User login
Once-Weekly Insulin Better Than Daily in Type 2 Diabetes
TOPLINE:
Once-weekly insulin icodec shows a higher glycated A1c reduction than once-daily basal insulin analogs in patients with type 2 diabetes (T2D), without major safety concerns.
METHODOLOGY:
- A meta-analysis of five phase 3 ONWARDS randomized controlled trials included 3764 patients with T2D.
- The trials compared the effects of the weekly insulin icodec with those of the daily basal insulin analogs glargine and degludec over 26-78 months.
- The primary outcome was the change in A1c levels.
- Secondary outcomes included fasting plasma glucose levels, A1c levels < 7%, time in target glycemic range, body weight changes, insulin dose, hypoglycemia events, and adverse events.
TAKEAWAY:
- A1c levels < 7% were observed in a higher percentage of patients in the insulin icodec group than in the comparator group (odds ratio, 1.51; P = .004).
- In subgroup analyses, insulin icodec was superior to insulin degludec by several measures but comparatively similar to glargine.
- Insulin icodec was associated with no major safety concerns and had a slightly higher incidence of levels 1, 2, and combined 2/3 than degludec but no significant differences compared with glargine.
IN PRACTICE:
“Sustained glycemic control with once-weekly injections of insulin icodec would lead to better patient acceptance and treatment satisfaction,” the authors wrote.
SOURCE:
This study, authored by Sahana Shetty, MD, and Renuka Suvarna, MSc, Manipal Academy of Higher Education, Department of Endocrinology, Kasturba Medical College, Manipal, Karnataka, was published online on January 8, 2024, in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The comparator group included individuals who used different basal insulin analogs. This heterogeneity in the comparator group introduced a potential source of variability, making it challenging to isolate the specific effects of insulin icodec compared with a standardized comparator. Blinding or masking of participants was performed in only one of the five trials.
DISCLOSURES:
The authors declared no conflicts of interest. All five clinical trials in the meta-analysis were sponsored by Novo Nordisk.
A version of this article appeared on Medscape.com.
TOPLINE:
Once-weekly insulin icodec shows a higher glycated A1c reduction than once-daily basal insulin analogs in patients with type 2 diabetes (T2D), without major safety concerns.
METHODOLOGY:
- A meta-analysis of five phase 3 ONWARDS randomized controlled trials included 3764 patients with T2D.
- The trials compared the effects of the weekly insulin icodec with those of the daily basal insulin analogs glargine and degludec over 26-78 months.
- The primary outcome was the change in A1c levels.
- Secondary outcomes included fasting plasma glucose levels, A1c levels < 7%, time in target glycemic range, body weight changes, insulin dose, hypoglycemia events, and adverse events.
TAKEAWAY:
- A1c levels < 7% were observed in a higher percentage of patients in the insulin icodec group than in the comparator group (odds ratio, 1.51; P = .004).
- In subgroup analyses, insulin icodec was superior to insulin degludec by several measures but comparatively similar to glargine.
- Insulin icodec was associated with no major safety concerns and had a slightly higher incidence of levels 1, 2, and combined 2/3 than degludec but no significant differences compared with glargine.
IN PRACTICE:
“Sustained glycemic control with once-weekly injections of insulin icodec would lead to better patient acceptance and treatment satisfaction,” the authors wrote.
SOURCE:
This study, authored by Sahana Shetty, MD, and Renuka Suvarna, MSc, Manipal Academy of Higher Education, Department of Endocrinology, Kasturba Medical College, Manipal, Karnataka, was published online on January 8, 2024, in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The comparator group included individuals who used different basal insulin analogs. This heterogeneity in the comparator group introduced a potential source of variability, making it challenging to isolate the specific effects of insulin icodec compared with a standardized comparator. Blinding or masking of participants was performed in only one of the five trials.
DISCLOSURES:
The authors declared no conflicts of interest. All five clinical trials in the meta-analysis were sponsored by Novo Nordisk.
A version of this article appeared on Medscape.com.
TOPLINE:
Once-weekly insulin icodec shows a higher glycated A1c reduction than once-daily basal insulin analogs in patients with type 2 diabetes (T2D), without major safety concerns.
METHODOLOGY:
- A meta-analysis of five phase 3 ONWARDS randomized controlled trials included 3764 patients with T2D.
- The trials compared the effects of the weekly insulin icodec with those of the daily basal insulin analogs glargine and degludec over 26-78 months.
- The primary outcome was the change in A1c levels.
- Secondary outcomes included fasting plasma glucose levels, A1c levels < 7%, time in target glycemic range, body weight changes, insulin dose, hypoglycemia events, and adverse events.
TAKEAWAY:
- A1c levels < 7% were observed in a higher percentage of patients in the insulin icodec group than in the comparator group (odds ratio, 1.51; P = .004).
- In subgroup analyses, insulin icodec was superior to insulin degludec by several measures but comparatively similar to glargine.
- Insulin icodec was associated with no major safety concerns and had a slightly higher incidence of levels 1, 2, and combined 2/3 than degludec but no significant differences compared with glargine.
IN PRACTICE:
“Sustained glycemic control with once-weekly injections of insulin icodec would lead to better patient acceptance and treatment satisfaction,” the authors wrote.
SOURCE:
This study, authored by Sahana Shetty, MD, and Renuka Suvarna, MSc, Manipal Academy of Higher Education, Department of Endocrinology, Kasturba Medical College, Manipal, Karnataka, was published online on January 8, 2024, in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The comparator group included individuals who used different basal insulin analogs. This heterogeneity in the comparator group introduced a potential source of variability, making it challenging to isolate the specific effects of insulin icodec compared with a standardized comparator. Blinding or masking of participants was performed in only one of the five trials.
DISCLOSURES:
The authors declared no conflicts of interest. All five clinical trials in the meta-analysis were sponsored by Novo Nordisk.
A version of this article appeared on Medscape.com.
Combo Tx Best in Metastatic Prostate Cancer with HRR Mutations
That’s the conclusion of investigators in the phase 2 BRCAAway trial, which compared a combination of abiraterone (Zytiga) and prednisone plus olaparib (Lynparza) against sequential therapy with the same agents.
At the time of data cutoff, median progression-free survival (PFS), the primary endpoint, was 39 months for patients randomized to the combination, compared with 8.4 months for those assigned to abiraterone/prednisone, and 14 months for those assigned to olaparib monotherapy, reported Maha Hussain, MD, of the Robert H. Lurie Comprehensive Cancer Center in Chicago.
“In patients with metastatic castration-resistant prostate cancer [mCRPC] and BRCA1/2 or ATM alterations, abiraterone and prednisone plus olaparib was well tolerated and resulted in better progression-free survival and response rates vs. single-agent olaparib or abiraterone/prednisone,” she said in an oral abstract presentation at the ASCO Genitourinary Cancers Symposium.
Although the study allowed crossover between the single-agent arms at the time of progression, only a few patients made the switch. Nonetheless, in these patients the PFS with the frontline combination was superior to that of sequential therapy, she noted.
Study Rationale and Design
Germline or somatic mutations in genes encoding for homologous recombination-repair occur in about 20% of men with mCRPC. Olaparib, a PARP1 (poly-adp ribose polymerase-1) inhibitor, interacts with androgen signaling, and preclinical studies have shown that castration-resistant prostate tumor cells have increased PARP1 activity. In addition, PARP1 has been shown preclinically to synergize with androgen receptor pathway inhibitors (ARPIs) such as abiraterone, Dr. Hussain explained.
The BRCAAway trial was designed to test whether co-targeting the androgen receptor and PARP1 could result in higher and more durable responses than current frontline therapies in patients with mCRPC with DNA-damage response mutations.
Patients with mCRPC with no prior exposure to either a PARP1 inhibitor, androgen receptor inhibitor, or mCRPC-directed chemotherapy underwent next-generation sequencing and germline testing of tumor tissues, and those patients found to have inactivating BRCA1/2 and/or ATM alterations were randomized on a 1:1:1 basis to either abiraterone 1000 mg daily plus prednisone 5 mg twice daily (19 patients); olaparib 300 mg twice daily (21 patients); or to the combination (21 patients).
The primary endpoint was radiographic PFS according to RECIST 1.1 criteria, Prostate Cancer Working Group 3 criteria, clinical assessment, or death.
As noted, the median PFS was 8.4 months with abiraterone/prednisone, 14 months with olaparib, and 39 months with the combination.
Secondary endpoints also favored the combination therapy arm, with objective response rates of 22%, 14%, and 33%, respectively; PSA response rates of 61%, 67% and 95%; and undetectable PSA response rates of 17%, 14%, and 33%.
A total of 8 of 19 patients on abiraterone were crossed over to olaparib, and 8 of 21 initially assigned to olaparib were crossed over to abiraterone. In these patients the median PFS from crossover was 8.3 and 7.2 months, respectively. In each crossover group the median PFS from the time of randomization was 16 months.
There were no grade 4 adverse events or treatment-related deaths reported in any of the study arms, and “essentially when you look at the adverse events, they pretty much are consistent with what you would expect to see with these particular agents,” Dr. Hussain said.
“Overall the patients were tolerating the treatment well,” she added.
Practice Changing with Caveats
Kim N. Chi, MD, FRCPC, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant, said that the strengths of the study included an olaparib monotherapy arm — something that was missing from phase 3 trials — that provides insights into how PARP inhibitors perform in this population. He also applauded the inclusion of clinical assessment as a primary endpoint, noting that “this is what we do in routine practice, and therefore, the generalizability of the trial becomes more evident.”
The crossover design provides important information about whether an upfront combination or a sequential therapy approach is more effective, as well, he added.
He pointed out, however, that the trial was limited by small sample size and by its “horse race” design rather than as a comparison trial.
“So how does the BRCAAway trial change our practice? Despite the limitations, I think it does support an upfront PARP inhibitor-ARPI combination as firstline therapy for HRR gene-mutated metastatic CRPC. These data suggest synergy, and most importantly, there is no loss of opportunity [for more effective therapies]. However, the limitations of the trial will not end this debate today,” he said.
The trial was funded by AstraZeneca. Both Dr. Hussain and Dr. Chi disclosed honoraria, consulting/advising, and institutional research funding from AstraZeneca and others.
That’s the conclusion of investigators in the phase 2 BRCAAway trial, which compared a combination of abiraterone (Zytiga) and prednisone plus olaparib (Lynparza) against sequential therapy with the same agents.
At the time of data cutoff, median progression-free survival (PFS), the primary endpoint, was 39 months for patients randomized to the combination, compared with 8.4 months for those assigned to abiraterone/prednisone, and 14 months for those assigned to olaparib monotherapy, reported Maha Hussain, MD, of the Robert H. Lurie Comprehensive Cancer Center in Chicago.
“In patients with metastatic castration-resistant prostate cancer [mCRPC] and BRCA1/2 or ATM alterations, abiraterone and prednisone plus olaparib was well tolerated and resulted in better progression-free survival and response rates vs. single-agent olaparib or abiraterone/prednisone,” she said in an oral abstract presentation at the ASCO Genitourinary Cancers Symposium.
Although the study allowed crossover between the single-agent arms at the time of progression, only a few patients made the switch. Nonetheless, in these patients the PFS with the frontline combination was superior to that of sequential therapy, she noted.
Study Rationale and Design
Germline or somatic mutations in genes encoding for homologous recombination-repair occur in about 20% of men with mCRPC. Olaparib, a PARP1 (poly-adp ribose polymerase-1) inhibitor, interacts with androgen signaling, and preclinical studies have shown that castration-resistant prostate tumor cells have increased PARP1 activity. In addition, PARP1 has been shown preclinically to synergize with androgen receptor pathway inhibitors (ARPIs) such as abiraterone, Dr. Hussain explained.
The BRCAAway trial was designed to test whether co-targeting the androgen receptor and PARP1 could result in higher and more durable responses than current frontline therapies in patients with mCRPC with DNA-damage response mutations.
Patients with mCRPC with no prior exposure to either a PARP1 inhibitor, androgen receptor inhibitor, or mCRPC-directed chemotherapy underwent next-generation sequencing and germline testing of tumor tissues, and those patients found to have inactivating BRCA1/2 and/or ATM alterations were randomized on a 1:1:1 basis to either abiraterone 1000 mg daily plus prednisone 5 mg twice daily (19 patients); olaparib 300 mg twice daily (21 patients); or to the combination (21 patients).
The primary endpoint was radiographic PFS according to RECIST 1.1 criteria, Prostate Cancer Working Group 3 criteria, clinical assessment, or death.
As noted, the median PFS was 8.4 months with abiraterone/prednisone, 14 months with olaparib, and 39 months with the combination.
Secondary endpoints also favored the combination therapy arm, with objective response rates of 22%, 14%, and 33%, respectively; PSA response rates of 61%, 67% and 95%; and undetectable PSA response rates of 17%, 14%, and 33%.
A total of 8 of 19 patients on abiraterone were crossed over to olaparib, and 8 of 21 initially assigned to olaparib were crossed over to abiraterone. In these patients the median PFS from crossover was 8.3 and 7.2 months, respectively. In each crossover group the median PFS from the time of randomization was 16 months.
There were no grade 4 adverse events or treatment-related deaths reported in any of the study arms, and “essentially when you look at the adverse events, they pretty much are consistent with what you would expect to see with these particular agents,” Dr. Hussain said.
“Overall the patients were tolerating the treatment well,” she added.
Practice Changing with Caveats
Kim N. Chi, MD, FRCPC, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant, said that the strengths of the study included an olaparib monotherapy arm — something that was missing from phase 3 trials — that provides insights into how PARP inhibitors perform in this population. He also applauded the inclusion of clinical assessment as a primary endpoint, noting that “this is what we do in routine practice, and therefore, the generalizability of the trial becomes more evident.”
The crossover design provides important information about whether an upfront combination or a sequential therapy approach is more effective, as well, he added.
He pointed out, however, that the trial was limited by small sample size and by its “horse race” design rather than as a comparison trial.
“So how does the BRCAAway trial change our practice? Despite the limitations, I think it does support an upfront PARP inhibitor-ARPI combination as firstline therapy for HRR gene-mutated metastatic CRPC. These data suggest synergy, and most importantly, there is no loss of opportunity [for more effective therapies]. However, the limitations of the trial will not end this debate today,” he said.
The trial was funded by AstraZeneca. Both Dr. Hussain and Dr. Chi disclosed honoraria, consulting/advising, and institutional research funding from AstraZeneca and others.
That’s the conclusion of investigators in the phase 2 BRCAAway trial, which compared a combination of abiraterone (Zytiga) and prednisone plus olaparib (Lynparza) against sequential therapy with the same agents.
At the time of data cutoff, median progression-free survival (PFS), the primary endpoint, was 39 months for patients randomized to the combination, compared with 8.4 months for those assigned to abiraterone/prednisone, and 14 months for those assigned to olaparib monotherapy, reported Maha Hussain, MD, of the Robert H. Lurie Comprehensive Cancer Center in Chicago.
“In patients with metastatic castration-resistant prostate cancer [mCRPC] and BRCA1/2 or ATM alterations, abiraterone and prednisone plus olaparib was well tolerated and resulted in better progression-free survival and response rates vs. single-agent olaparib or abiraterone/prednisone,” she said in an oral abstract presentation at the ASCO Genitourinary Cancers Symposium.
