User login
Hospital medicine leaders offer tips for gender equity
When Marisha Burden, MD, division head of hospital medicine at the University of Colorado at Denver, Aurora, would go to medical conferences, it seemed as if very few women were giving talks. She wondered if she could be wrong.
“I started doing my own assessments at every conference I would go to, just to make sure I wasn’t biased in my own belief system,” she said in a session at SHM Converge 2021, the annual conference of the Society of Hospital Medicine.
She wasn’t wrong.
In 2015, only 35% of all speakers at the SHM annual conference were women, and only 23% of the plenary speakers were women. In the years after that, when the society put out open calls for speakers, the numbers of women who spoke increased substantially, to 47% overall and 45% of plenary speakers.
The results – part of the SPEAK UP study Dr. Burden led in 2020 – show how gender disparity can be improved with a systematic process that is designed to improve it. The results of the study also showed that as the percentages of female speakers increased, the attendee ratings of the sessions did, too.
“You can do these things, and the quality of your conference doesn’t get negatively impacted – and in this case, actually improved,” Dr. Burden said.
That study marked progress toward leveling a traditionally uneven playing field when it comes to men and women in medicine, and the panelists in the session called on the field to use a variety of tools and strategies to continue toward something closer to equality.
Sara Spilseth, MD, MBA, chief of staff at Regions Hospital, in St. Paul, Minn., said it’s well established that although almost 50% of medical school students are women, the percentage shrinks each step from faculty to full professor to dean – of which only 16% are women. She referred to what’s known as the “leaky pipe.”
In what Dr. Spilseth said was one of her favorite studies, researchers in 2015 found that only 13% of clinical department leaders at the top 50 U.S. medical schools were women – they were outnumbered by the percentage of department leaders with mustaches, at 19%, even though mustaches are dwindling in popularity.
“Why does this exist? Why did we end up like this?” Part of the problem is a “respect gap,” she said, pointing to a study on the tendency of women to use the formal title of “doctor” when introducing male colleagues, whereas men who introduce women use that title less than half the time.
The COVID-19 pandemic has only made these disparities worse. Women are responsible for childcare much more frequently than men, Dr. Burden said, although the pandemic has brought caregiving duties to the forefront.
Dr. Spilseth said mentoring can help women navigate the workplace so as to help overcome these disparities. At Regions, the mentoring program is robust.
“Even before a new hire steps foot in the hospital, we have established them with a mentor,” she said. Sponsoring – the “ability of someone with political capital to use it to help colleagues” – can also help boost women’s careers, she said.
Her hospital also has a Women in Medicine Cooperative, which provides a way for women to talk about common struggles and to network.
Flexible work opportunities – working in transitional care units, being a physician advisor, and doing research – can all help boost a career as well, Dr. Spilseth said.
She said that at the University of Colorado, leaders set out to reach salary equity in a year and a half – and “it was a painful, painful process.” They found that different people held different beliefs about how people were paid, which led to a lot of unnecessary stress as they tried to construct a fairer system.
“On the back end of having done that, while it was a rough year and half, it has saved so much time – and I think built a culture of trust and transparency,” she said.
Recruiting in a more thoughtful way can also have a big impact, Dr. Spilseth said. The manner in which people are told about opportunities could exclude people without intending to.
“Are you casting a wide net?” she asked.
Adia Ross, MD, MHA, chief medical officer at Duke Regional Hospital, Durham, N.C., said that even in the face of obvious disparities, women can take steps on their own to boost their careers. She encouraged taking on “stretch assignments,” a project or task that is a bit beyond one’s current comfort level or level of experience or knowledge. “It can be a little scary, and sometimes there are bumps along the way,” she said.
All of these measures, though incremental, are the way to make bigger change, she said. “We want to take small steps but big strides forward.”
A version of this article first appeared on Medscape.com.
When Marisha Burden, MD, division head of hospital medicine at the University of Colorado at Denver, Aurora, would go to medical conferences, it seemed as if very few women were giving talks. She wondered if she could be wrong.
“I started doing my own assessments at every conference I would go to, just to make sure I wasn’t biased in my own belief system,” she said in a session at SHM Converge 2021, the annual conference of the Society of Hospital Medicine.
She wasn’t wrong.
In 2015, only 35% of all speakers at the SHM annual conference were women, and only 23% of the plenary speakers were women. In the years after that, when the society put out open calls for speakers, the numbers of women who spoke increased substantially, to 47% overall and 45% of plenary speakers.
The results – part of the SPEAK UP study Dr. Burden led in 2020 – show how gender disparity can be improved with a systematic process that is designed to improve it. The results of the study also showed that as the percentages of female speakers increased, the attendee ratings of the sessions did, too.
“You can do these things, and the quality of your conference doesn’t get negatively impacted – and in this case, actually improved,” Dr. Burden said.
That study marked progress toward leveling a traditionally uneven playing field when it comes to men and women in medicine, and the panelists in the session called on the field to use a variety of tools and strategies to continue toward something closer to equality.
Sara Spilseth, MD, MBA, chief of staff at Regions Hospital, in St. Paul, Minn., said it’s well established that although almost 50% of medical school students are women, the percentage shrinks each step from faculty to full professor to dean – of which only 16% are women. She referred to what’s known as the “leaky pipe.”
In what Dr. Spilseth said was one of her favorite studies, researchers in 2015 found that only 13% of clinical department leaders at the top 50 U.S. medical schools were women – they were outnumbered by the percentage of department leaders with mustaches, at 19%, even though mustaches are dwindling in popularity.
“Why does this exist? Why did we end up like this?” Part of the problem is a “respect gap,” she said, pointing to a study on the tendency of women to use the formal title of “doctor” when introducing male colleagues, whereas men who introduce women use that title less than half the time.
The COVID-19 pandemic has only made these disparities worse. Women are responsible for childcare much more frequently than men, Dr. Burden said, although the pandemic has brought caregiving duties to the forefront.
Dr. Spilseth said mentoring can help women navigate the workplace so as to help overcome these disparities. At Regions, the mentoring program is robust.
“Even before a new hire steps foot in the hospital, we have established them with a mentor,” she said. Sponsoring – the “ability of someone with political capital to use it to help colleagues” – can also help boost women’s careers, she said.
Her hospital also has a Women in Medicine Cooperative, which provides a way for women to talk about common struggles and to network.
Flexible work opportunities – working in transitional care units, being a physician advisor, and doing research – can all help boost a career as well, Dr. Spilseth said.
She said that at the University of Colorado, leaders set out to reach salary equity in a year and a half – and “it was a painful, painful process.” They found that different people held different beliefs about how people were paid, which led to a lot of unnecessary stress as they tried to construct a fairer system.
“On the back end of having done that, while it was a rough year and half, it has saved so much time – and I think built a culture of trust and transparency,” she said.
Recruiting in a more thoughtful way can also have a big impact, Dr. Spilseth said. The manner in which people are told about opportunities could exclude people without intending to.
“Are you casting a wide net?” she asked.
Adia Ross, MD, MHA, chief medical officer at Duke Regional Hospital, Durham, N.C., said that even in the face of obvious disparities, women can take steps on their own to boost their careers. She encouraged taking on “stretch assignments,” a project or task that is a bit beyond one’s current comfort level or level of experience or knowledge. “It can be a little scary, and sometimes there are bumps along the way,” she said.
All of these measures, though incremental, are the way to make bigger change, she said. “We want to take small steps but big strides forward.”
A version of this article first appeared on Medscape.com.
When Marisha Burden, MD, division head of hospital medicine at the University of Colorado at Denver, Aurora, would go to medical conferences, it seemed as if very few women were giving talks. She wondered if she could be wrong.
“I started doing my own assessments at every conference I would go to, just to make sure I wasn’t biased in my own belief system,” she said in a session at SHM Converge 2021, the annual conference of the Society of Hospital Medicine.
She wasn’t wrong.
In 2015, only 35% of all speakers at the SHM annual conference were women, and only 23% of the plenary speakers were women. In the years after that, when the society put out open calls for speakers, the numbers of women who spoke increased substantially, to 47% overall and 45% of plenary speakers.
The results – part of the SPEAK UP study Dr. Burden led in 2020 – show how gender disparity can be improved with a systematic process that is designed to improve it. The results of the study also showed that as the percentages of female speakers increased, the attendee ratings of the sessions did, too.
“You can do these things, and the quality of your conference doesn’t get negatively impacted – and in this case, actually improved,” Dr. Burden said.
That study marked progress toward leveling a traditionally uneven playing field when it comes to men and women in medicine, and the panelists in the session called on the field to use a variety of tools and strategies to continue toward something closer to equality.
Sara Spilseth, MD, MBA, chief of staff at Regions Hospital, in St. Paul, Minn., said it’s well established that although almost 50% of medical school students are women, the percentage shrinks each step from faculty to full professor to dean – of which only 16% are women. She referred to what’s known as the “leaky pipe.”
In what Dr. Spilseth said was one of her favorite studies, researchers in 2015 found that only 13% of clinical department leaders at the top 50 U.S. medical schools were women – they were outnumbered by the percentage of department leaders with mustaches, at 19%, even though mustaches are dwindling in popularity.
“Why does this exist? Why did we end up like this?” Part of the problem is a “respect gap,” she said, pointing to a study on the tendency of women to use the formal title of “doctor” when introducing male colleagues, whereas men who introduce women use that title less than half the time.
The COVID-19 pandemic has only made these disparities worse. Women are responsible for childcare much more frequently than men, Dr. Burden said, although the pandemic has brought caregiving duties to the forefront.
Dr. Spilseth said mentoring can help women navigate the workplace so as to help overcome these disparities. At Regions, the mentoring program is robust.
“Even before a new hire steps foot in the hospital, we have established them with a mentor,” she said. Sponsoring – the “ability of someone with political capital to use it to help colleagues” – can also help boost women’s careers, she said.
Her hospital also has a Women in Medicine Cooperative, which provides a way for women to talk about common struggles and to network.
Flexible work opportunities – working in transitional care units, being a physician advisor, and doing research – can all help boost a career as well, Dr. Spilseth said.
