User login
Age cutoff suggested for STI screening in HIV patients
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
mlesney@mdedge.com
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
mlesney@mdedge.com
WASHINGTON – Current guidelines recommend a minimum of annual screening for gonorrhea and chlamydia in all sexually active individuals with HIV infection. However, this goal, which is frequently not attained in HIV clinics, may be excessive in certain populations with HIV infection.
In particular, women as well as men who have sex exclusively with women (MSW) may best be served by targeted, age-based screening rather than universal screening, according to a presentation by Susan A. Tuddenham, MD, an assistant professor of medicine at Johns Hopkins University, Baltimore.
“Detection and treatment of gonorrhea and chlamydia in HIV-positive patients in the United States is a priority both because of patient morbidity and because of the potential for these infections to enhance transmission of HIV,” said Dr. Tuddenham.
She and her colleagues assessed data from 16,864 gonorrhea and chlamydia tests of all adults in care at three HIV Research Network sites during 2011-2014. She presented the data at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
They assessed the number needed to screen (NNS) in order to identify a single infection across three risk populations of individuals with HIV infection: 1,123 women, 1,236 men who have sex only with women (MSW), and 3,501 men who have sex with men (MSM). NNS was defined as the number of persons tested divided by the number who tested positive and was calculated for the three risk groups for urogenital and extragenital (rectal and pharyngeal) sampling and by age.
Dr. Tuddenham and her colleagues found that NNS based on urogenital screening was similar in all three groups for those individuals aged younger than or equal to 25 years: 15 for women (95% confidence interval, 9-71); 21 for MSW (95% CI, 6-171); and 20 for MSM (95% CI, 12-36). However at ages greater than 25 years, the picture changed, with urogenital NNS increasing to 363 for women (95% CI, 167-1000); 160 for MSW (95% CI, 100-333). For MSM over the age of 25 years, however, the NNS only increased to 46 (95% CI, 38-56).
There were insufficient numbers of extragenital screenings of women and MSW for analysis. But for MSM, rectal NNS was 5 and 10 for those men aged 25 years and younger and those aged over age 25 years, respectively, and pharyngeal NNS was 8 and 20 for the two groups, respectively.
“Our results provide some support for age-based screening cutoffs for women and MSW, with universal screening appropriate for those less than or equal to 25 years of age, and targeted screening for those over 25,” said Dr. Tuddenham. She emphasized the importance of continued universal screening of MSM of all ages for gonorrhea/chlamydia, in particular using extragenital screening as well in order to capture those missed by urogenital screening alone.
Dr. Tuddenham reported that she had no disclosures.
mlesney@mdedge.com
REPORTING FROM THE 2018 STD PREVENTION CONFERENCE
Key clinical point: Men who have sex with men with HIV should be regularly screened for STIs regardless of age.
Major finding: The number needed to screen to detect an STI in individuals infected with HIV aged over 25 years were 363 (women); 160 (men who have sex exclusively with women); and 46 (men who have sex with men).
Study details: Gonorrhea/chlamydia tests were assessed from 16,864 individuals infected with HIV and number needed to screen calculated.
Disclosures: Dr. Tuddenham reported that she had no disclosures.
Baseline patient-reported physical function predicts SLE-related mortality
The baseline physical component score of the Medical Outcomes Short Form 36 (SF-36) was an independent predictor of mortality for patients with systemic lupus erythematosus (SLE), according to recent analysis of data from the University of California, San Francisco, (UCSF) Lupus Outcomes Study.
Desiree R. Azizoddin, PsyD, of Stanford Health Care in Palo Alto, Calif., and her colleagues analyzed patient-reported outcomes (PROs) of 728 patients with SLE from the UCSF study, which included measures such as self-rated health, the Center for Epidemiologic Studies Depression scale (CESD), and SF-36 mental and physical component scores between 2007 and 2015. Patients had a mean age of 50.6 years and consisted of mostly whites (68.5%) and women (92.2%), with a mean SLE disease duration of 16.7 years.
“As PROs can be measured easily, quickly, reliably in a systematic manner, without use of many resources (including cost) and experts, PROs can serve as an important tool in community settings that provide care for individuals with SLE,” Dr. Azizoddin and her colleagues wrote in Arthritis Care & Research. “Implementation and integration of PROs may potentially help decrease mortality among patients with SLE in the long run.”
The researchers reported 71 deaths (9.8%) in the study. A univariate analysis showed that self-rated health as fair or poor and all SF-36 subscale scores, except for mental health and role emotional subscales, were predictors of mortality. After the investigators performed a multivariate analysis, they found that baseline SF-36 physical component scores alone were associated with an increased mortality risk (hazard ratio, 0.97; 95% confidence interval, 0.94-0.99; P less than .01), with a 3.5% lower risk for each point-rating increase in the score.
“Insight to predictors of mortality, above and beyond those that require physician resources such as assessments of renal involvement and traditional physician-completed measures of disease activity and damage in this complex disease, affords rheumatologists and SLE providers with additional tools to assess potential patient health trajectories,” Dr. Azizoddin and her colleagues wrote in their study. “Identification of such predictors will expand opportunities for early interventions aimed at the reduction of such risk, including allocation of appropriate medical resources in areas with large numbers of patients with compromised health.”
The researchers noted that bias in self-reporting, a lack of prospective evaluations at each visit, and clinical applicability to only similar cohorts were limitations in the study.
This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SOURCE: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
The baseline physical component score of the Medical Outcomes Short Form 36 (SF-36) was an independent predictor of mortality for patients with systemic lupus erythematosus (SLE), according to recent analysis of data from the University of California, San Francisco, (UCSF) Lupus Outcomes Study.
Desiree R. Azizoddin, PsyD, of Stanford Health Care in Palo Alto, Calif., and her colleagues analyzed patient-reported outcomes (PROs) of 728 patients with SLE from the UCSF study, which included measures such as self-rated health, the Center for Epidemiologic Studies Depression scale (CESD), and SF-36 mental and physical component scores between 2007 and 2015. Patients had a mean age of 50.6 years and consisted of mostly whites (68.5%) and women (92.2%), with a mean SLE disease duration of 16.7 years.
“As PROs can be measured easily, quickly, reliably in a systematic manner, without use of many resources (including cost) and experts, PROs can serve as an important tool in community settings that provide care for individuals with SLE,” Dr. Azizoddin and her colleagues wrote in Arthritis Care & Research. “Implementation and integration of PROs may potentially help decrease mortality among patients with SLE in the long run.”
The researchers reported 71 deaths (9.8%) in the study. A univariate analysis showed that self-rated health as fair or poor and all SF-36 subscale scores, except for mental health and role emotional subscales, were predictors of mortality. After the investigators performed a multivariate analysis, they found that baseline SF-36 physical component scores alone were associated with an increased mortality risk (hazard ratio, 0.97; 95% confidence interval, 0.94-0.99; P less than .01), with a 3.5% lower risk for each point-rating increase in the score.
“Insight to predictors of mortality, above and beyond those that require physician resources such as assessments of renal involvement and traditional physician-completed measures of disease activity and damage in this complex disease, affords rheumatologists and SLE providers with additional tools to assess potential patient health trajectories,” Dr. Azizoddin and her colleagues wrote in their study. “Identification of such predictors will expand opportunities for early interventions aimed at the reduction of such risk, including allocation of appropriate medical resources in areas with large numbers of patients with compromised health.”