Although the study allowed crossover between the single-agent arms at the time of progression, only a few patients made the switch. Nonetheless, in these patients the PFS with the frontline combination was superior to that of sequential therapy, she noted.
Study Rationale and Design
Germline or somatic mutations in genes encoding for homologous recombination-repair occur in about 20% of men with mCRPC. Olaparib, a PARP1 (poly-adp ribose polymerase-1) inhibitor, interacts with androgen signaling, and preclinical studies have shown that castration-resistant prostate tumor cells have increased PARP1 activity. In addition, PARP1 has been shown preclinically to synergize with androgen receptor pathway inhibitors (ARPIs) such as abiraterone, Dr. Hussain explained.
The BRCAAway trial was designed to test whether co-targeting the androgen receptor and PARP1 could result in higher and more durable responses than current frontline therapies in patients with mCRPC with DNA-damage response mutations.
Patients with mCRPC with no prior exposure to either a PARP1 inhibitor, androgen receptor inhibitor, or mCRPC-directed chemotherapy underwent next-generation sequencing and germline testing of tumor tissues, and those patients found to have inactivating BRCA1/2 and/or ATM alterations were randomized on a 1:1:1 basis to either abiraterone 1000 mg daily plus prednisone 5 mg twice daily (19 patients); olaparib 300 mg twice daily (21 patients); or to the combination (21 patients).
The primary endpoint was radiographic PFS according to RECIST 1.1 criteria, Prostate Cancer Working Group 3 criteria, clinical assessment, or death.
As noted, the median PFS was 8.4 months with abiraterone/prednisone, 14 months with olaparib, and 39 months with the combination.
Secondary endpoints also favored the combination therapy arm, with objective response rates of 22%, 14%, and 33%, respectively; PSA response rates of 61%, 67% and 95%; and undetectable PSA response rates of 17%, 14%, and 33%.
A total of 8 of 19 patients on abiraterone were crossed over to olaparib, and 8 of 21 initially assigned to olaparib were crossed over to abiraterone. In these patients the median PFS from crossover was 8.3 and 7.2 months, respectively. In each crossover group the median PFS from the time of randomization was 16 months.
There were no grade 4 adverse events or treatment-related deaths reported in any of the study arms, and “essentially when you look at the adverse events, they pretty much are consistent with what you would expect to see with these particular agents,” Dr. Hussain said.
“Overall the patients were tolerating the treatment well,” she added.
Practice Changing with Caveats
Kim N. Chi, MD, FRCPC, of the University of British Columbia in Vancouver, BC, Canada, the invited discussant, said that the strengths of the study included an olaparib monotherapy arm — something that was missing from phase 3 trials — that provides insights into how PARP inhibitors perform in this population. He also applauded the inclusion of clinical assessment as a primary endpoint, noting that “this is what we do in routine practice, and therefore, the generalizability of the trial becomes more evident.”
The crossover design provides important information about whether an upfront combination or a sequential therapy approach is more effective, as well, he added.
He pointed out, however, that the trial was limited by small sample size and by its “horse race” design rather than as a comparison trial.
“So how does the BRCAAway trial change our practice? Despite the limitations, I think it does support an upfront PARP inhibitor-ARPI combination as firstline therapy for HRR gene-mutated metastatic CRPC. These data suggest synergy, and most importantly, there is no loss of opportunity [for more effective therapies]. However, the limitations of the trial will not end this debate today,” he said.
The trial was funded by AstraZeneca. Both Dr. Hussain and Dr. Chi disclosed honoraria, consulting/advising, and institutional research funding from AstraZeneca and others.
FROM ASCO GU 2024
New Criteria Identify Sepsis in Children With Infection
New criteria for pediatric sepsis, based on a novel score that predicts mortality in children with suspected or confirmed infection, perform better than existing organ dysfunction scores and criteria and have the potential to improve clinical care globally, researchers say.
Current pediatric-specific criteria for sepsis were published in 2005, based on expert opinion. In 2016, sepsis was redefined for adults as life-threatening organ dysfunction caused by a dysregulated host response to infection, as opposed to an earlier focus on systemic inflammation. But the paradigm-shifting changes were not extended to children (< 18 years, but not newborns), setting the stage for the new initiative.
The new criteria, and their development and validation, were published in JAMA and presented the same day at the Society of Critical Care Medicine’s 2024 Critical Care Congress in Phoenix, Arizona.
International Consensus
“The new criteria we derived are based on data from electronic health records and analysis of more than 3 million pediatric healthcare encounters from 10 hospitals around the world, including in low-resource settings,” L. Nelson Sanchez-Pinto, MD, MBI, a critical care physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, told this news organization.
Dr. Sanchez-Pinto co-led the data group of the international expert task force convened by the Society of Critical Care Medicine (SCCM) to develop and validate the criteria, which are based on evidence from an international survey, systematic review and meta-analysis, a newly created organ dysfunction score (Phoenix Sepsis Score), and sites on four continents.
Based on the findings, the task force now suggests that pediatric sepsis be defined by a Phoenix Sepsis Score of at least 2 points in children with suspected infection, which indicates potentially life-threatening dysfunction of the respiratory, cardiovascular, coagulation, and/or neurological systems. Septic shock is defined as sepsis with at least 1 cardiovascular point in the score.
Disparities Across Settings
To derive and validate the new criteria across differently resourced settings, the researchers conducted a multicenter, international, retrospective cohort study involving 10 health systems in the United States, Colombia, Bangladesh, China, and Kenya, 3 of which were used as external validation sites.
Data were collected from pediatric emergency and inpatient encounters from 2010 to 2019. The development set comprised 3,049,699 children, and the external validation set included 581,317.
Stacked regression models to predict mortality in children with suspected infection were derived and validated using the best-performing organ dysfunction subscores from eight existing scores.
The final model was then translated into the integer-based Phoenix Sepsis Score and used to establish binary criteria for sepsis and septic shock.
Among 172,984 children with suspected infection in the first 24 hours (development set; 1.2% mortality), a four-organ-system model performed best. The Phoenix Sepsis Score — the integer version of the model — had areas under the precision recall curve of 0.23 to 0.38, and areas under the receiver operating characteristic curve of 0.71 to 0.92 to predict mortality in the validation sets.
A Phoenix Sepsis Score of 2 points or higher in children with suspected infection as criteria for sepsis, plus 1 or more cardiovascular points as criteria for septic shock, resulted in a higher positive predictive value and higher or similar sensitivity compared with the 2005 International Pediatric Sepsis Consensus Conference criteria across differently resourced settings.
Specifically, children with a Phoenix Sepsis Score of at least 2 points had in-hospital mortality of 7.1% in higher-resource settings and 28.5% in lower-resource settings — more than 8 times that of children with suspected infection not meeting these criteria.
Mortality also was higher in children who had organ dysfunction in at least one of four organ systems — respiratory, cardiovascular, coagulation, and/or neurological — that was not the primary site of infection.
Children with septic shock, indicated by at least 1 cardiovascular point in the Phoenix Sepsis Score, had severe hypotension for age, blood lactate exceeding 5 mmol/L, or need for vasoactive medication. These children had an in-hospital mortality rate of 10.8% in higher-resource settings and 33.5% in lower-resource settings.
A Better Score
Given the findings, the task force recommends that “the former criteria based on systemic inflammatory response syndrome should not be used to diagnose sepsis in children [and] the former term severe sepsis should no longer be used because sepsis is life-threatening organ dysfunction associated with infection and is thus indicative of a severe disease state.”
The task force cautions that although the four organs in the Phoenix Sepsis Score are most commonly involved in sepsis, “this does not diminish the crucial importance of the assessment and management of other organ dysfunction.”
Furthermore, they emphasize that the Phoenix score was designed to identify sepsis in children, not to screen children at risk for developing sepsis or early identification of children with suspected sepsis.
Additional Considerations
In related editorials, commentators noted some caveats and concerns with regard to the study design and the new criteria.
Roberto Jabornisky, MD, PhD, of National University of the Northeast, Corrientes, Argentina, and colleagues pointed out that “all the low-resource validation sites were institutions with electronic health records and most had PICUs [pediatric intensive care units], which does not adequately reflect conditions in most low-resource settings. These factors introduce a distinct bias favoring a ‘PICU-based consensus,’ potentially limiting the generalizability and adoption of the new criteria by health care practitioners in non-PICU and nonhospital settings responsible for recognizing and managing children with sepsis.” The editorialists called for additional prospective validation in differently resourced settings, especially those with the highest disease burdens.
“Until then,” they wrote, “it is essential to refrain from considering these criteria as an inflexible directive governing medical interventions for pediatric sepsis. No definition can fully substitute for the clinical judgment of an experienced, vigilant clinician caring for an unwell child.”
Erin F. Carlton, MD, MSc of the University of Michigan, Ann Arbor, and colleagues added in a separate editorial, “The Phoenix criteria identify a sicker subset of patients than prior SIRS [systemic inflammatory response syndrome]-based criteria. Some may worry this higher threshold could delay management of patients not meeting sepsis criteria. Just as patients with chest pain and a troponin leak warrant monitoring and treatment (but are not prioritized for immediate heart catheterization), patients with infection need monitoring and treatment. Improvements in care should thus be judged not only by improved outcomes among patients with sepsis but also by decreased progression to sepsis among patients with infection.”
The International Consensus Criteria paper was supported by the Society of Critical Care Medicine and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to Tellen C. Bennett, MD, MS, and Nelson Sanchez-Pinto, MD. Data for the Kenya site were collected with support of the Wellcome Trust to the Kenya Major Overseas Programme. Dr. Jabornisky reported no conflicts of interest. Dr. Carlton reported serving on the Pediatric Surviving Sepsis Campaign Guideline committee and receiving grant support from the NIH.
New criteria for pediatric sepsis, based on a novel score that predicts mortality in children with suspected or confirmed infection, perform better than existing organ dysfunction scores and criteria and have the potential to improve clinical care globally, researchers say.
Current pediatric-specific criteria for sepsis were published in 2005, based on expert opinion. In 2016, sepsis was redefined for adults as life-threatening organ dysfunction caused by a dysregulated host response to infection, as opposed to an earlier focus on systemic inflammation. But the paradigm-shifting changes were not extended to children (< 18 years, but not newborns), setting the stage for the new initiative.
The new criteria, and their development and validation, were published in JAMA and presented the same day at the Society of Critical Care Medicine’s 2024 Critical Care Congress in Phoenix, Arizona.
International Consensus
“The new criteria we derived are based on data from electronic health records and analysis of more than 3 million pediatric healthcare encounters from 10 hospitals around the world, including in low-resource settings,” L. Nelson Sanchez-Pinto, MD, MBI, a critical care physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, told this news organization.
Dr. Sanchez-Pinto co-led the data group of the international expert task force convened by the Society of Critical Care Medicine (SCCM) to develop and validate the criteria, which are based on evidence from an international survey, systematic review and meta-analysis, a newly created organ dysfunction score (Phoenix Sepsis Score), and sites on four continents.
Based on the findings, the task force now suggests that pediatric sepsis be defined by a Phoenix Sepsis Score of at least 2 points in children with suspected infection, which indicates potentially life-threatening dysfunction of the respiratory, cardiovascular, coagulation, and/or neurological systems. Septic shock is defined as sepsis with at least 1 cardiovascular point in the score.
Disparities Across Settings
To derive and validate the new criteria across differently resourced settings, the researchers conducted a multicenter, international, retrospective cohort study involving 10 health systems in the United States, Colombia, Bangladesh, China, and Kenya, 3 of which were used as external validation sites.
Data were collected from pediatric emergency and inpatient encounters from 2010 to 2019. The development set comprised 3,049,699 children, and the external validation set included 581,317.
Stacked regression models to predict mortality in children with suspected infection were derived and validated using the best-performing organ dysfunction subscores from eight existing scores.
The final model was then translated into the integer-based Phoenix Sepsis Score and used to establish binary criteria for sepsis and septic shock.
Among 172,984 children with suspected infection in the first 24 hours (development set; 1.2% mortality), a four-organ-system model performed best. The Phoenix Sepsis Score — the integer version of the model — had areas under the precision recall curve of 0.23 to 0.38, and areas under the receiver operating characteristic curve of 0.71 to 0.92 to predict mortality in the validation sets.
A Phoenix Sepsis Score of 2 points or higher in children with suspected infection as criteria for sepsis, plus 1 or more cardiovascular points as criteria for septic shock, resulted in a higher positive predictive value and higher or similar sensitivity compared with the 2005 International Pediatric Sepsis Consensus Conference criteria across differently resourced settings.
Specifically, children with a Phoenix Sepsis Score of at least 2 points had in-hospital mortality of 7.1% in higher-resource settings and 28.5% in lower-resource settings — more than 8 times that of children with suspected infection not meeting these criteria.
Mortality also was higher in children who had organ dysfunction in at least one of four organ systems — respiratory, cardiovascular, coagulation, and/or neurological — that was not the primary site of infection.
Children with septic shock, indicated by at least 1 cardiovascular point in the Phoenix Sepsis Score, had severe hypotension for age, blood lactate exceeding 5 mmol/L, or need for vasoactive medication. These children had an in-hospital mortality rate of 10.8% in higher-resource settings and 33.5% in lower-resource settings.
A Better Score
Given the findings, the task force recommends that “the former criteria based on systemic inflammatory response syndrome should not be used to diagnose sepsis in children [and] the former term severe sepsis should no longer be used because sepsis is life-threatening organ dysfunction associated with infection and is thus indicative of a severe disease state.”
The task force cautions that although the four organs in the Phoenix Sepsis Score are most commonly involved in sepsis, “this does not diminish the crucial importance of the assessment and management of other organ dysfunction.”
Furthermore, they emphasize that the Phoenix score was designed to identify sepsis in children, not to screen children at risk for developing sepsis or early identification of children with suspected sepsis.
Additional Considerations
In related editorials, commentators noted some caveats and concerns with regard to the study design and the new criteria.
Roberto Jabornisky, MD, PhD, of National University of the Northeast, Corrientes, Argentina, and colleagues pointed out that “all the low-resource validation sites were institutions with electronic health records and most had PICUs [pediatric intensive care units], which does not adequately reflect conditions in most low-resource settings. These factors introduce a distinct bias favoring a ‘PICU-based consensus,’ potentially limiting the generalizability and adoption of the new criteria by health care practitioners in non-PICU and nonhospital settings responsible for recognizing and managing children with sepsis.” The editorialists called for additional prospective validation in differently resourced settings, especially those with the highest disease burdens.
“Until then,” they wrote, “it is essential to refrain from considering these criteria as an inflexible directive governing medical interventions for pediatric sepsis. No definition can fully substitute for the clinical judgment of an experienced, vigilant clinician caring for an unwell child.”
Erin F. Carlton, MD, MSc of the University of Michigan, Ann Arbor, and colleagues added in a separate editorial, “The Phoenix criteria identify a sicker subset of patients than prior SIRS [systemic inflammatory response syndrome]-based criteria. Some may worry this higher threshold could delay management of patients not meeting sepsis criteria. Just as patients with chest pain and a troponin leak warrant monitoring and treatment (but are not prioritized for immediate heart catheterization), patients with infection need monitoring and treatment. Improvements in care should thus be judged not only by improved outcomes among patients with sepsis but also by decreased progression to sepsis among patients with infection.”
