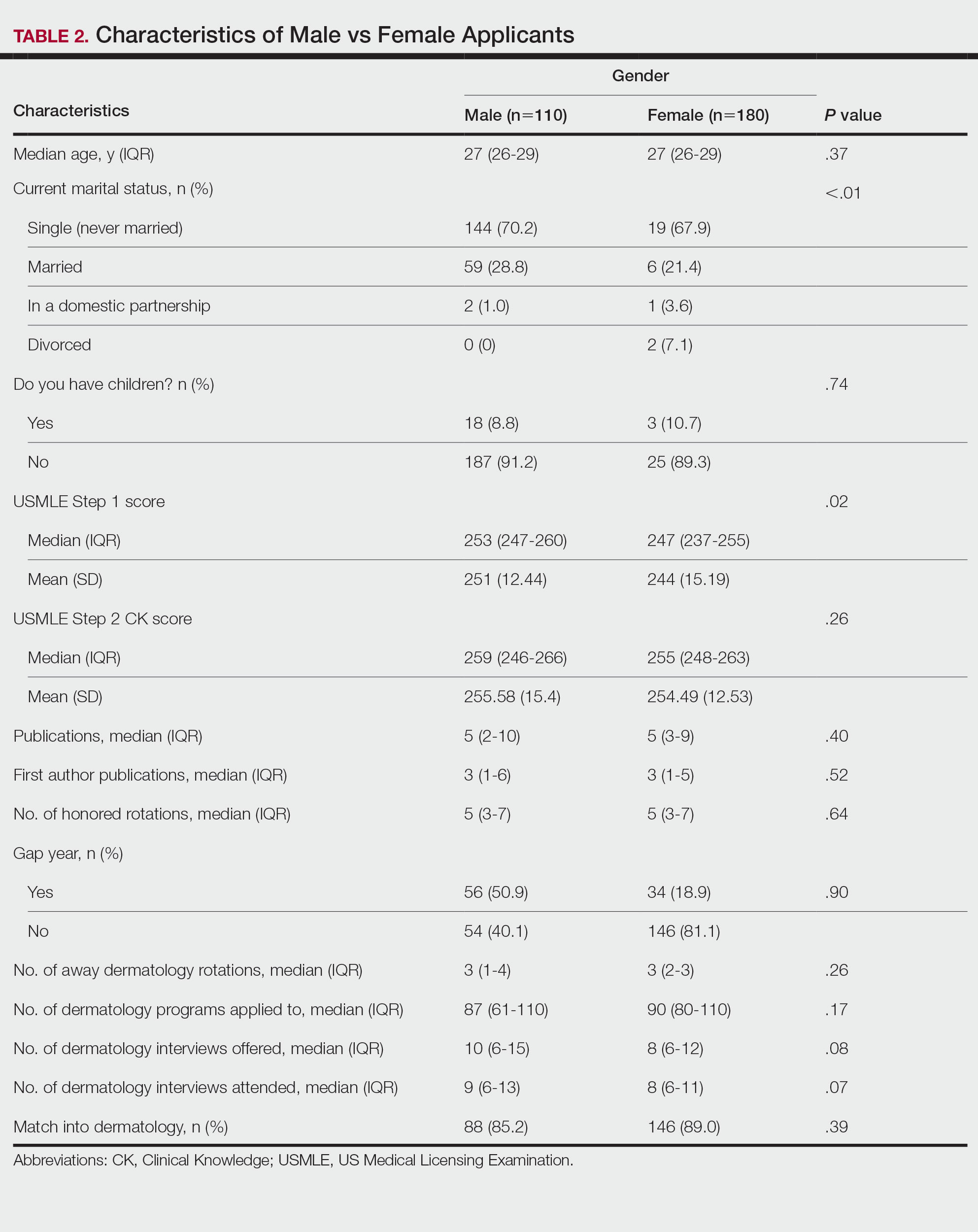
Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.
