User login
How Is the Colorectal Cancer Control Program Doing?
The CDC developed the Colorectal Cancer Control Program (CRCCP) to provide direct colorectal cancer (CRC) screening services to low-income, uninsured, or underinsured populations known to have low CRC screening rates. However, early evaluators found the program was insufficient to detect impact at the state level. In response to those findings, the CDC redesigned CRCCP and funded a new 5-year grant period beginning in 2015. How did the program fare this time? CDC researchers say it “shows promise.”
The CRCCP funds 23 states, 6 universities, and 1 tribal organization to partner with health care systems, implementing evidence-based interventions (EBIs). In this study, the researchers analyzed data reported by 387 of 413 clinics of varying sizes, representing 3,438 providers, and serving a screening-eligible population of 722,925 patients.
The researchers say their evaluation suggests that the CRCCP is working as intended: Program reach was measurable and “substantial,” clinics enhanced EBIs in place or implemented new ones, and the overall average screening rate rose.
At baseline, the screening rate was low (43%), and lowest in rural clinics—although evidence indicates that death rates for CRC are highest among people living in rural areas. In the first year, the overall screening rate increased by 4.4 percentage points. Still, that 47.3% is “much lower” than the commonly cited 67.3% from the 2016 Behavioral Risk Factor Surveillance System, the researchers note. They add, though, that the results confirm that grantees are working with clinics serving the intended populations and indicate the significant gap in CRC screening rates between those reached by the CRCCP and the US population overall.
Many clinics had ≥ 1 EBI or supporting activity (SA) already in place. Grantees used CRCCP resources to implement new or to enhance EBIs in 95% of the clinics, most often patient reminder activities and provider assessment and feedback. Most of the clinics used CRCCP resources for SAs, such as small media and provider education. Only 12% of clinics used resources for supporting community health workers. However, nearly half the clinics conducted planning activities for future implementation of community health workers and patient navigators.
Nearly 80% of the clinics reported having a CRC screening champion, 73% had a CRC screening policy, and 50% had either 3 or 4 EBIs in place at the end of the first year—all factors that the researchers suggest may support greater screening rate increases.
The CDC developed the Colorectal Cancer Control Program (CRCCP) to provide direct colorectal cancer (CRC) screening services to low-income, uninsured, or underinsured populations known to have low CRC screening rates. However, early evaluators found the program was insufficient to detect impact at the state level. In response to those findings, the CDC redesigned CRCCP and funded a new 5-year grant period beginning in 2015. How did the program fare this time? CDC researchers say it “shows promise.”
The CRCCP funds 23 states, 6 universities, and 1 tribal organization to partner with health care systems, implementing evidence-based interventions (EBIs). In this study, the researchers analyzed data reported by 387 of 413 clinics of varying sizes, representing 3,438 providers, and serving a screening-eligible population of 722,925 patients.
The researchers say their evaluation suggests that the CRCCP is working as intended: Program reach was measurable and “substantial,” clinics enhanced EBIs in place or implemented new ones, and the overall average screening rate rose.
At baseline, the screening rate was low (43%), and lowest in rural clinics—although evidence indicates that death rates for CRC are highest among people living in rural areas. In the first year, the overall screening rate increased by 4.4 percentage points. Still, that 47.3% is “much lower” than the commonly cited 67.3% from the 2016 Behavioral Risk Factor Surveillance System, the researchers note. They add, though, that the results confirm that grantees are working with clinics serving the intended populations and indicate the significant gap in CRC screening rates between those reached by the CRCCP and the US population overall.
Many clinics had ≥ 1 EBI or supporting activity (SA) already in place. Grantees used CRCCP resources to implement new or to enhance EBIs in 95% of the clinics, most often patient reminder activities and provider assessment and feedback. Most of the clinics used CRCCP resources for SAs, such as small media and provider education. Only 12% of clinics used resources for supporting community health workers. However, nearly half the clinics conducted planning activities for future implementation of community health workers and patient navigators.
Nearly 80% of the clinics reported having a CRC screening champion, 73% had a CRC screening policy, and 50% had either 3 or 4 EBIs in place at the end of the first year—all factors that the researchers suggest may support greater screening rate increases.
The CDC developed the Colorectal Cancer Control Program (CRCCP) to provide direct colorectal cancer (CRC) screening services to low-income, uninsured, or underinsured populations known to have low CRC screening rates. However, early evaluators found the program was insufficient to detect impact at the state level. In response to those findings, the CDC redesigned CRCCP and funded a new 5-year grant period beginning in 2015. How did the program fare this time? CDC researchers say it “shows promise.”
The CRCCP funds 23 states, 6 universities, and 1 tribal organization to partner with health care systems, implementing evidence-based interventions (EBIs). In this study, the researchers analyzed data reported by 387 of 413 clinics of varying sizes, representing 3,438 providers, and serving a screening-eligible population of 722,925 patients.
The researchers say their evaluation suggests that the CRCCP is working as intended: Program reach was measurable and “substantial,” clinics enhanced EBIs in place or implemented new ones, and the overall average screening rate rose.
At baseline, the screening rate was low (43%), and lowest in rural clinics—although evidence indicates that death rates for CRC are highest among people living in rural areas. In the first year, the overall screening rate increased by 4.4 percentage points. Still, that 47.3% is “much lower” than the commonly cited 67.3% from the 2016 Behavioral Risk Factor Surveillance System, the researchers note. They add, though, that the results confirm that grantees are working with clinics serving the intended populations and indicate the significant gap in CRC screening rates between those reached by the CRCCP and the US population overall.
Many clinics had ≥ 1 EBI or supporting activity (SA) already in place. Grantees used CRCCP resources to implement new or to enhance EBIs in 95% of the clinics, most often patient reminder activities and provider assessment and feedback. Most of the clinics used CRCCP resources for SAs, such as small media and provider education. Only 12% of clinics used resources for supporting community health workers. However, nearly half the clinics conducted planning activities for future implementation of community health workers and patient navigators.
Nearly 80% of the clinics reported having a CRC screening champion, 73% had a CRC screening policy, and 50% had either 3 or 4 EBIs in place at the end of the first year—all factors that the researchers suggest may support greater screening rate increases.
HIV-associated Kaposi sarcoma responds to checkpoint inhibitors
Checkpoint inhibitor therapy is effective for patients with HIV-associated Kaposi sarcoma (KS), a recent study has found.
Partial or complete remission was achieved by a majority of patients; others currently have stable disease lasting longer than 6 months, reported Natalie Galanina, MD, of Rebecca and John Moores Cancer Center at the University of California, San Diego, and her colleagues. Earlier this year, investigators reported similar responses to checkpoint inhibitors in two patients with KS that wasn’t associated with HIV.
“An association has been demonstrated between chronic viral infection, malignancy, and up-regulation of programmed death receptor 1 (PD-1) on CD8+ cytotoxic T-lymphocytes,” the authors wrote in Cancer Immunology Research. In particular, “HIV-specific CD8+ T cells have increased PD-1 expression, which … promotes a cellular milieu conducive to oncogenesis.” These factors, together with the results from the previous study, have suggested that checkpoint inhibitors may be effective for patients with HIV-associated KS.
The retrospective study involved 320 patients treated with immunotherapy at Moores Cancer Center from August 2013 through December 2017. From this group, nine cases of HIV-associated KS were found. Median CD4 count was 256 cells/mcL and median viral load was 20 copies/mL. Eight patients were treated with nivolumab and one was treated with pembrolizumab. Median age was 44 years. All patients were male and receiving antiretroviral therapy.
Six patients (67%) achieved remission, with five attaining partial remission and one attaining complete remission (gastrointestinal disease). Of the remaining three patients, two currently have stable disease lasting longer than 6 months, and one has stable disease lasting longer than 3 months.
Muscle aches, pruritus, and low-grade fever were the most common adverse events. No grade 3 or higher drug-related adverse events occurred.
“Most of our patients received one to four prior lines of therapy but still responded to checkpoint blockade,” the authors wrote. “Our observations suggest that patients with HIV-associated KS have high [response rates] to PD-1 checkpoint blockade, without significant toxicity, even in the presence of low [tumor mutational burden] and/or lack of PD-L1 expression.”
Authors reported compensation from Incyte, Genentech, Merck, Pfizer, and others.
SOURCE: Galanina et al. Cancer Immunol Res. doi: 10.1158/2326-6066.CIR-18-0121.
Checkpoint inhibitor therapy is effective for patients with HIV-associated Kaposi sarcoma (KS), a recent study has found.
Partial or complete remission was achieved by a majority of patients; others currently have stable disease lasting longer than 6 months, reported Natalie Galanina, MD, of Rebecca and John Moores Cancer Center at the University of California, San Diego, and her colleagues. Earlier this year, investigators reported similar responses to checkpoint inhibitors in two patients with KS that wasn’t associated with HIV.
“An association has been demonstrated between chronic viral infection, malignancy, and up-regulation of programmed death receptor 1 (PD-1) on CD8+ cytotoxic T-lymphocytes,” the authors wrote in Cancer Immunology Research. In particular, “HIV-specific CD8+ T cells have increased PD-1 expression, which … promotes a cellular milieu conducive to oncogenesis.” These factors, together with the results from the previous study, have suggested that checkpoint inhibitors may be effective for patients with HIV-associated KS.
The retrospective study involved 320 patients treated with immunotherapy at Moores Cancer Center from August 2013 through December 2017. From this group, nine cases of HIV-associated KS were found. Median CD4 count was 256 cells/mcL and median viral load was 20 copies/mL. Eight patients were treated with nivolumab and one was treated with pembrolizumab. Median age was 44 years. All patients were male and receiving antiretroviral therapy.
Six patients (67%) achieved remission, with five attaining partial remission and one attaining complete remission (gastrointestinal disease). Of the remaining three patients, two currently have stable disease lasting longer than 6 months, and one has stable disease lasting longer than 3 months.
Muscle aches, pruritus, and low-grade fever were the most common adverse events. No grade 3 or higher drug-related adverse events occurred.
“Most of our patients received one to four prior lines of therapy but still responded to checkpoint blockade,” the authors wrote. “Our observations suggest that patients with HIV-associated KS have high [response rates] to PD-1 checkpoint blockade, without significant toxicity, even in the presence of low [tumor mutational burden] and/or lack of PD-L1 expression.”
Authors reported compensation from Incyte, Genentech, Merck, Pfizer, and others.
SOURCE: Galanina et al. Cancer Immunol Res. doi: 10.1158/2326-6066.CIR-18-0121.
Checkpoint inhibitor therapy is effective for patients with HIV-associated Kaposi sarcoma (KS), a recent study has found.
Partial or complete remission was achieved by a majority of patients; others currently have stable disease lasting longer than 6 months, reported Natalie Galanina, MD, of Rebecca and John Moores Cancer Center at the University of California, San Diego, and her colleagues. Earlier this year, investigators reported similar responses to checkpoint inhibitors in two patients with KS that wasn’t associated with HIV.
“An association has been demonstrated between chronic viral infection, malignancy, and up-regulation of programmed death receptor 1 (PD-1) on CD8+ cytotoxic T-lymphocytes,” the authors wrote in Cancer Immunology Research. In particular, “HIV-specific CD8+ T cells have increased PD-1 expression, which … promotes a cellular milieu conducive to oncogenesis.” These factors, together with the results from the previous study, have suggested that checkpoint inhibitors may be effective for patients with HIV-associated KS.
The retrospective study involved 320 patients treated with immunotherapy at Moores Cancer Center from August 2013 through December 2017. From this group, nine cases of HIV-associated KS were found. Median CD4 count was 256 cells/mcL and median viral load was 20 copies/mL. Eight patients were treated with nivolumab and one was treated with pembrolizumab. Median age was 44 years. All patients were male and receiving antiretroviral therapy.
Six patients (67%) achieved remission, with five attaining partial remission and one attaining complete remission (gastrointestinal disease). Of the remaining three patients, two currently have stable disease lasting longer than 6 months, and one has stable disease lasting longer than 3 months.
Muscle aches, pruritus, and low-grade fever were the most common adverse events. No grade 3 or higher drug-related adverse events occurred.
“Most of our patients received one to four prior lines of therapy but still responded to checkpoint blockade,” the authors wrote. “Our observations suggest that patients with HIV-associated KS have high [response rates] to PD-1 checkpoint blockade, without significant toxicity, even in the presence of low [tumor mutational burden] and/or lack of PD-L1 expression.”