She said that at the University of Colorado, leaders set out to reach salary equity in a year and a half – and “it was a painful, painful process.” They found that different people held different beliefs about how people were paid, which led to a lot of unnecessary stress as they tried to construct a fairer system.
“On the back end of having done that, while it was a rough year and half, it has saved so much time – and I think built a culture of trust and transparency,” she said.
Recruiting in a more thoughtful way can also have a big impact, Dr. Spilseth said. The manner in which people are told about opportunities could exclude people without intending to.
“Are you casting a wide net?” she asked.
Adia Ross, MD, MHA, chief medical officer at Duke Regional Hospital, Durham, N.C., said that even in the face of obvious disparities, women can take steps on their own to boost their careers. She encouraged taking on “stretch assignments,” a project or task that is a bit beyond one’s current comfort level or level of experience or knowledge. “It can be a little scary, and sometimes there are bumps along the way,” she said.
All of these measures, though incremental, are the way to make bigger change, she said. “We want to take small steps but big strides forward.”
A version of this article first appeared on Medscape.com.
FROM SHM CONVERGE 2021
FDA approves secukinumab in psoriasis patients age six and older
The who are candidates for systemic therapy or phototherapy. The expanded indication marks the first time the drug has been available for a pediatric population in the United States.
Children with plaque psoriasis are often undertreated because of fear of side effects of therapies, according to Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco. “Now, more and more medicines are being tested for safety and efficacy in children, and we no longer have to rely on adult studies to inform treatment choices for children,” Dr. Cordoro told this news organization.
The FDA approval of secukinumab for children aged 6 and older with moderate to severe psoriasis “is a welcome addition to the therapeutic toolbox for pediatric psoriasis,” she said. “We’ve entered an era where severe pediatric psoriasis has become a condition that can be adequately controlled with minimal risk and with the convenience of intermittent injections. This has changed the playing field for these children and their families completely. Given the potential short- and long-term negative impact of chronic inflammation on the body of a growing child, we now have approved treatments that can safely offset the risks of undertreated severe psoriasis on the functional and psychological health of the child.”
The approved pediatric dosing for secukinumab is 75 mg or 150 mg depending on the child’s weight at the time of dosing, and it is administered by subcutaneous injection every 4 weeks after an initial loading regimen. According to a press release from Novartis, the FDA approval came on the heels of two phase 3 studies that evaluated the use of secukinumab in children aged 6 to younger than 18 years with plaque psoriasis. The first was a 52-week, randomized, double-blind, placebo- and active-controlled study which included 162 children 6 years of age and older with severe plaque psoriasis. The doses evaluated were 75 mg for children who weighed less than 50 kg and 150 mg for those 50 kg or greater.
At week 12, the Psoriasis Area Severity Index (PASI)-75 response was 55% among children in the 75-mg dosing group vs. 10% in the placebo group and 86% in the 150-mg dosing group vs. 19% in the placebo group.
Meanwhile, the Investigator’s Global Assessment modified 2011 (IGA) “clear” response was achieved in 32% of children in the 75-mg dosing group vs. 5% in the placebo group and in 81% of children in the 150-mg dosing group vs. 5% in the placebo group. An IGA “almost clear” skin response was achieved in 81% of children in the 75-mg dosing group vs. 5% in the placebo group.
The second phase 3 study was a randomized open-label, 208-week trial of 84 subjects 6 years of age and older with moderate to severe plaque psoriasis. According to the Novartis press release, the safety profile reported in both trials was consistent with the safety profile reported in adult plaque psoriasis trials and no new safety signals were observed. The updated prescribing information for secukinumab can be found here.
“When considering treatment with a systemic agent such as a biologic, it is important to consider objective measures of severity, such as extent of disease and involvement of joints but also subjective indicators of severity such as impact beyond the skin on psychological well-being,” Dr. Cordoro said in the interview. “Kids with psoriasis in visible locations may socially isolate themselves due to embarrassment or bullying. Therefore, the impact of moderate to severe psoriasis not only on overall health but on self-esteem and identity formation can be significant, and therefore adequately treating children of all ages to prevent the downstream negative consequences of childhood psoriasis is critical.”
Dr. Cordoro reported having no financial disclosures.
The who are candidates for systemic therapy or phototherapy. The expanded indication marks the first time the drug has been available for a pediatric population in the United States.
Children with plaque psoriasis are often undertreated because of fear of side effects of therapies, according to Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco. “Now, more and more medicines are being tested for safety and efficacy in children, and we no longer have to rely on adult studies to inform treatment choices for children,” Dr. Cordoro told this news organization.
The FDA approval of secukinumab for children aged 6 and older with moderate to severe psoriasis “is a welcome addition to the therapeutic toolbox for pediatric psoriasis,” she said. “We’ve entered an era where severe pediatric psoriasis has become a condition that can be adequately controlled with minimal risk and with the convenience of intermittent injections. This has changed the playing field for these children and their families completely. Given the potential short- and long-term negative impact of chronic inflammation on the body of a growing child, we now have approved treatments that can safely offset the risks of undertreated severe psoriasis on the functional and psychological health of the child.”
The approved pediatric dosing for secukinumab is 75 mg or 150 mg depending on the child’s weight at the time of dosing, and it is administered by subcutaneous injection every 4 weeks after an initial loading regimen. According to a press release from Novartis, the FDA approval came on the heels of two phase 3 studies that evaluated the use of secukinumab in children aged 6 to younger than 18 years with plaque psoriasis. The first was a 52-week, randomized, double-blind, placebo- and active-controlled study which included 162 children 6 years of age and older with severe plaque psoriasis. The doses evaluated were 75 mg for children who weighed less than 50 kg and 150 mg for those 50 kg or greater.
At week 12, the Psoriasis Area Severity Index (PASI)-75 response was 55% among children in the 75-mg dosing group vs. 10% in the placebo group and 86% in the 150-mg dosing group vs. 19% in the placebo group.
Meanwhile, the Investigator’s Global Assessment modified 2011 (IGA) “clear” response was achieved in 32% of children in the 75-mg dosing group vs. 5% in the placebo group and in 81% of children in the 150-mg dosing group vs. 5% in the placebo group. An IGA “almost clear” skin response was achieved in 81% of children in the 75-mg dosing group vs. 5% in the placebo group.
The second phase 3 study was a randomized open-label, 208-week trial of 84 subjects 6 years of age and older with moderate to severe plaque psoriasis. According to the Novartis press release, the safety profile reported in both trials was consistent with the safety profile reported in adult plaque psoriasis trials and no new safety signals were observed. The updated prescribing information for secukinumab can be found here.
“When considering treatment with a systemic agent such as a biologic, it is important to consider objective measures of severity, such as extent of disease and involvement of joints but also subjective indicators of severity such as impact beyond the skin on psychological well-being,” Dr. Cordoro said in the interview. “Kids with psoriasis in visible locations may socially isolate themselves due to embarrassment or bullying. Therefore, the impact of moderate to severe psoriasis not only on overall health but on self-esteem and identity formation can be significant, and therefore adequately treating children of all ages to prevent the downstream negative consequences of childhood psoriasis is critical.”
Dr. Cordoro reported having no financial disclosures.
The who are candidates for systemic therapy or phototherapy. The expanded indication marks the first time the drug has been available for a pediatric population in the United States.
Children with plaque psoriasis are often undertreated because of fear of side effects of therapies, according to Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco. “Now, more and more medicines are being tested for safety and efficacy in children, and we no longer have to rely on adult studies to inform treatment choices for children,” Dr. Cordoro told this news organization.
The FDA approval of secukinumab for children aged 6 and older with moderate to severe psoriasis “is a welcome addition to the therapeutic toolbox for pediatric psoriasis,” she said. “We’ve entered an era where severe pediatric psoriasis has become a condition that can be adequately controlled with minimal risk and with the convenience of intermittent injections. This has changed the playing field for these children and their families completely. Given the potential short- and long-term negative impact of chronic inflammation on the body of a growing child, we now have approved treatments that can safely offset the risks of undertreated severe psoriasis on the functional and psychological health of the child.”
The approved pediatric dosing for secukinumab is 75 mg or 150 mg depending on the child’s weight at the time of dosing, and it is administered by subcutaneous injection every 4 weeks after an initial loading regimen. According to a press release from Novartis, the FDA approval came on the heels of two phase 3 studies that evaluated the use of secukinumab in children aged 6 to younger than 18 years with plaque psoriasis. The first was a 52-week, randomized, double-blind, placebo- and active-controlled study which included 162 children 6 years of age and older with severe plaque psoriasis. The doses evaluated were 75 mg for children who weighed less than 50 kg and 150 mg for those 50 kg or greater.
At week 12, the Psoriasis Area Severity Index (PASI)-75 response was 55% among children in the 75-mg dosing group vs. 10% in the placebo group and 86% in the 150-mg dosing group vs. 19% in the placebo group.
Meanwhile, the Investigator’s Global Assessment modified 2011 (IGA) “clear” response was achieved in 32% of children in the 75-mg dosing group vs. 5% in the placebo group and in 81% of children in the 150-mg dosing group vs. 5% in the placebo group. An IGA “almost clear” skin response was achieved in 81% of children in the 75-mg dosing group vs. 5% in the placebo group.
The second phase 3 study was a randomized open-label, 208-week trial of 84 subjects 6 years of age and older with moderate to severe plaque psoriasis. According to the Novartis press release, the safety profile reported in both trials was consistent with the safety profile reported in adult plaque psoriasis trials and no new safety signals were observed. The updated prescribing information for secukinumab can be found here.
“When considering treatment with a systemic agent such as a biologic, it is important to consider objective measures of severity, such as extent of disease and involvement of joints but also subjective indicators of severity such as impact beyond the skin on psychological well-being,” Dr. Cordoro said in the interview. “Kids with psoriasis in visible locations may socially isolate themselves due to embarrassment or bullying. Therefore, the impact of moderate to severe psoriasis not only on overall health but on self-esteem and identity formation can be significant, and therefore adequately treating children of all ages to prevent the downstream negative consequences of childhood psoriasis is critical.”