The researchers noted that bias in self-reporting, a lack of prospective evaluations at each visit, and clinical applicability to only similar cohorts were limitations in the study.
This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SOURCE: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
The baseline physical component score of the Medical Outcomes Short Form 36 (SF-36) was an independent predictor of mortality for patients with systemic lupus erythematosus (SLE), according to recent analysis of data from the University of California, San Francisco, (UCSF) Lupus Outcomes Study.
Desiree R. Azizoddin, PsyD, of Stanford Health Care in Palo Alto, Calif., and her colleagues analyzed patient-reported outcomes (PROs) of 728 patients with SLE from the UCSF study, which included measures such as self-rated health, the Center for Epidemiologic Studies Depression scale (CESD), and SF-36 mental and physical component scores between 2007 and 2015. Patients had a mean age of 50.6 years and consisted of mostly whites (68.5%) and women (92.2%), with a mean SLE disease duration of 16.7 years.
“As PROs can be measured easily, quickly, reliably in a systematic manner, without use of many resources (including cost) and experts, PROs can serve as an important tool in community settings that provide care for individuals with SLE,” Dr. Azizoddin and her colleagues wrote in Arthritis Care & Research. “Implementation and integration of PROs may potentially help decrease mortality among patients with SLE in the long run.”
The researchers reported 71 deaths (9.8%) in the study. A univariate analysis showed that self-rated health as fair or poor and all SF-36 subscale scores, except for mental health and role emotional subscales, were predictors of mortality. After the investigators performed a multivariate analysis, they found that baseline SF-36 physical component scores alone were associated with an increased mortality risk (hazard ratio, 0.97; 95% confidence interval, 0.94-0.99; P less than .01), with a 3.5% lower risk for each point-rating increase in the score.
“Insight to predictors of mortality, above and beyond those that require physician resources such as assessments of renal involvement and traditional physician-completed measures of disease activity and damage in this complex disease, affords rheumatologists and SLE providers with additional tools to assess potential patient health trajectories,” Dr. Azizoddin and her colleagues wrote in their study. “Identification of such predictors will expand opportunities for early interventions aimed at the reduction of such risk, including allocation of appropriate medical resources in areas with large numbers of patients with compromised health.”
The researchers noted that bias in self-reporting, a lack of prospective evaluations at each visit, and clinical applicability to only similar cohorts were limitations in the study.
This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
SOURCE: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: There was a 3.5% lower risk of mortality per each increased point rating on the SF-36 physical component score.
Study details: An analysis of 728 patients with SLE from the University of California, San Francisco, Lupus Outcomes Study.
Disclosures: This study was funded by the Robert Wood Johnson Foundation Investigator Awards in Health Policy Research and individually was awarded grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Source: Azizoddin DR et al. Arthritis Care Res. 2018 Aug 24. doi: 10.1002/acr.23734.
England green-lights coverage of one CAR T-cell therapy
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
Researchers find drug target in anaplastic large-cell lymphoma
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
FROM LEUKEMIA
Key clinical point:
Major finding: TYK2 was expressed in all types of ALCL studied and mediated the same anti-apoptotic response across ALCLs.
Study details: A preclinical study of mouse and human ALCL cell lines.
Disclosures: The researchers received grant funding from various organizations but reported having no conflicts of interest.
Source: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
ESMO scale offers guidance on cancer targets
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
The European Society for Medical Oncology (ESMO) has published a proposed scale that would rank molecular targets for various cancers by how well they can be treated with new or emerging drugs.
The ESMO Scale of Clinical Actionability for Molecular Targets is designed to “harmonize and standardize the reporting and interpretation of clinically relevant genomics data,” according to Joaquin Mateo, MD, PhD, from the Vall d’Hebron Institute of Oncology in Barcelona, Spain, and his fellow members of the ESMO Translational Research and Precision Medicine Working Group.
“A major challenge for oncologists in the clinic is to distinguish between findings that represent proven clinical value or potential value based on preliminary clinical or preclinical evidence from hypothetical gene-drug matches and findings that are currently irrelevant for clinical practice,” they wrote in Annals of Oncology.
The scale groups targets into one of six tiers based on levels of evidence ranging from the gold standard of prospective, randomized clinical trials to targets for which there are no evidence and only hypothetical actionability. The primary goal is to help oncologists assign priority to potential targets when they review results of gene-sequencing panels for individual patients, according to the developers.
Briefly, the six tiers are:
Tier I includes targets that are agreed to be suitable for routine use and a recommended specific drug when a specific molecular alteration is detected. Examples include trastuzumab for human epidermal growth factor receptor 2 (HER2)–positive breast cancer, and inhibitors of epidermal growth factor receptor (EGFR) in patients with non–small cell lung cancer positive for EGFR mutations.
Tier II includes “investigational targets that likely define a patient population that benefits from a targeted drug but additional data are needed.” This tier includes agents that work in the phosphatidylinostiol 3-kinase pathway.
Tier III is similar to Tier II, in that it includes investigational targets that define a patient population with proven benefit from a targeted therapy, but in this case the target is detected in a different tumor type that has not previously been studied. For example, the targeted agent vemurafenib (Zelboraf), which extends survival of patients with metastatic melanomas carrying the BRAF V600E mutation, has only limited activity against BRAF-mutated colorectal cancers.
Tier IV includes targets with preclinical evidence of actionability.
Tier V includes targets with “evidence of relevant antitumor activity, not resulting in clinical meaningful benefit as single treatment but supporting development of cotargeting approaches.” The authors cite the example of PIK3CA inhibitors in patients with estrogen receptor–positive, HER2-negative breast cancers who also have PIK3CA activating mutations. In clinical trials, this strategy led to objective responses but not change outcomes.
The final tier is not Tier VI, as might be expected, but Tier X, with the X in this case being the unknown – that is, alterations/mutations for which there is neither preclinical nor clinical evidence to support their hypothetical use as a drug target.
“This clinical benefit–centered classification system offers a common language for all the actors involved in clinical cancer drug development. Its implementation in sequencing reports, tumor boards, and scientific communication can enable precise treatment decisions and facilitate discussions with patients about novel therapeutic options,” Dr. Mateo and his associates wrote in their conclusion.
The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
SOURCE: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
FROM ANNALS OF ONCOLOGY
Key clinical point: The scale is intended to standardize reporting and interpretation of cancer gene panel results to help oncologists plan treatment.
Major finding: The scale divides current and future therapeutic targets into tiers based on levels of clinical and preclinical evidence.
Study details: Proposed guiding principles for a classification system developed by the Translational Research and Precision Medicine Working Group of the European Society of Medical Oncology.
Disclosures: The development process was supported by ESMO. Multiple coauthors reported financial relationships with various companies as well as grants/support from other foundations or charities.
Source: Mateo J et al. Ann Oncol. 2018 Aug 21. doi: 10.1093/annonc/mdy263.
MRI doubles rate of observation in low-risk prostate cancer
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Men who undergo MRI of the prostate around the time of a low-risk prostate cancer diagnosis are nearly twice as likely to be managed with active surveillance as are men who do not get MRI, investigators found.
The findings suggest that MRI at the time of diagnosis can enhance patient and physician confidence in the decision to choose active surveillance (AS) over immediate surgery or radiation therapy in men with low-risk disease, according to Michael S. Leapman, MD, and his colleagues from Yale University, New Haven, Conn.