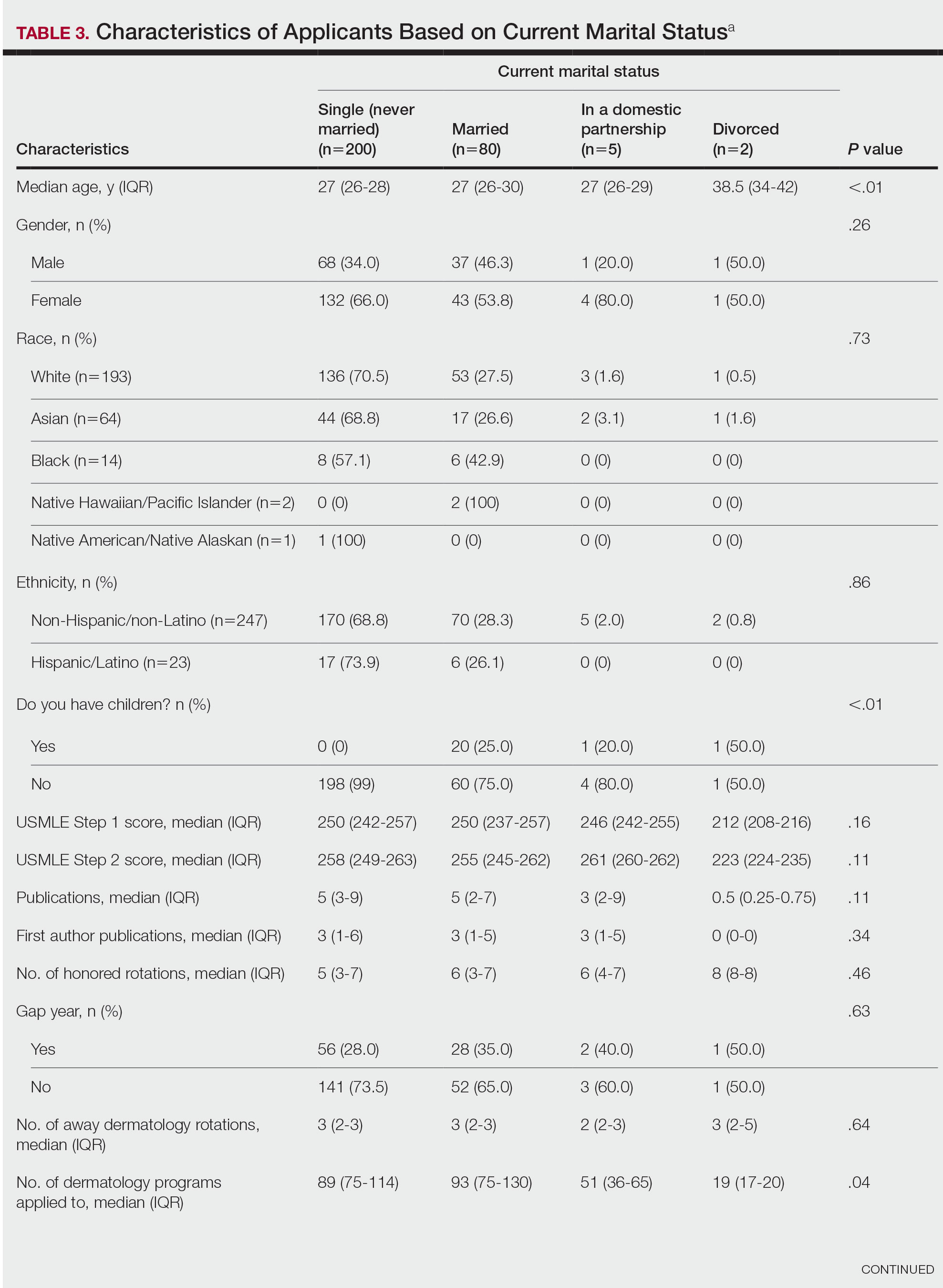
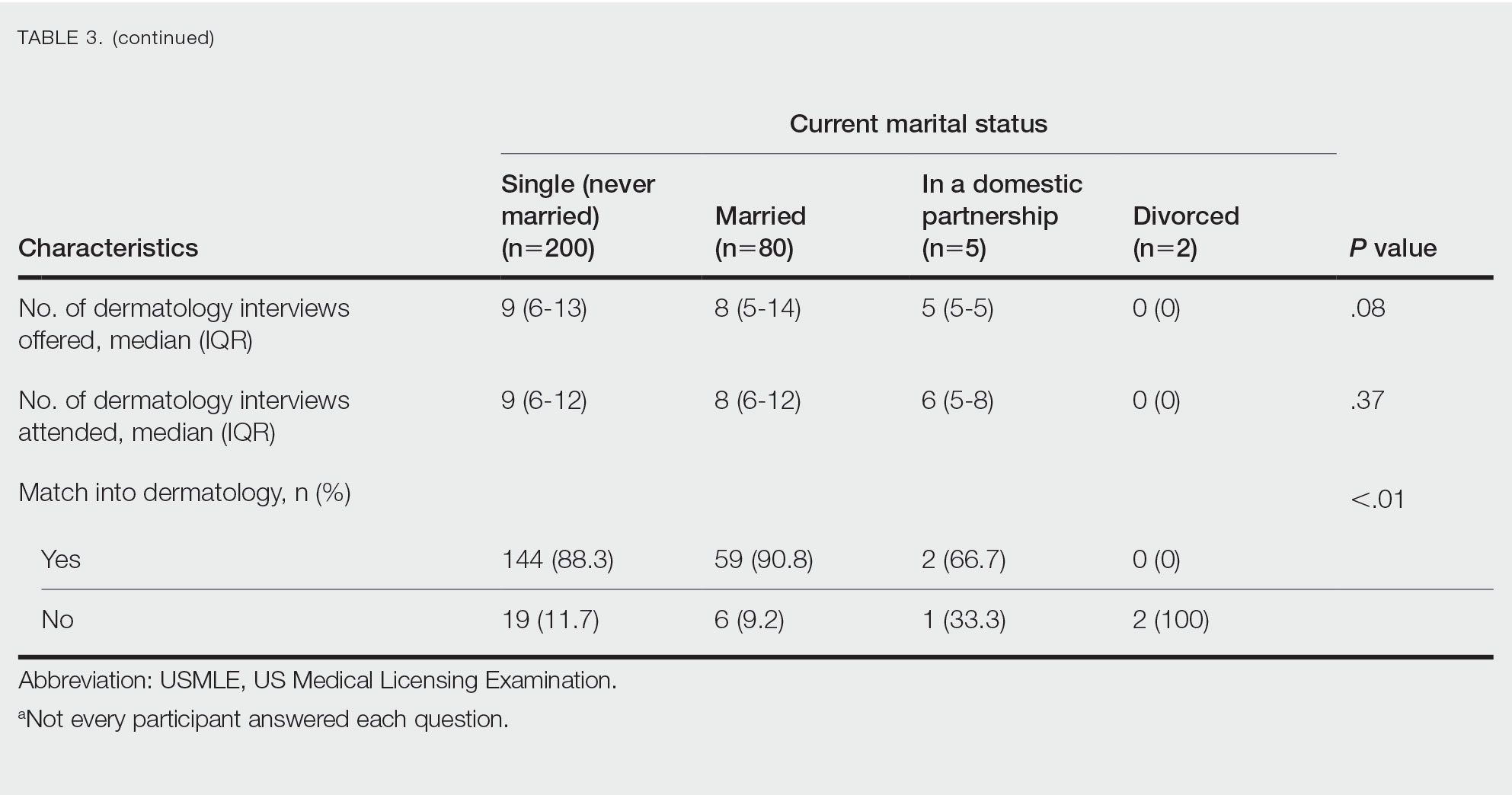
On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).