Dr. Cordoro reported having no financial disclosures.
Phacomatosis Pigmentokeratotica Associated With Raynaud Phenomenon, Segmental Nevi, Hyperhidrosis, and Scoliosis
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
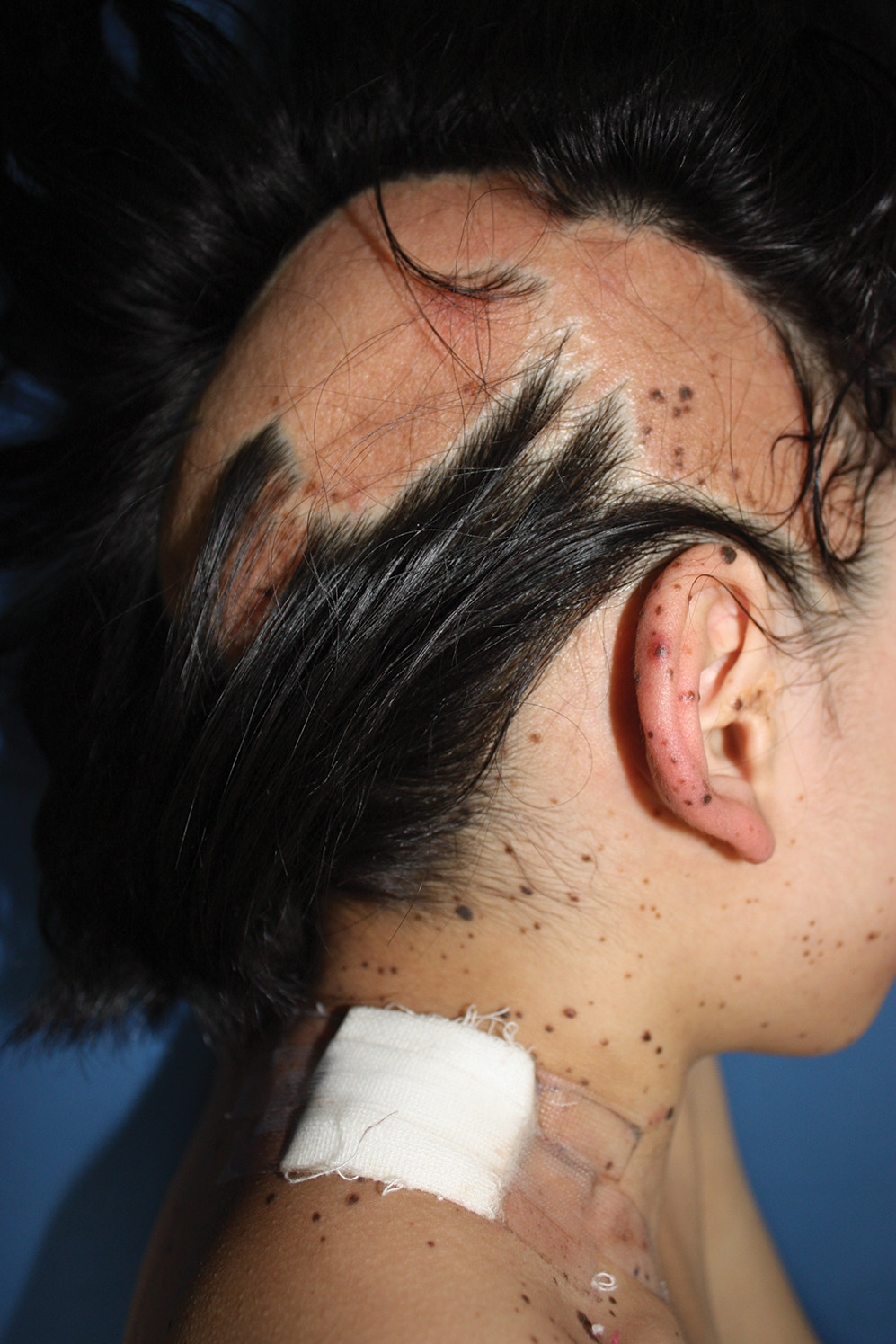
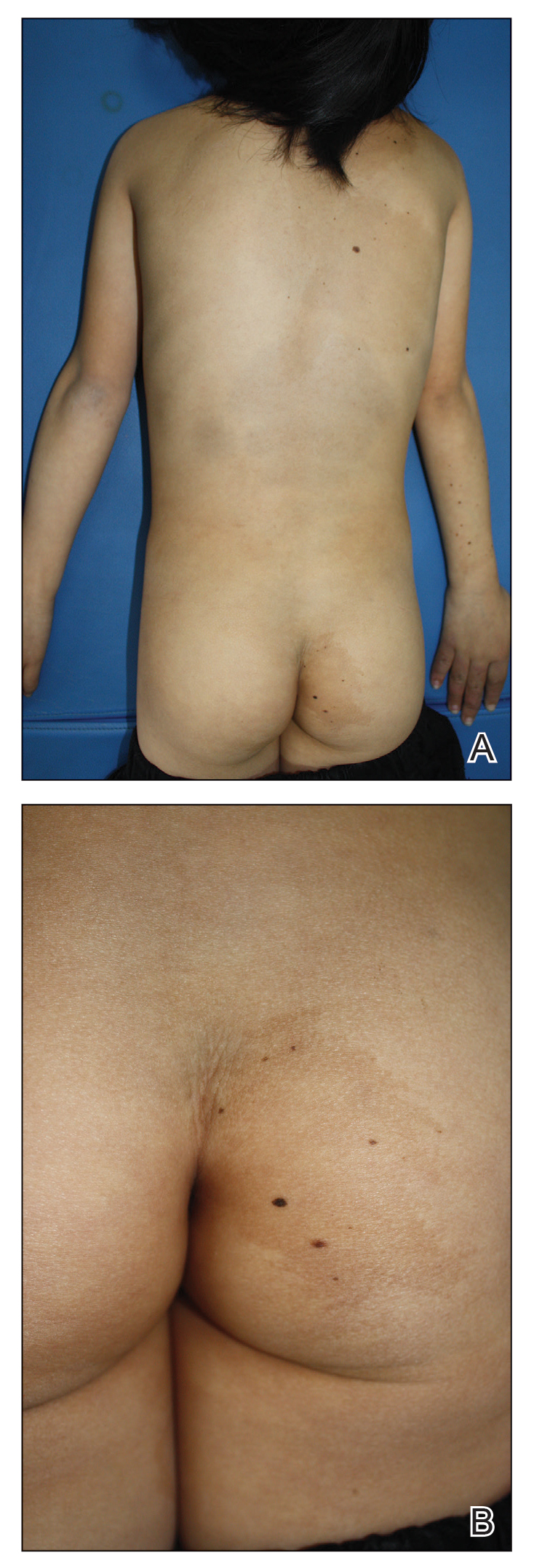
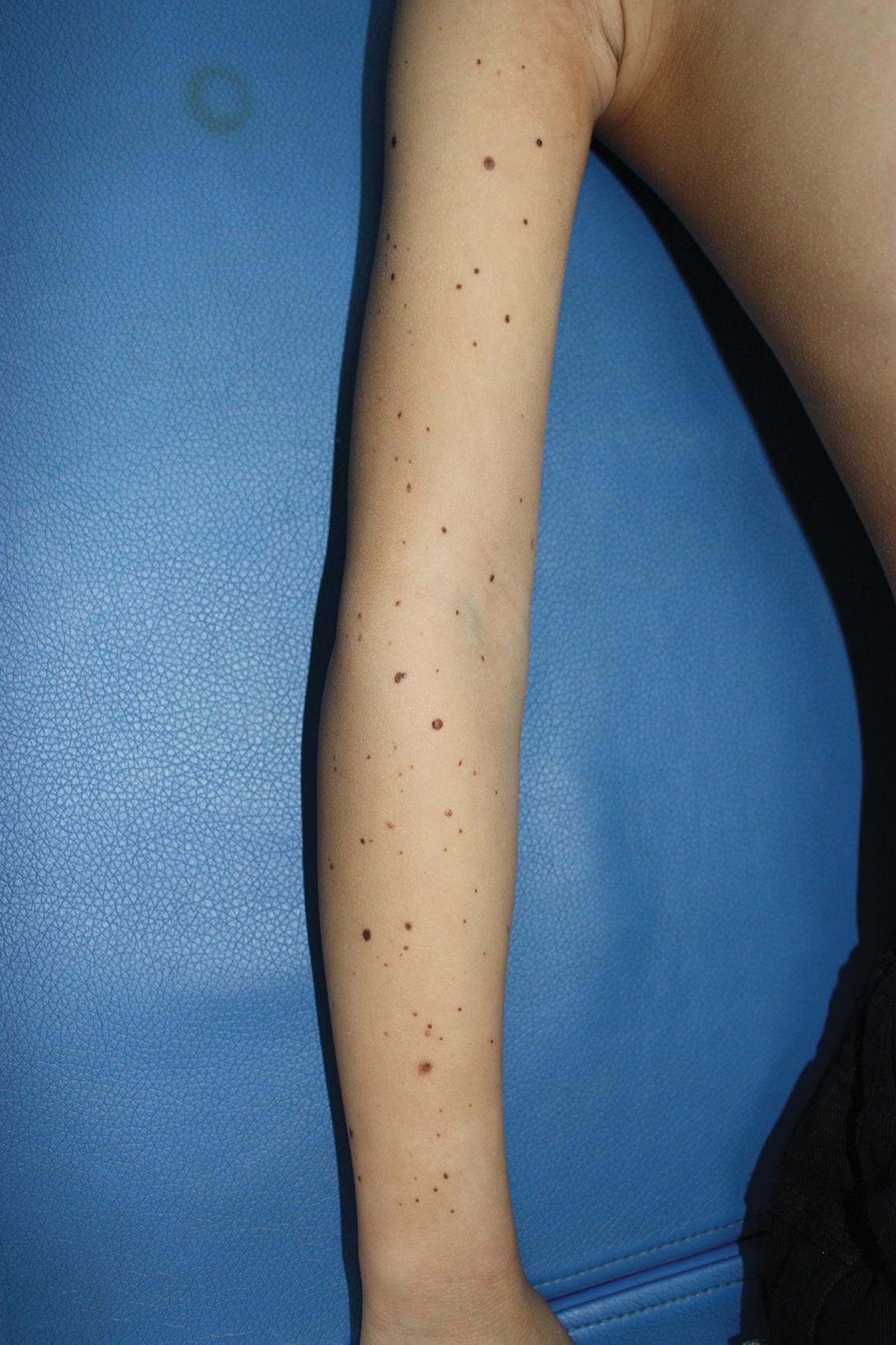
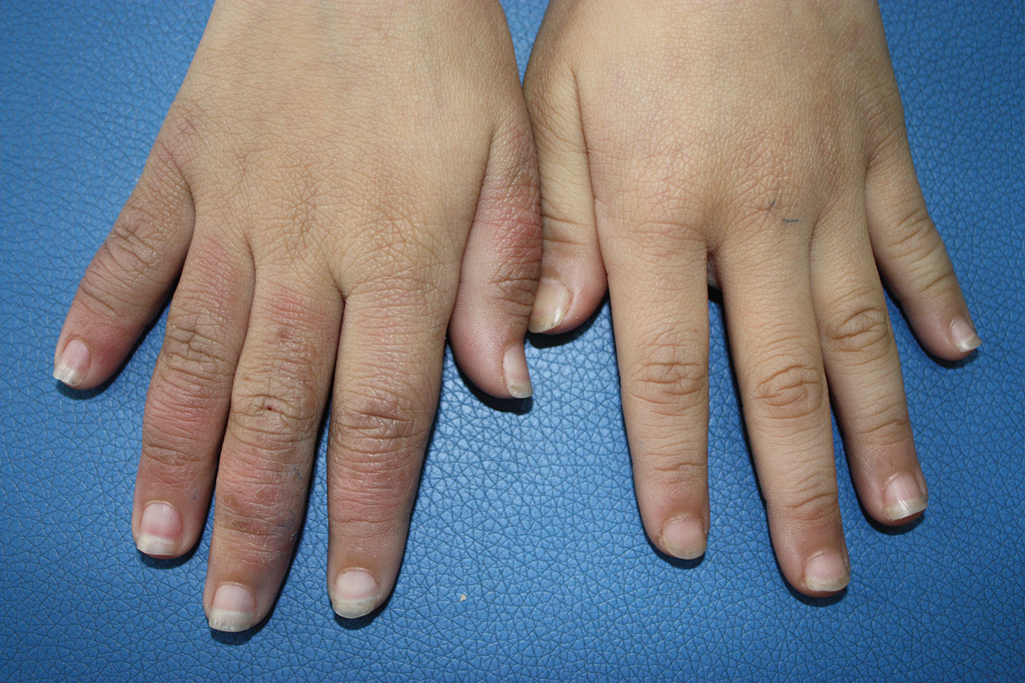
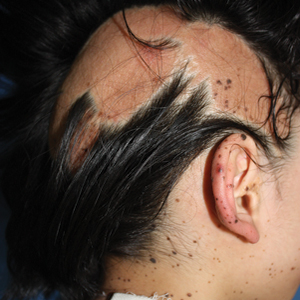
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
Practice Points
- Phacomatosis pigmentokeratotica (PPK) is characterized by the coexistence of speckled lentiginous nevus and nevus sebaceous.
- Raynaud phenomenon may be an unreported association with PPK.
FDA approves diagnostic device for autism spectrum disorder
The Food and Drug Administration has approved marketing for a device that will help diagnose autism spectrum disorder (ASD) in children between the ages of 18 months and 5 years old who exhibit potential symptoms.
Cognoa ASD Diagnosis Aid is a machine learning–based software program that receives information from parents or caregivers, video analysts, and health care providers to assist physicians in evaluating whether a child is at risk of having autism.
Autism is a developmental disorder that can cause social, communication, and behavioral challenges, according to the Centers for Disease Control and Prevention. The disorder affects about 1 in 54 children. The disorder is difficult to diagnose because there isn’t a medical test to diagnose the it. Instead, physicians have to look at a child’s developmental history and behavior to make a diagnosis.
Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the FDA.
“[ASD] can delay a child’s physical, cognitive, and social development, including motor skill development, learning, communication, and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s marketing authorization provides a new tool for helping diagnose children with ASD.”
The safety and efficacy of the Cognoa ASD Diagnosis Aid was assessed in a study of 425 patients between the ages of 18 months and 5 years old. For the study, researchers compared the diagnostic assessments made by the device to those made by a panel of clinical experts who used the current standard ASD diagnostic process. The device diagnosed 32% of the children with either a “Positive for ASD” or a “Negative for ASD” result. Researchers found that the device matched the panel’s conclusions for 81% of the patients who received a positive diagnosis. For those who received a negative diagnosis, the device matched the panel’s conclusions for 98% of the patients. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Cognoa ASD Diagnosis Aid has three main components. One component includes a mobile app for caregivers to answer questions about the child’s behavioral problems and to upload videos of the child. The next component is a video analysis portal for specialists to view and analyze uploaded videos of patients. Another component is a portal for health care providers that allows them to enter answers to preloaded questions about behavior problems, track the information provided by parents, and review a report of the results.
After the machine learning–based device processes the information provided by parents and health care providers, it reports either a positive or a negative diagnosis. If there is insufficient information to make either a positive or a negative diagnosis, the ASD Diagnostic AID will report that no result can be generated.
Some of the risks associated with this device include misdiagnosis and delayed diagnosis of ASD because of a false-positive or false-negative result, or when no result is generated. Researchers said a false-positive result occurred in 15 out of 303 study subjects without ASD and a false-negative result occurred in 1 out of 122 study subjects with ASD.
The FDA emphasized that the device is indicated to aid physicians in the process of diagnosing ASD in children. This means it shouldn’t be treated as a standalone diagnostic device, but as an adjunct to the diagnostic process.
The Food and Drug Administration has approved marketing for a device that will help diagnose autism spectrum disorder (ASD) in children between the ages of 18 months and 5 years old who exhibit potential symptoms.
Cognoa ASD Diagnosis Aid is a machine learning–based software program that receives information from parents or caregivers, video analysts, and health care providers to assist physicians in evaluating whether a child is at risk of having autism.