Authors reported compensation from Incyte, Genentech, Merck, Pfizer, and others.
SOURCE: Galanina et al. Cancer Immunol Res. doi: 10.1158/2326-6066.CIR-18-0121.
FROM CANCER IMMUNOLOGY RESEARCH
Key clinical point: Checkpoint inhibitor therapy is effective for patients with HIV-associated Kaposi sarcoma.
Major finding: Two-thirds of patients (67%) with HIV-associated Kaposi sarcoma achieved partial or complete remission when treated with immune checkpoint blockade.
Study details: A retrospective study involving nine patients with Kaposi sarcoma treated with either nivolumab or pembrolizumab at the Rebecca and John Moores Cancer Center at the University of California, San Diego, (UCSD) from August 2013 through December 2017.
Disclosures: Authors reported compensation from Incyte, Genentech, Merck, Pfizer, and others.
Source: Galanina et al. Cancer Immunol Res. 2018 Sept 7. doi: 10.1158/2326-6066.CIR-18-0121.
First CAR T-cell therapy approved in Canada
Health Canada has authorized use of tisagenlecleucel (Kymriah™), making it the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel (formerly CTL019) is approved to treat patients ages 3 to 25 with B-cell acute lymphoblastic leukemia (ALL) who have relapsed after allogeneic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers, and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
JULIET enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
These results were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
ELIANA trial
ELIANA included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi. All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
Ninety-five percent of patients had AEs thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
These results were published in The New England Journal of Medicine in February.
Health Canada has authorized use of tisagenlecleucel (Kymriah™), making it the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel (formerly CTL019) is approved to treat patients ages 3 to 25 with B-cell acute lymphoblastic leukemia (ALL) who have relapsed after allogeneic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers, and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
JULIET enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
These results were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
ELIANA trial
ELIANA included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi. All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
Ninety-five percent of patients had AEs thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
These results were published in The New England Journal of Medicine in February.
Health Canada has authorized use of tisagenlecleucel (Kymriah™), making it the first chimeric antigen receptor (CAR) T-cell therapy to receive regulatory approval in Canada.
Tisagenlecleucel (formerly CTL019) is approved to treat patients ages 3 to 25 with B-cell acute lymphoblastic leukemia (ALL) who have relapsed after allogeneic stem cell transplant (SCT) or are otherwise ineligible for SCT, have experienced second or later relapse, or have refractory disease.
Tisagenlecleucel is also approved in Canada to treat adults who have received two or more lines of systemic therapy and have relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, or DLBCL arising from follicular lymphoma.
Novartis, the company marketing tisagenlecleucel, said it is working with qualified treatment centers in Canada to prepare for the delivery of tisagenlecleucel. Certification and training are underway at these centers, and Novartis is enhancing manufacturing capacity to meet patient needs.
Tisagenlecleucel has been studied in a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
JULIET enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached with a median follow-up of 13.9 months. At last follow-up, none of the responders had gone on to SCT.
The 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
These results were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
ELIANA trial
ELIANA included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi. All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to SCT while in remission. At last follow-up, four were still in remission, and four had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
Ninety-five percent of patients had AEs thought to be related to tisagenlecleucel. The incidence of treatment-related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
These results were published in The New England Journal of Medicine in February.
Regimens produce similar results in FL
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in the treatment of follicular lymphoma (FL) in a phase 3 trial.
Patients with previously untreated FL had similar complete response (CR) rates and progression-free survival (PFS) rates whether they received rituximab-based chemotherapy or rituximab plus lenalidomide.
These results were published in The New England Journal of Medicine.
The trial, RELEVANCE, included 1,030 patients with previously untreated FL. They were randomized to receive rituximab plus chemotherapy (n=517) or rituximab plus lenalidomide (n=513) for 18 cycles.
Patients in the chemotherapy arm received one of three regimens—R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), rituximab and bendamustine, or R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone).
Patients in both treatment arms went on to receive rituximab maintenance every 8 weeks for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years.
The coprimary endpoints were CR (confirmed or unconfirmed) and PFS. After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the treatment arms.
CR was observed in 48% of the rituximab-lenalidomide arm and 53% of the rituximab-chemotherapy arm (P=0.13).
The interim 3-year PFS rate was 77% in the rituximab-lenalidomide arm and 78% in the rituximab-chemotherapy arm. The hazard ratio for progression or death from any cause was 1.10 (P=0.48).
The efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose disease was Ann Arbor stage I or II, whereas the efficacy of rituximab-lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some adverse events (AEs) being more common in one arm than the other.
AEs that were more common with rituximab-lenalidomide include cutaneous reactions (43% vs 24%), diarrhea (37% vs 19%), rash (29% vs 8%), abdominal pain (15% vs 9%), peripheral edema (14% vs 9%), muscle spasms (13% vs 4%), myalgia (14% vs 6%), and tumor flare reaction (6% vs <1%).
AEs that were more common with rituximab-chemotherapy were anemia (89% vs 66%), fatigue (29% vs 23%), nausea (42% vs 20%), vomiting (19% vs 7%), febrile neutropenia (7% vs 2%), leukopenia (10% vs 4%), and peripheral neuropathy (16% vs 7%).
Grade 3/4 cutaneous reactions were more common with rituximab-lenalidomide (7% vs 1%), and grade 3/4 neutropenia was more common with rituximab-chemotherapy (50% vs 32%).
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in the treatment of follicular lymphoma (FL) in a phase 3 trial.
Patients with previously untreated FL had similar complete response (CR) rates and progression-free survival (PFS) rates whether they received rituximab-based chemotherapy or rituximab plus lenalidomide.
These results were published in The New England Journal of Medicine.
The trial, RELEVANCE, included 1,030 patients with previously untreated FL. They were randomized to receive rituximab plus chemotherapy (n=517) or rituximab plus lenalidomide (n=513) for 18 cycles.
Patients in the chemotherapy arm received one of three regimens—R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), rituximab and bendamustine, or R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone).
Patients in both treatment arms went on to receive rituximab maintenance every 8 weeks for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years.
The coprimary endpoints were CR (confirmed or unconfirmed) and PFS. After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the treatment arms.
CR was observed in 48% of the rituximab-lenalidomide arm and 53% of the rituximab-chemotherapy arm (P=0.13).
The interim 3-year PFS rate was 77% in the rituximab-lenalidomide arm and 78% in the rituximab-chemotherapy arm. The hazard ratio for progression or death from any cause was 1.10 (P=0.48).
The efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose disease was Ann Arbor stage I or II, whereas the efficacy of rituximab-lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some adverse events (AEs) being more common in one arm than the other.
AEs that were more common with rituximab-lenalidomide include cutaneous reactions (43% vs 24%), diarrhea (37% vs 19%), rash (29% vs 8%), abdominal pain (15% vs 9%), peripheral edema (14% vs 9%), muscle spasms (13% vs 4%), myalgia (14% vs 6%), and tumor flare reaction (6% vs <1%).
AEs that were more common with rituximab-chemotherapy were anemia (89% vs 66%), fatigue (29% vs 23%), nausea (42% vs 20%), vomiting (19% vs 7%), febrile neutropenia (7% vs 2%), leukopenia (10% vs 4%), and peripheral neuropathy (16% vs 7%).
Grade 3/4 cutaneous reactions were more common with rituximab-lenalidomide (7% vs 1%), and grade 3/4 neutropenia was more common with rituximab-chemotherapy (50% vs 32%).
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in the treatment of follicular lymphoma (FL) in a phase 3 trial.
Patients with previously untreated FL had similar complete response (CR) rates and progression-free survival (PFS) rates whether they received rituximab-based chemotherapy or rituximab plus lenalidomide.
These results were published in The New England Journal of Medicine.
The trial, RELEVANCE, included 1,030 patients with previously untreated FL. They were randomized to receive rituximab plus chemotherapy (n=517) or rituximab plus lenalidomide (n=513) for 18 cycles.
Patients in the chemotherapy arm received one of three regimens—R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), rituximab and bendamustine, or R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone).
Patients in both treatment arms went on to receive rituximab maintenance every 8 weeks for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years.
The coprimary endpoints were CR (confirmed or unconfirmed) and PFS. After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the treatment arms.
CR was observed in 48% of the rituximab-lenalidomide arm and 53% of the rituximab-chemotherapy arm (P=0.13).
The interim 3-year PFS rate was 77% in the rituximab-lenalidomide arm and 78% in the rituximab-chemotherapy arm. The hazard ratio for progression or death from any cause was 1.10 (P=0.48).
The efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose disease was Ann Arbor stage I or II, whereas the efficacy of rituximab-lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some adverse events (AEs) being more common in one arm than the other.
AEs that were more common with rituximab-lenalidomide include cutaneous reactions (43% vs 24%), diarrhea (37% vs 19%), rash (29% vs 8%), abdominal pain (15% vs 9%), peripheral edema (14% vs 9%), muscle spasms (13% vs 4%), myalgia (14% vs 6%), and tumor flare reaction (6% vs <1%).
AEs that were more common with rituximab-chemotherapy were anemia (89% vs 66%), fatigue (29% vs 23%), nausea (42% vs 20%), vomiting (19% vs 7%), febrile neutropenia (7% vs 2%), leukopenia (10% vs 4%), and peripheral neuropathy (16% vs 7%).
Grade 3/4 cutaneous reactions were more common with rituximab-lenalidomide (7% vs 1%), and grade 3/4 neutropenia was more common with rituximab-chemotherapy (50% vs 32%).
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
Ibrutinib maintains efficacy over time
Extended follow-up of the RESONATE-2 trial showed that first-line ibrutinib sustained efficacy in elderly patients with chronic lymphocytic leukemia (CLL).
Patients who received ibrutinib had a long-term progression-free survival benefit over those who received chlorambucil.
The depth of response to ibrutinib improved over time, which meant there was a substantial increase in the proportion of patients achieving complete response.
Additionally, rates of some serious adverse events associated with ibrutinib decreased over time.
Paul M. Barr, MD, of the University of Rochester in New York, and his colleagues reported these findings in Haematologica.
Previously reported results of the RESONATE-2 trial, which showed an 84% reduction in the risk of death for ibrutinib versus chlorambucil, led to the approval of ibrutinib for first-line CLL treatment, the authors said.
The study included 269 patients with untreated CLL or small lymphocytic lymphoma who had active disease and were at least 65 years of age. They were randomized to receive ibrutinib (n=136) or chlorambucil (n=133).
At a median follow-up of 29 months, 79% (107/136) of patients remained on ibrutinib.
There was an 88% reduction in the risk of progression or death for patients randomized to ibrutinib (P<0.0001).
The rate of complete response improved over time in ibrutinib-treated patients, from 7% at 12 months to 15% at 24 months and 18% at 36 months (maximum follow-up).
The overall response rate (ORR) with ibrutinib was 92%, with comparable findings in high-risk subgroups. The ORR was 100% in patients with del(11q) and 95% in those with unmutated IGHV.
Lymphadenopathy improved in most ibrutinib-treated patients, with complete resolution in 42%, compared to 7% of patients who received chlorambucil.
Splenomegaly improved by at least 50% in 95% of ibrutinib-treated patients and 52% in chlorambucil recipients, with complete resolution in 56% and 22%, respectively.
Adverse events of grade 3 or greater were generally seen more often in the first year of ibrutinib therapy and decreased over time.
The rate of grade 3 or higher neutropenia decreased from 8.1% in the first 12 months of treatment to 0% in the third year. The rate of grade 3 or higher anemia decreased from 5.9% to 1%. And the rate of grade 3 or higher thrombocytopenia decreased from 2.2% to 0%.
The rate of atrial fibrillation increased from 6% in the primary analysis to 10% in extended follow-up. However, investigators said ibrutinib dose reductions and discontinuations because of this adverse event were uncommon and less frequent with extended treatment.
“Atrial fibrillation therefore appears manageable and does not frequently necessitate ibrutinib discontinuation,” they concluded.
This study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data. Several study authors reported funding from various companies, including Pharmacyclics.
Extended follow-up of the RESONATE-2 trial showed that first-line ibrutinib sustained efficacy in elderly patients with chronic lymphocytic leukemia (CLL).
Patients who received ibrutinib had a long-term progression-free survival benefit over those who received chlorambucil.