“Despite initial high costs associated with obtaining and interpreting MRI studies of the prostate, economic modeling studies imply that MRI would be cost effective if it resulted in increased utilization of AS for low- and very-low-risk PCa [prostate cancer]. The association identified in our study between MRI use and initial observation may serve as an informative basis for examining strategies to improve the quality of PCa care with the anticipated growing use of this technology,” they wrote in Urology.
Although active surveillance is increasingly accepted as an initial management strategy for patients with low-risk (Gleason score 6 or less) localized prostate cancer, the majority of patients with low-risk disease still receive definitive treatment.
“Although longitudinal studies support the safety of AS, uncertainty about the possibility of underestimating an indvidual’s risk of harboring aggressive disease remains a strong motivator to treat,” Dr. Leapman and his associates noted.
To see whether MRI of the prostate may have an effect on the use of active surveillance in men with low-risk disease, the investigators reviewed records from the Surveillance, Epidemiology and End Results (SEER) Medicare database to identify men diagnosed with low-risk prostate cancer during 2010-2013.
They looked at the association between MRI and patient management (ascertained by claims) and evaluated clinical and demographic factors associated with the receipt of MRI.
They identified 8,144 patients with low-risk prostate cancer during the study period, of whom 495 (6.1%) had undergone MRI scans. They found that the use of MRI in patients with low-risk cancer increased from 3.4% in 2010 to 10.5% in 2013.
MRI was performed significantly more frequently among 3,060 patients who were managed with observation, with 265 (8.7%) receiving scans, compared with 230 (4.5%) of the 5,084 patients who underwent treatment within a year of diagnosis.
In multivariable analysis that controlled for demographics, factors significantly associated with increased likelihood of undergoing observation versus definitive therapy included MRI, white vs. nonwhite race, later years of diagnosis, higher income status (by ZIP code), unmarried vs. married, treatment region (more common in the West and Midwest versus Northeast or South), and in referral regions with higher population density of urologists.
In a propensity score–matched analysis designed to smooth out potential confounders, the investigators found that receipt of MRI around the time of diagnosis was associated with a significantly higher likelihood of active surveillance, with an odds ratio of 1.90 (95% confidence interval, 1.56-2.32).
“Efforts to facilitate observational approaches for low-risk PCa are highly valuable to improving the quality of cancer care. Because the use of prostate MRI has grown, and is likely to continue expanding, the cost-effectiveness of MRI-driven pathways are increasingly relevant to the sustainability of the practice,” the authors wrote.
SOURCE: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
FROM UROLOGY
Key clinical point: MRI at screening or diagnosis of low-risk prostate cancer is associated with a higher likelihood of observation versus immediate definitive therapy.
Major finding: MRI was associated with a near doubling of the likelihood of observation.
Study details: Review of SEER Medicare data on 8,144 men diagnosed with low-risk prostate cancers during 2010-2013.
Disclosures: The study was supported by the National Cancer Institute, California Department of Public Health, and Centers for Disease Control and Prevention. The authors reported no relevant conflicts of interest.
Source: Leapman MS et al. Urology. 2018 Aug 11. doi: 10.1016/j.urology.2018.07.041.
Rituximab/lenalidomide similar to rituximab/chemotherapy for follicular lymphoma
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in treatment of follicular lymphoma, according to results from a phase 3 trial.
RELEVANCE (NCT01476787) was a multicenter, international, randomized, open-label trial designed to determine the superiority of rituximab/lenalidomide over rituximab/chemotherapy.
This trial randomized 1,030 patients with previously untreated follicular lymphoma to receive either rituximab plus lenalidomide (n = 513) or rituximab plus chemotherapy (n = 517) for 18 cycles; both groups then went on to receive rituximab maintenance therapy for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years. The study was published in the New England Journal of Medicine.
One of the coprimary endpoints was complete response (confirmed or unconfirmed) by the end of the treatment period; the other was progression-free survival, which was planned to be assessed through three analyses, including two interim analyses, the first of which was reported in this study.
After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the two groups. Complete response (confirmed or unconfirmed) was seen in 48% of the rituximab/lenalidomide group (95% confidence interval [CI], 44-53) and in 53% of the rituximab/chemotherapy group (95% CI, 49-57; P = .13). The hazard ratio for progression or death from any cause was 1.10 (95% CI, 0.85-1.43; P = .48).
In the subgroup analyses, the efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose follicular lymphoma was Ann Arbor stage I or II, whereas efficacy of rituximab/lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some events being more common in one group than in the other. For example, cutaneous reactions, diarrhea, rash, and myalgia were more common with rituximab/lenalidomide treatment, whereas anemia, fatigue, nausea, and febrile neutropenia were more common with rituximab/chemotherapy treatment. Among grade 3 or 4 events, cutaneous reactions were more common with rituximab/lenalidomide, and grade 3 or 4 neutropenia was more common with rituximab/chemotherapy.
“Overall, both treatment groups showed good outcomes, and a median has not yet been reached for either progression-free survival or overall survival,” the study authors wrote.
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
SOURCE: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in treatment of follicular lymphoma, according to results from a phase 3 trial.
RELEVANCE (NCT01476787) was a multicenter, international, randomized, open-label trial designed to determine the superiority of rituximab/lenalidomide over rituximab/chemotherapy.
This trial randomized 1,030 patients with previously untreated follicular lymphoma to receive either rituximab plus lenalidomide (n = 513) or rituximab plus chemotherapy (n = 517) for 18 cycles; both groups then went on to receive rituximab maintenance therapy for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years. The study was published in the New England Journal of Medicine.
One of the coprimary endpoints was complete response (confirmed or unconfirmed) by the end of the treatment period; the other was progression-free survival, which was planned to be assessed through three analyses, including two interim analyses, the first of which was reported in this study.
After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the two groups. Complete response (confirmed or unconfirmed) was seen in 48% of the rituximab/lenalidomide group (95% confidence interval [CI], 44-53) and in 53% of the rituximab/chemotherapy group (95% CI, 49-57; P = .13). The hazard ratio for progression or death from any cause was 1.10 (95% CI, 0.85-1.43; P = .48).
In the subgroup analyses, the efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose follicular lymphoma was Ann Arbor stage I or II, whereas efficacy of rituximab/lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some events being more common in one group than in the other. For example, cutaneous reactions, diarrhea, rash, and myalgia were more common with rituximab/lenalidomide treatment, whereas anemia, fatigue, nausea, and febrile neutropenia were more common with rituximab/chemotherapy treatment. Among grade 3 or 4 events, cutaneous reactions were more common with rituximab/lenalidomide, and grade 3 or 4 neutropenia was more common with rituximab/chemotherapy.
“Overall, both treatment groups showed good outcomes, and a median has not yet been reached for either progression-free survival or overall survival,” the study authors wrote.
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
SOURCE: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
Rituximab plus lenalidomide had efficacy similar to that of rituximab plus chemotherapy in treatment of follicular lymphoma, according to results from a phase 3 trial.
RELEVANCE (NCT01476787) was a multicenter, international, randomized, open-label trial designed to determine the superiority of rituximab/lenalidomide over rituximab/chemotherapy.
This trial randomized 1,030 patients with previously untreated follicular lymphoma to receive either rituximab plus lenalidomide (n = 513) or rituximab plus chemotherapy (n = 517) for 18 cycles; both groups then went on to receive rituximab maintenance therapy for 12 cycles. The total duration of treatment was 120 weeks. The median age of the combined groups was 59 years. The study was published in the New England Journal of Medicine.