Autism is a developmental disorder that can cause social, communication, and behavioral challenges, according to the Centers for Disease Control and Prevention. The disorder affects about 1 in 54 children. The disorder is difficult to diagnose because there isn’t a medical test to diagnose the it. Instead, physicians have to look at a child’s developmental history and behavior to make a diagnosis.
Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the FDA.
“[ASD] can delay a child’s physical, cognitive, and social development, including motor skill development, learning, communication, and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s marketing authorization provides a new tool for helping diagnose children with ASD.”
The safety and efficacy of the Cognoa ASD Diagnosis Aid was assessed in a study of 425 patients between the ages of 18 months and 5 years old. For the study, researchers compared the diagnostic assessments made by the device to those made by a panel of clinical experts who used the current standard ASD diagnostic process. The device diagnosed 32% of the children with either a “Positive for ASD” or a “Negative for ASD” result. Researchers found that the device matched the panel’s conclusions for 81% of the patients who received a positive diagnosis. For those who received a negative diagnosis, the device matched the panel’s conclusions for 98% of the patients. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Cognoa ASD Diagnosis Aid has three main components. One component includes a mobile app for caregivers to answer questions about the child’s behavioral problems and to upload videos of the child. The next component is a video analysis portal for specialists to view and analyze uploaded videos of patients. Another component is a portal for health care providers that allows them to enter answers to preloaded questions about behavior problems, track the information provided by parents, and review a report of the results.
After the machine learning–based device processes the information provided by parents and health care providers, it reports either a positive or a negative diagnosis. If there is insufficient information to make either a positive or a negative diagnosis, the ASD Diagnostic AID will report that no result can be generated.
Some of the risks associated with this device include misdiagnosis and delayed diagnosis of ASD because of a false-positive or false-negative result, or when no result is generated. Researchers said a false-positive result occurred in 15 out of 303 study subjects without ASD and a false-negative result occurred in 1 out of 122 study subjects with ASD.
The FDA emphasized that the device is indicated to aid physicians in the process of diagnosing ASD in children. This means it shouldn’t be treated as a standalone diagnostic device, but as an adjunct to the diagnostic process.
The Food and Drug Administration has approved marketing for a device that will help diagnose autism spectrum disorder (ASD) in children between the ages of 18 months and 5 years old who exhibit potential symptoms.
Cognoa ASD Diagnosis Aid is a machine learning–based software program that receives information from parents or caregivers, video analysts, and health care providers to assist physicians in evaluating whether a child is at risk of having autism.
Autism is a developmental disorder that can cause social, communication, and behavioral challenges, according to the Centers for Disease Control and Prevention. The disorder affects about 1 in 54 children. The disorder is difficult to diagnose because there isn’t a medical test to diagnose the it. Instead, physicians have to look at a child’s developmental history and behavior to make a diagnosis.
Many children are not diagnosed with ASD until later in childhood, which in some cases delays treatment and early intervention. ASD may be detected as early as 18 months, but the average age of diagnosis for ASD is 4.3 years, according to the FDA.
“[ASD] can delay a child’s physical, cognitive, and social development, including motor skill development, learning, communication, and interacting with others. The earlier ASD can be diagnosed, the more quickly intervention strategies and appropriate therapies can begin,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, said in a statement. “Today’s marketing authorization provides a new tool for helping diagnose children with ASD.”