The depth of response to ibrutinib improved over time, which meant there was a substantial increase in the proportion of patients achieving complete response.
Additionally, rates of some serious adverse events associated with ibrutinib decreased over time.
Paul M. Barr, MD, of the University of Rochester in New York, and his colleagues reported these findings in Haematologica.
Previously reported results of the RESONATE-2 trial, which showed an 84% reduction in the risk of death for ibrutinib versus chlorambucil, led to the approval of ibrutinib for first-line CLL treatment, the authors said.
The study included 269 patients with untreated CLL or small lymphocytic lymphoma who had active disease and were at least 65 years of age. They were randomized to receive ibrutinib (n=136) or chlorambucil (n=133).
At a median follow-up of 29 months, 79% (107/136) of patients remained on ibrutinib.
There was an 88% reduction in the risk of progression or death for patients randomized to ibrutinib (P<0.0001).
The rate of complete response improved over time in ibrutinib-treated patients, from 7% at 12 months to 15% at 24 months and 18% at 36 months (maximum follow-up).
The overall response rate (ORR) with ibrutinib was 92%, with comparable findings in high-risk subgroups. The ORR was 100% in patients with del(11q) and 95% in those with unmutated IGHV.
Lymphadenopathy improved in most ibrutinib-treated patients, with complete resolution in 42%, compared to 7% of patients who received chlorambucil.
Splenomegaly improved by at least 50% in 95% of ibrutinib-treated patients and 52% in chlorambucil recipients, with complete resolution in 56% and 22%, respectively.
Adverse events of grade 3 or greater were generally seen more often in the first year of ibrutinib therapy and decreased over time.
The rate of grade 3 or higher neutropenia decreased from 8.1% in the first 12 months of treatment to 0% in the third year. The rate of grade 3 or higher anemia decreased from 5.9% to 1%. And the rate of grade 3 or higher thrombocytopenia decreased from 2.2% to 0%.
The rate of atrial fibrillation increased from 6% in the primary analysis to 10% in extended follow-up. However, investigators said ibrutinib dose reductions and discontinuations because of this adverse event were uncommon and less frequent with extended treatment.
“Atrial fibrillation therefore appears manageable and does not frequently necessitate ibrutinib discontinuation,” they concluded.
This study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data. Several study authors reported funding from various companies, including Pharmacyclics.
Extended follow-up of the RESONATE-2 trial showed that first-line ibrutinib sustained efficacy in elderly patients with chronic lymphocytic leukemia (CLL).
Patients who received ibrutinib had a long-term progression-free survival benefit over those who received chlorambucil.
The depth of response to ibrutinib improved over time, which meant there was a substantial increase in the proportion of patients achieving complete response.
Additionally, rates of some serious adverse events associated with ibrutinib decreased over time.
Paul M. Barr, MD, of the University of Rochester in New York, and his colleagues reported these findings in Haematologica.
Previously reported results of the RESONATE-2 trial, which showed an 84% reduction in the risk of death for ibrutinib versus chlorambucil, led to the approval of ibrutinib for first-line CLL treatment, the authors said.
The study included 269 patients with untreated CLL or small lymphocytic lymphoma who had active disease and were at least 65 years of age. They were randomized to receive ibrutinib (n=136) or chlorambucil (n=133).
At a median follow-up of 29 months, 79% (107/136) of patients remained on ibrutinib.
There was an 88% reduction in the risk of progression or death for patients randomized to ibrutinib (P<0.0001).
The rate of complete response improved over time in ibrutinib-treated patients, from 7% at 12 months to 15% at 24 months and 18% at 36 months (maximum follow-up).
The overall response rate (ORR) with ibrutinib was 92%, with comparable findings in high-risk subgroups. The ORR was 100% in patients with del(11q) and 95% in those with unmutated IGHV.
Lymphadenopathy improved in most ibrutinib-treated patients, with complete resolution in 42%, compared to 7% of patients who received chlorambucil.
Splenomegaly improved by at least 50% in 95% of ibrutinib-treated patients and 52% in chlorambucil recipients, with complete resolution in 56% and 22%, respectively.
Adverse events of grade 3 or greater were generally seen more often in the first year of ibrutinib therapy and decreased over time.
The rate of grade 3 or higher neutropenia decreased from 8.1% in the first 12 months of treatment to 0% in the third year. The rate of grade 3 or higher anemia decreased from 5.9% to 1%. And the rate of grade 3 or higher thrombocytopenia decreased from 2.2% to 0%.
The rate of atrial fibrillation increased from 6% in the primary analysis to 10% in extended follow-up. However, investigators said ibrutinib dose reductions and discontinuations because of this adverse event were uncommon and less frequent with extended treatment.
“Atrial fibrillation therefore appears manageable and does not frequently necessitate ibrutinib discontinuation,” they concluded.
This study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data. Several study authors reported funding from various companies, including Pharmacyclics.
RESONATE-2 update: First-line ibrutinib has sustained efficacy in older CLL patients
In older patients with chronic lymphocytic leukemia (CLL), first-line treatment with ibrutinib resulted in a long-term progression-free survival benefit versus chemotherapy, according to extended follow-up results of a phase 3 trial.
The quality of response to ibrutinib continued to improve over time in the study, including a substantial increase in the proportion of patients achieving complete response, the updated results of the RESONATE-2 trial show.
Rates of serious adverse events decreased over time in the study, while common reasons for initiating treatment, such as marrow failure and disease symptoms, all improved to a greater extent than with chlorambucil, reported Paul M. Barr, MD, of the University of Rochester (N.Y.) and colleagues.
“These data support the use of ibrutinib in the first-line treatment of CLL as a chemotherapy-free option that can be taken continuously, achieving long-term disease control for the majority of patients, including those with high-risk features,” Dr. Barr and coauthors said in the journal Haematologica.
Previously reported primary results of the RESONATE-2 trial, which showed an 84% reduction in risk of death for ibrutinib versus chlorambucil with a median follow-up of 18 months, led to the approval of ibrutinib for first-line CLL treatment, the authors said.
The study included 269 patients with untreated CLL or small lymphocytic lymphoma who had active disease and were at least 65 years of age. They were randomized 1:1 to ibrutinib or chlorambucil.
Out of 136 ibrutinib-treated patients, 107 (79%) remained on therapy at this extended analysis, which had a median follow-up of 29 months.
The extended analysis also showed an 88% reduction in risk of progression or death for those patients randomized to ibrutinib (P less than .0001), with significant improvements in subgroups evaluated, which include groups typically considered high risk, according to Dr. Barr and colleagues.
The rate of complete response improved over time in ibrutinib-treated patients, from 7% at 12 months, to 15% at 24 months, and to 18% with a maximum of 36 months’ follow-up, they said.
The overall response rate for ibrutinib was 92% in this extended analysis, with comparable findings in high-risk subgroups, including those with del(11q) at 100% and unmutated IGHV at 95%, according to the report.
Lymphadenopathy improved in most ibrutinib-treated patients, with complete resolution in 42% versus 7% with chlorambucil. Splenomegaly improved by at least 50% in 95% of ibrutinib-treated patients versus 52% for chlorambucil, with complete resolution in 56% of ibrutinib-treated patients and 22% of chlorambucil-treated patients.
Adverse events of grade 3 or greater were generally seen more often in the first year of ibrutinib therapy and decreased over time. Rates of grade 3 or greater neutropenia, anemia, and thrombocytopenia were 8.1%, 5.9%, and 2.2%, respectively, in the first 12 months of treatment; those decreased to 0%, 1%, and 0% in the third year.
The rate of atrial fibrillation increased from 6% in the primary analysis to 10% in extended follow-up; however, investigators said ibrutinib dose reductions and discontinuations because of this adverse effect were uncommon and less frequent with extended treatment.
“Atrial fibrillation therefore appears manageable and does not frequently necessitate ibrutinib discontinuation,” they concluded.
The study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data. Several study authors reported funding from various companies, including Pharmacyclics.
SOURCE: Barr PM, et al. Haematologica. 2018;103(9):1502-10.
In older patients with chronic lymphocytic leukemia (CLL), first-line treatment with ibrutinib resulted in a long-term progression-free survival benefit versus chemotherapy, according to extended follow-up results of a phase 3 trial.
The quality of response to ibrutinib continued to improve over time in the study, including a substantial increase in the proportion of patients achieving complete response, the updated results of the RESONATE-2 trial show.
Rates of serious adverse events decreased over time in the study, while common reasons for initiating treatment, such as marrow failure and disease symptoms, all improved to a greater extent than with chlorambucil, reported Paul M. Barr, MD, of the University of Rochester (N.Y.) and colleagues.
“These data support the use of ibrutinib in the first-line treatment of CLL as a chemotherapy-free option that can be taken continuously, achieving long-term disease control for the majority of patients, including those with high-risk features,” Dr. Barr and coauthors said in the journal Haematologica.
Previously reported primary results of the RESONATE-2 trial, which showed an 84% reduction in risk of death for ibrutinib versus chlorambucil with a median follow-up of 18 months, led to the approval of ibrutinib for first-line CLL treatment, the authors said.
The study included 269 patients with untreated CLL or small lymphocytic lymphoma who had active disease and were at least 65 years of age. They were randomized 1:1 to ibrutinib or chlorambucil.
Out of 136 ibrutinib-treated patients, 107 (79%) remained on therapy at this extended analysis, which had a median follow-up of 29 months.
The extended analysis also showed an 88% reduction in risk of progression or death for those patients randomized to ibrutinib (P less than .0001), with significant improvements in subgroups evaluated, which include groups typically considered high risk, according to Dr. Barr and colleagues.
The rate of complete response improved over time in ibrutinib-treated patients, from 7% at 12 months, to 15% at 24 months, and to 18% with a maximum of 36 months’ follow-up, they said.
The overall response rate for ibrutinib was 92% in this extended analysis, with comparable findings in high-risk subgroups, including those with del(11q) at 100% and unmutated IGHV at 95%, according to the report.
Lymphadenopathy improved in most ibrutinib-treated patients, with complete resolution in 42% versus 7% with chlorambucil. Splenomegaly improved by at least 50% in 95% of ibrutinib-treated patients versus 52% for chlorambucil, with complete resolution in 56% of ibrutinib-treated patients and 22% of chlorambucil-treated patients.
Adverse events of grade 3 or greater were generally seen more often in the first year of ibrutinib therapy and decreased over time. Rates of grade 3 or greater neutropenia, anemia, and thrombocytopenia were 8.1%, 5.9%, and 2.2%, respectively, in the first 12 months of treatment; those decreased to 0%, 1%, and 0% in the third year.
The rate of atrial fibrillation increased from 6% in the primary analysis to 10% in extended follow-up; however, investigators said ibrutinib dose reductions and discontinuations because of this adverse effect were uncommon and less frequent with extended treatment.
“Atrial fibrillation therefore appears manageable and does not frequently necessitate ibrutinib discontinuation,” they concluded.
The study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data. Several study authors reported funding from various companies, including Pharmacyclics.
SOURCE: Barr PM, et al. Haematologica. 2018;103(9):1502-10.
In older patients with chronic lymphocytic leukemia (CLL), first-line treatment with ibrutinib resulted in a long-term progression-free survival benefit versus chemotherapy, according to extended follow-up results of a phase 3 trial.
The quality of response to ibrutinib continued to improve over time in the study, including a substantial increase in the proportion of patients achieving complete response, the updated results of the RESONATE-2 trial show.
Rates of serious adverse events decreased over time in the study, while common reasons for initiating treatment, such as marrow failure and disease symptoms, all improved to a greater extent than with chlorambucil, reported Paul M. Barr, MD, of the University of Rochester (N.Y.) and colleagues.
“These data support the use of ibrutinib in the first-line treatment of CLL as a chemotherapy-free option that can be taken continuously, achieving long-term disease control for the majority of patients, including those with high-risk features,” Dr. Barr and coauthors said in the journal Haematologica.
Previously reported primary results of the RESONATE-2 trial, which showed an 84% reduction in risk of death for ibrutinib versus chlorambucil with a median follow-up of 18 months, led to the approval of ibrutinib for first-line CLL treatment, the authors said.
The study included 269 patients with untreated CLL or small lymphocytic lymphoma who had active disease and were at least 65 years of age. They were randomized 1:1 to ibrutinib or chlorambucil.