The International Consensus Criteria paper was supported by the Society of Critical Care Medicine and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to Tellen C. Bennett, MD, MS, and Nelson Sanchez-Pinto, MD. Data for the Kenya site were collected with support of the Wellcome Trust to the Kenya Major Overseas Programme. Dr. Jabornisky reported no conflicts of interest. Dr. Carlton reported serving on the Pediatric Surviving Sepsis Campaign Guideline committee and receiving grant support from the NIH.
New criteria for pediatric sepsis, based on a novel score that predicts mortality in children with suspected or confirmed infection, perform better than existing organ dysfunction scores and criteria and have the potential to improve clinical care globally, researchers say.
Current pediatric-specific criteria for sepsis were published in 2005, based on expert opinion. In 2016, sepsis was redefined for adults as life-threatening organ dysfunction caused by a dysregulated host response to infection, as opposed to an earlier focus on systemic inflammation. But the paradigm-shifting changes were not extended to children (< 18 years, but not newborns), setting the stage for the new initiative.
The new criteria, and their development and validation, were published in JAMA and presented the same day at the Society of Critical Care Medicine’s 2024 Critical Care Congress in Phoenix, Arizona.
International Consensus
“The new criteria we derived are based on data from electronic health records and analysis of more than 3 million pediatric healthcare encounters from 10 hospitals around the world, including in low-resource settings,” L. Nelson Sanchez-Pinto, MD, MBI, a critical care physician at the Ann and Robert H. Lurie Children’s Hospital of Chicago, told this news organization.
Dr. Sanchez-Pinto co-led the data group of the international expert task force convened by the Society of Critical Care Medicine (SCCM) to develop and validate the criteria, which are based on evidence from an international survey, systematic review and meta-analysis, a newly created organ dysfunction score (Phoenix Sepsis Score), and sites on four continents.
Based on the findings, the task force now suggests that pediatric sepsis be defined by a Phoenix Sepsis Score of at least 2 points in children with suspected infection, which indicates potentially life-threatening dysfunction of the respiratory, cardiovascular, coagulation, and/or neurological systems. Septic shock is defined as sepsis with at least 1 cardiovascular point in the score.
Disparities Across Settings
To derive and validate the new criteria across differently resourced settings, the researchers conducted a multicenter, international, retrospective cohort study involving 10 health systems in the United States, Colombia, Bangladesh, China, and Kenya, 3 of which were used as external validation sites.
Data were collected from pediatric emergency and inpatient encounters from 2010 to 2019. The development set comprised 3,049,699 children, and the external validation set included 581,317.
Stacked regression models to predict mortality in children with suspected infection were derived and validated using the best-performing organ dysfunction subscores from eight existing scores.
The final model was then translated into the integer-based Phoenix Sepsis Score and used to establish binary criteria for sepsis and septic shock.
Among 172,984 children with suspected infection in the first 24 hours (development set; 1.2% mortality), a four-organ-system model performed best. The Phoenix Sepsis Score — the integer version of the model — had areas under the precision recall curve of 0.23 to 0.38, and areas under the receiver operating characteristic curve of 0.71 to 0.92 to predict mortality in the validation sets.
A Phoenix Sepsis Score of 2 points or higher in children with suspected infection as criteria for sepsis, plus 1 or more cardiovascular points as criteria for septic shock, resulted in a higher positive predictive value and higher or similar sensitivity compared with the 2005 International Pediatric Sepsis Consensus Conference criteria across differently resourced settings.
Specifically, children with a Phoenix Sepsis Score of at least 2 points had in-hospital mortality of 7.1% in higher-resource settings and 28.5% in lower-resource settings — more than 8 times that of children with suspected infection not meeting these criteria.
Mortality also was higher in children who had organ dysfunction in at least one of four organ systems — respiratory, cardiovascular, coagulation, and/or neurological — that was not the primary site of infection.
Children with septic shock, indicated by at least 1 cardiovascular point in the Phoenix Sepsis Score, had severe hypotension for age, blood lactate exceeding 5 mmol/L, or need for vasoactive medication. These children had an in-hospital mortality rate of 10.8% in higher-resource settings and 33.5% in lower-resource settings.
A Better Score
Given the findings, the task force recommends that “the former criteria based on systemic inflammatory response syndrome should not be used to diagnose sepsis in children [and] the former term severe sepsis should no longer be used because sepsis is life-threatening organ dysfunction associated with infection and is thus indicative of a severe disease state.”
The task force cautions that although the four organs in the Phoenix Sepsis Score are most commonly involved in sepsis, “this does not diminish the crucial importance of the assessment and management of other organ dysfunction.”
Furthermore, they emphasize that the Phoenix score was designed to identify sepsis in children, not to screen children at risk for developing sepsis or early identification of children with suspected sepsis.
Additional Considerations
In related editorials, commentators noted some caveats and concerns with regard to the study design and the new criteria.
Roberto Jabornisky, MD, PhD, of National University of the Northeast, Corrientes, Argentina, and colleagues pointed out that “all the low-resource validation sites were institutions with electronic health records and most had PICUs [pediatric intensive care units], which does not adequately reflect conditions in most low-resource settings. These factors introduce a distinct bias favoring a ‘PICU-based consensus,’ potentially limiting the generalizability and adoption of the new criteria by health care practitioners in non-PICU and nonhospital settings responsible for recognizing and managing children with sepsis.” The editorialists called for additional prospective validation in differently resourced settings, especially those with the highest disease burdens.
“Until then,” they wrote, “it is essential to refrain from considering these criteria as an inflexible directive governing medical interventions for pediatric sepsis. No definition can fully substitute for the clinical judgment of an experienced, vigilant clinician caring for an unwell child.”
Erin F. Carlton, MD, MSc of the University of Michigan, Ann Arbor, and colleagues added in a separate editorial, “The Phoenix criteria identify a sicker subset of patients than prior SIRS [systemic inflammatory response syndrome]-based criteria. Some may worry this higher threshold could delay management of patients not meeting sepsis criteria. Just as patients with chest pain and a troponin leak warrant monitoring and treatment (but are not prioritized for immediate heart catheterization), patients with infection need monitoring and treatment. Improvements in care should thus be judged not only by improved outcomes among patients with sepsis but also by decreased progression to sepsis among patients with infection.”
The International Consensus Criteria paper was supported by the Society of Critical Care Medicine and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to Tellen C. Bennett, MD, MS, and Nelson Sanchez-Pinto, MD. Data for the Kenya site were collected with support of the Wellcome Trust to the Kenya Major Overseas Programme. Dr. Jabornisky reported no conflicts of interest. Dr. Carlton reported serving on the Pediatric Surviving Sepsis Campaign Guideline committee and receiving grant support from the NIH.
FROM JAMA
Breaking the Diagnostic Bottleneck in RA
As head of the clinical laboratory at the San Juan University Hospital in Alicante, Spain, Maria Salinas, PhD, is passionate about the role she and her colleagues can play in clinical decision-making.
Her mission is the identification of “hidden diseases,” as she calls them, chronic conditions for which early identification and intervention can change the course of the illness. Her lab has been a leader over the past decade in using technology to partner with clinicians to promote the appropriate use of testing and clinical decision-making.
An example of a disease ripe for this type of intervention is rheumatoid arthritis (RA), the most common form of autoimmune arthritis, affecting around 1.3 million people in the United States. The prognosis for patients is better the earlier treatment begins.
But the
Amy S. Kehl, MD, an attending rheumatologist at Cedars-Sinai Medical Center in Los Angeles, who also sees patients at Saint John’s Physician Partners in Santa Monica, California, recommends a workup for inflammatory arthritis for patients presenting with the new onset of joint pain and swelling, primarily of small joints, although larger joints can be involved. The workup includes markers of inflammation such as an erythrocyte sedimentation rate and C-reactive protein, which are typically elevated and can be used to monitor the progression of the disease. Similarly, the presence of anemia is consistent with RA and helpful in tracking response to treatment.
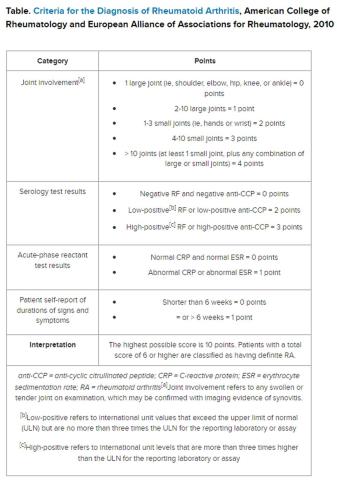
But pinning down the diagnosis requires the presence of autoimmune antibodies. Guidelines from the American College of Rheumatology require a positive result for either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibody to definitively determine whether a patient has RA (Table).
“Classically, I find that the primary care physicians include a rheumatoid factor, not always a CCP, and may include other antibodies, including an ANA [antinuclear antibody] test, as part of that workup,” Dr. Kehl said. The problem with that strategy is that although the RF test does detect 60%-80% of patients with RA, it is positive in many other autoimmune conditions. Although the ANA might be positive in patients with RA, it is nonspecific and does not confirm the diagnosis of RA.
Up to 50% of autoimmune antibody tests are inappropriately ordered. And for rheumatologists, that leads to unnecessary referrals of patients with musculoskeletal complaints who do not meet objective clinical criteria for joint disease.
“These tests get ordered almost reflexively, and sometimes they’re ordered as part of a panel that includes a rheumatoid factor and an ANA, and it’s not necessarily going to be a high-yield test,” Dr. Kehl said. Superfluous tests and referrals often cause unnecessary anxiety in patients, as well as drive up costs, she added.
Dr. Salinas made the same observation in her hospital lab, which also serves nine primary care centers. She documented an upward trend in orders for RF testing in her hospital lab between 2011 and 2019. Dr. Salinas also noted that the anti-CCP antibody test was not commonly requested, although it has more utility in the diagnosis. Like the RF, it detects 50%-70% of patients with RA but has 95% specificity, resulting in far fewer false-positive results.
The appearance of both RF and anti-CCP antibodies often predicts a rapid progression to clinical disease. Dr. Kehl wants symptomatic patients with positive results for both markers to be seen right away by a rheumatologist. “We do know from studies that bony erosions can develop as early as a month or months after the onset of an inflammatory arthritis,” she said.
To address this need, Dr. Salinas worked with rheumatologists and primary care clinicians to develop an algorithm that called for reflex testing of samples from patients with positive RF results (> 30 IU/mL) for anti-CCP antibodies. If the anti-CCP antibody result was > 40 IU/mL, a comment in the lab report suggested rheumatology referral. The lab turned down requests to test sample for RF if the patient had a negative result in the previous 12 months — but it would perform the test if the clinician repeated the request.
The results were encouraging, Dr. Salinas said. “The main result in this study was that we really identified more hidden cases of patients with rheumatoid arthritis,” she told this news organization.
Compared with baseline trends, during the study period from April 2019 to January 2021 her lab demonstrated:
- Reduced RF tests conducted by canceling 16% of tests ordered for patients with negative RF result in the previous 12 months
- Fewer unnecessary referrals, from 22% in the baseline period to 8% during the intervention period
- A smaller percentage of missed patients, from 21% to 16%
To be sure, pre- and post-implementation comparisons are difficult when the implementation period happens to coincide with the emergence of SARS-CoV-2.
Although fewer patients were seen and fewer lab tests were ordered overall in Alicante during the COVID-19 pandemic, the proportion of tests ordered for RF testing dropped, and all the patients identified with double positives for RF and anti-CCP antibodies were referred to rheumatology, suggesting evidence of benefit.
Dr. Kehl said the practice of using clinical decision support systems could be used in the United States. “I thought this was an important study,” she said. Electronic health records systems “have all these capabilities where we can include best practice alerts when you order a test to make sure that it’s clinically warranted and cost-effective.”
Dr. Salinas and Dr. Kehl reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
As head of the clinical laboratory at the San Juan University Hospital in Alicante, Spain, Maria Salinas, PhD, is passionate about the role she and her colleagues can play in clinical decision-making.
Her mission is the identification of “hidden diseases,” as she calls them, chronic conditions for which early identification and intervention can change the course of the illness. Her lab has been a leader over the past decade in using technology to partner with clinicians to promote the appropriate use of testing and clinical decision-making.
An example of a disease ripe for this type of intervention is rheumatoid arthritis (RA), the most common form of autoimmune arthritis, affecting around 1.3 million people in the United States. The prognosis for patients is better the earlier treatment begins.
But the
Amy S. Kehl, MD, an attending rheumatologist at Cedars-Sinai Medical Center in Los Angeles, who also sees patients at Saint John’s Physician Partners in Santa Monica, California, recommends a workup for inflammatory arthritis for patients presenting with the new onset of joint pain and swelling, primarily of small joints, although larger joints can be involved. The workup includes markers of inflammation such as an erythrocyte sedimentation rate and C-reactive protein, which are typically elevated and can be used to monitor the progression of the disease. Similarly, the presence of anemia is consistent with RA and helpful in tracking response to treatment.
But pinning down the diagnosis requires the presence of autoimmune antibodies. Guidelines from the American College of Rheumatology require a positive result for either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibody to definitively determine whether a patient has RA (Table).
“Classically, I find that the primary care physicians include a rheumatoid factor, not always a CCP, and may include other antibodies, including an ANA [antinuclear antibody] test, as part of that workup,” Dr. Kehl said. The problem with that strategy is that although the RF test does detect 60%-80% of patients with RA, it is positive in many other autoimmune conditions. Although the ANA might be positive in patients with RA, it is nonspecific and does not confirm the diagnosis of RA.
Up to 50% of autoimmune antibody tests are inappropriately ordered. And for rheumatologists, that leads to unnecessary referrals of patients with musculoskeletal complaints who do not meet objective clinical criteria for joint disease.
“These tests get ordered almost reflexively, and sometimes they’re ordered as part of a panel that includes a rheumatoid factor and an ANA, and it’s not necessarily going to be a high-yield test,” Dr. Kehl said. Superfluous tests and referrals often cause unnecessary anxiety in patients, as well as drive up costs, she added.
Dr. Salinas made the same observation in her hospital lab, which also serves nine primary care centers. She documented an upward trend in orders for RF testing in her hospital lab between 2011 and 2019. Dr. Salinas also noted that the anti-CCP antibody test was not commonly requested, although it has more utility in the diagnosis. Like the RF, it detects 50%-70% of patients with RA but has 95% specificity, resulting in far fewer false-positive results.
The appearance of both RF and anti-CCP antibodies often predicts a rapid progression to clinical disease. Dr. Kehl wants symptomatic patients with positive results for both markers to be seen right away by a rheumatologist. “We do know from studies that bony erosions can develop as early as a month or months after the onset of an inflammatory arthritis,” she said.
To address this need, Dr. Salinas worked with rheumatologists and primary care clinicians to develop an algorithm that called for reflex testing of samples from patients with positive RF results (> 30 IU/mL) for anti-CCP antibodies. If the anti-CCP antibody result was > 40 IU/mL, a comment in the lab report suggested rheumatology referral. The lab turned down requests to test sample for RF if the patient had a negative result in the previous 12 months — but it would perform the test if the clinician repeated the request.