One of the coprimary endpoints was complete response (confirmed or unconfirmed) by the end of the treatment period; the other was progression-free survival, which was planned to be assessed through three analyses, including two interim analyses, the first of which was reported in this study.
After a median follow-up of 37.9 months, the rates of coprimary endpoints were similar between the two groups. Complete response (confirmed or unconfirmed) was seen in 48% of the rituximab/lenalidomide group (95% confidence interval [CI], 44-53) and in 53% of the rituximab/chemotherapy group (95% CI, 49-57; P = .13). The hazard ratio for progression or death from any cause was 1.10 (95% CI, 0.85-1.43; P = .48).
In the subgroup analyses, the efficacy of rituximab plus chemotherapy was greater in low-risk patients (based on Follicular Lymphoma International Prognostic Index scores) and in patients whose follicular lymphoma was Ann Arbor stage I or II, whereas efficacy of rituximab/lenalidomide was independent of prognostic factors.
Safety was the biggest area of difference, with some events being more common in one group than in the other. For example, cutaneous reactions, diarrhea, rash, and myalgia were more common with rituximab/lenalidomide treatment, whereas anemia, fatigue, nausea, and febrile neutropenia were more common with rituximab/chemotherapy treatment. Among grade 3 or 4 events, cutaneous reactions were more common with rituximab/lenalidomide, and grade 3 or 4 neutropenia was more common with rituximab/chemotherapy.
“Overall, both treatment groups showed good outcomes, and a median has not yet been reached for either progression-free survival or overall survival,” the study authors wrote.
The RELEVANCE trial was sponsored by Celgene and the Lymphoma Academic Research Organisation. The study authors reported various disclosures, including financial ties to Celgene.
SOURCE: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Complete responses were seen in 48% of rituximab/lenalidomide patients versus 53% in the rituximab/chemotherapy patients (P = .13).
Study details: A phase 3 superiority trial of 1,030 patients with previously untreated follicular lymphoma.
Disclosures: Celgene and the Lymphoma Academic Research Organization funded the study. The authors reported various disclosures, including financial ties to Celgene.
Source: Morschhauser F et al. N Engl J Med. 2018;379:934-47.
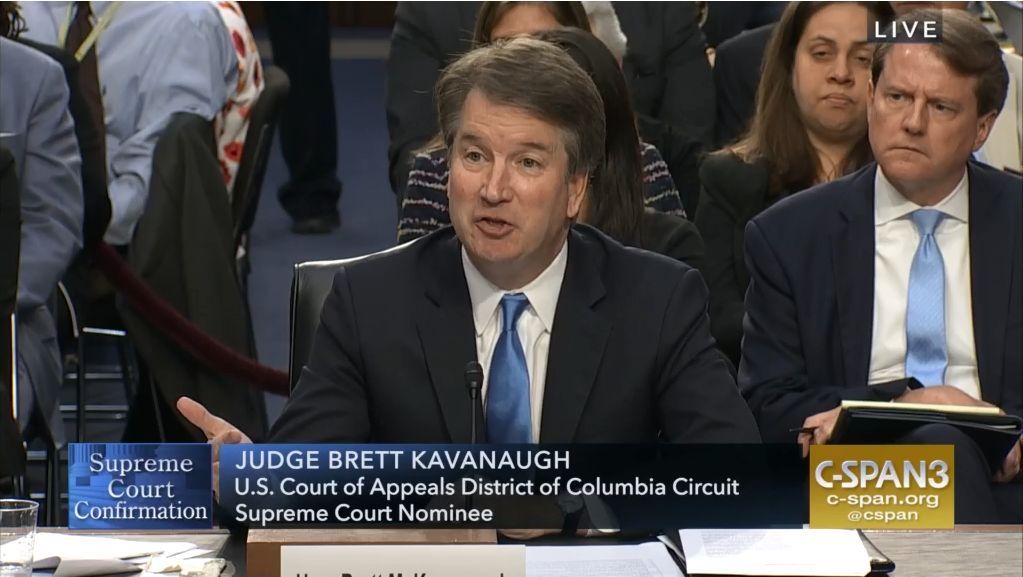
SCOTUS: Kavanaugh suggests precedent will govern future abortion decisions
Supreme Court justice nominee Brett Kavanaugh suggested precedent would guide his thinking when it comes to deliberating on any Roe v. Wade challenges, but he came short of saying that he would not support overturning the landmark abortion rights case.
Testifying Sept. 5 before the Senate Judiciary Committee during a confirmation hearing, Judge Kavanaugh noted that the decision of Roe v. Wade has been reaffirmed in follow-up cases, and that would play a significant role in his approach to future challenges.
Sen. Dianne Feinstein (D-Calif.), the ranking Democrat on the panel, asked Judge Kavanaugh if he agreed with Justice Sandra Day O’Connor that “a woman’s right to control her reproductive life impacts her ability to ‘participate equally in the economic and social life of the nation?’ ”
“As a general proposition, I understand the importance of the precedent set forth in Roe v. Wade,” Judge Kavanaugh replied. “So Roe v. Wade held, of course, and is reaffirmed in Planned Parenthood v. Casey, that a woman has a constitutional right to obtain an abortion before viability, subject to reasonable regulation by the state up to the point where that regulation constitutes an undue burden on the woman’s right to obtain an abortion. One of the reasons for that holding, as explained by the court in Roe and also in Planned Parenthood v. Casey more fully, is along the lines of what you said, Sen. Feinstein, about the quote from Justice O’Connor.”
He continued: “That’s one of the rationales that undergirds Roe v. Wade. It’s one of the rationales that undergirds Planned Parenthood v. Casey.”
Sen. Feinstein followed by noting reports quoting Judge Kavanaugh as saying Roe v. Wade was “settled law,” inquired what that term means, and asked for further clarification as to whether it was settled precedent and whether it could be overturned.
“Senator, I said that it’s settled as a precedent of the Supreme Court, entitled the respect of principles of stare decisis,” he responded, referencing the legal principle of determining points in litigation according to precedent. “One of the important things to keep in mind about Roe v. Wade is that it has been reaffirmed many times over the past 45 years, as you know, and most prominently, most importantly reaffirmed in Planned Parenthood v. Casey in 1992. And as you well recall, Senator, I know when that case came up, the Supreme Court didn’t just reaffirm it in passing, the court specifically went through all factors of stare decisis in considering whether to overrule it.”
He noted that the joint opinion of Justice Anthony Kennedy, Justice O’Connor, and Justice David Souter, went to “great length” to go through those factors.
Judge Kavanaugh called Planned Parenthood v. Casey a “precedent-on-precedent. It is not as if it’s just a run-of-the-mill case that was decided and never been reconsidered. Casey specifically reconsidered [Roe v. Wade], applied the stare decisis factors and decided to reaffirm it. That makes Casey precedent-on-precedent.”
That said, Judge Kavanaugh did not say outright that Row v. Wade would not be overturned because of these factors.
The first 2 days of Judge Kavanaugh’s confirmation hearing were frequently interrupted by protesters shouting objections to his nomination. At press time, more than 120 protesters had been arrested, according to the U.S. Capitol Police.
Health care did surface in questions about the Affordable Care Act and in specific the current legal action in Texas that is looking to kill the health care law, but Judge Kavanaugh declined to answer any questions surrounding it, stating that he did not want to prejudge any potential case that could come before him.