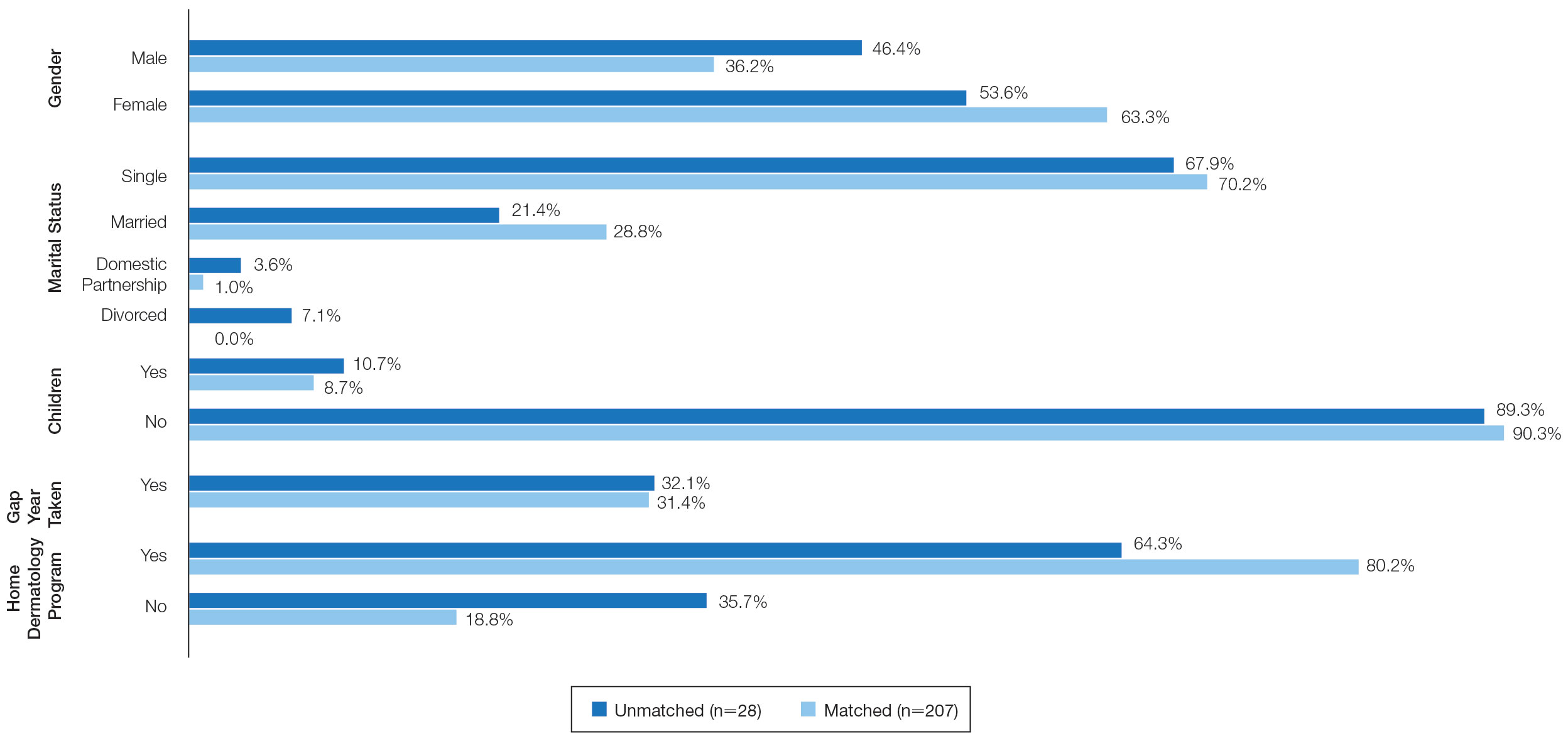
Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
PRACTICE POINTS
- Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% in 2019.