The results were encouraging, Dr. Salinas said. “The main result in this study was that we really identified more hidden cases of patients with rheumatoid arthritis,” she told this news organization.
Compared with baseline trends, during the study period from April 2019 to January 2021 her lab demonstrated:
- Reduced RF tests conducted by canceling 16% of tests ordered for patients with negative RF result in the previous 12 months
- Fewer unnecessary referrals, from 22% in the baseline period to 8% during the intervention period
- A smaller percentage of missed patients, from 21% to 16%
To be sure, pre- and post-implementation comparisons are difficult when the implementation period happens to coincide with the emergence of SARS-CoV-2.
Although fewer patients were seen and fewer lab tests were ordered overall in Alicante during the COVID-19 pandemic, the proportion of tests ordered for RF testing dropped, and all the patients identified with double positives for RF and anti-CCP antibodies were referred to rheumatology, suggesting evidence of benefit.
Dr. Kehl said the practice of using clinical decision support systems could be used in the United States. “I thought this was an important study,” she said. Electronic health records systems “have all these capabilities where we can include best practice alerts when you order a test to make sure that it’s clinically warranted and cost-effective.”
Dr. Salinas and Dr. Kehl reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
As head of the clinical laboratory at the San Juan University Hospital in Alicante, Spain, Maria Salinas, PhD, is passionate about the role she and her colleagues can play in clinical decision-making.
Her mission is the identification of “hidden diseases,” as she calls them, chronic conditions for which early identification and intervention can change the course of the illness. Her lab has been a leader over the past decade in using technology to partner with clinicians to promote the appropriate use of testing and clinical decision-making.
An example of a disease ripe for this type of intervention is rheumatoid arthritis (RA), the most common form of autoimmune arthritis, affecting around 1.3 million people in the United States. The prognosis for patients is better the earlier treatment begins.
But the
Amy S. Kehl, MD, an attending rheumatologist at Cedars-Sinai Medical Center in Los Angeles, who also sees patients at Saint John’s Physician Partners in Santa Monica, California, recommends a workup for inflammatory arthritis for patients presenting with the new onset of joint pain and swelling, primarily of small joints, although larger joints can be involved. The workup includes markers of inflammation such as an erythrocyte sedimentation rate and C-reactive protein, which are typically elevated and can be used to monitor the progression of the disease. Similarly, the presence of anemia is consistent with RA and helpful in tracking response to treatment.
But pinning down the diagnosis requires the presence of autoimmune antibodies. Guidelines from the American College of Rheumatology require a positive result for either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (anti-CCP) antibody to definitively determine whether a patient has RA (Table).
“Classically, I find that the primary care physicians include a rheumatoid factor, not always a CCP, and may include other antibodies, including an ANA [antinuclear antibody] test, as part of that workup,” Dr. Kehl said. The problem with that strategy is that although the RF test does detect 60%-80% of patients with RA, it is positive in many other autoimmune conditions. Although the ANA might be positive in patients with RA, it is nonspecific and does not confirm the diagnosis of RA.
Up to 50% of autoimmune antibody tests are inappropriately ordered. And for rheumatologists, that leads to unnecessary referrals of patients with musculoskeletal complaints who do not meet objective clinical criteria for joint disease.
“These tests get ordered almost reflexively, and sometimes they’re ordered as part of a panel that includes a rheumatoid factor and an ANA, and it’s not necessarily going to be a high-yield test,” Dr. Kehl said. Superfluous tests and referrals often cause unnecessary anxiety in patients, as well as drive up costs, she added.
Dr. Salinas made the same observation in her hospital lab, which also serves nine primary care centers. She documented an upward trend in orders for RF testing in her hospital lab between 2011 and 2019. Dr. Salinas also noted that the anti-CCP antibody test was not commonly requested, although it has more utility in the diagnosis. Like the RF, it detects 50%-70% of patients with RA but has 95% specificity, resulting in far fewer false-positive results.
The appearance of both RF and anti-CCP antibodies often predicts a rapid progression to clinical disease. Dr. Kehl wants symptomatic patients with positive results for both markers to be seen right away by a rheumatologist. “We do know from studies that bony erosions can develop as early as a month or months after the onset of an inflammatory arthritis,” she said.
To address this need, Dr. Salinas worked with rheumatologists and primary care clinicians to develop an algorithm that called for reflex testing of samples from patients with positive RF results (> 30 IU/mL) for anti-CCP antibodies. If the anti-CCP antibody result was > 40 IU/mL, a comment in the lab report suggested rheumatology referral. The lab turned down requests to test sample for RF if the patient had a negative result in the previous 12 months — but it would perform the test if the clinician repeated the request.
The results were encouraging, Dr. Salinas said. “The main result in this study was that we really identified more hidden cases of patients with rheumatoid arthritis,” she told this news organization.
Compared with baseline trends, during the study period from April 2019 to January 2021 her lab demonstrated:
- Reduced RF tests conducted by canceling 16% of tests ordered for patients with negative RF result in the previous 12 months
- Fewer unnecessary referrals, from 22% in the baseline period to 8% during the intervention period
- A smaller percentage of missed patients, from 21% to 16%
To be sure, pre- and post-implementation comparisons are difficult when the implementation period happens to coincide with the emergence of SARS-CoV-2.
Although fewer patients were seen and fewer lab tests were ordered overall in Alicante during the COVID-19 pandemic, the proportion of tests ordered for RF testing dropped, and all the patients identified with double positives for RF and anti-CCP antibodies were referred to rheumatology, suggesting evidence of benefit.
Dr. Kehl said the practice of using clinical decision support systems could be used in the United States. “I thought this was an important study,” she said. Electronic health records systems “have all these capabilities where we can include best practice alerts when you order a test to make sure that it’s clinically warranted and cost-effective.”
Dr. Salinas and Dr. Kehl reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Noduloplaque on the Forehead
The Diagnosis: Giant Apocrine Hidrocystoma
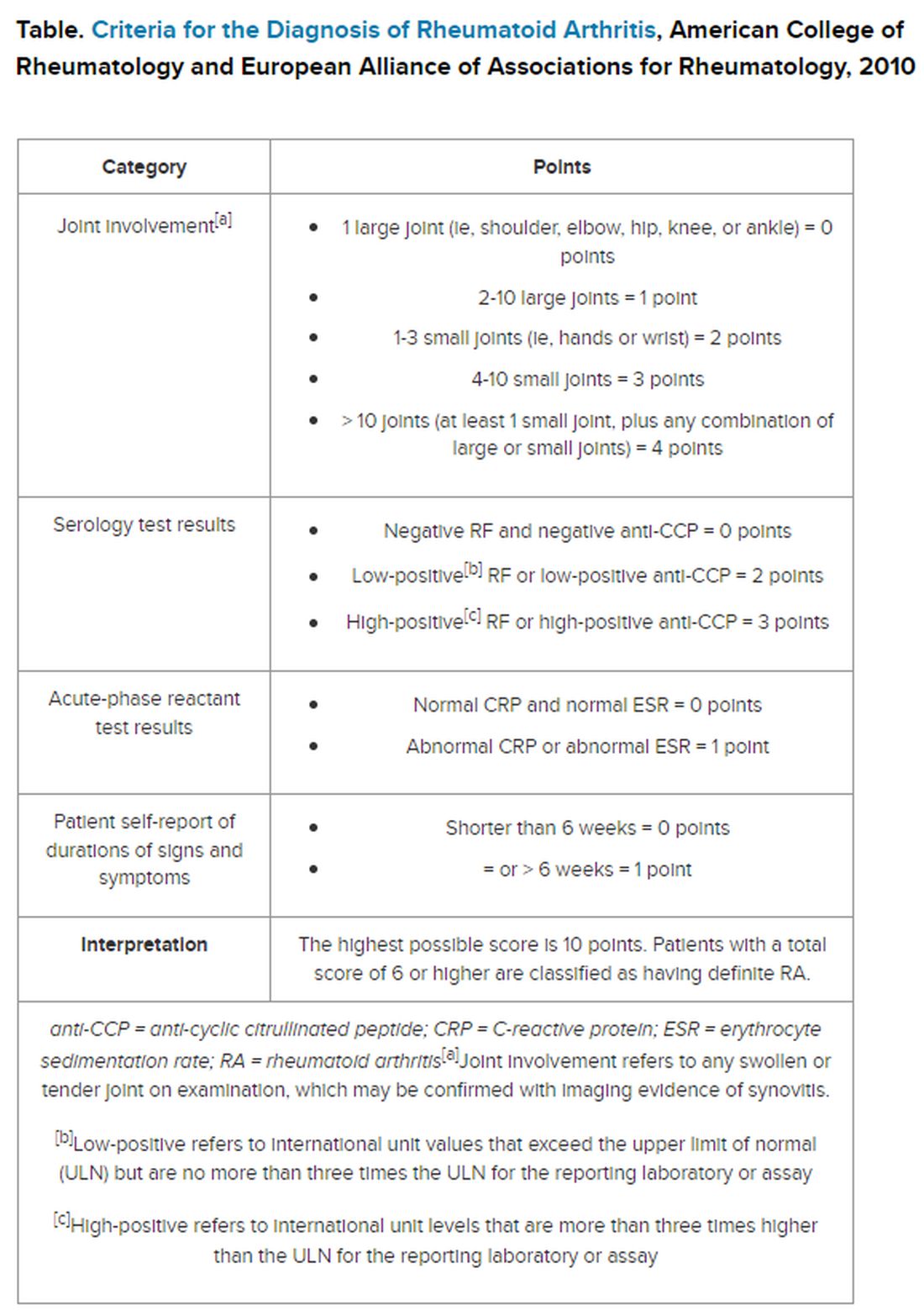
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.
Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
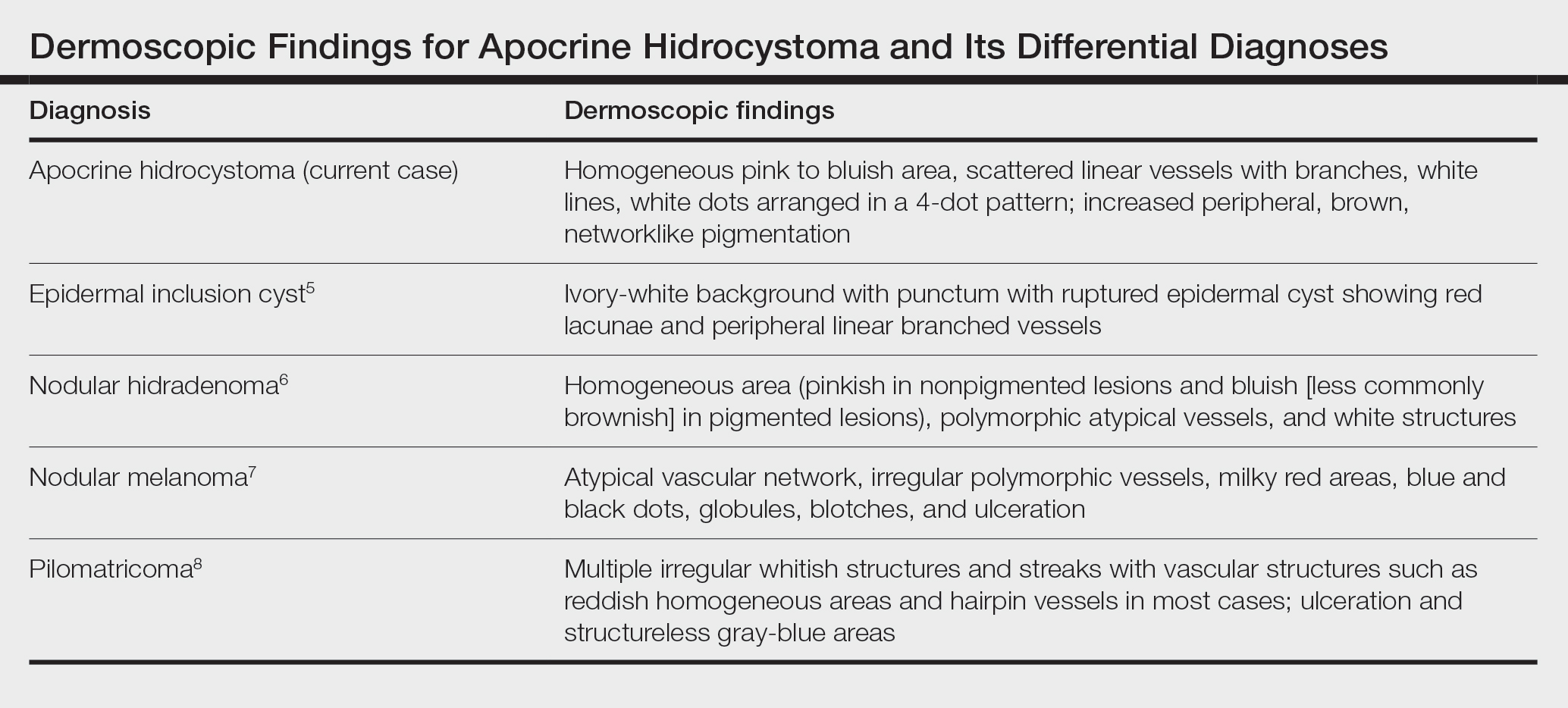
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.
The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
A 21-year-old man presented with a raised lesion on the forehead that had started as a single papule 16 years prior and gradually increased in number and size. There were no associated symptoms and no history of seasonal variation in the size of the lesions. Physical examination revealed multiple erythematous to slightly bluish translucent papules that coalesced to form a 3×3-cm noduloplaque with cystic consistency on the right side of the forehead (top). Dermoscopic examination (middle) (polarized noncontact mode) revealed a homogeneous pink to bluish background, scattered linear vessels with branches (black arrows), multiple chrysalislike shiny white lines (blue arrows), and dots arranged in a 4-dot pattern (black circle) resembling a four-leaf clover. Increased peripheral, brown, networklike pigmentation (black stars) also was noted on dermoscopy. Histopathologic examination of the noduloplaque was performed (bottom).