Much of the questioning he faced was more geared toward opinions on presidential powers, including whether presidents could avoid subpoenas and whether a president could pardon himself, but he declined to answer those questions because they are ones that could come before him given the current political environment.
Judge Kavanaugh painted himself as of independent thought, pushing back at those who are saying that he will serve to protect the president and act as his puppet on the bench, citing cases where he went against former President George W. Bush, for whom he served as White House staff secretary and who eventually was appointed him to the D.C. Circuit Court in May 2006.
Supreme Court justice nominee Brett Kavanaugh suggested precedent would guide his thinking when it comes to deliberating on any Roe v. Wade challenges, but he came short of saying that he would not support overturning the landmark abortion rights case.
Testifying Sept. 5 before the Senate Judiciary Committee during a confirmation hearing, Judge Kavanaugh noted that the decision of Roe v. Wade has been reaffirmed in follow-up cases, and that would play a significant role in his approach to future challenges.
Sen. Dianne Feinstein (D-Calif.), the ranking Democrat on the panel, asked Judge Kavanaugh if he agreed with Justice Sandra Day O’Connor that “a woman’s right to control her reproductive life impacts her ability to ‘participate equally in the economic and social life of the nation?’ ”
“As a general proposition, I understand the importance of the precedent set forth in Roe v. Wade,” Judge Kavanaugh replied. “So Roe v. Wade held, of course, and is reaffirmed in Planned Parenthood v. Casey, that a woman has a constitutional right to obtain an abortion before viability, subject to reasonable regulation by the state up to the point where that regulation constitutes an undue burden on the woman’s right to obtain an abortion. One of the reasons for that holding, as explained by the court in Roe and also in Planned Parenthood v. Casey more fully, is along the lines of what you said, Sen. Feinstein, about the quote from Justice O’Connor.”
He continued: “That’s one of the rationales that undergirds Roe v. Wade. It’s one of the rationales that undergirds Planned Parenthood v. Casey.”
Sen. Feinstein followed by noting reports quoting Judge Kavanaugh as saying Roe v. Wade was “settled law,” inquired what that term means, and asked for further clarification as to whether it was settled precedent and whether it could be overturned.
“Senator, I said that it’s settled as a precedent of the Supreme Court, entitled the respect of principles of stare decisis,” he responded, referencing the legal principle of determining points in litigation according to precedent. “One of the important things to keep in mind about Roe v. Wade is that it has been reaffirmed many times over the past 45 years, as you know, and most prominently, most importantly reaffirmed in Planned Parenthood v. Casey in 1992. And as you well recall, Senator, I know when that case came up, the Supreme Court didn’t just reaffirm it in passing, the court specifically went through all factors of stare decisis in considering whether to overrule it.”
He noted that the joint opinion of Justice Anthony Kennedy, Justice O’Connor, and Justice David Souter, went to “great length” to go through those factors.
Judge Kavanaugh called Planned Parenthood v. Casey a “precedent-on-precedent. It is not as if it’s just a run-of-the-mill case that was decided and never been reconsidered. Casey specifically reconsidered [Roe v. Wade], applied the stare decisis factors and decided to reaffirm it. That makes Casey precedent-on-precedent.”
That said, Judge Kavanaugh did not say outright that Row v. Wade would not be overturned because of these factors.
The first 2 days of Judge Kavanaugh’s confirmation hearing were frequently interrupted by protesters shouting objections to his nomination. At press time, more than 120 protesters had been arrested, according to the U.S. Capitol Police.
Health care did surface in questions about the Affordable Care Act and in specific the current legal action in Texas that is looking to kill the health care law, but Judge Kavanaugh declined to answer any questions surrounding it, stating that he did not want to prejudge any potential case that could come before him.
Much of the questioning he faced was more geared toward opinions on presidential powers, including whether presidents could avoid subpoenas and whether a president could pardon himself, but he declined to answer those questions because they are ones that could come before him given the current political environment.
Judge Kavanaugh painted himself as of independent thought, pushing back at those who are saying that he will serve to protect the president and act as his puppet on the bench, citing cases where he went against former President George W. Bush, for whom he served as White House staff secretary and who eventually was appointed him to the D.C. Circuit Court in May 2006.
Supreme Court justice nominee Brett Kavanaugh suggested precedent would guide his thinking when it comes to deliberating on any Roe v. Wade challenges, but he came short of saying that he would not support overturning the landmark abortion rights case.
Testifying Sept. 5 before the Senate Judiciary Committee during a confirmation hearing, Judge Kavanaugh noted that the decision of Roe v. Wade has been reaffirmed in follow-up cases, and that would play a significant role in his approach to future challenges.
Sen. Dianne Feinstein (D-Calif.), the ranking Democrat on the panel, asked Judge Kavanaugh if he agreed with Justice Sandra Day O’Connor that “a woman’s right to control her reproductive life impacts her ability to ‘participate equally in the economic and social life of the nation?’ ”
“As a general proposition, I understand the importance of the precedent set forth in Roe v. Wade,” Judge Kavanaugh replied. “So Roe v. Wade held, of course, and is reaffirmed in Planned Parenthood v. Casey, that a woman has a constitutional right to obtain an abortion before viability, subject to reasonable regulation by the state up to the point where that regulation constitutes an undue burden on the woman’s right to obtain an abortion. One of the reasons for that holding, as explained by the court in Roe and also in Planned Parenthood v. Casey more fully, is along the lines of what you said, Sen. Feinstein, about the quote from Justice O’Connor.”
He continued: “That’s one of the rationales that undergirds Roe v. Wade. It’s one of the rationales that undergirds Planned Parenthood v. Casey.”
Sen. Feinstein followed by noting reports quoting Judge Kavanaugh as saying Roe v. Wade was “settled law,” inquired what that term means, and asked for further clarification as to whether it was settled precedent and whether it could be overturned.
“Senator, I said that it’s settled as a precedent of the Supreme Court, entitled the respect of principles of stare decisis,” he responded, referencing the legal principle of determining points in litigation according to precedent. “One of the important things to keep in mind about Roe v. Wade is that it has been reaffirmed many times over the past 45 years, as you know, and most prominently, most importantly reaffirmed in Planned Parenthood v. Casey in 1992. And as you well recall, Senator, I know when that case came up, the Supreme Court didn’t just reaffirm it in passing, the court specifically went through all factors of stare decisis in considering whether to overrule it.”
He noted that the joint opinion of Justice Anthony Kennedy, Justice O’Connor, and Justice David Souter, went to “great length” to go through those factors.
Judge Kavanaugh called Planned Parenthood v. Casey a “precedent-on-precedent. It is not as if it’s just a run-of-the-mill case that was decided and never been reconsidered. Casey specifically reconsidered [Roe v. Wade], applied the stare decisis factors and decided to reaffirm it. That makes Casey precedent-on-precedent.”
That said, Judge Kavanaugh did not say outright that Row v. Wade would not be overturned because of these factors.
The first 2 days of Judge Kavanaugh’s confirmation hearing were frequently interrupted by protesters shouting objections to his nomination. At press time, more than 120 protesters had been arrested, according to the U.S. Capitol Police.
Health care did surface in questions about the Affordable Care Act and in specific the current legal action in Texas that is looking to kill the health care law, but Judge Kavanaugh declined to answer any questions surrounding it, stating that he did not want to prejudge any potential case that could come before him.
Much of the questioning he faced was more geared toward opinions on presidential powers, including whether presidents could avoid subpoenas and whether a president could pardon himself, but he declined to answer those questions because they are ones that could come before him given the current political environment.