Out of 136 ibrutinib-treated patients, 107 (79%) remained on therapy at this extended analysis, which had a median follow-up of 29 months.
The extended analysis also showed an 88% reduction in risk of progression or death for those patients randomized to ibrutinib (P less than .0001), with significant improvements in subgroups evaluated, which include groups typically considered high risk, according to Dr. Barr and colleagues.
The rate of complete response improved over time in ibrutinib-treated patients, from 7% at 12 months, to 15% at 24 months, and to 18% with a maximum of 36 months’ follow-up, they said.
The overall response rate for ibrutinib was 92% in this extended analysis, with comparable findings in high-risk subgroups, including those with del(11q) at 100% and unmutated IGHV at 95%, according to the report.
Lymphadenopathy improved in most ibrutinib-treated patients, with complete resolution in 42% versus 7% with chlorambucil. Splenomegaly improved by at least 50% in 95% of ibrutinib-treated patients versus 52% for chlorambucil, with complete resolution in 56% of ibrutinib-treated patients and 22% of chlorambucil-treated patients.
Adverse events of grade 3 or greater were generally seen more often in the first year of ibrutinib therapy and decreased over time. Rates of grade 3 or greater neutropenia, anemia, and thrombocytopenia were 8.1%, 5.9%, and 2.2%, respectively, in the first 12 months of treatment; those decreased to 0%, 1%, and 0% in the third year.
The rate of atrial fibrillation increased from 6% in the primary analysis to 10% in extended follow-up; however, investigators said ibrutinib dose reductions and discontinuations because of this adverse effect were uncommon and less frequent with extended treatment.
“Atrial fibrillation therefore appears manageable and does not frequently necessitate ibrutinib discontinuation,” they concluded.
The study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data. Several study authors reported funding from various companies, including Pharmacyclics.
SOURCE: Barr PM, et al. Haematologica. 2018;103(9):1502-10.
FROM HAEMATOLOGICA
Key clinical point:
Major finding: There was an 88% reduction in risk of progression-free survival events for those patients randomized to ibrutinib (P less than .0001).
Study details: Extended phase 3 results from the RESONATE-2 trial, including 269 older patients with untreated CLL or small lymphocytic lymphoma.
Disclosures: This study was supported by Pharmacyclics, an AbbVie company, and by grants from the National Institutes of Health and the MD Anderson Moon Shot Program in CLL. Pharmacyclics designed the study and performed analysis of the data.
Source: Barr PM et al. Haematologica. 2018;103(9):1502-10.
FILM: Rave review for indocyanine green in lymphatic mapping
Green is just as good – make that better – than blue at identifying sentinel lymph nodes in women with cervical and uterine cancers, results of the multicenter FILM (Fluorescence Imaging for Lymphatic Mapping) study indicate.
Among 176 patients randomly assigned to first have lymphatic mapping with indocyanine green fluorescent dye visualized with near infrared imaging followed by isosulfan blue dye visualized with white light, or the two modalities in the reverse order, indocyanine green identified 50% more lymph nodes in both modified intention-to-treat and per-protocol analyses, reported Michael Frumovitz, MD, from the University of Texas MD Anderson Cancer Center in Houston and colleagues.
“Indocyanine green dye with near-infrared imaging identified significantly more sentinel nodes and more bilateral sentinel nodes than did isosulfan blue dye. It also identified all sentinel nodes with metastatic disease, whereas isosulfan blue dye missed a large proportion of them,” they wrote in the Lancet Oncology.
The FILM study was designed to determine whether fluorescent indocyanine green dye would be noninferior to isosulfan blue dye for accurately identifying sentinel lymph nodes in patients with cancer.
Although several single-center retrospective studies have reported on the use of interstitial injection of indocyanine green for lymphatic mapping in various solid tumors, including uterine and cervical cancers, there were no published studies comparing indocyanine green mapping to isosulfan blue mapping, the standard of care, the authors noted.
They enrolled 180 women aged 18 or older with clinical stage I endometrial or cervical cancers who were undergoing curative surgery and randomly assigned them as described above to have lymphatic mapping with each of the imaging modalities assigned in random order.
The patients but not the operators were masked as to the order of randomization.
Of the 180 patients enrolled, 176 received the intervention, and 13 of these patients were excluded because of major protocol violations, leaving 163 for a per-protocol analysis.
In the per-protocol analysis, 517 sentinel nodes were identified in the 163 patients, and of these, 478 (92%) were confirmed to be lymph nodes on pathological examination. This sample included 219 of 238 nodes identified with both blue and green dyes, all seven nodes revealed by blue dye alone, and 252 of 265 nodes identified by only green dye. Seven sentinel lymph nodes that were not identified by either dye were removed because they were enlarged or appeared suspicious on visual inspection.
In total, green dye identified 97% of lymph nodes in the per-protocol population, and blue dye identified 47%, an absolute difference of 50% (P less than .0001).
In the modified intention-to-treat population, which included all 176 patients randomized and treated, 545 nodes were identified, and 513 (94%) were confirmed to be lymph nodes on pathology. In this sample, 229 (92%) of 248 nodes showed both blue and green, nine nodes were blue only, and 266 (95%) of 279 were green only. Nine sentinel lymph nodes that were not revealed by either blue or green were removed for appearing suspicious or enlarged visually.
In total, in the modified-ITT analysis, 495 of 513 (96%) nodes were identified with the green dye and 238 (46%) were identified with the blue dye, again for an absolute difference of 50% (P less than .0001).
Based on the results of the study, the green dye’s maker, Novadaq Technologies, is submitting an application to the Food and Drug Administration for on-label use of interstitial injection of indocyanine green combined with near-infrared imaging for lymphatic mapping.
The study was funded by Novadaq. Dr. Frumovitz reported grants from Novadaq/Stryker during the conduct of the study, as well as personal fees; grants from Navidea; personal fees from Johnson & Johnson; and personal fees from Genentech outside the submitted work. The other authors declared no competing interests.
SOURCE: Frumovitz M et al. Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30448-0.
In endometrial cancer, the site of injection is still debated; intracervical injection is simple and effective, but hysteroscopic peritumoral injection of the tracer might lead to increased detection of para-aortic sentinel lymph nodes. A combination of pericervical and hysteroscopic peritumoral injection could be useful in selected cases with a higher incidence, such as poorly differentiated carcinomas, or metastasis to isolated para-aortic lymph nodes. The oncologic significance of low-volume metastasis to sentinel lymph nodes and the role of systematic lymphadenectomy in patients with positive sentinel lymph nodes still remain unclear.
In cervical cancer, preliminary data from the LACC trial (NCT00614211) of long-term outcomes of different surgical methods need to be integrated with sentinel lymph node mapping. These data show a detrimental oncologic effect of a minimally invasive approach. If the LACC data are confirmed, two options might be considered: minimally invasive sentinel lymph node mapping as triage to an open radical hysterectomy or the adoption of dedicated near-infrared technology hardware for open surgery.
Through its user-friendliness and effectiveness, indocyanine green is enabling surgeons to transition from systematic lymphadenectomy to sentinel lymph node biopsy. After all, innovation is not only about new technologies, it is also about changing how people think about alternative treatment approaches.
Maria Luisa Gasparri, MD, Michael D. Mueller, MD, and Andrea Papadia, MD, are with the department of obstetrics and gynecology, University Hospital of Bern and University of Bern, Switzerland. Their remarks are adapted and condensed from an editorial accompanying the study (Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30514-X.) The authors declared no competing financial interests.
In endometrial cancer, the site of injection is still debated; intracervical injection is simple and effective, but hysteroscopic peritumoral injection of the tracer might lead to increased detection of para-aortic sentinel lymph nodes. A combination of pericervical and hysteroscopic peritumoral injection could be useful in selected cases with a higher incidence, such as poorly differentiated carcinomas, or metastasis to isolated para-aortic lymph nodes. The oncologic significance of low-volume metastasis to sentinel lymph nodes and the role of systematic lymphadenectomy in patients with positive sentinel lymph nodes still remain unclear.
In cervical cancer, preliminary data from the LACC trial (NCT00614211) of long-term outcomes of different surgical methods need to be integrated with sentinel lymph node mapping. These data show a detrimental oncologic effect of a minimally invasive approach. If the LACC data are confirmed, two options might be considered: minimally invasive sentinel lymph node mapping as triage to an open radical hysterectomy or the adoption of dedicated near-infrared technology hardware for open surgery.
Through its user-friendliness and effectiveness, indocyanine green is enabling surgeons to transition from systematic lymphadenectomy to sentinel lymph node biopsy. After all, innovation is not only about new technologies, it is also about changing how people think about alternative treatment approaches.
Maria Luisa Gasparri, MD, Michael D. Mueller, MD, and Andrea Papadia, MD, are with the department of obstetrics and gynecology, University Hospital of Bern and University of Bern, Switzerland. Their remarks are adapted and condensed from an editorial accompanying the study (Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30514-X.) The authors declared no competing financial interests.
In endometrial cancer, the site of injection is still debated; intracervical injection is simple and effective, but hysteroscopic peritumoral injection of the tracer might lead to increased detection of para-aortic sentinel lymph nodes. A combination of pericervical and hysteroscopic peritumoral injection could be useful in selected cases with a higher incidence, such as poorly differentiated carcinomas, or metastasis to isolated para-aortic lymph nodes. The oncologic significance of low-volume metastasis to sentinel lymph nodes and the role of systematic lymphadenectomy in patients with positive sentinel lymph nodes still remain unclear.
In cervical cancer, preliminary data from the LACC trial (NCT00614211) of long-term outcomes of different surgical methods need to be integrated with sentinel lymph node mapping. These data show a detrimental oncologic effect of a minimally invasive approach. If the LACC data are confirmed, two options might be considered: minimally invasive sentinel lymph node mapping as triage to an open radical hysterectomy or the adoption of dedicated near-infrared technology hardware for open surgery.
Through its user-friendliness and effectiveness, indocyanine green is enabling surgeons to transition from systematic lymphadenectomy to sentinel lymph node biopsy. After all, innovation is not only about new technologies, it is also about changing how people think about alternative treatment approaches.
Maria Luisa Gasparri, MD, Michael D. Mueller, MD, and Andrea Papadia, MD, are with the department of obstetrics and gynecology, University Hospital of Bern and University of Bern, Switzerland. Their remarks are adapted and condensed from an editorial accompanying the study (Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30514-X.) The authors declared no competing financial interests.
Green is just as good – make that better – than blue at identifying sentinel lymph nodes in women with cervical and uterine cancers, results of the multicenter FILM (Fluorescence Imaging for Lymphatic Mapping) study indicate.
Among 176 patients randomly assigned to first have lymphatic mapping with indocyanine green fluorescent dye visualized with near infrared imaging followed by isosulfan blue dye visualized with white light, or the two modalities in the reverse order, indocyanine green identified 50% more lymph nodes in both modified intention-to-treat and per-protocol analyses, reported Michael Frumovitz, MD, from the University of Texas MD Anderson Cancer Center in Houston and colleagues.
“Indocyanine green dye with near-infrared imaging identified significantly more sentinel nodes and more bilateral sentinel nodes than did isosulfan blue dye. It also identified all sentinel nodes with metastatic disease, whereas isosulfan blue dye missed a large proportion of them,” they wrote in the Lancet Oncology.
The FILM study was designed to determine whether fluorescent indocyanine green dye would be noninferior to isosulfan blue dye for accurately identifying sentinel lymph nodes in patients with cancer.
Although several single-center retrospective studies have reported on the use of interstitial injection of indocyanine green for lymphatic mapping in various solid tumors, including uterine and cervical cancers, there were no published studies comparing indocyanine green mapping to isosulfan blue mapping, the standard of care, the authors noted.
They enrolled 180 women aged 18 or older with clinical stage I endometrial or cervical cancers who were undergoing curative surgery and randomly assigned them as described above to have lymphatic mapping with each of the imaging modalities assigned in random order.
The patients but not the operators were masked as to the order of randomization.
Of the 180 patients enrolled, 176 received the intervention, and 13 of these patients were excluded because of major protocol violations, leaving 163 for a per-protocol analysis.
In the per-protocol analysis, 517 sentinel nodes were identified in the 163 patients, and of these, 478 (92%) were confirmed to be lymph nodes on pathological examination. This sample included 219 of 238 nodes identified with both blue and green dyes, all seven nodes revealed by blue dye alone, and 252 of 265 nodes identified by only green dye. Seven sentinel lymph nodes that were not identified by either dye were removed because they were enlarged or appeared suspicious on visual inspection.