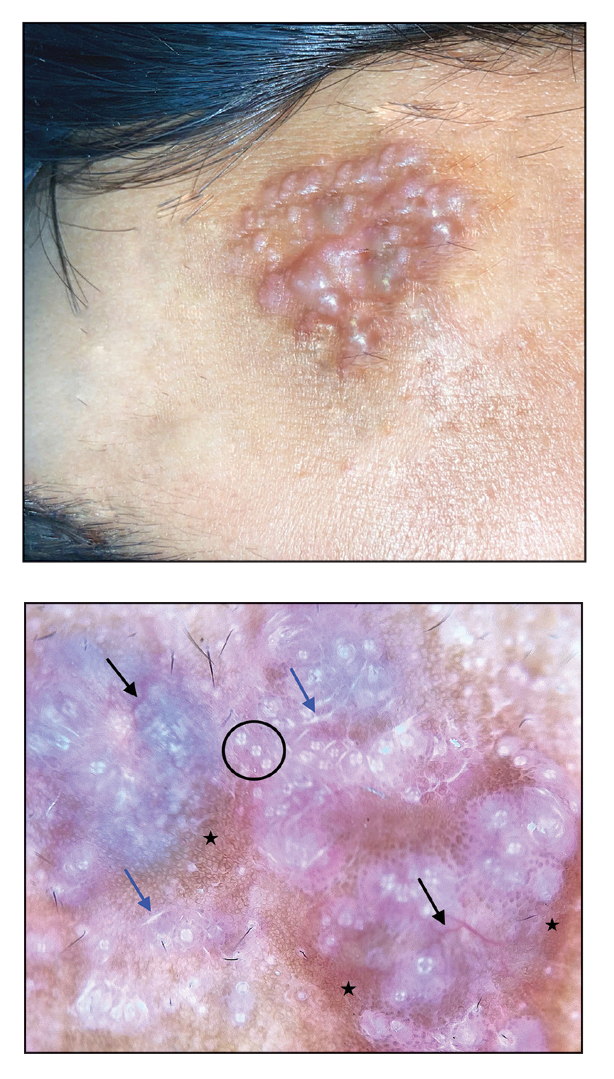
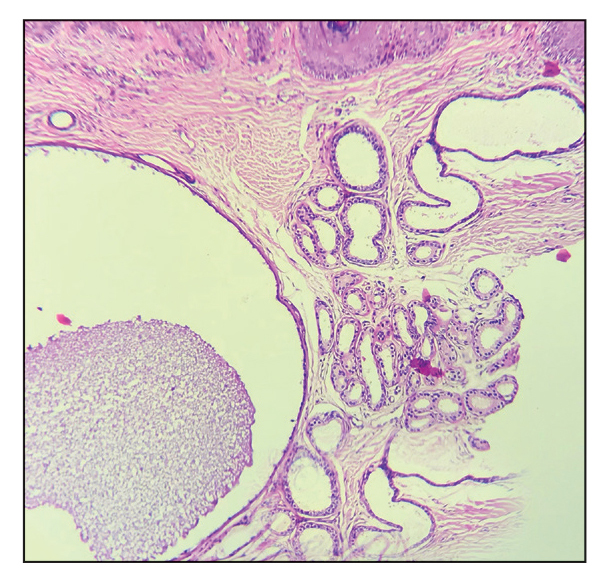
Cell-Free DNA May Inform IBD Diagnosis
LAS VEGAS —
“We think for indeterminate colitis, our assay can be quite beneficial in helping a clinician resolve or provide some additional insight and information. We have a very robust ability to predict from just the plasma microbial cell free DNA alone [whether] individuals are in remission or mild or moderate or active disease,” Shiv Kale, PhD, said during a presentation of the results at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. Dr. Kale is director of computational biomarker discovery at Karius Inc., a company whose Karius Test is also being developed as a rapid test for various infections and febrile neutropenia.
cfDNA has proved to be a useful biomarker for a range of conditions, including cancer screening, diagnosis and monitoring, prenatal testing, and organ monitoring following transplantation. The tests rely on the fact that both human and microbial cells release DNA after cell death, and it is readily detectable in plasma.
Dr. Kale described the company’s efforts to identify microbial species biomarkers and a classification scheme that he said leads to consistent performance.
The latest study included 196 patients with Crohn’s disease and 196 with ulcerative colitis, with each group including individuals in remission and with mild, moderate, and severe disease. All patients had an endoscopic assessment within 30 days of plasma measurements.
cfDNA distinguished between patients with Crohn’s disease, ulcerative colitis, and those who were asymptomatic. It distinguished between patients with IBD and those who were asymptomatic with a sensitivity of 99.5% and a specificity of 90%, which are equivalent to or better than other traditional measures to distinguish active IBD versus asymptomatic patients.
The researchers plan a follow-up study of 1,800 samples in partnership with the Crohn’s and Colitis Foundation to examine the ability of cfDNA to determine disease severity, as well as location of disease and Crohn’s disease subtypes.
An ‘Intriguing’ Method
During the Q&A session, one questioner pointed out that colorectal cancer and infections can produce microbial signatures that could be confounding the results. He asked: “Do you have a data set to compare [cfDNA findings] to serum from C. diff, norovirus, and colorectal cancer? And if you don’t, I strongly encourage you to do so.” Dr. Kale responded that the group is working on that.
Asked for comment, session moderator Dana Lukin, MD, AGAF, offered praise for the work.
“I think it’s an intriguing method that we haven’t seen used as a predictor in IBD. Using this as a biomarker of disease type is very intriguing for confirming a diagnosis. I think probably one of the most compelling points is the distinction between IBD and non-IBD. Our current serologic-based assays do not really do that very well. I think this is a big improvement on that,” said Dr. Lukin, associate professor of clinical medicine at Weill Cornell Medical College, New York.
He echoed the questioner’s comments, suggesting that a key question will be whether cfDNA can correlate with disease activity over time.
He also called for prospective studies to validate the approach.
If successful, the technology could have broad application. “Certainly in kids this might be useful or in folks that either aren’t as inclined to have invasive testing done, or for whatever logistic reasons it’s not easy,” said Dr. Lukin.
Dr. Kale is an employee of Karius. Dr. Lukin has no relevant financial disclosures..
LAS VEGAS —
“We think for indeterminate colitis, our assay can be quite beneficial in helping a clinician resolve or provide some additional insight and information. We have a very robust ability to predict from just the plasma microbial cell free DNA alone [whether] individuals are in remission or mild or moderate or active disease,” Shiv Kale, PhD, said during a presentation of the results at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. Dr. Kale is director of computational biomarker discovery at Karius Inc., a company whose Karius Test is also being developed as a rapid test for various infections and febrile neutropenia.
cfDNA has proved to be a useful biomarker for a range of conditions, including cancer screening, diagnosis and monitoring, prenatal testing, and organ monitoring following transplantation. The tests rely on the fact that both human and microbial cells release DNA after cell death, and it is readily detectable in plasma.
Dr. Kale described the company’s efforts to identify microbial species biomarkers and a classification scheme that he said leads to consistent performance.
The latest study included 196 patients with Crohn’s disease and 196 with ulcerative colitis, with each group including individuals in remission and with mild, moderate, and severe disease. All patients had an endoscopic assessment within 30 days of plasma measurements.
cfDNA distinguished between patients with Crohn’s disease, ulcerative colitis, and those who were asymptomatic. It distinguished between patients with IBD and those who were asymptomatic with a sensitivity of 99.5% and a specificity of 90%, which are equivalent to or better than other traditional measures to distinguish active IBD versus asymptomatic patients.
The researchers plan a follow-up study of 1,800 samples in partnership with the Crohn’s and Colitis Foundation to examine the ability of cfDNA to determine disease severity, as well as location of disease and Crohn’s disease subtypes.
An ‘Intriguing’ Method
During the Q&A session, one questioner pointed out that colorectal cancer and infections can produce microbial signatures that could be confounding the results. He asked: “Do you have a data set to compare [cfDNA findings] to serum from C. diff, norovirus, and colorectal cancer? And if you don’t, I strongly encourage you to do so.” Dr. Kale responded that the group is working on that.
Asked for comment, session moderator Dana Lukin, MD, AGAF, offered praise for the work.
“I think it’s an intriguing method that we haven’t seen used as a predictor in IBD. Using this as a biomarker of disease type is very intriguing for confirming a diagnosis. I think probably one of the most compelling points is the distinction between IBD and non-IBD. Our current serologic-based assays do not really do that very well. I think this is a big improvement on that,” said Dr. Lukin, associate professor of clinical medicine at Weill Cornell Medical College, New York.
He echoed the questioner’s comments, suggesting that a key question will be whether cfDNA can correlate with disease activity over time.
He also called for prospective studies to validate the approach.
If successful, the technology could have broad application. “Certainly in kids this might be useful or in folks that either aren’t as inclined to have invasive testing done, or for whatever logistic reasons it’s not easy,” said Dr. Lukin.
Dr. Kale is an employee of Karius. Dr. Lukin has no relevant financial disclosures..
LAS VEGAS —
“We think for indeterminate colitis, our assay can be quite beneficial in helping a clinician resolve or provide some additional insight and information. We have a very robust ability to predict from just the plasma microbial cell free DNA alone [whether] individuals are in remission or mild or moderate or active disease,” Shiv Kale, PhD, said during a presentation of the results at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. Dr. Kale is director of computational biomarker discovery at Karius Inc., a company whose Karius Test is also being developed as a rapid test for various infections and febrile neutropenia.
cfDNA has proved to be a useful biomarker for a range of conditions, including cancer screening, diagnosis and monitoring, prenatal testing, and organ monitoring following transplantation. The tests rely on the fact that both human and microbial cells release DNA after cell death, and it is readily detectable in plasma.
Dr. Kale described the company’s efforts to identify microbial species biomarkers and a classification scheme that he said leads to consistent performance.
The latest study included 196 patients with Crohn’s disease and 196 with ulcerative colitis, with each group including individuals in remission and with mild, moderate, and severe disease. All patients had an endoscopic assessment within 30 days of plasma measurements.
cfDNA distinguished between patients with Crohn’s disease, ulcerative colitis, and those who were asymptomatic. It distinguished between patients with IBD and those who were asymptomatic with a sensitivity of 99.5% and a specificity of 90%, which are equivalent to or better than other traditional measures to distinguish active IBD versus asymptomatic patients.
The researchers plan a follow-up study of 1,800 samples in partnership with the Crohn’s and Colitis Foundation to examine the ability of cfDNA to determine disease severity, as well as location of disease and Crohn’s disease subtypes.
An ‘Intriguing’ Method
During the Q&A session, one questioner pointed out that colorectal cancer and infections can produce microbial signatures that could be confounding the results. He asked: “Do you have a data set to compare [cfDNA findings] to serum from C. diff, norovirus, and colorectal cancer? And if you don’t, I strongly encourage you to do so.” Dr. Kale responded that the group is working on that.
Asked for comment, session moderator Dana Lukin, MD, AGAF, offered praise for the work.
“I think it’s an intriguing method that we haven’t seen used as a predictor in IBD. Using this as a biomarker of disease type is very intriguing for confirming a diagnosis. I think probably one of the most compelling points is the distinction between IBD and non-IBD. Our current serologic-based assays do not really do that very well. I think this is a big improvement on that,” said Dr. Lukin, associate professor of clinical medicine at Weill Cornell Medical College, New York.
He echoed the questioner’s comments, suggesting that a key question will be whether cfDNA can correlate with disease activity over time.
He also called for prospective studies to validate the approach.
If successful, the technology could have broad application. “Certainly in kids this might be useful or in folks that either aren’t as inclined to have invasive testing done, or for whatever logistic reasons it’s not easy,” said Dr. Lukin.
Dr. Kale is an employee of Karius. Dr. Lukin has no relevant financial disclosures..
FROM CROHN’S AND COLITIS CONGRESS
Targeting Fetus-derived Gdf15 May Curb Nausea and Vomiting of Pregnancy
, and targeting the hormone prophylactically may reduce this common gestational condition.
This protein acts on the brainstem to cause emesis, and, significantly, a mother’s prior exposure to it determines the degree of NVP severity she will experience, according to international researchers including Marlena Fejzo, PhD, a clinical assistant professor of population and public Health at Keck School of Medicine, University of Southern California, Los Angeles.
“GDF15 is at the mechanistic heart of NVP and HG [hyperemesis gravidarum],” Dr. Fejzo and colleagues wrote in Nature, pointing to the need for preventive and therapeutic strategies.
“My previous research showed an association between variation in the GDF15 gene and nausea and vomiting of pregnancy and HG, and this study takes it one step further by elucidating the mechanism. It confirms that the nausea and vomiting (N/V) hormone GDF15 is a major cause of NVP and HG,” Dr. Fejzo said.
The etiology of NVP remains poorly understood although it affects up to 80% of pregnancies. In the US, its severe form, HG, is the leading cause of hospitalization in early pregnancy and the second-leading reason for pregnancy hospitalization overall.
The immunoassay-based study showed that the majority of GDF15 in maternal blood during pregnancy comes from the fetal part of the placenta, and confirms previous studies reporting higher levels in pregnancies with more severe NVP, said Dr. Fejzo, who is who is a board member of the Hyperemesis Education and Research Foundation.
“However, what was really fascinating and surprising is that prior to pregnancy the women who have more severe NVP symptoms actually have lower levels of the hormone.”
Although the gene variant linked to HG was previously associated with higher circulating levels in maternal blood, counterintuitively, this new research showed that women with abnormally high levels prior to pregnancy have either no or very little NVP, said Dr. Fejzo. “That suggests that in humans higher levels may lead to a desensitization to the high levels of the hormone in pregnancy. Then we also proved that desensitization can occur in a mouse model.”
According to Erin Higgins, MD, a clinical assistant professor of obgyn and reproductive biology at the Cleveland Clinic, Cleveland, Ohio, who was not involved in the study, “This is an exciting finding that may help us to better target treatment of N/V in pregnancy. Factors for NVP have been identified, but to my knowledge there has not been a clear etiology.”
Dr. Higgins cautioned, however, that the GDF15 gene seems important in normal placentation, “so it’s not as simple as blocking the gene or its receptor.” But since preconception exposure to GDF15 might decrease nausea and vomiting once a woman is pregnant, prophylactic treatment may be possible, and metformin has been suggested as a possibility, she said.
The study findings emerged from immunoassays on maternal blood samples collected at about 15 weeks (first trimester and early second trimester), from women with NVP (n = 168) or seen at a hospital for HG (n = 57). Results were compared with those from controls having similar characteristics but no significant symptoms.
Interestingly, GDF15 is also associated with cachexia, a condition similar to HG and characterized by loss of appetite and weight loss, Dr. Fejzo noted. “The hormone can be produced by malignant tumors at levels similar to those seen in pregnancy, and symptoms can be reduced by blocking GDF15 or its receptor, GFRAL. Clinical trials are already underway in cancer patients to test this.”
She is seeking funding to test the impact of increasing GDF15 levels prior to pregnancy in patients who previously experienced HG. “I am confident that desensitizing patients by increasing GDF15 prior to pregnancy and by lowering GDF15 levels during pregnancy will work. But we need to make sure we do safety studies and get the dosing and duration right, and that will take some time.”
Desensitization will need testing first in HG, where the risk for adverse maternal and fetal outcomes is high, so the benefit will outweigh any possible risk of testing medication in pregnancy, she continued. “It will take some time before we get to patients with normal NVP, but I do believe eventually the new findings will result in game-changing therapeutics for the condition.”
Dr. Higgins added, “Even if this isn’t the golden ticket, researchers and clinicians are working toward improvements in the treatment of NVP. We’ve already come a long way in recent years with the development of treatment algorithms and the advent of doxylamine/pyridoxine.”
This study was supported primarily by the Medical Research Council UK and National Institute for Health and Care Research UK, with additional support from various research funding organizations, including Novo Nordisk Foundation.
Dr. Fejzo is a paid consultant for Materna Biosciences and NGM Biopharmaceuticals, and a board member and science adviser for the Hyperemesis Education and Research Foundation.
Numerous study co-authors disclosed financial relationships with private-sector companies, including employment and patent ownership.
Dr. Higgins disclosed no competing interests relevant to her comments but is an instructor for Organon.