Judge Kavanaugh painted himself as of independent thought, pushing back at those who are saying that he will serve to protect the president and act as his puppet on the bench, citing cases where he went against former President George W. Bush, for whom he served as White House staff secretary and who eventually was appointed him to the D.C. Circuit Court in May 2006.
Maryland gets an A on ‘Rheumatic Disease Report Card’
Maryland is alone at the top of the rheumatology care class, but the number of failing states is even smaller, according to the American College of Rheumatology.
Maryland was the only state to earn an A on the “Rheumatic Disease Report Card,” and while no state failed, two – Alabama and Oklahoma – did receive Ds. Among the 47 other states and the District of Columbia, there were 14 Bs and 34 Cs.
Maryland posted strong scores in all three of the report card’s broad categories of care: 38.25 out of 50 points (third among all states) for access, 35 out of 50 (tied for first with New York) for affordability, and 40 out of 50 (tied for ninth) for activity/lifestyle. Arkansas had the highest score (42.25) for access and Nebraska got 50 out of 50 for activity/lifestyle. Inferiority, however, turned out to be a lot more widespread, as eight states were tied for the low of 10 points in the access category, 26 states got a 0 for affordability, and six states earned 15 points for activity/lifestyle, the ACR said.
Arkansas’s high marks for access were based primarily on “state lawmakers’ recent efforts to address [pharmacy benefit manager] transparency by enacting legislation that should serve as a model for future action in other states looking to address this issue,” ACR officials said in a statement. Nebraska did well in both of the measures used in the activity/lifestyle category – age-adjusted prevalence of arthritis attributable activity limitations among adults and percent of adults who are physically inactive; it also did well because it is home to at least one YMCA-sponsored and one National Recreation and Park Association–sponsored arthritis intervention program funded in part by the Centers for Disease Control and Prevention.
as demand increases and supply decreases. The college’s projections show that almost 6,800 rheumatologists will be needed by 2020 but less than 4,500 will be available, and by 2030 the demand will rise to need for almost 8,200 rheumatologists, while supply is expected to drop below 3,500, according to the report.
“We are at a critical juncture in rheumatology care. The rheumatology workforce is not growing fast enough to keep up with demand and too many of our patients struggle to access and afford the breakthrough therapies they need to manage pain and avoid long-term disability,” ACR President David Daikh, MD, PhD wrote in the report.
Maryland is alone at the top of the rheumatology care class, but the number of failing states is even smaller, according to the American College of Rheumatology.

Maryland was the only state to earn an A on the “Rheumatic Disease Report Card,” and while no state failed, two – Alabama and Oklahoma – did receive Ds. Among the 47 other states and the District of Columbia, there were 14 Bs and 34 Cs.
Maryland posted strong scores in all three of the report card’s broad categories of care: 38.25 out of 50 points (third among all states) for access, 35 out of 50 (tied for first with New York) for affordability, and 40 out of 50 (tied for ninth) for activity/lifestyle. Arkansas had the highest score (42.25) for access and Nebraska got 50 out of 50 for activity/lifestyle. Inferiority, however, turned out to be a lot more widespread, as eight states were tied for the low of 10 points in the access category, 26 states got a 0 for affordability, and six states earned 15 points for activity/lifestyle, the ACR said.
Arkansas’s high marks for access were based primarily on “state lawmakers’ recent efforts to address [pharmacy benefit manager] transparency by enacting legislation that should serve as a model for future action in other states looking to address this issue,” ACR officials said in a statement. Nebraska did well in both of the measures used in the activity/lifestyle category – age-adjusted prevalence of arthritis attributable activity limitations among adults and percent of adults who are physically inactive; it also did well because it is home to at least one YMCA-sponsored and one National Recreation and Park Association–sponsored arthritis intervention program funded in part by the Centers for Disease Control and Prevention.
as demand increases and supply decreases. The college’s projections show that almost 6,800 rheumatologists will be needed by 2020 but less than 4,500 will be available, and by 2030 the demand will rise to need for almost 8,200 rheumatologists, while supply is expected to drop below 3,500, according to the report.
“We are at a critical juncture in rheumatology care. The rheumatology workforce is not growing fast enough to keep up with demand and too many of our patients struggle to access and afford the breakthrough therapies they need to manage pain and avoid long-term disability,” ACR President David Daikh, MD, PhD wrote in the report.
Maryland is alone at the top of the rheumatology care class, but the number of failing states is even smaller, according to the American College of Rheumatology.

Maryland was the only state to earn an A on the “Rheumatic Disease Report Card,” and while no state failed, two – Alabama and Oklahoma – did receive Ds. Among the 47 other states and the District of Columbia, there were 14 Bs and 34 Cs.
Maryland posted strong scores in all three of the report card’s broad categories of care: 38.25 out of 50 points (third among all states) for access, 35 out of 50 (tied for first with New York) for affordability, and 40 out of 50 (tied for ninth) for activity/lifestyle. Arkansas had the highest score (42.25) for access and Nebraska got 50 out of 50 for activity/lifestyle. Inferiority, however, turned out to be a lot more widespread, as eight states were tied for the low of 10 points in the access category, 26 states got a 0 for affordability, and six states earned 15 points for activity/lifestyle, the ACR said.
Arkansas’s high marks for access were based primarily on “state lawmakers’ recent efforts to address [pharmacy benefit manager] transparency by enacting legislation that should serve as a model for future action in other states looking to address this issue,” ACR officials said in a statement. Nebraska did well in both of the measures used in the activity/lifestyle category – age-adjusted prevalence of arthritis attributable activity limitations among adults and percent of adults who are physically inactive; it also did well because it is home to at least one YMCA-sponsored and one National Recreation and Park Association–sponsored arthritis intervention program funded in part by the Centers for Disease Control and Prevention.
as demand increases and supply decreases. The college’s projections show that almost 6,800 rheumatologists will be needed by 2020 but less than 4,500 will be available, and by 2030 the demand will rise to need for almost 8,200 rheumatologists, while supply is expected to drop below 3,500, according to the report.
“We are at a critical juncture in rheumatology care. The rheumatology workforce is not growing fast enough to keep up with demand and too many of our patients struggle to access and afford the breakthrough therapies they need to manage pain and avoid long-term disability,” ACR President David Daikh, MD, PhD wrote in the report.
Children born from ART at increased risk of developing arterial hypertension
Children born from assisted reproductive technologies such as in vitro fertilization and intracytoplasmic sperm injection may be at risk of developing arterial hypertension due to premature vascular aging, according to a study published in the Journal of the American College of Cardiology.
In a previous study, Emrush Rexhaj, MD, director of arterial hypertension and altitude medicine at Inselspital, University Hospital, Bern, Switzerland, and his colleagues assessed vascular function in participants who were born with assisted reproductive technology (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI); the investigators found vascular dysfunction in this patient population not “related to parental factors but to the ART procedure itself,” they said.
Dr. Rexhaj and his colleagues then reassessed vascular function in 54 participants (mean age 16.5 years old) who returned from the previous study 5 years after the initial assessment and compared the results with 43 matched patients in a control group (mean age, 17.4 years). There were no significant differences regarding body mass index, lipid, creatinine, electrolyte plasma concentrations, high-sensitive C-reactive protein, birth weight, and gestational age between children in either group, as well as no significant differences in maternal BMI, cardiovascular risk profile, and smoking status.