The safety and efficacy of the Cognoa ASD Diagnosis Aid was assessed in a study of 425 patients between the ages of 18 months and 5 years old. For the study, researchers compared the diagnostic assessments made by the device to those made by a panel of clinical experts who used the current standard ASD diagnostic process. The device diagnosed 32% of the children with either a “Positive for ASD” or a “Negative for ASD” result. Researchers found that the device matched the panel’s conclusions for 81% of the patients who received a positive diagnosis. For those who received a negative diagnosis, the device matched the panel’s conclusions for 98% of the patients. In addition, the device made an accurate ASD determination in 98.4% of patients with the condition and in 78.9% of patients without the condition.
Cognoa ASD Diagnosis Aid has three main components. One component includes a mobile app for caregivers to answer questions about the child’s behavioral problems and to upload videos of the child. The next component is a video analysis portal for specialists to view and analyze uploaded videos of patients. Another component is a portal for health care providers that allows them to enter answers to preloaded questions about behavior problems, track the information provided by parents, and review a report of the results.
After the machine learning–based device processes the information provided by parents and health care providers, it reports either a positive or a negative diagnosis. If there is insufficient information to make either a positive or a negative diagnosis, the ASD Diagnostic AID will report that no result can be generated.
Some of the risks associated with this device include misdiagnosis and delayed diagnosis of ASD because of a false-positive or false-negative result, or when no result is generated. Researchers said a false-positive result occurred in 15 out of 303 study subjects without ASD and a false-negative result occurred in 1 out of 122 study subjects with ASD.
The FDA emphasized that the device is indicated to aid physicians in the process of diagnosing ASD in children. This means it shouldn’t be treated as a standalone diagnostic device, but as an adjunct to the diagnostic process.
The Power of a Multidisciplinary Tumor Board: Managing Unresectable and/or High-Risk Skin Cancers
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
Multidisciplinary tumor boards are composed of providers from many fields who deliver coordinated care for patients with unresectable and high-risk skin cancers. Providers who comprise the tumor board often are radiation oncologists, hematologists/oncologists, general surgeons, dermatologists, dermatologic surgeons, and pathologists. The benefit of having a tumor board is that each patient is evaluated simultaneously by a group of physicians from various specialties who bring diverse perspectives that will contribute to the overall treatment plan. The cases often encompass high-risk tumors including unresectable basal cell carcinomas or invasive melanomas. By combining knowledge from each specialty in a team approach, the tumor board can effectively and holistically develop a care plan for each patient.
For the tumor board at the Warren Alpert Medical School of Brown University (Providence, Rhode Island), we often prepare a presentation with comprehensive details about the patient and tumor. During the presentation, we also propose a treatment plan prior to describing each patient at the weekly conference and amend the plans during the discussion. Tumor boards also provide a consulting role to the community and hospital providers in which patients are being referred by their primary provider and are seeking a second opinion or guidance.
In many ways, the tumor board is a multidisciplinary approach for patient advocacy in the form of treatment. These physicians meet on a regular basis to check on the patient’s progress and continually reevaluate how to have discussions about the patient’s care. There are many reasons why it is important to refer patients to a multidisciplinary tumor board.
Improved Workup and Diagnosis
One of the values of a tumor board is that it allows for patient data to be collected and assembled in a way that tells a story. The specialist from each field can then discuss and weigh the benefits and risks for each diagnostic test that should be performed for the workup in each patient. Physicians who refer their patients to the tumor board use their recommendations to both confirm the diagnosis and shift their treatment plans, depending on the information presented during the meeting.1 There may be a change in the tumor type, decision to refer for surgery, cancer staging, and list of viable options, especially after reviewing pathology and imaging.2 The discussion of the treatment plan may consider not only surgical considerations but also the patient’s quality of life. At times, noninvasive interventions are more appropriate and align with the patient’s goals of care. In addition, during the tumor board clinic there may be new tumors that are identified and biopsied, providing increased diagnosis and surveillance for patients who may have a higher risk for developing skin cancer.
Education for Residents and Providers
The multidisciplinary tumor board not only helps patients but also educates both residents and providers on the evidence-based therapeutic management of high-risk tumors.2 Research literature on cutaneous oncology is dynamic, and the weekly tumor board meetings help providers stay informed about the best and most effective treatments for their patients.3 In addition to the attending specialists, participants of the tumor board also may include residents, medical students, medical assistance staff, nurses, physician assistants, and fellows. Furthermore, the recommendations given by the tumor board serve to educate both the patient and the provider who referred them to the tumor board. Although we have access to excellent dermatology textbooks as residents, the most impactful educational experience is seeing the patients in tumor board clinic and participating in the immensely educational discussions at the weekly conferences. Through this experience, I have learned that treatment plans should be personalized to the patient. There are many factors to take into consideration when deciphering what the best course of treatment will be for a patient. Sometimes the best option is Mohs micrographic surgery, while other times it may be scheduling several sessions of palliative radiation oncology. Treatment depends on the individual patient and their condition.
Coordination of Care
During a week that I was on call, I was consulted to biopsy a patient with a giant hemorrhagic basal cell carcinoma that caused substantial cheek and nose distortion as well as anemia secondary to acute blood loss. The patient not only did not have a dermatologist but also did not have a primary care physician given he had not had contact with the health care system in more than 30 years. The reason for him not seeking care was multifactorial, but the approach to his care became multidisciplinary. We sought to connect him with the right providers to help him in any way that we could. We presented him at our multidisciplinary tumor board and started him on sonedigib, a medication that binds to and inhibits the smoothened protein.4 Through the tumor board, we were able to establish sustained contact with the patient. The tumor board created effective communication between providers to get him the referrals that he needed for dermatology, pathology, radiation oncology, hematology/oncology, and otolaryngology. The discussions centered around being cognizant of the patient’s apprehension with the health care system as well as providing medical and surgical treatment that would help his quality of life. We built a consensus on what the best plan was for the patient and his family. This consensus would have been more difficult had it not been for the combined specialties of the tumor board. In general, studies have shown that weekly tumor boards have resulted in decreased mortality rates for patients with advanced cancers.5
Final Thoughts
The multidisciplinary tumor board is a powerful resource for hospitals and the greater medical community. At these weekly conferences you realize there may still be hope that begins at the line where your expertise ends. It represents a team of providers who compassionately refuse to give up on patients when they are the last refuge.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
- Foster TJ, Bouchard-Fortier A, Olivotto IA, et al. Effect of multidisciplinary case conferences on physician decision making: breast diagnostic rounds. Cureus. 2016;8:E895.
- El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology international survey. J Glob Oncol. 2015;1:57-64.
- Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16:1209-1215.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor board participation among physicians caring for patients with lung or colorectal cancer. J Oncol Pract. 2015;11:E267-E278.
Resident Pearl
- Participating in a multidisciplinary tumor board allows residents to learn more about how to manage and treat high-risk skin cancers. The multidisciplinary team approach provides high-quality care for challenging patients.
Hospitalists play key role in advance care planning
Advance care planning (ACP) is a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences for future medical care, according to Meredith A. MacMartin, MD, director of inpatient palliative care at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
ACP “is really about planning for care in advance,” and in many ways, the inpatient setting is uniquely suited to this process, Dr. MacMartin said in a presentation at SHM Converge 2021, the annual conference of the Society of Hospital Medicine. “The key part is the advance part. You want conversations to happen before the care is actually needed,” she said.
Dr. MacMartin emphasized the importance of distinguishing between ACP and advance directives (ADs). ACP is a process, whereas ADs are documentation, “ideally of the content of advance care planning discussions,” she explained. ACP involves discussion about what is important to the patients, their goals, what information is helpful for them, and whether their current care is aligned with their goals, Dr. MacMartin said. ADs might involve a designated power of attorney for health care, a living will, and, in some states, specific clinician-signed orders regarding resuscitation or transport to hospital.
ACP is “more than whether a patient wants CPR [cardiopulmonary resuscitation] or not,” said Dr. MacMartin. ACP matters because it helps ensure that the care a patient receives aligns with the patient’s wishes and values, she said. ACP increases the likelihood that patients will die in their preferred locations, it allows them to discuss their wishes and prepare for decline, and it relieves family members of the burden of decision making, she said. From a hospital perspective, data show that use of an ACP can decrease intensive care unit (ICU) utilization and overall health care costs. “Often, when people are given the opportunity to express their wishes, they get less unnecessary care,” Dr. MacMartin noted.
Although ACP often takes place in an outpatient setting, hospitalists are in a unique position to conduct some ACP conversations with their patients, Dr. MacMartin said. “Hospitalists are available” and are physically present at least once a day, so there is a pragmatic advantage. Also, some data suggest that patients may feel more comfortable having ACP conversations with a hospitalist than with a primary care provider with whom they have a long-standing relationship, Dr. MacMartin added.
Another important advantage of ACP in the hospital setting is that, “as hospitalists, you are the expert on inpatient illness; you know what sick looks like, and you have a unique perspective on prognostication that may be harder to recreate in the outpatient setting,” Dr. MacMartin said.
Barriers to ACP include patient identification, logistics, attitudes
Settings in which ACP is appropriate include those in which a patient is undergoing “sentinel hospitalization,” meaning that the patient is at a transition point in the disease course. Examples are a patient newly diagnosed with metastatic solid cancer, a patient with progressive chronic kidney disease who is considering hemodialysis, or a patient who receives treatment in the ICU for longer than 7 days, Dr. MacMartin said.
Guidelines for identifying patients who might benefit from ACP include the use of the “surprise question” (“would you be surprised if this patient dies in the next year?”) as well as functional status assessments using tools such as the Australia-modified Karnofsky Performance Status or the Eastern Cooperative Oncology Group score, said Dr. MacMartin. Some studies suggest that any hospitalized patient older than 65 years should have an ACP discussion, she added.
Time pressure remains a significant barrier to ACP conversations. Some strategies to overcome this problem include enlisting help from other specialists, particularly social workers, Dr. MacMartin said. Social workers report a higher comfort level for talking to patients about death than any other medical specialty; “this is something they want to be doing,” she said. Also, the possibility of reimbursement may act as a buffer to create more time to have ACP conversations with patients, she noted.