, and targeting the hormone prophylactically may reduce this common gestational condition.
This protein acts on the brainstem to cause emesis, and, significantly, a mother’s prior exposure to it determines the degree of NVP severity she will experience, according to international researchers including Marlena Fejzo, PhD, a clinical assistant professor of population and public Health at Keck School of Medicine, University of Southern California, Los Angeles.
“GDF15 is at the mechanistic heart of NVP and HG [hyperemesis gravidarum],” Dr. Fejzo and colleagues wrote in Nature, pointing to the need for preventive and therapeutic strategies.
“My previous research showed an association between variation in the GDF15 gene and nausea and vomiting of pregnancy and HG, and this study takes it one step further by elucidating the mechanism. It confirms that the nausea and vomiting (N/V) hormone GDF15 is a major cause of NVP and HG,” Dr. Fejzo said.
The etiology of NVP remains poorly understood although it affects up to 80% of pregnancies. In the US, its severe form, HG, is the leading cause of hospitalization in early pregnancy and the second-leading reason for pregnancy hospitalization overall.
The immunoassay-based study showed that the majority of GDF15 in maternal blood during pregnancy comes from the fetal part of the placenta, and confirms previous studies reporting higher levels in pregnancies with more severe NVP, said Dr. Fejzo, who is who is a board member of the Hyperemesis Education and Research Foundation.
“However, what was really fascinating and surprising is that prior to pregnancy the women who have more severe NVP symptoms actually have lower levels of the hormone.”
Although the gene variant linked to HG was previously associated with higher circulating levels in maternal blood, counterintuitively, this new research showed that women with abnormally high levels prior to pregnancy have either no or very little NVP, said Dr. Fejzo. “That suggests that in humans higher levels may lead to a desensitization to the high levels of the hormone in pregnancy. Then we also proved that desensitization can occur in a mouse model.”
According to Erin Higgins, MD, a clinical assistant professor of obgyn and reproductive biology at the Cleveland Clinic, Cleveland, Ohio, who was not involved in the study, “This is an exciting finding that may help us to better target treatment of N/V in pregnancy. Factors for NVP have been identified, but to my knowledge there has not been a clear etiology.”
Dr. Higgins cautioned, however, that the GDF15 gene seems important in normal placentation, “so it’s not as simple as blocking the gene or its receptor.” But since preconception exposure to GDF15 might decrease nausea and vomiting once a woman is pregnant, prophylactic treatment may be possible, and metformin has been suggested as a possibility, she said.
The study findings emerged from immunoassays on maternal blood samples collected at about 15 weeks (first trimester and early second trimester), from women with NVP (n = 168) or seen at a hospital for HG (n = 57). Results were compared with those from controls having similar characteristics but no significant symptoms.
Interestingly, GDF15 is also associated with cachexia, a condition similar to HG and characterized by loss of appetite and weight loss, Dr. Fejzo noted. “The hormone can be produced by malignant tumors at levels similar to those seen in pregnancy, and symptoms can be reduced by blocking GDF15 or its receptor, GFRAL. Clinical trials are already underway in cancer patients to test this.”
She is seeking funding to test the impact of increasing GDF15 levels prior to pregnancy in patients who previously experienced HG. “I am confident that desensitizing patients by increasing GDF15 prior to pregnancy and by lowering GDF15 levels during pregnancy will work. But we need to make sure we do safety studies and get the dosing and duration right, and that will take some time.”
Desensitization will need testing first in HG, where the risk for adverse maternal and fetal outcomes is high, so the benefit will outweigh any possible risk of testing medication in pregnancy, she continued. “It will take some time before we get to patients with normal NVP, but I do believe eventually the new findings will result in game-changing therapeutics for the condition.”
Dr. Higgins added, “Even if this isn’t the golden ticket, researchers and clinicians are working toward improvements in the treatment of NVP. We’ve already come a long way in recent years with the development of treatment algorithms and the advent of doxylamine/pyridoxine.”
This study was supported primarily by the Medical Research Council UK and National Institute for Health and Care Research UK, with additional support from various research funding organizations, including Novo Nordisk Foundation.
Dr. Fejzo is a paid consultant for Materna Biosciences and NGM Biopharmaceuticals, and a board member and science adviser for the Hyperemesis Education and Research Foundation.
Numerous study co-authors disclosed financial relationships with private-sector companies, including employment and patent ownership.
Dr. Higgins disclosed no competing interests relevant to her comments but is an instructor for Organon.
, and targeting the hormone prophylactically may reduce this common gestational condition.
This protein acts on the brainstem to cause emesis, and, significantly, a mother’s prior exposure to it determines the degree of NVP severity she will experience, according to international researchers including Marlena Fejzo, PhD, a clinical assistant professor of population and public Health at Keck School of Medicine, University of Southern California, Los Angeles.
“GDF15 is at the mechanistic heart of NVP and HG [hyperemesis gravidarum],” Dr. Fejzo and colleagues wrote in Nature, pointing to the need for preventive and therapeutic strategies.
“My previous research showed an association between variation in the GDF15 gene and nausea and vomiting of pregnancy and HG, and this study takes it one step further by elucidating the mechanism. It confirms that the nausea and vomiting (N/V) hormone GDF15 is a major cause of NVP and HG,” Dr. Fejzo said.
The etiology of NVP remains poorly understood although it affects up to 80% of pregnancies. In the US, its severe form, HG, is the leading cause of hospitalization in early pregnancy and the second-leading reason for pregnancy hospitalization overall.
The immunoassay-based study showed that the majority of GDF15 in maternal blood during pregnancy comes from the fetal part of the placenta, and confirms previous studies reporting higher levels in pregnancies with more severe NVP, said Dr. Fejzo, who is who is a board member of the Hyperemesis Education and Research Foundation.
“However, what was really fascinating and surprising is that prior to pregnancy the women who have more severe NVP symptoms actually have lower levels of the hormone.”
Although the gene variant linked to HG was previously associated with higher circulating levels in maternal blood, counterintuitively, this new research showed that women with abnormally high levels prior to pregnancy have either no or very little NVP, said Dr. Fejzo. “That suggests that in humans higher levels may lead to a desensitization to the high levels of the hormone in pregnancy. Then we also proved that desensitization can occur in a mouse model.”
According to Erin Higgins, MD, a clinical assistant professor of obgyn and reproductive biology at the Cleveland Clinic, Cleveland, Ohio, who was not involved in the study, “This is an exciting finding that may help us to better target treatment of N/V in pregnancy. Factors for NVP have been identified, but to my knowledge there has not been a clear etiology.”
Dr. Higgins cautioned, however, that the GDF15 gene seems important in normal placentation, “so it’s not as simple as blocking the gene or its receptor.” But since preconception exposure to GDF15 might decrease nausea and vomiting once a woman is pregnant, prophylactic treatment may be possible, and metformin has been suggested as a possibility, she said.
The study findings emerged from immunoassays on maternal blood samples collected at about 15 weeks (first trimester and early second trimester), from women with NVP (n = 168) or seen at a hospital for HG (n = 57). Results were compared with those from controls having similar characteristics but no significant symptoms.
Interestingly, GDF15 is also associated with cachexia, a condition similar to HG and characterized by loss of appetite and weight loss, Dr. Fejzo noted. “The hormone can be produced by malignant tumors at levels similar to those seen in pregnancy, and symptoms can be reduced by blocking GDF15 or its receptor, GFRAL. Clinical trials are already underway in cancer patients to test this.”
She is seeking funding to test the impact of increasing GDF15 levels prior to pregnancy in patients who previously experienced HG. “I am confident that desensitizing patients by increasing GDF15 prior to pregnancy and by lowering GDF15 levels during pregnancy will work. But we need to make sure we do safety studies and get the dosing and duration right, and that will take some time.”
Desensitization will need testing first in HG, where the risk for adverse maternal and fetal outcomes is high, so the benefit will outweigh any possible risk of testing medication in pregnancy, she continued. “It will take some time before we get to patients with normal NVP, but I do believe eventually the new findings will result in game-changing therapeutics for the condition.”
Dr. Higgins added, “Even if this isn’t the golden ticket, researchers and clinicians are working toward improvements in the treatment of NVP. We’ve already come a long way in recent years with the development of treatment algorithms and the advent of doxylamine/pyridoxine.”
This study was supported primarily by the Medical Research Council UK and National Institute for Health and Care Research UK, with additional support from various research funding organizations, including Novo Nordisk Foundation.
Dr. Fejzo is a paid consultant for Materna Biosciences and NGM Biopharmaceuticals, and a board member and science adviser for the Hyperemesis Education and Research Foundation.
Numerous study co-authors disclosed financial relationships with private-sector companies, including employment and patent ownership.
Dr. Higgins disclosed no competing interests relevant to her comments but is an instructor for Organon.
FROM NATURE
Nonblanching, Erythematous, Cerebriform Plaques on the Foot
The Diagnosis: Coral Dermatitis
At 3-week follow-up, the patient demonstrated remarkable improvement in the intensity and size of the erythematous cerebriform plaques following daily application of triamcinolone acetonide cream 0.1% (Figure). The lesion disappeared after several months and did not recur. The delayed presentation of symptoms with a history of incidental coral contact during snorkeling most likely represents the type IV hypersensitivity reaction seen in the diagnosis of coral dermatitis, an extraordinarily rare form of contact dermatitis.1 Not all coral trigger skin reactions. Species of coral that contain nematocysts in their tentacles (aptly named stinging capsules) are responsible for the sting preceding coral dermatitis, as the nematocysts eject a coiled filament in response to human tactile stimulation that injects toxins into the epidermis.2
Acute, delayed, or chronic cutaneous changes follow envenomation. Acute responses arise immediately to a few hours after initial contact and are considered an irritant contact dermatitis.3 Local tissue histamine release and cascades of cytotoxic reactions often result in the characteristic urticarial or vesiculobullous plaques in addition to necrosis, piloerection, and localized lymphadenopathy.2-4 Although relatively uncommon, there may be rapid onset of systemic symptoms such as fever, malaise, hives, nausea, or emesis. Cardiopulmonary events, hepatotoxicity, renal failure, or anaphylaxis are rare.2 Histopathology of biopsy specimens reveals epidermal spongiosis with microvesicles and papillary dermal edema.1,5 In comparison, delayed reactions occur within days to weeks and exhibit epidermal parakeratosis, spongiosis, basal layer vacuolization, focal necrosis, lymphocyte exocytosis, and papillary dermal edema with extravasated erythrocytes.1,6 Clinically, it may present as linear rows of erythematous papules with burning and pruritus.6 Chronic reactions manifest after months as difficult-to-treat, persistent lichenoid dermatitis occasionally accompanied by granulomatous changes.1,2,4 Primary prevention measures after initial contact include an acetic acid rinse and cold compression to wash away residual nematocysts in the affected area.4,7,8 If a rash develops, topical steroids are the mainstay of treatment.3,8
In tandem with toxic nematocysts, the rigid calcified bodies of coral provide an additional self-defense mechanism against human contact.2,4 The irregular haphazard nature of coral may catch novice divers off guard and lead to laceration of a mispositioned limb, thereby increasing the risk for secondary infections due to the introduction of calcium carbonate and toxic mucinous deposits at the wound site, warranting antibiotic treatment.2,4,7 Because tropical locales are home to other natural dangers that inflict disease and mimic early signs of coral dermatitis, reaching an accurate diagnosis can be difficult, particularly for lower limb lesions. In summary, the diagnosis of coral dermatitis can be rendered based on morphology of the lesion and clinical context (exposure to corals and delayed symptoms) as well as response to topical steroids.
The differential diagnosis includes accidental trauma. Variations in impact force and patient skin integrity lead to a number of possible cutaneous manifestations seen in accidental trauma,9 which includes contusions resulting from burst capillaries underneath intact skin, abrasions due to the superficial epidermis scuffing away, and lacerations caused by enough force to rip and split the skin, leaving subcutaneous tissue between the intact tissue.9,10 Typically, the pattern of injury can provide hints to match what object or organism caused the wound.9 However, delayed response and worsening symptoms, as seen in coral dermatitis, would be unusual in accidental trauma unless it is complicated by secondary infection (infectious dermatitis), which does not respond to topical steroids and requires antibiotic treatment.
Another differential diagnosis includes cutaneous larva migrans, which infests domesticated and stray animals. For example, hookworm larvae propagate their eggs inside the intestines of their host before fecal-soil transmission in sandy locales.11 Unexpecting beachgoers travel barefoot on this contaminated soil, offering ample opportunity for the parasite to burrow into the upper dermis.11,12 The clinical presentation includes signs and symptoms of creeping eruption such as pruritic, linear, serpiginous tracks. Topical treatment with thiabendazole requires application 3 times daily for 15 days, which increases the risk for nonadherence, yet this therapy proves advantageous if a patient does not tolerate oral agents due to systemic adverse effects.11,12 Oral agents (eg, ivermectin, albendazole) offer improved adherence with a single dose11,13; the cure rate was higher with a single dose of ivermectin 12 mg vs a single dose of albendazole 400 mg.13 The current suggested treatment is ivermectin 200 μg/kg by mouth daily for 1 or 2 days.14
The incidence of seabather’s eruption (also known as chinkui dermatitis) is highest during the summer season and fluctuates between epidemic and nonepidemic years.15,16 It occurs sporadically worldwide mostly in tropical climates due to trapping of larvae spawn of sea animals such as crustaceans in swimwear. Initially, it presents as a pruritic and burning sensation after exiting the water, manifesting as a macular, papular, or maculopapular rash on areas covered by the swimsuit.15,16 The sensation is worse in areas that are tightly banded on the swimsuit, including the waistband and elastic straps.15 Commonly, the affected individual will seek relief via a shower, which intensifies the burning, especially if the swimsuit has not been removed. The contaminated swimwear should be immediately discarded, as the trapped sea larvae’s nematocysts activate with the pressure and friction of movement.15 Seabather’s eruption typically resolves spontaneously within a week, but symptom management can be achieved with topical steroids (triamcinolone 0.1% or clobetasol 0.05%).15,16 Unlike coral dermatitis, in seabather’s eruption the symptoms are immediate and the location of the eruption coincides with areas covered by the swimsuit.