- A high US Medical Licensing Examination (USMLE) Step 1 score and having a home dermatology program and a greater number of interviews may lead to higher likeliness of matching in dermatology.
- Most applicants (82.4%) applied to programs their partner had interviews at, suggesting this may be a helpful strategy.
FDA OKs zanubrutinib for CLL or SLL
By giving the nod to these uses of this second-generation Bruton’s tyrosine kinase inhibitor, the FDA expanded on its previous approvals of this drug in mantle cell and marginal zone lymphoma.
“We have seen striking data from the Brukinsa development program demonstrating significant and consistent efficacy across CLL patient subtypes, including the high-risk del17p/TP53-mutated population, and regardless of treatment setting,” Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, said in a press release from drug developer BeiGene.
The FDA’s decision was based on two phase 3 trials – SEQUOIA and ALPINE. The SEQUOIA trial assessed 479 patients with treatment-naive CLL/SLL who either received zanubrutinib until disease progression or unacceptable toxicity or bendamustine plus rituximab for six cycles. Median progression-free survival was not reached in the zanubrutinib arm and was 33.7 months in the bendamustine plus rituximab arm (hazard ratio, 0.42).
In a separate, nonrandomized SEQUOIA cohort, investigators assessed zanubrutinib in patients with a 17p deletion and found an overall response rate of 88%. In addition, over the 25-month follow-up, the median duration of response was not reached.
The ALPINE trial included 652 patients with relapsed or refractory CLL/SLL who received either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm versus 73% in the ibrutinib arm, and the median duration of response was not reached in either arm over the 14-month follow-up period. Median progression-free survival was not reached in the zanubrutinib arm and was 35 months in the ibrutinib group.
Dr. Brown, a lead investigator on both drug trials, suggested that, given the improvements observed in progression-free survival, zanubrutinib could become the standard of care in this setting.
In the ALPINE trial, treatment discontinuation rate was lower among patients receiving zanubrutinib (26%) versus ibrutinib (41.2%), with most discontinuations a result of adverse events or progressive disease.
And across both trials, the most common adverse reactions were decreased neutrophil count (42%), upper respiratory tract infection (39%), decreased platelet count (34%), hemorrhage (30%), and musculoskeletal pain (30%).
A version of this article first appeared on Medscape.com.
By giving the nod to these uses of this second-generation Bruton’s tyrosine kinase inhibitor, the FDA expanded on its previous approvals of this drug in mantle cell and marginal zone lymphoma.
“We have seen striking data from the Brukinsa development program demonstrating significant and consistent efficacy across CLL patient subtypes, including the high-risk del17p/TP53-mutated population, and regardless of treatment setting,” Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, said in a press release from drug developer BeiGene.
The FDA’s decision was based on two phase 3 trials – SEQUOIA and ALPINE. The SEQUOIA trial assessed 479 patients with treatment-naive CLL/SLL who either received zanubrutinib until disease progression or unacceptable toxicity or bendamustine plus rituximab for six cycles. Median progression-free survival was not reached in the zanubrutinib arm and was 33.7 months in the bendamustine plus rituximab arm (hazard ratio, 0.42).
In a separate, nonrandomized SEQUOIA cohort, investigators assessed zanubrutinib in patients with a 17p deletion and found an overall response rate of 88%. In addition, over the 25-month follow-up, the median duration of response was not reached.
The ALPINE trial included 652 patients with relapsed or refractory CLL/SLL who received either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm versus 73% in the ibrutinib arm, and the median duration of response was not reached in either arm over the 14-month follow-up period. Median progression-free survival was not reached in the zanubrutinib arm and was 35 months in the ibrutinib group.
Dr. Brown, a lead investigator on both drug trials, suggested that, given the improvements observed in progression-free survival, zanubrutinib could become the standard of care in this setting.
In the ALPINE trial, treatment discontinuation rate was lower among patients receiving zanubrutinib (26%) versus ibrutinib (41.2%), with most discontinuations a result of adverse events or progressive disease.