Addressing clinicians’ discomfort with ACP conversations can be “a tougher nut to crack,” Dr. MacMartin acknowledged. Clinicians report that they don’t want to cause their patients distress, and some report that having conversations about end-of-life care is distressing for them as well. Some of these barriers can be overcome with skills training, including use of a prepared guideline or framework to help increase the comfort level for both clinicians and patients, said Dr. MacMartin.
A look ahead: Training strategies and COVID-19 impact
“For hospitalists interested in developing their ACP skills, I highly recommend two resources,” Dr. MacMartin said in an interview. “The Serious Illness Conversation Guide, from Ariadne Labs, is an excellent tool for any clinician to guide discussion about a patient’s goals and values,” she said.
“For clinicians wanting to build or improve their communication, including advance care planning discussions but also topics like responding to patient’s emotions, VitalTalk training offers a deeper dive into core communications skills,” she added.
“If your hospital has a palliative care team, they may also have more local resources available to you. To learn more about billing for ACP discussions, I recommend starting with your institutional billing and coding group, as these practices vary some between practices, and they will be able to provide the best guidance for clinicians. These are new codes that aren’t yet being very widely used so it’s a chance to innovate,” Dr. MacMartin noted.
“The hospital setting is an opportunity for patients to reflect on their health, both present and in the future, with a physician who has expertise in acute illness and prognostication and who is available for discussion on a daily basis during the hospitalization,” Dr. MacMartin emphasized.
As for whether the COVID-19 pandemic has affected ACP in the inpatient setting, the data are limited, but more information is forthcoming, Dr. MacMartin said. “In my personal experience and in talking to colleagues elsewhere, the pandemic has highlighted the need for ACP in some ways, as we have tried to ensure that people who wouldn’t want things like intensive care are identified early,” she said. “I hope that some of the workflows developed to identify patients who should get ACP in the hospital stay in practice and are strengthened over time,” she added.
Dr. MacMartin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Advance care planning (ACP) is a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences for future medical care, according to Meredith A. MacMartin, MD, director of inpatient palliative care at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
ACP “is really about planning for care in advance,” and in many ways, the inpatient setting is uniquely suited to this process, Dr. MacMartin said in a presentation at SHM Converge 2021, the annual conference of the Society of Hospital Medicine. “The key part is the advance part. You want conversations to happen before the care is actually needed,” she said.
Dr. MacMartin emphasized the importance of distinguishing between ACP and advance directives (ADs). ACP is a process, whereas ADs are documentation, “ideally of the content of advance care planning discussions,” she explained. ACP involves discussion about what is important to the patients, their goals, what information is helpful for them, and whether their current care is aligned with their goals, Dr. MacMartin said. ADs might involve a designated power of attorney for health care, a living will, and, in some states, specific clinician-signed orders regarding resuscitation or transport to hospital.
ACP is “more than whether a patient wants CPR [cardiopulmonary resuscitation] or not,” said Dr. MacMartin. ACP matters because it helps ensure that the care a patient receives aligns with the patient’s wishes and values, she said. ACP increases the likelihood that patients will die in their preferred locations, it allows them to discuss their wishes and prepare for decline, and it relieves family members of the burden of decision making, she said. From a hospital perspective, data show that use of an ACP can decrease intensive care unit (ICU) utilization and overall health care costs. “Often, when people are given the opportunity to express their wishes, they get less unnecessary care,” Dr. MacMartin noted.
Although ACP often takes place in an outpatient setting, hospitalists are in a unique position to conduct some ACP conversations with their patients, Dr. MacMartin said. “Hospitalists are available” and are physically present at least once a day, so there is a pragmatic advantage. Also, some data suggest that patients may feel more comfortable having ACP conversations with a hospitalist than with a primary care provider with whom they have a long-standing relationship, Dr. MacMartin added.
Another important advantage of ACP in the hospital setting is that, “as hospitalists, you are the expert on inpatient illness; you know what sick looks like, and you have a unique perspective on prognostication that may be harder to recreate in the outpatient setting,” Dr. MacMartin said.
Barriers to ACP include patient identification, logistics, attitudes
Settings in which ACP is appropriate include those in which a patient is undergoing “sentinel hospitalization,” meaning that the patient is at a transition point in the disease course. Examples are a patient newly diagnosed with metastatic solid cancer, a patient with progressive chronic kidney disease who is considering hemodialysis, or a patient who receives treatment in the ICU for longer than 7 days, Dr. MacMartin said.
Guidelines for identifying patients who might benefit from ACP include the use of the “surprise question” (“would you be surprised if this patient dies in the next year?”) as well as functional status assessments using tools such as the Australia-modified Karnofsky Performance Status or the Eastern Cooperative Oncology Group score, said Dr. MacMartin. Some studies suggest that any hospitalized patient older than 65 years should have an ACP discussion, she added.
Time pressure remains a significant barrier to ACP conversations. Some strategies to overcome this problem include enlisting help from other specialists, particularly social workers, Dr. MacMartin said. Social workers report a higher comfort level for talking to patients about death than any other medical specialty; “this is something they want to be doing,” she said. Also, the possibility of reimbursement may act as a buffer to create more time to have ACP conversations with patients, she noted.
Addressing clinicians’ discomfort with ACP conversations can be “a tougher nut to crack,” Dr. MacMartin acknowledged. Clinicians report that they don’t want to cause their patients distress, and some report that having conversations about end-of-life care is distressing for them as well. Some of these barriers can be overcome with skills training, including use of a prepared guideline or framework to help increase the comfort level for both clinicians and patients, said Dr. MacMartin.
A look ahead: Training strategies and COVID-19 impact
“For hospitalists interested in developing their ACP skills, I highly recommend two resources,” Dr. MacMartin said in an interview. “The Serious Illness Conversation Guide, from Ariadne Labs, is an excellent tool for any clinician to guide discussion about a patient’s goals and values,” she said.
“For clinicians wanting to build or improve their communication, including advance care planning discussions but also topics like responding to patient’s emotions, VitalTalk training offers a deeper dive into core communications skills,” she added.
“If your hospital has a palliative care team, they may also have more local resources available to you. To learn more about billing for ACP discussions, I recommend starting with your institutional billing and coding group, as these practices vary some between practices, and they will be able to provide the best guidance for clinicians. These are new codes that aren’t yet being very widely used so it’s a chance to innovate,” Dr. MacMartin noted.
“The hospital setting is an opportunity for patients to reflect on their health, both present and in the future, with a physician who has expertise in acute illness and prognostication and who is available for discussion on a daily basis during the hospitalization,” Dr. MacMartin emphasized.
As for whether the COVID-19 pandemic has affected ACP in the inpatient setting, the data are limited, but more information is forthcoming, Dr. MacMartin said. “In my personal experience and in talking to colleagues elsewhere, the pandemic has highlighted the need for ACP in some ways, as we have tried to ensure that people who wouldn’t want things like intensive care are identified early,” she said. “I hope that some of the workflows developed to identify patients who should get ACP in the hospital stay in practice and are strengthened over time,” she added.
Dr. MacMartin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Advance care planning (ACP) is a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences for future medical care, according to Meredith A. MacMartin, MD, director of inpatient palliative care at the Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
ACP “is really about planning for care in advance,” and in many ways, the inpatient setting is uniquely suited to this process, Dr. MacMartin said in a presentation at SHM Converge 2021, the annual conference of the Society of Hospital Medicine. “The key part is the advance part. You want conversations to happen before the care is actually needed,” she said.
Dr. MacMartin emphasized the importance of distinguishing between ACP and advance directives (ADs). ACP is a process, whereas ADs are documentation, “ideally of the content of advance care planning discussions,” she explained. ACP involves discussion about what is important to the patients, their goals, what information is helpful for them, and whether their current care is aligned with their goals, Dr. MacMartin said. ADs might involve a designated power of attorney for health care, a living will, and, in some states, specific clinician-signed orders regarding resuscitation or transport to hospital.
ACP is “more than whether a patient wants CPR [cardiopulmonary resuscitation] or not,” said Dr. MacMartin. ACP matters because it helps ensure that the care a patient receives aligns with the patient’s wishes and values, she said. ACP increases the likelihood that patients will die in their preferred locations, it allows them to discuss their wishes and prepare for decline, and it relieves family members of the burden of decision making, she said. From a hospital perspective, data show that use of an ACP can decrease intensive care unit (ICU) utilization and overall health care costs. “Often, when people are given the opportunity to express their wishes, they get less unnecessary care,” Dr. MacMartin noted.
Although ACP often takes place in an outpatient setting, hospitalists are in a unique position to conduct some ACP conversations with their patients, Dr. MacMartin said. “Hospitalists are available” and are physically present at least once a day, so there is a pragmatic advantage. Also, some data suggest that patients may feel more comfortable having ACP conversations with a hospitalist than with a primary care provider with whom they have a long-standing relationship, Dr. MacMartin added.
Another important advantage of ACP in the hospital setting is that, “as hospitalists, you are the expert on inpatient illness; you know what sick looks like, and you have a unique perspective on prognostication that may be harder to recreate in the outpatient setting,” Dr. MacMartin said.
Barriers to ACP include patient identification, logistics, attitudes
Settings in which ACP is appropriate include those in which a patient is undergoing “sentinel hospitalization,” meaning that the patient is at a transition point in the disease course. Examples are a patient newly diagnosed with metastatic solid cancer, a patient with progressive chronic kidney disease who is considering hemodialysis, or a patient who receives treatment in the ICU for longer than 7 days, Dr. MacMartin said.
Guidelines for identifying patients who might benefit from ACP include the use of the “surprise question” (“would you be surprised if this patient dies in the next year?”) as well as functional status assessments using tools such as the Australia-modified Karnofsky Performance Status or the Eastern Cooperative Oncology Group score, said Dr. MacMartin. Some studies suggest that any hospitalized patient older than 65 years should have an ACP discussion, she added.
Time pressure remains a significant barrier to ACP conversations. Some strategies to overcome this problem include enlisting help from other specialists, particularly social workers, Dr. MacMartin said. Social workers report a higher comfort level for talking to patients about death than any other medical specialty; “this is something they want to be doing,” she said. Also, the possibility of reimbursement may act as a buffer to create more time to have ACP conversations with patients, she noted.