- Ahn HS, Yoon SY, Park HJ, et al. A patient with delayed contact dermatitis to coral and she displayed superficial granuloma. Ann Dermatol. 2009;21:95-97. doi:10.5021/ad.2009.21.1.95
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-752. doi:10.1016/j.jaad.2009.01.046
- Salik J, Tang R. Images in clinical medicine. Coral dermatitis. N Engl J Med. 2015;373:E2. doi:10.1056/NEJMicm1412907
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131. doi:10.1249/JSR.0000000000000042
- Addy JH. Red sea coral contact dermatitis. Int J Dermatol. 1991; 30:271-273. doi:10.1111/j.1365-4362.1991.tb04636.x
- Miracco C, Lalinga AV, Sbano P, et al. Delayed skin reaction to Red Sea coral injury showing superficial granulomas and atypical CD30+ lymphocytes: report of a case. Br J Dermatol. 2001;145:849-851. doi:10.1046/j.1365-2133.2001.04454.x
- Ceponis PJ, Cable R, Weaver LK. Don’t kick the coral! Wilderness Environ Med. 2017;28:153-155. doi:10.1016/j.wem.2017.01.025
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dematoses. part 2-in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002. doi:10.1111/j.1365-4632.2010.04476.x
- Simon LV, Lopez RA, King KC. Blunt force trauma. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed January 12, 2034. https://www.ncbi.nlm.nih.gov/books/NBK470338/
- Gentile S, Kneubuehl BP, Barrera V, et al. Fracture energy threshold in parry injuries due to sharp and blunt force. Int J Legal Med. 2019;133:1429-1435.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814. doi:10.1086/313787
- Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588-591. doi:10.1001 /archderm.129.5.588
- Caumes E, Carriere J, Datry A, et al. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg. 1993;49:641-644. doi:10.4269 /ajtmh.1993.49.641
- Schuster A, Lesshafft H, Reichert F, et al. Hookworm-related cutaneous larva migrans in northern Brazil: resolution of clinical pathology after a single dose of ivermectin. Clin Infect Dis. 2013;57:1155-1157. doi:10.1093/cid/cit440
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Odagawa S, Watari T, Yoshida M. Chinkui dermatitis: the sea bather’s eruption. QJM. 2022;115:100-101. doi:10.1093/qjmed/hcab277
The Diagnosis: Coral Dermatitis
At 3-week follow-up, the patient demonstrated remarkable improvement in the intensity and size of the erythematous cerebriform plaques following daily application of triamcinolone acetonide cream 0.1% (Figure). The lesion disappeared after several months and did not recur. The delayed presentation of symptoms with a history of incidental coral contact during snorkeling most likely represents the type IV hypersensitivity reaction seen in the diagnosis of coral dermatitis, an extraordinarily rare form of contact dermatitis.1 Not all coral trigger skin reactions. Species of coral that contain nematocysts in their tentacles (aptly named stinging capsules) are responsible for the sting preceding coral dermatitis, as the nematocysts eject a coiled filament in response to human tactile stimulation that injects toxins into the epidermis.2

Acute, delayed, or chronic cutaneous changes follow envenomation. Acute responses arise immediately to a few hours after initial contact and are considered an irritant contact dermatitis.3 Local tissue histamine release and cascades of cytotoxic reactions often result in the characteristic urticarial or vesiculobullous plaques in addition to necrosis, piloerection, and localized lymphadenopathy.2-4 Although relatively uncommon, there may be rapid onset of systemic symptoms such as fever, malaise, hives, nausea, or emesis. Cardiopulmonary events, hepatotoxicity, renal failure, or anaphylaxis are rare.2 Histopathology of biopsy specimens reveals epidermal spongiosis with microvesicles and papillary dermal edema.1,5 In comparison, delayed reactions occur within days to weeks and exhibit epidermal parakeratosis, spongiosis, basal layer vacuolization, focal necrosis, lymphocyte exocytosis, and papillary dermal edema with extravasated erythrocytes.1,6 Clinically, it may present as linear rows of erythematous papules with burning and pruritus.6 Chronic reactions manifest after months as difficult-to-treat, persistent lichenoid dermatitis occasionally accompanied by granulomatous changes.1,2,4 Primary prevention measures after initial contact include an acetic acid rinse and cold compression to wash away residual nematocysts in the affected area.4,7,8 If a rash develops, topical steroids are the mainstay of treatment.3,8
In tandem with toxic nematocysts, the rigid calcified bodies of coral provide an additional self-defense mechanism against human contact.2,4 The irregular haphazard nature of coral may catch novice divers off guard and lead to laceration of a mispositioned limb, thereby increasing the risk for secondary infections due to the introduction of calcium carbonate and toxic mucinous deposits at the wound site, warranting antibiotic treatment.2,4,7 Because tropical locales are home to other natural dangers that inflict disease and mimic early signs of coral dermatitis, reaching an accurate diagnosis can be difficult, particularly for lower limb lesions. In summary, the diagnosis of coral dermatitis can be rendered based on morphology of the lesion and clinical context (exposure to corals and delayed symptoms) as well as response to topical steroids.
The differential diagnosis includes accidental trauma. Variations in impact force and patient skin integrity lead to a number of possible cutaneous manifestations seen in accidental trauma,9 which includes contusions resulting from burst capillaries underneath intact skin, abrasions due to the superficial epidermis scuffing away, and lacerations caused by enough force to rip and split the skin, leaving subcutaneous tissue between the intact tissue.9,10 Typically, the pattern of injury can provide hints to match what object or organism caused the wound.9 However, delayed response and worsening symptoms, as seen in coral dermatitis, would be unusual in accidental trauma unless it is complicated by secondary infection (infectious dermatitis), which does not respond to topical steroids and requires antibiotic treatment.
Another differential diagnosis includes cutaneous larva migrans, which infests domesticated and stray animals. For example, hookworm larvae propagate their eggs inside the intestines of their host before fecal-soil transmission in sandy locales.11 Unexpecting beachgoers travel barefoot on this contaminated soil, offering ample opportunity for the parasite to burrow into the upper dermis.11,12 The clinical presentation includes signs and symptoms of creeping eruption such as pruritic, linear, serpiginous tracks. Topical treatment with thiabendazole requires application 3 times daily for 15 days, which increases the risk for nonadherence, yet this therapy proves advantageous if a patient does not tolerate oral agents due to systemic adverse effects.11,12 Oral agents (eg, ivermectin, albendazole) offer improved adherence with a single dose11,13; the cure rate was higher with a single dose of ivermectin 12 mg vs a single dose of albendazole 400 mg.13 The current suggested treatment is ivermectin 200 μg/kg by mouth daily for 1 or 2 days.14
The incidence of seabather’s eruption (also known as chinkui dermatitis) is highest during the summer season and fluctuates between epidemic and nonepidemic years.15,16 It occurs sporadically worldwide mostly in tropical climates due to trapping of larvae spawn of sea animals such as crustaceans in swimwear. Initially, it presents as a pruritic and burning sensation after exiting the water, manifesting as a macular, papular, or maculopapular rash on areas covered by the swimsuit.15,16 The sensation is worse in areas that are tightly banded on the swimsuit, including the waistband and elastic straps.15 Commonly, the affected individual will seek relief via a shower, which intensifies the burning, especially if the swimsuit has not been removed. The contaminated swimwear should be immediately discarded, as the trapped sea larvae’s nematocysts activate with the pressure and friction of movement.15 Seabather’s eruption typically resolves spontaneously within a week, but symptom management can be achieved with topical steroids (triamcinolone 0.1% or clobetasol 0.05%).15,16 Unlike coral dermatitis, in seabather’s eruption the symptoms are immediate and the location of the eruption coincides with areas covered by the swimsuit.
The Diagnosis: Coral Dermatitis
At 3-week follow-up, the patient demonstrated remarkable improvement in the intensity and size of the erythematous cerebriform plaques following daily application of triamcinolone acetonide cream 0.1% (Figure). The lesion disappeared after several months and did not recur. The delayed presentation of symptoms with a history of incidental coral contact during snorkeling most likely represents the type IV hypersensitivity reaction seen in the diagnosis of coral dermatitis, an extraordinarily rare form of contact dermatitis.1 Not all coral trigger skin reactions. Species of coral that contain nematocysts in their tentacles (aptly named stinging capsules) are responsible for the sting preceding coral dermatitis, as the nematocysts eject a coiled filament in response to human tactile stimulation that injects toxins into the epidermis.2

Acute, delayed, or chronic cutaneous changes follow envenomation. Acute responses arise immediately to a few hours after initial contact and are considered an irritant contact dermatitis.3 Local tissue histamine release and cascades of cytotoxic reactions often result in the characteristic urticarial or vesiculobullous plaques in addition to necrosis, piloerection, and localized lymphadenopathy.2-4 Although relatively uncommon, there may be rapid onset of systemic symptoms such as fever, malaise, hives, nausea, or emesis. Cardiopulmonary events, hepatotoxicity, renal failure, or anaphylaxis are rare.2 Histopathology of biopsy specimens reveals epidermal spongiosis with microvesicles and papillary dermal edema.1,5 In comparison, delayed reactions occur within days to weeks and exhibit epidermal parakeratosis, spongiosis, basal layer vacuolization, focal necrosis, lymphocyte exocytosis, and papillary dermal edema with extravasated erythrocytes.1,6 Clinically, it may present as linear rows of erythematous papules with burning and pruritus.6 Chronic reactions manifest after months as difficult-to-treat, persistent lichenoid dermatitis occasionally accompanied by granulomatous changes.1,2,4 Primary prevention measures after initial contact include an acetic acid rinse and cold compression to wash away residual nematocysts in the affected area.4,7,8 If a rash develops, topical steroids are the mainstay of treatment.3,8
In tandem with toxic nematocysts, the rigid calcified bodies of coral provide an additional self-defense mechanism against human contact.2,4 The irregular haphazard nature of coral may catch novice divers off guard and lead to laceration of a mispositioned limb, thereby increasing the risk for secondary infections due to the introduction of calcium carbonate and toxic mucinous deposits at the wound site, warranting antibiotic treatment.2,4,7 Because tropical locales are home to other natural dangers that inflict disease and mimic early signs of coral dermatitis, reaching an accurate diagnosis can be difficult, particularly for lower limb lesions. In summary, the diagnosis of coral dermatitis can be rendered based on morphology of the lesion and clinical context (exposure to corals and delayed symptoms) as well as response to topical steroids.
The differential diagnosis includes accidental trauma. Variations in impact force and patient skin integrity lead to a number of possible cutaneous manifestations seen in accidental trauma,9 which includes contusions resulting from burst capillaries underneath intact skin, abrasions due to the superficial epidermis scuffing away, and lacerations caused by enough force to rip and split the skin, leaving subcutaneous tissue between the intact tissue.9,10 Typically, the pattern of injury can provide hints to match what object or organism caused the wound.9 However, delayed response and worsening symptoms, as seen in coral dermatitis, would be unusual in accidental trauma unless it is complicated by secondary infection (infectious dermatitis), which does not respond to topical steroids and requires antibiotic treatment.
Another differential diagnosis includes cutaneous larva migrans, which infests domesticated and stray animals. For example, hookworm larvae propagate their eggs inside the intestines of their host before fecal-soil transmission in sandy locales.11 Unexpecting beachgoers travel barefoot on this contaminated soil, offering ample opportunity for the parasite to burrow into the upper dermis.11,12 The clinical presentation includes signs and symptoms of creeping eruption such as pruritic, linear, serpiginous tracks. Topical treatment with thiabendazole requires application 3 times daily for 15 days, which increases the risk for nonadherence, yet this therapy proves advantageous if a patient does not tolerate oral agents due to systemic adverse effects.11,12 Oral agents (eg, ivermectin, albendazole) offer improved adherence with a single dose11,13; the cure rate was higher with a single dose of ivermectin 12 mg vs a single dose of albendazole 400 mg.13 The current suggested treatment is ivermectin 200 μg/kg by mouth daily for 1 or 2 days.14
The incidence of seabather’s eruption (also known as chinkui dermatitis) is highest during the summer season and fluctuates between epidemic and nonepidemic years.15,16 It occurs sporadically worldwide mostly in tropical climates due to trapping of larvae spawn of sea animals such as crustaceans in swimwear. Initially, it presents as a pruritic and burning sensation after exiting the water, manifesting as a macular, papular, or maculopapular rash on areas covered by the swimsuit.15,16 The sensation is worse in areas that are tightly banded on the swimsuit, including the waistband and elastic straps.15 Commonly, the affected individual will seek relief via a shower, which intensifies the burning, especially if the swimsuit has not been removed. The contaminated swimwear should be immediately discarded, as the trapped sea larvae’s nematocysts activate with the pressure and friction of movement.15 Seabather’s eruption typically resolves spontaneously within a week, but symptom management can be achieved with topical steroids (triamcinolone 0.1% or clobetasol 0.05%).15,16 Unlike coral dermatitis, in seabather’s eruption the symptoms are immediate and the location of the eruption coincides with areas covered by the swimsuit.
- Ahn HS, Yoon SY, Park HJ, et al. A patient with delayed contact dermatitis to coral and she displayed superficial granuloma. Ann Dermatol. 2009;21:95-97. doi:10.5021/ad.2009.21.1.95
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-752. doi:10.1016/j.jaad.2009.01.046
- Salik J, Tang R. Images in clinical medicine. Coral dermatitis. N Engl J Med. 2015;373:E2. doi:10.1056/NEJMicm1412907
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131. doi:10.1249/JSR.0000000000000042
- Addy JH. Red sea coral contact dermatitis. Int J Dermatol. 1991; 30:271-273. doi:10.1111/j.1365-4362.1991.tb04636.x
- Miracco C, Lalinga AV, Sbano P, et al. Delayed skin reaction to Red Sea coral injury showing superficial granulomas and atypical CD30+ lymphocytes: report of a case. Br J Dermatol. 2001;145:849-851. doi:10.1046/j.1365-2133.2001.04454.x
- Ceponis PJ, Cable R, Weaver LK. Don’t kick the coral! Wilderness Environ Med. 2017;28:153-155. doi:10.1016/j.wem.2017.01.025
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dematoses. part 2-in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002. doi:10.1111/j.1365-4632.2010.04476.x
- Simon LV, Lopez RA, King KC. Blunt force trauma. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed January 12, 2034. https://www.ncbi.nlm.nih.gov/books/NBK470338/
- Gentile S, Kneubuehl BP, Barrera V, et al. Fracture energy threshold in parry injuries due to sharp and blunt force. Int J Legal Med. 2019;133:1429-1435.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814. doi:10.1086/313787
- Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588-591. doi:10.1001 /archderm.129.5.588
- Caumes E, Carriere J, Datry A, et al. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg. 1993;49:641-644. doi:10.4269 /ajtmh.1993.49.641
- Schuster A, Lesshafft H, Reichert F, et al. Hookworm-related cutaneous larva migrans in northern Brazil: resolution of clinical pathology after a single dose of ivermectin. Clin Infect Dis. 2013;57:1155-1157. doi:10.1093/cid/cit440
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Odagawa S, Watari T, Yoshida M. Chinkui dermatitis: the sea bather’s eruption. QJM. 2022;115:100-101. doi:10.1093/qjmed/hcab277
- Ahn HS, Yoon SY, Park HJ, et al. A patient with delayed contact dermatitis to coral and she displayed superficial granuloma. Ann Dermatol. 2009;21:95-97. doi:10.5021/ad.2009.21.1.95
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-752. doi:10.1016/j.jaad.2009.01.046
- Salik J, Tang R. Images in clinical medicine. Coral dermatitis. N Engl J Med. 2015;373:E2. doi:10.1056/NEJMicm1412907
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131. doi:10.1249/JSR.0000000000000042
- Addy JH. Red sea coral contact dermatitis. Int J Dermatol. 1991; 30:271-273. doi:10.1111/j.1365-4362.1991.tb04636.x
- Miracco C, Lalinga AV, Sbano P, et al. Delayed skin reaction to Red Sea coral injury showing superficial granulomas and atypical CD30+ lymphocytes: report of a case. Br J Dermatol. 2001;145:849-851. doi:10.1046/j.1365-2133.2001.04454.x
- Ceponis PJ, Cable R, Weaver LK. Don’t kick the coral! Wilderness Environ Med. 2017;28:153-155. doi:10.1016/j.wem.2017.01.025
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dematoses. part 2-in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002. doi:10.1111/j.1365-4632.2010.04476.x
- Simon LV, Lopez RA, King KC. Blunt force trauma. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed January 12, 2034. https://www.ncbi.nlm.nih.gov/books/NBK470338/
- Gentile S, Kneubuehl BP, Barrera V, et al. Fracture energy threshold in parry injuries due to sharp and blunt force. Int J Legal Med. 2019;133:1429-1435.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814. doi:10.1086/313787
- Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588-591. doi:10.1001 /archderm.129.5.588
- Caumes E, Carriere J, Datry A, et al. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg. 1993;49:641-644. doi:10.4269 /ajtmh.1993.49.641
- Schuster A, Lesshafft H, Reichert F, et al. Hookworm-related cutaneous larva migrans in northern Brazil: resolution of clinical pathology after a single dose of ivermectin. Clin Infect Dis. 2013;57:1155-1157. doi:10.1093/cid/cit440
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Odagawa S, Watari T, Yoshida M. Chinkui dermatitis: the sea bather’s eruption. QJM. 2022;115:100-101. doi:10.1093/qjmed/hcab277
A 48-year-old otherwise healthy man presented with a tender lesion on the dorsal aspect of the right foot with dysesthesia and progressive pruritus that he originally noticed 9 days prior after snorkeling in the Caribbean. He recalled kicking what he assumed was a rock while swimming. Initially there was negligible discomfort; however, on day 7 the symptoms started to worsen and the lesion started to swell. Application of a gauze pad soaked in hydrogen peroxide 3% failed to alleviate symptoms. Physical examination revealed a 4-cm region of well-demarcated, nonblanching, erythematous plaques in a lattice pattern accompanied by edematous and bullous changes. Triamcinolone acetonide cream 0.1% was prescribed.