The investigators – with Théo A. Meister, MD, also of the university, as a joint lead author with Dr. Rexhaj – performed blinded endothelium-dependent and endothelium-independent vasodilation of the brachial artery in a supine position at room temperature and after 15 minutes of rest. They also measured carotid intima-media thickness (IMT), large artery stiffness, 24-hour ambulatory blood pressure monitoring, and short-term blood pressure variability.
“It only took five years for differences in arterial blood pressure to show,” Dr. Rexhaj stated in a press release. “This is a rapidly growing population and apparently healthy children are showing serious signs of concern for early cardiovascular risk, especially when it comes to arterial hypertension.”
Specifically, there was an approximately 25% reduction in flow-mediated dilation in the ART group (7%) compared with the control group (9%), which the investigators attributed to endothelial dysfunction (P less than .001). In ART patients, carotid IMT (463 mm) and carotid pulse-wave velocity (7.7 m/s) was significantly increased, compared with carotid IMT (435 mm; P less than .01) and pulse-wave velocity (7.2 m/s; P equals .033) in the control group.
With regard to arterial hypertension, 24-hour systolic blood pressure in the ART group (120 mm Hg) was “markedly” higher than in the control group (116 mm Hg; P equals .02); 24-hour diastolic blood pressure was also significantly higher in the ART group (71 mm Hg) compared with the control group (69 mm Hg; P equals .03). Investigators noted 8 of the 52 patients (15%) in the ART group met clinical criteria for arterial hypertension according to ambulatory blood pressure monitoring, compared with 1 of the 40 patients (2%) in the control group.
“The increased prevalence of arterial hypertension in ART participants is what is most concerning,” Dr. Rexhaj stated in the release. “There is growing evidence that ART alters the blood vessels in children, but the long-term consequences were not known. We now know that this places ART children at a six times higher rate of hypertension than children conceived naturally.”
The investigators cited as a limitation the fact that they studied only children born from singleton births recruited from a single center, which may have a lower cardiovascular risk profile than other patient populations.
This study was supported by the Swiss National Science Foundation, the Placide Nicod Foundation, the Swiss Society of Hypertension, the Swiss Society of Cardiology and Mach-Gaensslen Stiftung (Schweiz). The authors reported no conflicts of interest.
SOURCE: Meister TA et al. J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.06.060.
Clinicians should be vigilant in detecting early cardiovascular problems in children born from ART, Larry A. Weinrauch, MD, of Harvard Medical School, Boston, and his colleagues wrote in a joint editorial comment. While the sixfold higher risk of arterial hypertension was obtained from an ambulatory blood pressure monitoring that was not repeated, the relative risk of cardiovascular problems for singleton births could be a sign that a greater risk for vascular aging exists with multiple births.
“This observation, derived from a relatively small cohort, may actually understate the importance of this problem for ART populations because higher risk populations for development of hypertension (e.g., multiple birth pregnancies) and those resulting from maternal factors of excess risk (e.g., eclampsia, chronic hypertension, diabetes, obesity) were excluded from the study,” Dr. Weinrauch and his colleagues said.
The authors cited the pediatric hypertension clinical practice guidelines of annual in-office hypertension screening after 3 years of age and noted that certain high-risk groups, such as patients with repaired aortic coarctation and chronic kidney disease, should be screened “at every health encounter.
“If adolescent hypertension risk is really sixfold higher in ART patients (and potentially subsequent generations), consequences for longevity will be vast given the millions of patients whose births were achieved by using ART methods,” wrote Dr. Weinrauch and his colleagues. “Early study, detection, and treatment of ART-conceived individuals may be the appropriate ounce of prevention.”
Dr. Weinrauch is with Harvard Medical School, Marie D. Gerhard-Herman, MD, is with Brigham and Women’s Hospital, and Michael M. Mendelson, MD, is with Boston Children’s Hospital, all in Boston. These comments summarize their editorial in response to Meister et al. (J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.07.013). Dr. Gerhard-Herman is supported by the Progeria Research Foundation and Dr. Mendelson is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health. They reported no other relevant conflicts of interest.
Clinicians should be vigilant in detecting early cardiovascular problems in children born from ART, Larry A. Weinrauch, MD, of Harvard Medical School, Boston, and his colleagues wrote in a joint editorial comment. While the sixfold higher risk of arterial hypertension was obtained from an ambulatory blood pressure monitoring that was not repeated, the relative risk of cardiovascular problems for singleton births could be a sign that a greater risk for vascular aging exists with multiple births.
“This observation, derived from a relatively small cohort, may actually understate the importance of this problem for ART populations because higher risk populations for development of hypertension (e.g., multiple birth pregnancies) and those resulting from maternal factors of excess risk (e.g., eclampsia, chronic hypertension, diabetes, obesity) were excluded from the study,” Dr. Weinrauch and his colleagues said.
The authors cited the pediatric hypertension clinical practice guidelines of annual in-office hypertension screening after 3 years of age and noted that certain high-risk groups, such as patients with repaired aortic coarctation and chronic kidney disease, should be screened “at every health encounter.
“If adolescent hypertension risk is really sixfold higher in ART patients (and potentially subsequent generations), consequences for longevity will be vast given the millions of patients whose births were achieved by using ART methods,” wrote Dr. Weinrauch and his colleagues. “Early study, detection, and treatment of ART-conceived individuals may be the appropriate ounce of prevention.”
Dr. Weinrauch is with Harvard Medical School, Marie D. Gerhard-Herman, MD, is with Brigham and Women’s Hospital, and Michael M. Mendelson, MD, is with Boston Children’s Hospital, all in Boston. These comments summarize their editorial in response to Meister et al. (J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.07.013). Dr. Gerhard-Herman is supported by the Progeria Research Foundation and Dr. Mendelson is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health. They reported no other relevant conflicts of interest.
Clinicians should be vigilant in detecting early cardiovascular problems in children born from ART, Larry A. Weinrauch, MD, of Harvard Medical School, Boston, and his colleagues wrote in a joint editorial comment. While the sixfold higher risk of arterial hypertension was obtained from an ambulatory blood pressure monitoring that was not repeated, the relative risk of cardiovascular problems for singleton births could be a sign that a greater risk for vascular aging exists with multiple births.
“This observation, derived from a relatively small cohort, may actually understate the importance of this problem for ART populations because higher risk populations for development of hypertension (e.g., multiple birth pregnancies) and those resulting from maternal factors of excess risk (e.g., eclampsia, chronic hypertension, diabetes, obesity) were excluded from the study,” Dr. Weinrauch and his colleagues said.
The authors cited the pediatric hypertension clinical practice guidelines of annual in-office hypertension screening after 3 years of age and noted that certain high-risk groups, such as patients with repaired aortic coarctation and chronic kidney disease, should be screened “at every health encounter.
“If adolescent hypertension risk is really sixfold higher in ART patients (and potentially subsequent generations), consequences for longevity will be vast given the millions of patients whose births were achieved by using ART methods,” wrote Dr. Weinrauch and his colleagues. “Early study, detection, and treatment of ART-conceived individuals may be the appropriate ounce of prevention.”
Dr. Weinrauch is with Harvard Medical School, Marie D. Gerhard-Herman, MD, is with Brigham and Women’s Hospital, and Michael M. Mendelson, MD, is with Boston Children’s Hospital, all in Boston. These comments summarize their editorial in response to Meister et al. (J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.07.013). Dr. Gerhard-Herman is supported by the Progeria Research Foundation and Dr. Mendelson is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health. They reported no other relevant conflicts of interest.