In total, green dye identified 97% of lymph nodes in the per-protocol population, and blue dye identified 47%, an absolute difference of 50% (P less than .0001).
In the modified intention-to-treat population, which included all 176 patients randomized and treated, 545 nodes were identified, and 513 (94%) were confirmed to be lymph nodes on pathology. In this sample, 229 (92%) of 248 nodes showed both blue and green, nine nodes were blue only, and 266 (95%) of 279 were green only. Nine sentinel lymph nodes that were not revealed by either blue or green were removed for appearing suspicious or enlarged visually.
In total, in the modified-ITT analysis, 495 of 513 (96%) nodes were identified with the green dye and 238 (46%) were identified with the blue dye, again for an absolute difference of 50% (P less than .0001).
Based on the results of the study, the green dye’s maker, Novadaq Technologies, is submitting an application to the Food and Drug Administration for on-label use of interstitial injection of indocyanine green combined with near-infrared imaging for lymphatic mapping.
The study was funded by Novadaq. Dr. Frumovitz reported grants from Novadaq/Stryker during the conduct of the study, as well as personal fees; grants from Navidea; personal fees from Johnson & Johnson; and personal fees from Genentech outside the submitted work. The other authors declared no competing interests.
SOURCE: Frumovitz M et al. Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30448-0.
Green is just as good – make that better – than blue at identifying sentinel lymph nodes in women with cervical and uterine cancers, results of the multicenter FILM (Fluorescence Imaging for Lymphatic Mapping) study indicate.
Among 176 patients randomly assigned to first have lymphatic mapping with indocyanine green fluorescent dye visualized with near infrared imaging followed by isosulfan blue dye visualized with white light, or the two modalities in the reverse order, indocyanine green identified 50% more lymph nodes in both modified intention-to-treat and per-protocol analyses, reported Michael Frumovitz, MD, from the University of Texas MD Anderson Cancer Center in Houston and colleagues.
“Indocyanine green dye with near-infrared imaging identified significantly more sentinel nodes and more bilateral sentinel nodes than did isosulfan blue dye. It also identified all sentinel nodes with metastatic disease, whereas isosulfan blue dye missed a large proportion of them,” they wrote in the Lancet Oncology.
The FILM study was designed to determine whether fluorescent indocyanine green dye would be noninferior to isosulfan blue dye for accurately identifying sentinel lymph nodes in patients with cancer.
Although several single-center retrospective studies have reported on the use of interstitial injection of indocyanine green for lymphatic mapping in various solid tumors, including uterine and cervical cancers, there were no published studies comparing indocyanine green mapping to isosulfan blue mapping, the standard of care, the authors noted.
They enrolled 180 women aged 18 or older with clinical stage I endometrial or cervical cancers who were undergoing curative surgery and randomly assigned them as described above to have lymphatic mapping with each of the imaging modalities assigned in random order.
The patients but not the operators were masked as to the order of randomization.
Of the 180 patients enrolled, 176 received the intervention, and 13 of these patients were excluded because of major protocol violations, leaving 163 for a per-protocol analysis.
In the per-protocol analysis, 517 sentinel nodes were identified in the 163 patients, and of these, 478 (92%) were confirmed to be lymph nodes on pathological examination. This sample included 219 of 238 nodes identified with both blue and green dyes, all seven nodes revealed by blue dye alone, and 252 of 265 nodes identified by only green dye. Seven sentinel lymph nodes that were not identified by either dye were removed because they were enlarged or appeared suspicious on visual inspection.
In total, green dye identified 97% of lymph nodes in the per-protocol population, and blue dye identified 47%, an absolute difference of 50% (P less than .0001).
In the modified intention-to-treat population, which included all 176 patients randomized and treated, 545 nodes were identified, and 513 (94%) were confirmed to be lymph nodes on pathology. In this sample, 229 (92%) of 248 nodes showed both blue and green, nine nodes were blue only, and 266 (95%) of 279 were green only. Nine sentinel lymph nodes that were not revealed by either blue or green were removed for appearing suspicious or enlarged visually.
In total, in the modified-ITT analysis, 495 of 513 (96%) nodes were identified with the green dye and 238 (46%) were identified with the blue dye, again for an absolute difference of 50% (P less than .0001).
Based on the results of the study, the green dye’s maker, Novadaq Technologies, is submitting an application to the Food and Drug Administration for on-label use of interstitial injection of indocyanine green combined with near-infrared imaging for lymphatic mapping.
The study was funded by Novadaq. Dr. Frumovitz reported grants from Novadaq/Stryker during the conduct of the study, as well as personal fees; grants from Navidea; personal fees from Johnson & Johnson; and personal fees from Genentech outside the submitted work. The other authors declared no competing interests.
SOURCE: Frumovitz M et al. Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30448-0.
FROM LANCET ONCOLOGY
Key clinical point: Fluorescent indocyanine green dye with near-infrared visualization was superior to isosulfan blue dye at identifying lymph nodes in patients with early-stage cervical and endometrial cancers.
Major finding: Indocyanine green identified 50% more lymph nodes than isosulfan blue, the standard of care.
Study details: Randomized, phase 3, within-patient, noninferiority trial in 176 women with clinical stage I endometrial or cervical cancers.
Disclosures: The study was funded by Novadaq. Dr. Frumovitz reported grants from Novadaq/Stryker during the conduct of the study, as well as personal fees; grants from Navidea, personal fees from Johnson & Johnson; and personal fees from Genentech outside the submitted work. The other authors declared no competing interests.
Source: Frumovitz M et al. Lancet Oncol. 2018 Aug 21. doi: 10.1016/S1470-2045(18)30448-0.
A Rare Case of Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type
CASE REPORT
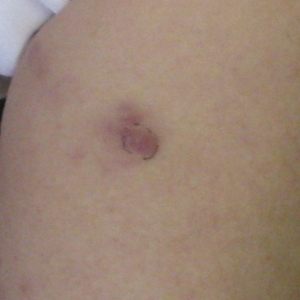
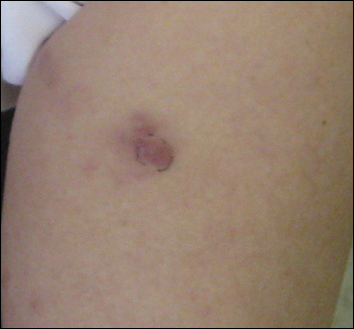
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.
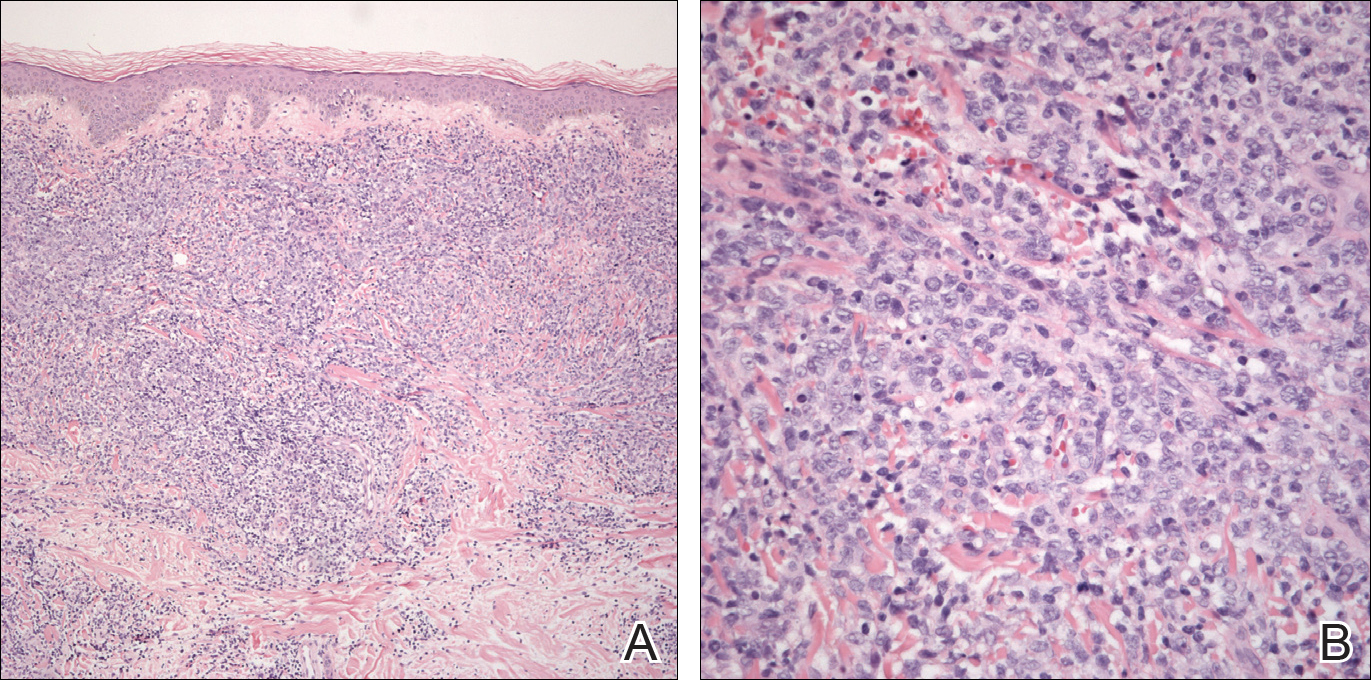
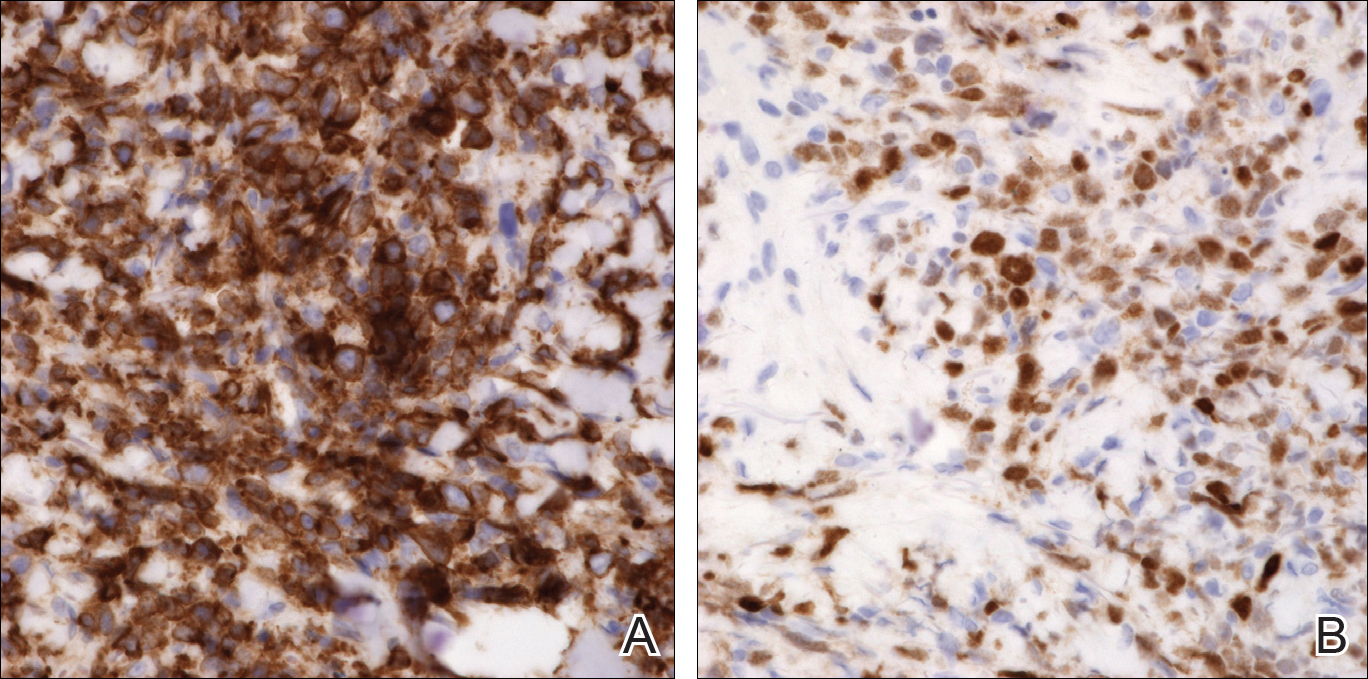
An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.
COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.

An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.


COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
CASE REPORT
A 74-year-old woman presented with a painful lesion on the left lower leg that was getting larger and more edematous and erythematous over the last 5 months. She experienced numbness and burning of the left lower leg 1 year prior to the development of the lesion. A review of her medical history revealed an otherwise healthy woman with no constitutional symptoms of fever, chills, nausea, vomiting, diarrhea, or chest pain. The patient did not exhibit mucosal, genital, or nail involvement. Physical examination revealed a group of four 1-cm, ill-defined, irregularly bordered, violaceous plaques on the left anterior tibial leg with faint surrounding erythematous to violaceous patches (Figure 1). The plaques were tender to palpation with no bleeding or drainage.

An 8.0-mm punch biopsy of the lesion was obtained. Hematoxylin and eosin staining on low-power magnification demonstrated a diffuse lymphocytic inflammatory infiltrate in the dermis and subcutis. Notable sparing of the subepidermal area (free grenz zone) was present (Figure 2A). On higher power, centroblasts and immunoblasts were visualized alongside extravasated red blood cells (Figure 2B). A diagnosis of primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) was made. Various immunohistochemical stains confirmed the diagnosis, including B-cell lymphoma 2 (BCL-2)(Figure 3A) and multiple myeloma oncogene 1 (MUM-1)(Figure 3B), which were highly positive in our patient. The patient had a negative bone marrow biopsy and positron emission tomography scan. She was started on rituximab infusions and multiple radiation treatments. At 2-year follow-up the lymphoma continued to recur despite radiation therapy.


COMMENT
Incidence and Clinical Characteristics
Primary cutaneous DLBCLLT is an intermediately aggressive form of primary cutaneous B-cell lymphoma (CBCL) that accounts for approximately 10% to 20% of all primary CBCLs and 1% to 3% of all cutaneous lymphomas.1 Diffuse large B-cell lymphoma, leg type primarily affects elderly patients (median age, 70 years). Women are more commonly affected. Clinically, primary cutaneous DLBCLLT presents as red-brown to bluish nodules or tumors on one or both distal legs.
Histopathology
The diagnosis of DLBCLLT is best made histologically. There is a dense inflammatory infiltrate present in the dermis and subcutis that may extend upward into the dermoepidermal junction. Often a subepidermal free grenz zone may be seen, and adnexal structures may be destroyed. This infiltrate is composed of confluent sheets of large round cells including centroblasts and immunoblasts.2 Centroblasts are large cells that have nuclei with several small nucleoli adhering to the membrane, while immunoblasts are large round cells containing nuclei with large central nucleoli. Both centroblasts and immunoblasts stain positively for BCL-2. Centrocytes typically are absent. Staining for BCL-2 can be important in distinguishing DLBCLLT from other forms of CBCL. Diffuse large B-cell lymphoma, leg type also can demonstrate clusters of large atypical cells in the epidermis simulating epidermotropism and Pautrier microabscesses. Neoplastic cells in this condition may express monoclonal surface and cytoplasmic immunoglobulins. Primary cutaneous DLBCLLT typically is positive for B-cell markers CD20 and CD79a. Additionally, MUM-1/IRF4 (interferon regulatory factor 4) and forkhead box protein 1 (FOXP1) are strongly expressed by most patients, which helps distinguish it from other forms of CBCL.
Treatment
Diffuse large B-cell lymphoma, leg type is a relatively aggressive form of CBCL that requires more aggressive treatment than the conservative watchful waiting of some of the more indolent forms of primary CBCL. One regimen involves using cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Local chemotherapy or radiation with rituximab is another treatment option.1,2 In patients with severe comorbidities, rituximab alone may be administered. The prognosis for DLBCLLT is not as favorable as other types of primary CBCL, with an estimated 5-year survival rate of approximately 50%.2
Differential Diagnosis
Lymphomas are malignancies of the lymphocytes that may be subdivided depending on the organ of origin. Both primary nodal lymphomas and primary cutaneous lymphomas exist. Primary nodal lymphomas arise from the lymph nodes and are divided into Hodgkin and non-Hodgkin lymphomas. There are 2 major types of primary cutaneous lymphomas: cutaneous T-cell lymphoma (CTCL) and CBCL. Most primary cutaneous lymphomas are CTCLs, accounting for 75% to 80%.3
Pseudolymphoma
Pseudolymphoma is an inflammatory condition that may histologically mimic cutaneous lymphoma but has a benign clinical course. Pseudolymphoma is not a specific disease but rather is a reactive lymphoproliferative response to a known or unknown stimulus.4 Pseudolymphoma can be broken down into 2 or 3 major categories: cutaneous B-cell pseudolymphoma; cutaneous T-cell pseudolymphoma; and debatably lymphomatoid papulosis, a chronic, self-remitting, papulonecrotic condition that resembles lymphoma histologically but clinically appears benign. It is unknown if lymphomatoid papulosis represents a pseudolymphoma or a true lymphoma. Lymphomatoid papulosis may represent an early indolent form of CTCL.4
Pseudolymphomas can be triggered by a variety of causes. Most cases are idiopathic, and a causative stimulus is never identified. Drugs are known to cause many cases of pseudolymphoma, either by a causing a hypersensitivity reaction or by depressing immunosurveillance.5 Pseudolymphomas may result from exogenous stimuli such as jewelry, tattoo dyes, injectable fillers (eg, silicone), insect bites, vaccines, and trauma.6,7 Lastly, infections in the form of Borrelia, varicella, and molluscum contagiosum can potentially cause pseudolymphomas.4
Clinically, pseudolymphomas may demonstrate a B-cell or T-cell pattern. In cutaneous B-cell pseudolymphomas, asymptomatic solitary erythematous, violaceous, or flesh-colored nodules appear on the face, followed by the chest and arms. Cutaneous T-cell pseudolymphomas present with erythematous patches that are more likely to be symptomatic.4
Histologically, pseudolymphomas also are classified as demonstrating B-cell or T-cell patterns. The nodular inflammatory infiltrate of cutaneous B-cell pseudolymphoma corresponds with its clinically apparent nodules. It can be distinguished from lymphoma in that it is not solely a lymphocytic infiltrate but rather a mixed infiltrate including histiocytes, lymphocytes, eosinophils, and plasma cells. Additionally, cutaneous B-cell pseudolymphoma does not penetrate the dermis as deeply as CBCL.8 Cutaneous T-cell pseudolymphoma is more difficult to distinguish from CTCL because it also demonstrates a bandlike lymphocytic infiltrate in the papillary dermis with epidermotropism.9
Treatment must address the underlying cause of pseudolymphoma for resolution. Other treatment options include surgery, cryotherapy, local radiotherapy, topical steroids, and topical immunomodulators. Spontaneous resolution also can occur. The prognosis is better when a known trigger is eliminated, though idiopathic pseudolymphomas may be chronic in nature. It is important to rule out concurrent cutaneous lymphoma or rare transformation into cutaneous lymphoma.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphomas are a diverse group of neoplasms that account for most cutaneous lymphomas seen by dermatologists. In 1806, the first case of CTCL in the form of mycosis fungoides (MF) was described by Jean Louis Alibert. Mycosis fungoides represents the most common form of CTCL, accounting for approximately 50% of all primary cutaneous lymphomas.10 Mycosis fungoides was named after its morphological resemblance to mushrooms. Although not all cases exhibit a classic progression, MF is known for its stepwise progression from patch stage to tumor stage.
Clinically, lesions typically begin as patches that progress to plaques and finally tumors. This progression may not always occur and often can take years to decades to progress. Patches are characterized by erythematous, finely scaling lesions that may be easily confused with eczema or psoriasis. Lesions occur primarily in a swimming trunk distribution.
Mycosis fungoides histologically demonstrates a bandlike lymphocytic infiltrate with epidermotropism, which occurs when lymphocytes infiltrate the epidermis without spongiosis. These lymphocytes are larger, darker, and more angulated than normal lymphocytes. Intraepidermal nests of these atypical lymphocytes creating Pautrier microabscesses may be present. Tumor-stage lesions demonstrate diminished epidermotropism with dense sheets of lymphocytes in the dermis, and fat cells with cerebriform nuclei are present.
Therapies for MF may control the disease but may not prolong patients’ lives. Topical corticosteroids, phototherapy, and radiotherapy are options for skin-targeting therapies. Systemic chemotherapy and biological response modifiers also are viable treatment options. Prognosis for MF is poor.
There are a few notable variants of MF that are important to consider. Sézary syndrome is an erythrodermic variant of MF characterized by atypical Sézary cells. Clinically, it presents with generalized erythroderma with leonine facies, facial edema, and alopecia with associated symptoms of burning and pruritus. Histologically, Sézary syndrome is similar to MF with an increased CD4:CD8 ratio.10 Sézary syndrome may be treated with methotrexate or photopheresis, but the prognosis remains poor with an average survival of 5 years.
Cutaneous B-Cell Lymphoma
There are 5 types of primary CBCL: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone B-cell lymphoma; primary cutaneous diffuse large B-cell lymphoma, other; precursor B-cell lymphoblastic lymphoma; and primary cutaneous DLBCLLT, which was seen in our patient.11
Primary cutaneous follicle center lymphoma is an indolent neoplastic proliferation in the skin. Clinically, it presents with solitary or grouped pinkish purple papules, plaques, or nodules on the trunk with surrounding patches of erythema.3 Lesions located on the back are referred to as Crosti lymphoma. Histopathology reveals a lymphocytic infiltrate with a diffuse follicular pattern and large round centroblasts, centrocytes, and immunoblasts with epidermal sparing. Tumor cells stain positively for κ or λ light chains, as well as CD20, CD79a, and B-cell lymphoma 6 (BCL-6); however, staining for the protein product of BCL-2 may be negative, which differentiates this form of CBCL from primary nodal B-cell lymphoma. Staining for MUM-1 may be negative, which contrasts with the strong expression seen in DLBCLLT. The follicular pattern of follicle center lymphoma stains positive for CD10, but the diffuse pattern may be CD10 negative. The prognosis for primary cutaneous follicle center lymphoma is favorable, but the recurrence rate is up to 50%.3 Treatment includes local radiotherapy or surgical excision.
Primary cutaneous marginal zone B-cell lymphoma is another indolent primary CBCL subtype that is closely related to mucosa-associated lymphoid tissue lymphomas and arises in areas of acrodermatitis chronica atrophicans and Borrelia infection. Clinically, it presents with recurrent, asymptomatic, red-brown papules, plaques, and nodules of the arms and legs. Histologically, there is a patchy infiltrate in the dermis and subcutis with sparing of the epidermis with pale-staining cells with indented nuclei, along with plasma cells and eosinophils. Primary cutaneous marginal zone B-cell lymphoma typically does not demonstrate epidermotropism. Centrocyte cells stain positively for CD20, CD79a, and BCL-2. The prognosis of primary cutaneous marginal zone B-cell lymphoma is favorable. Treatment is similar to primary cutaneous follicle center lymphoma with surgical excision, radiotherapy, and surveillance being the main modalities.
Primary cutaneous diffuse large B-cell lymphoma, other is an intermediately aggressive form of primary CBCL that is thought to be related to primary cutaneous DLBCLLT. Clinically, it presents with indurated erythematous to violaceous plaques on the trunk and thighs that may resemble a vascular tumor or panniculitis.2,12 Histopathologically, this form of lymphoma presents with a round cell morphology without BCL-2 expression, which distinguishes it from DLBCLLT. If limited to skin, the prognosis is better than the systemic form but is still less favorable than other forms of CBCL.
Precursor B-cell lymphoblastic lymphoma is an extremely rare type of CBCL that potentially can occur in the skin. It primarily affects children and young adults. Clinically, it presents as a solitary large erythematous tumor of the head. Histol
CONCLUSION
We present a rare case of primary cutaneous DLBCLLT. Our case demonstrates the classic presentation of primary cutaneous DLBCLLT in a 74-year-old woman with a tumor on the lower left leg. Histologically, a dense dermal and subcutis infiltrate of centroblasts and immunoblasts with a grenz zone was present. Immunostaining in our patient was consistent with characteristic findings in the literature, staining highly positive for BCL-2 and MUM-1. Primary cutaneous DLBCLLT is an extremely rare and unique form of cutaneous lymphoma that can have potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244.