Addressing clinicians’ discomfort with ACP conversations can be “a tougher nut to crack,” Dr. MacMartin acknowledged. Clinicians report that they don’t want to cause their patients distress, and some report that having conversations about end-of-life care is distressing for them as well. Some of these barriers can be overcome with skills training, including use of a prepared guideline or framework to help increase the comfort level for both clinicians and patients, said Dr. MacMartin.
A look ahead: Training strategies and COVID-19 impact
“For hospitalists interested in developing their ACP skills, I highly recommend two resources,” Dr. MacMartin said in an interview. “The Serious Illness Conversation Guide, from Ariadne Labs, is an excellent tool for any clinician to guide discussion about a patient’s goals and values,” she said.
“For clinicians wanting to build or improve their communication, including advance care planning discussions but also topics like responding to patient’s emotions, VitalTalk training offers a deeper dive into core communications skills,” she added.
“If your hospital has a palliative care team, they may also have more local resources available to you. To learn more about billing for ACP discussions, I recommend starting with your institutional billing and coding group, as these practices vary some between practices, and they will be able to provide the best guidance for clinicians. These are new codes that aren’t yet being very widely used so it’s a chance to innovate,” Dr. MacMartin noted.
“The hospital setting is an opportunity for patients to reflect on their health, both present and in the future, with a physician who has expertise in acute illness and prognostication and who is available for discussion on a daily basis during the hospitalization,” Dr. MacMartin emphasized.
As for whether the COVID-19 pandemic has affected ACP in the inpatient setting, the data are limited, but more information is forthcoming, Dr. MacMartin said. “In my personal experience and in talking to colleagues elsewhere, the pandemic has highlighted the need for ACP in some ways, as we have tried to ensure that people who wouldn’t want things like intensive care are identified early,” she said. “I hope that some of the workflows developed to identify patients who should get ACP in the hospital stay in practice and are strengthened over time,” she added.
Dr. MacMartin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SHM CONVERGE 2021
CDC: New botulism guidelines focus on mass casualty events
Botulinum toxin is said to be the most lethal substance known. Inhaling just 1-3 nanograms of toxin per kilogram of body mass constitutes a lethal dose.
The CDC has been working on these guidelines since 2015, initially establishing a technical development group and steering committee to prioritize topics for review and make recommendations. Since then, the agency published 15 systematic reviews in Clinical Infectious Diseases early in 2018. The reviews addressed the recognition of botulism clinically, treatment with botulinum antitoxin, and complications from that treatment. They also looked at the epidemiology of botulism outbreaks and botulism in the special populations of vulnerable pediatric and pregnant patients.
In 2016, the CDC held two extended forums and convened a workshop with 72 experts. In addition to the more standard topics of diagnosis and treatment, attention was given to crisis standards of care, caring for multiple patients at once, and ethical considerations in management.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, said in an interview that the new guidance “was really specific [and] was meant to address the gap in guidance for mass casualty settings.”
While clinicians are used to focusing on an individual patient, in times of crises, with multiple patients from a food-borne outbreak or a bioterrorism attack, the focus must shift to the population rather than the individual. The workshop explored issues of triaging, adding beds, and caring for patients when a hospital is overwhelmed with an acute influx of severely ill patients.
Such a mass casualty event is similar to the stress encountered this past year with COVID-19 patients swamping the hospitals, which had too little oxygen, too few ventilators, and too few staff members to care for the sudden influx of critically ill patients.
Diagnosis
Leslie Edwards, MHS, BSN, a CDC epidemiologist and botulism expert, said that “botulism is rare and [so] could be difficult to diagnose.” The CDC “wanted to highlight some of those key clinical factors” to speed recognition.
Hospitals and health officials are being urged to develop crisis protocols as part of emergency preparedness plans. And clinicians should be able to recognize four major syndromes: botulism from food, wounds, and inhalation, as well as iatrogenic botulism (from exposure via injection of the neurotoxin).
Botulism has a characteristic and unusual pattern of symptoms, which begin with cranial nerve palsies. Then there is typically a descending, symmetric flaccid paralysis. Symptoms might progress to respiratory failure and death. Other critical clues that implicate botulism include a lack of sensory deficits and the absence of pain.
Symptoms are most likely to be mistaken for myasthenia gravis or Guillain-Barré syndrome, but the latter has an ascending paralysis. Cranial nerve involvement can present as blurred vision, ptosis (drooping lid), diplopia (double vision), ophthalmoplegia (weak eye muscles), or difficulty with speech and swallowing. Shortness of breath and abdominal discomfort can also occur. Respiratory failure may occur from weakness or paralysis of cranial nerves. Cranial nerve signs and symptoms in the absence of fever, along with a descending paralysis, should strongly suggest the diagnosis.
With food-borne botulism, vomiting occurs in half the patients. Improperly sterilized home-canned food is the major risk factor. While the toxin is rapidly destroyed by heat, the bacterial spores are not. Wound botulism is most commonly associated with the injection of drugs, particularly black tar heroin.
Dr. Edwards stressed that “time is of the essence when it comes to botulism diagnostics and treating. Timely administration of the botulism antitoxin early in the course of illness can arrest the progression of paralysis and possibly avert the need for intubation or ventilation.”
It’s essential to note that botulism is an urgent diagnosis that has to be made on clinical grounds. Lab assays for botulinum neurotoxins take too long and are only conducted in public health laboratories. The decision to use antitoxin must not be delayed to wait for confirmation.
Clinicians should immediately contact the local or state health department’s emergency on-call team if botulism is suspected. They will arrange for expert consultation.
Treatment
Botulinum antitoxin is the only specific therapy for this infection. If given early – preferably within 24-48 hours of symptom onset – it can stop the progression of paralysis. But antitoxin will not reverse existing paralysis. If paralysis is still progressing outside of that 24- to 48-hour window, the antitoxin should still provide benefit. The antitoxin is available only through state health departments and a request to the CDC.
Botulism antitoxin is made from horse serum and therefore may cause a variety of allergic reactions. The risk for anaphylaxis is less than 2%, far lower than the mortality from untreated botulism.
While these guidelines have an important focus on triaging and treating mass casualties from botulism, it’s important to note that food-borne outbreaks and prevention issues are covered elsewhere on the CDC site.
Dr. Edwards has disclosed no relevant financial relationships. Dr. Adalja is a consultant for Emergent BioSolutions, which makes the heptavalent botulism antitoxin.
Dr. Stone is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research,” the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
Botulinum toxin is said to be the most lethal substance known. Inhaling just 1-3 nanograms of toxin per kilogram of body mass constitutes a lethal dose.
The CDC has been working on these guidelines since 2015, initially establishing a technical development group and steering committee to prioritize topics for review and make recommendations. Since then, the agency published 15 systematic reviews in Clinical Infectious Diseases early in 2018. The reviews addressed the recognition of botulism clinically, treatment with botulinum antitoxin, and complications from that treatment. They also looked at the epidemiology of botulism outbreaks and botulism in the special populations of vulnerable pediatric and pregnant patients.
In 2016, the CDC held two extended forums and convened a workshop with 72 experts. In addition to the more standard topics of diagnosis and treatment, attention was given to crisis standards of care, caring for multiple patients at once, and ethical considerations in management.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, said in an interview that the new guidance “was really specific [and] was meant to address the gap in guidance for mass casualty settings.”
While clinicians are used to focusing on an individual patient, in times of crises, with multiple patients from a food-borne outbreak or a bioterrorism attack, the focus must shift to the population rather than the individual. The workshop explored issues of triaging, adding beds, and caring for patients when a hospital is overwhelmed with an acute influx of severely ill patients.
Such a mass casualty event is similar to the stress encountered this past year with COVID-19 patients swamping the hospitals, which had too little oxygen, too few ventilators, and too few staff members to care for the sudden influx of critically ill patients.
Diagnosis
Leslie Edwards, MHS, BSN, a CDC epidemiologist and botulism expert, said that “botulism is rare and [so] could be difficult to diagnose.” The CDC “wanted to highlight some of those key clinical factors” to speed recognition.
Hospitals and health officials are being urged to develop crisis protocols as part of emergency preparedness plans. And clinicians should be able to recognize four major syndromes: botulism from food, wounds, and inhalation, as well as iatrogenic botulism (from exposure via injection of the neurotoxin).
Botulism has a characteristic and unusual pattern of symptoms, which begin with cranial nerve palsies. Then there is typically a descending, symmetric flaccid paralysis. Symptoms might progress to respiratory failure and death. Other critical clues that implicate botulism include a lack of sensory deficits and the absence of pain.
Symptoms are most likely to be mistaken for myasthenia gravis or Guillain-Barré syndrome, but the latter has an ascending paralysis. Cranial nerve involvement can present as blurred vision, ptosis (drooping lid), diplopia (double vision), ophthalmoplegia (weak eye muscles), or difficulty with speech and swallowing. Shortness of breath and abdominal discomfort can also occur. Respiratory failure may occur from weakness or paralysis of cranial nerves. Cranial nerve signs and symptoms in the absence of fever, along with a descending paralysis, should strongly suggest the diagnosis.
With food-borne botulism, vomiting occurs in half the patients. Improperly sterilized home-canned food is the major risk factor. While the toxin is rapidly destroyed by heat, the bacterial spores are not. Wound botulism is most commonly associated with the injection of drugs, particularly black tar heroin.
Dr. Edwards stressed that “time is of the essence when it comes to botulism diagnostics and treating. Timely administration of the botulism antitoxin early in the course of illness can arrest the progression of paralysis and possibly avert the need for intubation or ventilation.”
It’s essential to note that botulism is an urgent diagnosis that has to be made on clinical grounds. Lab assays for botulinum neurotoxins take too long and are only conducted in public health laboratories. The decision to use antitoxin must not be delayed to wait for confirmation.
Clinicians should immediately contact the local or state health department’s emergency on-call team if botulism is suspected. They will arrange for expert consultation.