Children born from assisted reproductive technologies such as in vitro fertilization and intracytoplasmic sperm injection may be at risk of developing arterial hypertension due to premature vascular aging, according to a study published in the Journal of the American College of Cardiology.
In a previous study, Emrush Rexhaj, MD, director of arterial hypertension and altitude medicine at Inselspital, University Hospital, Bern, Switzerland, and his colleagues assessed vascular function in participants who were born with assisted reproductive technology (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI); the investigators found vascular dysfunction in this patient population not “related to parental factors but to the ART procedure itself,” they said.
Dr. Rexhaj and his colleagues then reassessed vascular function in 54 participants (mean age 16.5 years old) who returned from the previous study 5 years after the initial assessment and compared the results with 43 matched patients in a control group (mean age, 17.4 years). There were no significant differences regarding body mass index, lipid, creatinine, electrolyte plasma concentrations, high-sensitive C-reactive protein, birth weight, and gestational age between children in either group, as well as no significant differences in maternal BMI, cardiovascular risk profile, and smoking status.
The investigators – with Théo A. Meister, MD, also of the university, as a joint lead author with Dr. Rexhaj – performed blinded endothelium-dependent and endothelium-independent vasodilation of the brachial artery in a supine position at room temperature and after 15 minutes of rest. They also measured carotid intima-media thickness (IMT), large artery stiffness, 24-hour ambulatory blood pressure monitoring, and short-term blood pressure variability.
“It only took five years for differences in arterial blood pressure to show,” Dr. Rexhaj stated in a press release. “This is a rapidly growing population and apparently healthy children are showing serious signs of concern for early cardiovascular risk, especially when it comes to arterial hypertension.”
Specifically, there was an approximately 25% reduction in flow-mediated dilation in the ART group (7%) compared with the control group (9%), which the investigators attributed to endothelial dysfunction (P less than .001). In ART patients, carotid IMT (463 mm) and carotid pulse-wave velocity (7.7 m/s) was significantly increased, compared with carotid IMT (435 mm; P less than .01) and pulse-wave velocity (7.2 m/s; P equals .033) in the control group.
With regard to arterial hypertension, 24-hour systolic blood pressure in the ART group (120 mm Hg) was “markedly” higher than in the control group (116 mm Hg; P equals .02); 24-hour diastolic blood pressure was also significantly higher in the ART group (71 mm Hg) compared with the control group (69 mm Hg; P equals .03). Investigators noted 8 of the 52 patients (15%) in the ART group met clinical criteria for arterial hypertension according to ambulatory blood pressure monitoring, compared with 1 of the 40 patients (2%) in the control group.
“The increased prevalence of arterial hypertension in ART participants is what is most concerning,” Dr. Rexhaj stated in the release. “There is growing evidence that ART alters the blood vessels in children, but the long-term consequences were not known. We now know that this places ART children at a six times higher rate of hypertension than children conceived naturally.”
The investigators cited as a limitation the fact that they studied only children born from singleton births recruited from a single center, which may have a lower cardiovascular risk profile than other patient populations.
This study was supported by the Swiss National Science Foundation, the Placide Nicod Foundation, the Swiss Society of Hypertension, the Swiss Society of Cardiology and Mach-Gaensslen Stiftung (Schweiz). The authors reported no conflicts of interest.
SOURCE: Meister TA et al. J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.06.060.
Children born from assisted reproductive technologies such as in vitro fertilization and intracytoplasmic sperm injection may be at risk of developing arterial hypertension due to premature vascular aging, according to a study published in the Journal of the American College of Cardiology.
In a previous study, Emrush Rexhaj, MD, director of arterial hypertension and altitude medicine at Inselspital, University Hospital, Bern, Switzerland, and his colleagues assessed vascular function in participants who were born with assisted reproductive technology (ART) such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI); the investigators found vascular dysfunction in this patient population not “related to parental factors but to the ART procedure itself,” they said.
Dr. Rexhaj and his colleagues then reassessed vascular function in 54 participants (mean age 16.5 years old) who returned from the previous study 5 years after the initial assessment and compared the results with 43 matched patients in a control group (mean age, 17.4 years). There were no significant differences regarding body mass index, lipid, creatinine, electrolyte plasma concentrations, high-sensitive C-reactive protein, birth weight, and gestational age between children in either group, as well as no significant differences in maternal BMI, cardiovascular risk profile, and smoking status.
The investigators – with Théo A. Meister, MD, also of the university, as a joint lead author with Dr. Rexhaj – performed blinded endothelium-dependent and endothelium-independent vasodilation of the brachial artery in a supine position at room temperature and after 15 minutes of rest. They also measured carotid intima-media thickness (IMT), large artery stiffness, 24-hour ambulatory blood pressure monitoring, and short-term blood pressure variability.
“It only took five years for differences in arterial blood pressure to show,” Dr. Rexhaj stated in a press release. “This is a rapidly growing population and apparently healthy children are showing serious signs of concern for early cardiovascular risk, especially when it comes to arterial hypertension.”
Specifically, there was an approximately 25% reduction in flow-mediated dilation in the ART group (7%) compared with the control group (9%), which the investigators attributed to endothelial dysfunction (P less than .001). In ART patients, carotid IMT (463 mm) and carotid pulse-wave velocity (7.7 m/s) was significantly increased, compared with carotid IMT (435 mm; P less than .01) and pulse-wave velocity (7.2 m/s; P equals .033) in the control group.
With regard to arterial hypertension, 24-hour systolic blood pressure in the ART group (120 mm Hg) was “markedly” higher than in the control group (116 mm Hg; P equals .02); 24-hour diastolic blood pressure was also significantly higher in the ART group (71 mm Hg) compared with the control group (69 mm Hg; P equals .03). Investigators noted 8 of the 52 patients (15%) in the ART group met clinical criteria for arterial hypertension according to ambulatory blood pressure monitoring, compared with 1 of the 40 patients (2%) in the control group.
“The increased prevalence of arterial hypertension in ART participants is what is most concerning,” Dr. Rexhaj stated in the release. “There is growing evidence that ART alters the blood vessels in children, but the long-term consequences were not known. We now know that this places ART children at a six times higher rate of hypertension than children conceived naturally.”
The investigators cited as a limitation the fact that they studied only children born from singleton births recruited from a single center, which may have a lower cardiovascular risk profile than other patient populations.
This study was supported by the Swiss National Science Foundation, the Placide Nicod Foundation, the Swiss Society of Hypertension, the Swiss Society of Cardiology and Mach-Gaensslen Stiftung (Schweiz). The authors reported no conflicts of interest.
SOURCE: Meister TA et al. J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.06.060.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: In the ART group, 24-hour systolic blood pressure was significantly higher than in the control group (120 mm Hg vs. 116 mm Hg), and 24-hour diastolic blood pressure was also significantly higher in the ART group compared with the control group (71 mm Hg vs. 69 mm Hg).
Study details: A reassessment of 54 children born from ART.
Disclosures: This study was supported by the Swiss National Science Foundation, the Placide Nicod Foundation, the Swiss Society of Hypertension, the Swiss Society of Cardiology and Mach-Gaensslen Stiftung (Schweiz). The authors reported no conflicts of interest.
Source: Meister TA et al. J Am Coll Cardiol. 2018 Sep 3. doi: 10.1016/j.jacc.2018.06.060.