And across both trials, the most common adverse reactions were decreased neutrophil count (42%), upper respiratory tract infection (39%), decreased platelet count (34%), hemorrhage (30%), and musculoskeletal pain (30%).
A version of this article first appeared on Medscape.com.
By giving the nod to these uses of this second-generation Bruton’s tyrosine kinase inhibitor, the FDA expanded on its previous approvals of this drug in mantle cell and marginal zone lymphoma.
“We have seen striking data from the Brukinsa development program demonstrating significant and consistent efficacy across CLL patient subtypes, including the high-risk del17p/TP53-mutated population, and regardless of treatment setting,” Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute in Boston, said in a press release from drug developer BeiGene.
The FDA’s decision was based on two phase 3 trials – SEQUOIA and ALPINE. The SEQUOIA trial assessed 479 patients with treatment-naive CLL/SLL who either received zanubrutinib until disease progression or unacceptable toxicity or bendamustine plus rituximab for six cycles. Median progression-free survival was not reached in the zanubrutinib arm and was 33.7 months in the bendamustine plus rituximab arm (hazard ratio, 0.42).
In a separate, nonrandomized SEQUOIA cohort, investigators assessed zanubrutinib in patients with a 17p deletion and found an overall response rate of 88%. In addition, over the 25-month follow-up, the median duration of response was not reached.
The ALPINE trial included 652 patients with relapsed or refractory CLL/SLL who received either zanubrutinib or ibrutinib. The overall response rate was 80% in the zanubrutinib arm versus 73% in the ibrutinib arm, and the median duration of response was not reached in either arm over the 14-month follow-up period. Median progression-free survival was not reached in the zanubrutinib arm and was 35 months in the ibrutinib group.
Dr. Brown, a lead investigator on both drug trials, suggested that, given the improvements observed in progression-free survival, zanubrutinib could become the standard of care in this setting.
In the ALPINE trial, treatment discontinuation rate was lower among patients receiving zanubrutinib (26%) versus ibrutinib (41.2%), with most discontinuations a result of adverse events or progressive disease.
And across both trials, the most common adverse reactions were decreased neutrophil count (42%), upper respiratory tract infection (39%), decreased platelet count (34%), hemorrhage (30%), and musculoskeletal pain (30%).
A version of this article first appeared on Medscape.com.
A freak impalement by a model rocket has this doctor scrambling
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
North central Washington state is a lot of nothing other than fields. Every year, the Federal Aviation Administration closes the airspace in a remote part of the area for a model rocket competition, the National Association of Rocketry Annual Meet. It’s a 2-day event and a pretty big deal. People come from all over the country to be there.
When you were a kid, you probably saw those rockets that are 3 feet tall. You launch them up in the air, they have a little parachute that comes out and they come back down to the ground. Well, picture that on ultimate steroids. There are anywhere from 3-foot to almost 20-foot-long rockets at this thing. People show up with horse trailers full of rockets and components. I mean, it’s an obsession.
Some of these rockets are super sophisticated. They have different stages where the first stage burns out and the second takes over. They go up thousands of feet to the edge of the stratosphere. Most of them have GoPro cameras, so you get to see when the rocket reaches the top of its trajectory and the last engine burns out. As it starts to descend, a parachute deploys and it can drift back anywhere from pretty close to where you launched it to a couple miles away. Then you use your little GPS to find it.
Why not? I drove out there and parked my Jeep and was walking over to the competition when I noticed something off. A bigger commotion than there should have been.
Here’s what happened 2 minutes before I got there:
A 5-foot-long rocket, 2½ inches in diameter, had reached the top of its several thousand–foot trajectory and was ready to come back to Earth. But its parachute didn’t deploy. It turned itself point-down and literally shot back to earth like a rocket.
It had gone up pretty darn straight and came down just as straight – right into a circle of people sitting in lawn chairs.