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:2768-3785.
- Brodell RT, Santa Cruz DJ. Cutaneous pseudolymphomas. Dermatol Clin. 1985;3:719-734.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244; vii.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Kluger N, Vermeulen C, Moguelet P, et al. Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: a case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-213.
- Burg G, Kerl H, Schmoeckel C. Differentiation between malignant B-cell lymphomas and pseudolymphomas of the skin. J Dermatol Surg Oncol. 1984;10:271-275.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38(6, pt 1):877-895; quiz 896-897.
- Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sézary syndrome. Blood. 1996;88:2385-2409.
- Kempf W, Ralfkiaer E, Duncan LM, et al. Cutaneous marginal zone B-cell lymphoma. In: LeBoit P, Burg G, Weedon D, et al, eds. Pathology and Genetics of Skin Tumors. Lyon, France: IARC Press; 2006:194-195.
- Grange F, Bekkenk MW, Wechsler J, et al. Prognostic factors in cutaneous large B-cell lymphomas: a European multicentric study. J Clin Oncol. 2001;19:3602-3610.
- Chimenti S, Fink-Puches R, Peris K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385.
Practice Points
- Primary cutaneous diffuse large B-cell lymphoma, leg type (DLBCLLT) is characterized by the presence of large round cells on histopathology.
- There are potentially fatal consequences if undiagnosed; therefore, clinicians must take great care to make the correct diagnosis based on a knowledge of the clinical and immunohistochemical findings of DLBCLLT.
Malpractice Counsel: Diverticulitis
A 44-year-old woman presented to the ED complaining of crampy lower abdominal pain with nausea and vomiting. The patient described gradual onset 2 days prior, with symptoms worsening over the previous 12 hours. She denied diarrhea, constipation, or blood in her stool. She did admit to frequency of urination, but no dysuria or hematuria. She was gravida 2, para 2, aborta 0, with a last menstrual period 3 weeks prior. She denied vaginal bleeding or discharge. Her past medical history was unremarkable, she was on no medications, and denied alcohol use. She did admit to smoking one pack of cigarettes per day.
On physical examination, the patient’s vital signs were: blood pressure, 132/68 mm Hg; heart rate, 96 beats/min; respiratory rate, 18 breaths/min; and temperature, 99.8°F. Oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat (HEENT) examination was completely normal, as was the heart and lung examination. The patient was tender to palpation in the lower abdomen, but without guarding or rebound. Bowel sounds were present and normoactive. A pelvic examination, including a bimanual examination, demonstrated mild left ovarian tenderness but without mass or cervical motion tenderness. The patient did not exhibit any costovertebral angle tenderness bilaterally. No rectal examination was performed.
The emergency physicians (EPs) ordered a complete blood count (CBC), basic metabolic profile (BMP), urinalysis, urine pregnancy test, and a vaginal wet preparation. In addition, the patient was administered 500 cc’s of normal saline intravenously (IV) and ondansetron (Zofran) 4 mg IV.
The urine pregnancy test result came back negative. The urinalysis was remarkable for positive leukocyte esterase, with five to 10 white cells and bacteria present. The CBC showed a mild leukocytosis, but with a normal hemoglobin and hematocrit. The BMP and vaginal wet preparation were completely normal.
The EP was concerned the patient might have something more serious than a simple urinary tract infection (UTI), so she ordered a computed tomography (CT) scan of the abdomen and pelvis with IV contrast.
The radiologist interpreted the CT scan as normal. The patient was discharged home with a prescription for an antibiotic for her UTI, encouraged to drink liquids, and instructed to follow-up with her primary care physician in 1 week.
The patient returned to the same ED approximately 48 hours later with worsening abdominal pain. On this presentation, she was tachycardic (110 beats/min) with a temperature of 101°F. The abdominal examination was remarkable for diffuse tenderness and voluntary guarding. The patient was administered IV fluids, morphine, and ondansetron. A repeat CT scan of the abdomen and pelvis with IV contrast showed a perforated sigmoid colon, with leakage of bowel contents into the peritoneum. The EP immediately started IV fluid resuscitation and administered IV antibiotics. The patient was taken emergently to the operating room by general surgery. The colon was repaired and a colostomy placed. The patient was able to be discharged home on day number 5.
The patient sued the hospital and the treating EP for failure to make the proper diagnosis on the initial ED visit, resulting in the patient having a long and difficult recovery, and the need for a colostomy. At trial, the jury returned a defense verdict.
Discussion
Diverticulitis, and its complications, account for a significant number of ED visits. It is the third most common inpatient gastrointestinal diagnosis in the United States, costing two billion dollars annually.1It is defined as clinically evident microscopic inflammation of a diverticulum or diverticula, and occurs in approximately 4% of patients with diverticulosis.1It is estimated that roughly 15% of these patients will experience a complication, defined as an abscess, perforation, fistula, or colonic obstruction; 15% to 30% will experience a recurrence.
The mean age of patients admitted to the hospital for diverticulitis is 63 years. While considered a disease of older patients, it should be included in the differential diagnosis for younger patients, as approximately 16% of admissions for acute diverticulitis are in patients less than 45 years.2Risk factors include poor diet (ie, low fiber, high fat, red meat), obesity, and smoking. The clinical presentation of diverticulitis has sometimes been referred to as “left-sided appendicitis” because of the similarities between the two entities. Patients will frequently complain of anorexia, change in bowel habits (either diarrhea or constipation), crampy abdominal pain (primarily in the left lower quadrant), low grade fever, and nausea with vomiting. Interestingly, 10% to 15% of patients with acute diverticulitis will complain of dysuria, urgency, or frequency (as in this patient) due to irritation of the bladder from an inflamed sigmoid colon.
Physical examination may reveal a low grade fever and tachycardia, if significant vomiting has been present. The abdomen is tender primarily in the left lower quadrant. The presence of severe tachycardia, hypotension, or a rigid abdomen with guarding and rebound suggests perforation. A pelvic examination should be performed on all women of child-bearing age. The rectal examination may reveal hemoccult positive stool; gross blood is rare.
Laboratory testing should include a CBC, BMP, urinalysis, and a urine pregnancy test (for women of child-bearing age). The CBC will usually reveal a mild leukocytosis. The urinalysis may reveal sterile pyuria for the reason previously described. Additional testing may be indicated by the history and physical examination.
A CT scan of the abdomen and pelvis is considered the gold standard with regards to imaging, with a reported sensitivity of 94% and specificity of 99%.3 Ideally, the CT scan should include both oral and IV contrast; however, IV alone is frequently used. In addition to identifying diverticulitis, CT can also visualize complications, including abscesses, perforation, and bowel obstruction. Ultrasound using high-resolution, graded compassion has a similar sensitivity and specificity as CT, with the advantage of less cost, can be performed at the bedside, and avoids radiation exposure.3 However, it is operator dependent and inferior to CT regarding visualizing complications.
Historically, antibiotics have been considered the treatment of choice for patients with acute uncomplicated diverticulitis, usually as an outpatient. Typically, this involves prescribing ciprofloxacin (or trimethoprim-sulfamethoxazole) plus metronidazole for 7 to 10 days. Monotherapy consisting of either moxifloxacin or amoxicillin/clavulanic acid is also acceptable. However, as our understanding of the important role of inflammation in this disease process, combined with the negative effects associated with antibiotic use, the role of antibiotics in uncomplicated diverticulitis has been called into question. In one recent study of 155 patients with acute uncomplicated diverticulitis, 97% were managed successfully as outpatients without antibiotics, admission, or complications.4 The American Gastroenterological Association (AGA) recommends that antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis. However, this is considered a “conditional recommendation with a low quality of evidence.”1In other words, this recommendation could easily change based on newer studies. Similarly, other “conditional recommendations” by the AGA include suggesting a fiber-rich diet, or fiber supplementation, and no need to avoid the consumption of nuts and popcorn.1 The majority of these patients begin to feel better in 2 to 3 days and have a good outcome.
Summary
For patients that appear ill, have significant comorbidities, are immunocompromised, or have a complication of acute diverticulitis, admission to the hospital with surgery consultation is recommended. For abscesses, interventional radiology has been used with success for CT-guided percutaneous drainage of diverticular abscesses. Intravenous antibiotics should be initiated; appropriate medications include metronidazole plus a third-generation cephalosporin (such as ceftriaxone or cefotaxime) or a fluoroquinolone (such as ciprofloxacin or levofloxacin). Monotherapy for the moderately ill patient includes piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanic acid, and imipenem. In addition, these patients should be placed at bowel rest (ie, nothing by mouth) with IV fluid resuscitation and hydration.
1. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944-1949. doi:10.1053/j.gastro.2015.10.003.
2. Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600-1605. doi:10.3748/wjg.v17.i12.1600.
3. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18(11):2498-2511. doi:10.1007/s00330-008-1018-6.
4. Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi:10.1007/s00384-015-2258-y.
A 44-year-old woman presented to the ED complaining of crampy lower abdominal pain with nausea and vomiting. The patient described gradual onset 2 days prior, with symptoms worsening over the previous 12 hours. She denied diarrhea, constipation, or blood in her stool. She did admit to frequency of urination, but no dysuria or hematuria. She was gravida 2, para 2, aborta 0, with a last menstrual period 3 weeks prior. She denied vaginal bleeding or discharge. Her past medical history was unremarkable, she was on no medications, and denied alcohol use. She did admit to smoking one pack of cigarettes per day.
On physical examination, the patient’s vital signs were: blood pressure, 132/68 mm Hg; heart rate, 96 beats/min; respiratory rate, 18 breaths/min; and temperature, 99.8°F. Oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat (HEENT) examination was completely normal, as was the heart and lung examination. The patient was tender to palpation in the lower abdomen, but without guarding or rebound. Bowel sounds were present and normoactive. A pelvic examination, including a bimanual examination, demonstrated mild left ovarian tenderness but without mass or cervical motion tenderness. The patient did not exhibit any costovertebral angle tenderness bilaterally. No rectal examination was performed.
The emergency physicians (EPs) ordered a complete blood count (CBC), basic metabolic profile (BMP), urinalysis, urine pregnancy test, and a vaginal wet preparation. In addition, the patient was administered 500 cc’s of normal saline intravenously (IV) and ondansetron (Zofran) 4 mg IV.
The urine pregnancy test result came back negative. The urinalysis was remarkable for positive leukocyte esterase, with five to 10 white cells and bacteria present. The CBC showed a mild leukocytosis, but with a normal hemoglobin and hematocrit. The BMP and vaginal wet preparation were completely normal.
The EP was concerned the patient might have something more serious than a simple urinary tract infection (UTI), so she ordered a computed tomography (CT) scan of the abdomen and pelvis with IV contrast.
The radiologist interpreted the CT scan as normal. The patient was discharged home with a prescription for an antibiotic for her UTI, encouraged to drink liquids, and instructed to follow-up with her primary care physician in 1 week.
The patient returned to the same ED approximately 48 hours later with worsening abdominal pain. On this presentation, she was tachycardic (110 beats/min) with a temperature of 101°F. The abdominal examination was remarkable for diffuse tenderness and voluntary guarding. The patient was administered IV fluids, morphine, and ondansetron. A repeat CT scan of the abdomen and pelvis with IV contrast showed a perforated sigmoid colon, with leakage of bowel contents into the peritoneum. The EP immediately started IV fluid resuscitation and administered IV antibiotics. The patient was taken emergently to the operating room by general surgery. The colon was repaired and a colostomy placed. The patient was able to be discharged home on day number 5.
The patient sued the hospital and the treating EP for failure to make the proper diagnosis on the initial ED visit, resulting in the patient having a long and difficult recovery, and the need for a colostomy. At trial, the jury returned a defense verdict.
Discussion
Diverticulitis, and its complications, account for a significant number of ED visits. It is the third most common inpatient gastrointestinal diagnosis in the United States, costing two billion dollars annually.1It is defined as clinically evident microscopic inflammation of a diverticulum or diverticula, and occurs in approximately 4% of patients with diverticulosis.1It is estimated that roughly 15% of these patients will experience a complication, defined as an abscess, perforation, fistula, or colonic obstruction; 15% to 30% will experience a recurrence.