More Side Effects With Local Therapies for Prostate Cancer
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
These were the findings of a retrospective cohort study in JAMA Network Open.
The standard treatment of advanced prostate cancer is androgen deprivation therapy (ADT). “The role of local therapy has been debated for several years. Studies have shown that radiation therapy or radical prostatectomy can improve patient survival under certain conditions,” said Hubert Kübler, MD, director of the Clinic and Polyclinic for Urology and Pediatric Urology at the University Hospital Würzburg in Germany. “At academic centers, a local therapy is pursued for oligometastatic patients if they are fit enough.”
The hope is to spare patients the side effects of ADT over an extended period and thus improve their quality of life. “But what impact does local therapy itself have on the men’s quality of life, especially considering that the survival advantage gained may be relatively small?” wrote study author Saira Khan, PhD, MPH, assistant professor of surgery at Washington University School of Medicine in St. Louis, Missouri, and her colleagues.
Examining Side Effects
This question has not been thoroughly examined yet. “To our knowledge, this is one of the first studies investigating the side effects of local therapy in men with advanced prostate cancer for up to 5 years after treatment,” wrote the authors.
The cohort study included 5500 US veterans who were diagnosed with advanced prostate cancer between January 1999 and December 2013. The tumors were in stage T4 (tumor is fixed or has spread to adjacent structures), with regional lymph node metastases (N1), and partially detectable distant metastases (M1).
The average age was 68.7 years, and 31% received local therapy (eg, radiation therapy, radical prostatectomy, or both), and 69% received systemic therapy (eg, hormone therapy, chemotherapy, or both).
Types of Local Therapy
Combining radiation therapy and radical prostatectomy “diminishes the meaningfulness of the study results,” according to Dr. Kübler. “The issue should have been analyzed in much finer detail. Studies clearly show, for example, that radiation therapy consistently performs slightly worse than prostatectomy in terms of gastrointestinal complaints.”
In their paper, the researchers reported that the prevalence of side effects was high, regardless of the therapy. Overall, 916 men (75.2%) with initial local therapy and 897 men (67.1%) with initial systemic therapy reported experiencing at least one side effect lasting more than 2 years and up to 5 years.
In the first year after the initial therapy, men who underwent local therapy, compared with those who underwent systemic therapy, experienced more of the following symptoms:
- Gastrointestinal issues (odds ratio [OR], 4.08)
- Pain (OR, 1.57)
- Sexual dysfunction (OR, 2.96)
- Urinary problems, predominantly incontinence (OR, 2.25)
Lasting Side Effects
Even up to year 5 after the initial therapy, men with local therapy reported more gastrointestinal and sexual issues, as well as more frequent incontinence, than those with systemic therapy. Only the frequency of pain equalized between the two groups in the second year.
“Our results are consistent with the known side effect profile [of local therapy] in patients with clinically localized prostate cancer receiving surgery or radiation therapy instead of active surveillance,” wrote the authors.
The comparison in advanced prostate cancer, however, is not with active surveillance but with ADT. “As the study confirmed, ADT is associated with various side effects,” said Dr. Kübler. Nevertheless, it was associated with fewer side effects than local therapy in this study. The concept behind local therapy (improving prognosis while avoiding local problems) is challenging to reconcile with these results.
Contradictory Data
The results also contradict findings from other studies. Dr. Kübler pointed to the recently presented PEACE-1 study, where “local complications and issues were reduced through local therapy in high-volume and high-risk patients.”
The study did not consider subsequent interventions, such as how many patients needed transurethral manipulation in the later course of the disease to address local problems. “There are older data showing that a radical prostatectomy can reduce the need for further resections,” Dr. Kübler added.
“I find it difficult to reconcile these data with other data and with my personal experience,” said Dr. Kübler. However, he agreed with the study authors’ conclusion, emphasizing the importance of informing patients about expected side effects of local therapy in the context of potentially marginal improvements in survival.
Different Situation in Germany
“As practitioners, we sometimes underestimate the side effects we subject our patients to. We need to talk to our patients about the prognosis improvement that comes with side effects,” said Dr. Kübler. He added that a similar study in Germany might yield different results. “Dr. Khan and her colleagues examined a very specific patient population: Namely, veterans. This patient clientele often faces many social difficulties, and the treatment structure in US veterans’ care differs significantly from ours.”
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Study Evaluates Aesthetic Concerns in Asian Women
CHICAGO — presented at the annual meeting of the American Society for Dermatologic Surgery (ASDS),
“Asian Americans represent the fastest-expanding racial group in the US, underscoring the need for a comprehensive understanding of their specific aesthetic goals and needs,” said Annie Chiu, MD, a cosmetic dermatologist in Redondo Beach, California, who presented the results at the meeting. In an interview, she noted that most of this type of research has been done in White or Eurocentric populations, “so we really wanted to identify the top aesthetic concerns for Asian women as they tend to be different.”
In the study, an online survey was administered to aesthetically-inclined adults — defined as those who care about improving their appearance and are willing to go to a professional to do so — across different demographic groups in the United States. Respondents were surveyed about 41 facial and 31 body characteristics, identifying those they have and find bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3,974 women surveyed, 652 self-identified as female and Asian. The majority of Asian female respondents self-reported a Fitzpatrick skin type IV (East/Southeast [SE]Asian: 58%; Indian/Central or Southwest [CSW] Asian: 24%) or type V (East/SE Asian: 59%; Indian/CSW Asian: 30%). The findings reported at the meeting are specific to aesthetic concerns in Asian women.
Among the Asian female participants, the top three facial concerns were uneven skin color (40%), dull/dry skin (35%), and hair loss/thinning (34%). Top facial concerns in younger patients (under 30 years) were related to skin quality, such as dull skin (54%), acne scarring (51%), and large pore size (51%), whereas the most common concerns among those older than 57 years were related to under-eye bags or sagginess (60%), uneven skin tone (55%), and hair loss/thinning (47%).
Of note, acne scarring was noted as a top concern by the Indian/CSW Asian cohort. While the lines between the brows and skin sagging were top concerns in White female participants in the overall study, these concerns were not nearly as high among Asian female participants.
For all Asian female participants, the top body concerns were related to stubborn body fat in the stomach, sides, bra or back area, and arms. Stubborn body fat in the stomach area was the most frequent concern across generations (41% to 64%). East/SE Asian participants were more interested in receiving cosmetic treatments (91%) than the Indian/CSW Asian group (47%).
“Given that injectable treatments of neuromodulators and fillers are often what we focus aesthetic treatments around and thus, what we often center cosmetic consults on, it is important to remember to customize patient consultations and address specific needs with cultural sensitivity,” Dr. Chiu said. “We may not be properly recognizing and prioritizing patient discussion around concerns of dyspigmentation, skin quality, or hair thinning,” she continued, adding: “Ultimately as experts, it’s important we use this data along with what we know about structural and cutaneous differences in patients of different cultural backgrounds to optimize and prioritize treatments.”
Allergan Aesthetics, an AbbVie company, funded the study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review. Dr. Chiu is a consultant, advisory board member, and investigator for Allergan, AbbVie,and Merz; and is a consultant and advisory board member for Galderma, Evolus, and Sofwave. Other authors disclosed ties with Allergan, Merz Aesthetics, Prollenium, Revance, Galderma, Alastin, Glo Pharma, and Teoxane. Two authors are AbbVie employees.
CHICAGO — presented at the annual meeting of the American Society for Dermatologic Surgery (ASDS),
“Asian Americans represent the fastest-expanding racial group in the US, underscoring the need for a comprehensive understanding of their specific aesthetic goals and needs,” said Annie Chiu, MD, a cosmetic dermatologist in Redondo Beach, California, who presented the results at the meeting. In an interview, she noted that most of this type of research has been done in White or Eurocentric populations, “so we really wanted to identify the top aesthetic concerns for Asian women as they tend to be different.”
In the study, an online survey was administered to aesthetically-inclined adults — defined as those who care about improving their appearance and are willing to go to a professional to do so — across different demographic groups in the United States. Respondents were surveyed about 41 facial and 31 body characteristics, identifying those they have and find bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3,974 women surveyed, 652 self-identified as female and Asian. The majority of Asian female respondents self-reported a Fitzpatrick skin type IV (East/Southeast [SE]Asian: 58%; Indian/Central or Southwest [CSW] Asian: 24%) or type V (East/SE Asian: 59%; Indian/CSW Asian: 30%). The findings reported at the meeting are specific to aesthetic concerns in Asian women.
Among the Asian female participants, the top three facial concerns were uneven skin color (40%), dull/dry skin (35%), and hair loss/thinning (34%). Top facial concerns in younger patients (under 30 years) were related to skin quality, such as dull skin (54%), acne scarring (51%), and large pore size (51%), whereas the most common concerns among those older than 57 years were related to under-eye bags or sagginess (60%), uneven skin tone (55%), and hair loss/thinning (47%).
Of note, acne scarring was noted as a top concern by the Indian/CSW Asian cohort. While the lines between the brows and skin sagging were top concerns in White female participants in the overall study, these concerns were not nearly as high among Asian female participants.
For all Asian female participants, the top body concerns were related to stubborn body fat in the stomach, sides, bra or back area, and arms. Stubborn body fat in the stomach area was the most frequent concern across generations (41% to 64%). East/SE Asian participants were more interested in receiving cosmetic treatments (91%) than the Indian/CSW Asian group (47%).
“Given that injectable treatments of neuromodulators and fillers are often what we focus aesthetic treatments around and thus, what we often center cosmetic consults on, it is important to remember to customize patient consultations and address specific needs with cultural sensitivity,” Dr. Chiu said. “We may not be properly recognizing and prioritizing patient discussion around concerns of dyspigmentation, skin quality, or hair thinning,” she continued, adding: “Ultimately as experts, it’s important we use this data along with what we know about structural and cutaneous differences in patients of different cultural backgrounds to optimize and prioritize treatments.”
Allergan Aesthetics, an AbbVie company, funded the study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review. Dr. Chiu is a consultant, advisory board member, and investigator for Allergan, AbbVie,and Merz; and is a consultant and advisory board member for Galderma, Evolus, and Sofwave. Other authors disclosed ties with Allergan, Merz Aesthetics, Prollenium, Revance, Galderma, Alastin, Glo Pharma, and Teoxane. Two authors are AbbVie employees.
CHICAGO — presented at the annual meeting of the American Society for Dermatologic Surgery (ASDS),
“Asian Americans represent the fastest-expanding racial group in the US, underscoring the need for a comprehensive understanding of their specific aesthetic goals and needs,” said Annie Chiu, MD, a cosmetic dermatologist in Redondo Beach, California, who presented the results at the meeting. In an interview, she noted that most of this type of research has been done in White or Eurocentric populations, “so we really wanted to identify the top aesthetic concerns for Asian women as they tend to be different.”
In the study, an online survey was administered to aesthetically-inclined adults — defined as those who care about improving their appearance and are willing to go to a professional to do so — across different demographic groups in the United States. Respondents were surveyed about 41 facial and 31 body characteristics, identifying those they have and find bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3,974 women surveyed, 652 self-identified as female and Asian. The majority of Asian female respondents self-reported a Fitzpatrick skin type IV (East/Southeast [SE]Asian: 58%; Indian/Central or Southwest [CSW] Asian: 24%) or type V (East/SE Asian: 59%; Indian/CSW Asian: 30%). The findings reported at the meeting are specific to aesthetic concerns in Asian women.
Among the Asian female participants, the top three facial concerns were uneven skin color (40%), dull/dry skin (35%), and hair loss/thinning (34%). Top facial concerns in younger patients (under 30 years) were related to skin quality, such as dull skin (54%), acne scarring (51%), and large pore size (51%), whereas the most common concerns among those older than 57 years were related to under-eye bags or sagginess (60%), uneven skin tone (55%), and hair loss/thinning (47%).
Of note, acne scarring was noted as a top concern by the Indian/CSW Asian cohort. While the lines between the brows and skin sagging were top concerns in White female participants in the overall study, these concerns were not nearly as high among Asian female participants.
For all Asian female participants, the top body concerns were related to stubborn body fat in the stomach, sides, bra or back area, and arms. Stubborn body fat in the stomach area was the most frequent concern across generations (41% to 64%). East/SE Asian participants were more interested in receiving cosmetic treatments (91%) than the Indian/CSW Asian group (47%).
“Given that injectable treatments of neuromodulators and fillers are often what we focus aesthetic treatments around and thus, what we often center cosmetic consults on, it is important to remember to customize patient consultations and address specific needs with cultural sensitivity,” Dr. Chiu said. “We may not be properly recognizing and prioritizing patient discussion around concerns of dyspigmentation, skin quality, or hair thinning,” she continued, adding: “Ultimately as experts, it’s important we use this data along with what we know about structural and cutaneous differences in patients of different cultural backgrounds to optimize and prioritize treatments.”
Allergan Aesthetics, an AbbVie company, funded the study and participated in the trial design, research, analysis, data collection, interpretation of data, and the review. Dr. Chiu is a consultant, advisory board member, and investigator for Allergan, AbbVie,and Merz; and is a consultant and advisory board member for Galderma, Evolus, and Sofwave. Other authors disclosed ties with Allergan, Merz Aesthetics, Prollenium, Revance, Galderma, Alastin, Glo Pharma, and Teoxane. Two authors are AbbVie employees.
AT ASDS 2023