It hit a middle-aged man. But you can’t imagine how. First of all, who knows how fast it was going. The point glanced off his forehead and ... how to describe the rest. The man was pretty heavy. So the rocket impaled him through the abdomen and stuck right into the ground. As in, the point entered the top of his belly just below chest level and came out the bottom of his belly. The rocket pinned him to the ground through his belly.
Well, this was not how I planned on spending my day. But my spectator time was over. There were a lot of people running around in circles where he was pinned, not really knowing what to do.
When I said I was an emergency physician, instantly 15 heads looked right at me for direction like, Oh my gosh, please take over! A lot of people were asking: “What can I do? What can I do?” I said: “Well, we don’t need to do CPR. What we really need to do is get this rocket out of the ground. We need to keep him still while we dig out the rocket and get him flat.”
People gently dug around the nose of the rocket. It was in about 6 or 8 inches, enough that we didn’t want to just yank on it (I still marvel at how fast it must have been traveling to both impale the man the way it did and also jam into the ground like that). We wanted to loosen it up and ease it out of the ground.
We managed to dig the nose out and get the guy on his back. Needless to say, he wasn’t particularly comfortable. He looked pretty ashen, like he was in pretty good trouble.
The festival had an EMS kit with some bandages in it, but not a whole lot else. There’s the old joke in emergency medicine: What can you do with duct tape, a Swiss army knife, and a paper clip? It’s like, what has anybody got that might work here?
What we really needed to do was keep both the rocket and the man from moving. We cut off his shirt and got his pants down so that I could better see where it entered and exited. Then we used a couple of clean T-shirts to stabilize the rocket so it didn’t move while he lay flat. It didn’t bleed all that much. And his belly wasn’t massively expanding like he was bleeding internally. I mean, he looked crappy. But so would I!
We were about an hour away from the closest EMS and only a couple people even had cell service out there. But we managed to get hold of EMS. It was also one of those 92-degree days with no shade for 50 miles in any direction.
There was a volunteer firefighter there to man the fire rig. He helped carry the guy into an air-conditioned trailer without moving him very much.
Basically, we stabilized him by keeping him super still and as comfortable as we could until EMS arrived. I rode with him about an hour and a half to the closest trauma center in Central Washington. He was conscious, which was lousy for him but reassuring for me. “You’re still talking to me,” I said. “I think you’re going to be okay.”
One of the take-home points from a medical point of view is never try to remove something sticking out of someone when you’re out in the field. If it’s pushing against something vital, you could do a lot of damage, and if it’s up against a blood vessel, that vessel’s going to bleed uncontrollably.
We got to the trauma center and they took him to the OR. By the grace of friendships, somebody got his wife to the hospital. She was calmer than I think I would have been if my spouse had been hit by a rocket.
The full diagnostic story: The rocket bouncing off his forehead gave him a small skull fracture and slight concussion. That was no big deal. But picture this: The rocket only went through his belly fat. It didn’t hit any of his abdominal organs! I still think this is absolutely amazing. If he had been leaning forward in his lawn chair even a few inches, the rocket would’ve gone through his head and that would’ve been all they wrote.
He stayed in the hospital for a couple of days. I never saw him again, but I received follow-up from the surgeon. And I read the paper the next day. Let me tell you, in Central Washington, this is pretty big news.
It wasn’t the way I’d planned my morning. But you just can’t predict that kind of thing. I don’t know, maybe spiritually or karma wise, I was meant to show up about 90 seconds after he’d been hit. The only emergency physician at the whole event, just by chance. My work blesses me with a certain skill set. I know when to really worry, how to go about keeping somebody safe until you can get them to the ED. It’s something I thank my stars for every single day.
As I said to the guy on the way to the hospital: “Well, it’s not your lucky day, but it sure as heck could have been a whole lot unluckier.”
Stephen Anderson, MD, is an emergency medicine physician in Auburn, Washington and is affiliated with MultiCare Auburn Medical Center.
A version of this article first appeared on Medscape.com.
Will your smartphone be the next doctor’s office?
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.