The mean age of patients admitted to the hospital for diverticulitis is 63 years. While considered a disease of older patients, it should be included in the differential diagnosis for younger patients, as approximately 16% of admissions for acute diverticulitis are in patients less than 45 years.2Risk factors include poor diet (ie, low fiber, high fat, red meat), obesity, and smoking. The clinical presentation of diverticulitis has sometimes been referred to as “left-sided appendicitis” because of the similarities between the two entities. Patients will frequently complain of anorexia, change in bowel habits (either diarrhea or constipation), crampy abdominal pain (primarily in the left lower quadrant), low grade fever, and nausea with vomiting. Interestingly, 10% to 15% of patients with acute diverticulitis will complain of dysuria, urgency, or frequency (as in this patient) due to irritation of the bladder from an inflamed sigmoid colon.
Physical examination may reveal a low grade fever and tachycardia, if significant vomiting has been present. The abdomen is tender primarily in the left lower quadrant. The presence of severe tachycardia, hypotension, or a rigid abdomen with guarding and rebound suggests perforation. A pelvic examination should be performed on all women of child-bearing age. The rectal examination may reveal hemoccult positive stool; gross blood is rare.
Laboratory testing should include a CBC, BMP, urinalysis, and a urine pregnancy test (for women of child-bearing age). The CBC will usually reveal a mild leukocytosis. The urinalysis may reveal sterile pyuria for the reason previously described. Additional testing may be indicated by the history and physical examination.
A CT scan of the abdomen and pelvis is considered the gold standard with regards to imaging, with a reported sensitivity of 94% and specificity of 99%.3 Ideally, the CT scan should include both oral and IV contrast; however, IV alone is frequently used. In addition to identifying diverticulitis, CT can also visualize complications, including abscesses, perforation, and bowel obstruction. Ultrasound using high-resolution, graded compassion has a similar sensitivity and specificity as CT, with the advantage of less cost, can be performed at the bedside, and avoids radiation exposure.3 However, it is operator dependent and inferior to CT regarding visualizing complications.
Historically, antibiotics have been considered the treatment of choice for patients with acute uncomplicated diverticulitis, usually as an outpatient. Typically, this involves prescribing ciprofloxacin (or trimethoprim-sulfamethoxazole) plus metronidazole for 7 to 10 days. Monotherapy consisting of either moxifloxacin or amoxicillin/clavulanic acid is also acceptable. However, as our understanding of the important role of inflammation in this disease process, combined with the negative effects associated with antibiotic use, the role of antibiotics in uncomplicated diverticulitis has been called into question. In one recent study of 155 patients with acute uncomplicated diverticulitis, 97% were managed successfully as outpatients without antibiotics, admission, or complications.4 The American Gastroenterological Association (AGA) recommends that antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis. However, this is considered a “conditional recommendation with a low quality of evidence.”1In other words, this recommendation could easily change based on newer studies. Similarly, other “conditional recommendations” by the AGA include suggesting a fiber-rich diet, or fiber supplementation, and no need to avoid the consumption of nuts and popcorn.1 The majority of these patients begin to feel better in 2 to 3 days and have a good outcome.
Summary
For patients that appear ill, have significant comorbidities, are immunocompromised, or have a complication of acute diverticulitis, admission to the hospital with surgery consultation is recommended. For abscesses, interventional radiology has been used with success for CT-guided percutaneous drainage of diverticular abscesses. Intravenous antibiotics should be initiated; appropriate medications include metronidazole plus a third-generation cephalosporin (such as ceftriaxone or cefotaxime) or a fluoroquinolone (such as ciprofloxacin or levofloxacin). Monotherapy for the moderately ill patient includes piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanic acid, and imipenem. In addition, these patients should be placed at bowel rest (ie, nothing by mouth) with IV fluid resuscitation and hydration.
A 44-year-old woman presented to the ED complaining of crampy lower abdominal pain with nausea and vomiting. The patient described gradual onset 2 days prior, with symptoms worsening over the previous 12 hours. She denied diarrhea, constipation, or blood in her stool. She did admit to frequency of urination, but no dysuria or hematuria. She was gravida 2, para 2, aborta 0, with a last menstrual period 3 weeks prior. She denied vaginal bleeding or discharge. Her past medical history was unremarkable, she was on no medications, and denied alcohol use. She did admit to smoking one pack of cigarettes per day.
On physical examination, the patient’s vital signs were: blood pressure, 132/68 mm Hg; heart rate, 96 beats/min; respiratory rate, 18 breaths/min; and temperature, 99.8°F. Oxygen saturation was 99% on room air.
The head, ears, eyes, nose, and throat (HEENT) examination was completely normal, as was the heart and lung examination. The patient was tender to palpation in the lower abdomen, but without guarding or rebound. Bowel sounds were present and normoactive. A pelvic examination, including a bimanual examination, demonstrated mild left ovarian tenderness but without mass or cervical motion tenderness. The patient did not exhibit any costovertebral angle tenderness bilaterally. No rectal examination was performed.
The emergency physicians (EPs) ordered a complete blood count (CBC), basic metabolic profile (BMP), urinalysis, urine pregnancy test, and a vaginal wet preparation. In addition, the patient was administered 500 cc’s of normal saline intravenously (IV) and ondansetron (Zofran) 4 mg IV.
The urine pregnancy test result came back negative. The urinalysis was remarkable for positive leukocyte esterase, with five to 10 white cells and bacteria present. The CBC showed a mild leukocytosis, but with a normal hemoglobin and hematocrit. The BMP and vaginal wet preparation were completely normal.
The EP was concerned the patient might have something more serious than a simple urinary tract infection (UTI), so she ordered a computed tomography (CT) scan of the abdomen and pelvis with IV contrast.
The radiologist interpreted the CT scan as normal. The patient was discharged home with a prescription for an antibiotic for her UTI, encouraged to drink liquids, and instructed to follow-up with her primary care physician in 1 week.
The patient returned to the same ED approximately 48 hours later with worsening abdominal pain. On this presentation, she was tachycardic (110 beats/min) with a temperature of 101°F. The abdominal examination was remarkable for diffuse tenderness and voluntary guarding. The patient was administered IV fluids, morphine, and ondansetron. A repeat CT scan of the abdomen and pelvis with IV contrast showed a perforated sigmoid colon, with leakage of bowel contents into the peritoneum. The EP immediately started IV fluid resuscitation and administered IV antibiotics. The patient was taken emergently to the operating room by general surgery. The colon was repaired and a colostomy placed. The patient was able to be discharged home on day number 5.
The patient sued the hospital and the treating EP for failure to make the proper diagnosis on the initial ED visit, resulting in the patient having a long and difficult recovery, and the need for a colostomy. At trial, the jury returned a defense verdict.
Discussion
Diverticulitis, and its complications, account for a significant number of ED visits. It is the third most common inpatient gastrointestinal diagnosis in the United States, costing two billion dollars annually.1It is defined as clinically evident microscopic inflammation of a diverticulum or diverticula, and occurs in approximately 4% of patients with diverticulosis.1It is estimated that roughly 15% of these patients will experience a complication, defined as an abscess, perforation, fistula, or colonic obstruction; 15% to 30% will experience a recurrence.
The mean age of patients admitted to the hospital for diverticulitis is 63 years. While considered a disease of older patients, it should be included in the differential diagnosis for younger patients, as approximately 16% of admissions for acute diverticulitis are in patients less than 45 years.2Risk factors include poor diet (ie, low fiber, high fat, red meat), obesity, and smoking. The clinical presentation of diverticulitis has sometimes been referred to as “left-sided appendicitis” because of the similarities between the two entities. Patients will frequently complain of anorexia, change in bowel habits (either diarrhea or constipation), crampy abdominal pain (primarily in the left lower quadrant), low grade fever, and nausea with vomiting. Interestingly, 10% to 15% of patients with acute diverticulitis will complain of dysuria, urgency, or frequency (as in this patient) due to irritation of the bladder from an inflamed sigmoid colon.
Physical examination may reveal a low grade fever and tachycardia, if significant vomiting has been present. The abdomen is tender primarily in the left lower quadrant. The presence of severe tachycardia, hypotension, or a rigid abdomen with guarding and rebound suggests perforation. A pelvic examination should be performed on all women of child-bearing age. The rectal examination may reveal hemoccult positive stool; gross blood is rare.
Laboratory testing should include a CBC, BMP, urinalysis, and a urine pregnancy test (for women of child-bearing age). The CBC will usually reveal a mild leukocytosis. The urinalysis may reveal sterile pyuria for the reason previously described. Additional testing may be indicated by the history and physical examination.
A CT scan of the abdomen and pelvis is considered the gold standard with regards to imaging, with a reported sensitivity of 94% and specificity of 99%.3 Ideally, the CT scan should include both oral and IV contrast; however, IV alone is frequently used. In addition to identifying diverticulitis, CT can also visualize complications, including abscesses, perforation, and bowel obstruction. Ultrasound using high-resolution, graded compassion has a similar sensitivity and specificity as CT, with the advantage of less cost, can be performed at the bedside, and avoids radiation exposure.3 However, it is operator dependent and inferior to CT regarding visualizing complications.
Historically, antibiotics have been considered the treatment of choice for patients with acute uncomplicated diverticulitis, usually as an outpatient. Typically, this involves prescribing ciprofloxacin (or trimethoprim-sulfamethoxazole) plus metronidazole for 7 to 10 days. Monotherapy consisting of either moxifloxacin or amoxicillin/clavulanic acid is also acceptable. However, as our understanding of the important role of inflammation in this disease process, combined with the negative effects associated with antibiotic use, the role of antibiotics in uncomplicated diverticulitis has been called into question. In one recent study of 155 patients with acute uncomplicated diverticulitis, 97% were managed successfully as outpatients without antibiotics, admission, or complications.4 The American Gastroenterological Association (AGA) recommends that antibiotics should be used selectively, rather than routinely, in patients with acute uncomplicated diverticulitis. However, this is considered a “conditional recommendation with a low quality of evidence.”1In other words, this recommendation could easily change based on newer studies. Similarly, other “conditional recommendations” by the AGA include suggesting a fiber-rich diet, or fiber supplementation, and no need to avoid the consumption of nuts and popcorn.1 The majority of these patients begin to feel better in 2 to 3 days and have a good outcome.
Summary
For patients that appear ill, have significant comorbidities, are immunocompromised, or have a complication of acute diverticulitis, admission to the hospital with surgery consultation is recommended. For abscesses, interventional radiology has been used with success for CT-guided percutaneous drainage of diverticular abscesses. Intravenous antibiotics should be initiated; appropriate medications include metronidazole plus a third-generation cephalosporin (such as ceftriaxone or cefotaxime) or a fluoroquinolone (such as ciprofloxacin or levofloxacin). Monotherapy for the moderately ill patient includes piperacillin/tazobactam, ampicillin/sulbactam, ticarcillin/clavulanic acid, and imipenem. In addition, these patients should be placed at bowel rest (ie, nothing by mouth) with IV fluid resuscitation and hydration.
1. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944-1949. doi:10.1053/j.gastro.2015.10.003.
2. Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600-1605. doi:10.3748/wjg.v17.i12.1600.
3. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18(11):2498-2511. doi:10.1007/s00330-008-1018-6.
4. Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi:10.1007/s00384-015-2258-y.
1. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149(7):1944-1949. doi:10.1053/j.gastro.2015.10.003.
2. Nguyen GC, Sam J, Anand N. Epidemiological trends and geographic variation in hospital admissions for diverticulitis in the United States. World J Gastroenterol. 2011;17(12):1600-1605. doi:10.3748/wjg.v17.i12.1600.
3. Laméris W, van Randen A, Bipat S, Bossuyt PM, Boermeester MA, Stoker J. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: meta-analysis of test accuracy. Eur Radiol. 2008;18(11):2498-2511. doi:10.1007/s00330-008-1018-6.
4. Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi:10.1007/s00384-015-2258-y.
New MI definition from ESC 2018
Also today, aspirin and fish oil flop in patients with diabetes, CT angiography cuts MI in patients with stable chest pain, and rivaroxaban is no help for heart failure outcomes.
Also today, aspirin and fish oil flop in patients with diabetes, CT angiography cuts MI in patients with stable chest pain, and rivaroxaban is no help for heart failure outcomes.
Also today, aspirin and fish oil flop in patients with diabetes, CT angiography cuts MI in patients with stable chest pain, and rivaroxaban is no help for heart failure outcomes.