Treatment
Botulinum antitoxin is the only specific therapy for this infection. If given early – preferably within 24-48 hours of symptom onset – it can stop the progression of paralysis. But antitoxin will not reverse existing paralysis. If paralysis is still progressing outside of that 24- to 48-hour window, the antitoxin should still provide benefit. The antitoxin is available only through state health departments and a request to the CDC.
Botulism antitoxin is made from horse serum and therefore may cause a variety of allergic reactions. The risk for anaphylaxis is less than 2%, far lower than the mortality from untreated botulism.
While these guidelines have an important focus on triaging and treating mass casualties from botulism, it’s important to note that food-borne outbreaks and prevention issues are covered elsewhere on the CDC site.
Dr. Edwards has disclosed no relevant financial relationships. Dr. Adalja is a consultant for Emergent BioSolutions, which makes the heptavalent botulism antitoxin.
Dr. Stone is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research,” the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
Botulinum toxin is said to be the most lethal substance known. Inhaling just 1-3 nanograms of toxin per kilogram of body mass constitutes a lethal dose.
The CDC has been working on these guidelines since 2015, initially establishing a technical development group and steering committee to prioritize topics for review and make recommendations. Since then, the agency published 15 systematic reviews in Clinical Infectious Diseases early in 2018. The reviews addressed the recognition of botulism clinically, treatment with botulinum antitoxin, and complications from that treatment. They also looked at the epidemiology of botulism outbreaks and botulism in the special populations of vulnerable pediatric and pregnant patients.
In 2016, the CDC held two extended forums and convened a workshop with 72 experts. In addition to the more standard topics of diagnosis and treatment, attention was given to crisis standards of care, caring for multiple patients at once, and ethical considerations in management.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, said in an interview that the new guidance “was really specific [and] was meant to address the gap in guidance for mass casualty settings.”
While clinicians are used to focusing on an individual patient, in times of crises, with multiple patients from a food-borne outbreak or a bioterrorism attack, the focus must shift to the population rather than the individual. The workshop explored issues of triaging, adding beds, and caring for patients when a hospital is overwhelmed with an acute influx of severely ill patients.
Such a mass casualty event is similar to the stress encountered this past year with COVID-19 patients swamping the hospitals, which had too little oxygen, too few ventilators, and too few staff members to care for the sudden influx of critically ill patients.
Diagnosis
Leslie Edwards, MHS, BSN, a CDC epidemiologist and botulism expert, said that “botulism is rare and [so] could be difficult to diagnose.” The CDC “wanted to highlight some of those key clinical factors” to speed recognition.
Hospitals and health officials are being urged to develop crisis protocols as part of emergency preparedness plans. And clinicians should be able to recognize four major syndromes: botulism from food, wounds, and inhalation, as well as iatrogenic botulism (from exposure via injection of the neurotoxin).
Botulism has a characteristic and unusual pattern of symptoms, which begin with cranial nerve palsies. Then there is typically a descending, symmetric flaccid paralysis. Symptoms might progress to respiratory failure and death. Other critical clues that implicate botulism include a lack of sensory deficits and the absence of pain.
Symptoms are most likely to be mistaken for myasthenia gravis or Guillain-Barré syndrome, but the latter has an ascending paralysis. Cranial nerve involvement can present as blurred vision, ptosis (drooping lid), diplopia (double vision), ophthalmoplegia (weak eye muscles), or difficulty with speech and swallowing. Shortness of breath and abdominal discomfort can also occur. Respiratory failure may occur from weakness or paralysis of cranial nerves. Cranial nerve signs and symptoms in the absence of fever, along with a descending paralysis, should strongly suggest the diagnosis.
With food-borne botulism, vomiting occurs in half the patients. Improperly sterilized home-canned food is the major risk factor. While the toxin is rapidly destroyed by heat, the bacterial spores are not. Wound botulism is most commonly associated with the injection of drugs, particularly black tar heroin.
Dr. Edwards stressed that “time is of the essence when it comes to botulism diagnostics and treating. Timely administration of the botulism antitoxin early in the course of illness can arrest the progression of paralysis and possibly avert the need for intubation or ventilation.”
It’s essential to note that botulism is an urgent diagnosis that has to be made on clinical grounds. Lab assays for botulinum neurotoxins take too long and are only conducted in public health laboratories. The decision to use antitoxin must not be delayed to wait for confirmation.
Clinicians should immediately contact the local or state health department’s emergency on-call team if botulism is suspected. They will arrange for expert consultation.
Treatment
Botulinum antitoxin is the only specific therapy for this infection. If given early – preferably within 24-48 hours of symptom onset – it can stop the progression of paralysis. But antitoxin will not reverse existing paralysis. If paralysis is still progressing outside of that 24- to 48-hour window, the antitoxin should still provide benefit. The antitoxin is available only through state health departments and a request to the CDC.
Botulism antitoxin is made from horse serum and therefore may cause a variety of allergic reactions. The risk for anaphylaxis is less than 2%, far lower than the mortality from untreated botulism.
While these guidelines have an important focus on triaging and treating mass casualties from botulism, it’s important to note that food-borne outbreaks and prevention issues are covered elsewhere on the CDC site.
Dr. Edwards has disclosed no relevant financial relationships. Dr. Adalja is a consultant for Emergent BioSolutions, which makes the heptavalent botulism antitoxin.
Dr. Stone is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research,” the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
Prediabetes linked to higher CVD and CKD rates
in a study of nearly 337,000 people included in the UK Biobank database.
The findings suggest that people with prediabetes have “heightened risk even without progression to type 2 diabetes,” Michael C. Honigberg, MD, said at the annual scientific sessions of the American College of Cardiology.
“Hemoglobin A1c may be better considered as a continuous measure of risk rather than dichotomized” as either less than 6.5%, or 6.5% or higher, the usual threshold defining people with type 2 diabetes, said Dr. Honigberg, a cardiologist at Massachusetts General Hospital in Boston.
‘Prediabetes is not a benign entity’
“Our findings reinforce the notion that A1c represents a continuum of risk, with elevated risks observed, especially for atherosclerotic cardiovascular disease [ASCVD], at levels where some clinicians wouldn’t think twice about them. Prediabetes is not a benign entity in the middle-aged population we studied,” Dr. Honigberg said in an interview. “Risks are higher in individuals with type 2 diabetes,” he stressed, “however, prediabetes is so much more common that it appears to confer similar cardio, renal, and metabolic risks at a population level.”
Results from prior observational studies also showed elevated incidence rate of cardiovascular disease events in people with prediabetes, including a 2010 report based on data from about 11,000 U.S. residents, and in a more recent meta-analysis of 129 studies involving more than 10 million people. The new report by Dr. Honigberg “is the first to comprehensively evaluate diverse cardio-renal-metabolic outcomes across a range of A1c levels using a very large, contemporary database,” he noted. In addition, most prior reports did not include chronic kidney disease as an examined outcome.
The primary endpoint examined in the new analysis was the combined incidence during a median follow-up of just over 11 years of ASCVD events (coronary artery disease, ischemic stroke, or peripheral artery disease), CKD, or heart failure among 336,709 adults in the UK Biobank who at baseline had none of these conditions nor type 1 diabetes.
The vast majority, 82%, were normoglycemic at baseline, based on having an A1c of less than 5.7%; 14% had prediabetes, with an A1c of 5.7%-6.4%; and 4% had type 2 diabetes based on an A1c of at least 6.5% or on insulin treatment. Patients averaged about 57 years of age, slightly more than half were women, and average body mass index was in the overweight category except for those with type 2 diabetes.
The primary endpoint, the combined incidence of ASCVD, CKD, and heart failure, was 24% among those with type 2 diabetes, 14% in those with prediabetes, and 8% in those who were normoglycemic at entry. Concurrently with the report, the results appeared online. Most of these events involved ASCVD, which occurred in 11% of those in the prediabetes subgroup (roughly four-fifths of the events in this subgroup), and in 17% of those with type 2 diabetes (nearly three-quarters of the events in this subgroup).
In an analysis that adjusted for more than a dozen demographic and clinical factors, the presence of prediabetes linked with significant increases in the incidence rate of all three outcomes compared with people who were normoglycemic at baseline. The analysis also identified an A1c level of 5.0% as linked with the lowest incidence of each of the three adverse outcomes. And a very granular analysis suggested that a significantly elevated risk for ASCVD first appeared when A1c levels were in the range of 5.4%-5.7%; a significantly increased incidence of CKD became apparent once A1c was in the range of 6.2%-6.5%; and a significantly increased incidence of heart failure began to manifest once A1c levels reached at least 7.0%.
Need for comprehensive cardiometabolic risk management
The findings “highlight the importance of identifying and comprehensively managing cardiometabolic risk in people with prediabetes, including dietary modification, exercise, weight loss and obesity management, smoking cessation, and attention to hypertension and hypercholesterolemia,” Dr. Honigberg said. While these data cannot address the appropriateness of using novel drug interventions in people with prediabetes, they suggest that people with prediabetes should be the focus of future prevention trials testing agents such as sodium-glucose cotransporter 2 inhibitors.
“These data help us discuss risk with patients [with prediabetes], and reemphasize the importance of guideline-directed preventive care,” said Vijay Nambi, MD, PhD, a preventive cardiologist and lipid specialist at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston, who was not involved with the study.
An additional analysis reported by Dr. Honigberg examined the risk among people with prediabetes who also were current or former smokers and in the top tertile of the prediabetes study population for systolic blood pressure, high non-HDL cholesterol, and C-reactive protein (a marker of inflammation). This very high-risk subgroup of people with prediabetes had incidence rates for ASCVD events and for heart failure that tracked identically to those with type 2 diabetes. However. the incidence rate for CKD in these high-risk people with prediabetes remained below that of patients with type 2 diabetes.
Dr. Honigberg had no disclosures. Dr. Nambi has received research funding from Amgen, Merck, and Roche.
in a study of nearly 337,000 people included in the UK Biobank database.
The findings suggest that people with prediabetes have “heightened risk even without progression to type 2 diabetes,” Michael C. Honigberg, MD, said at the annual scientific sessions of the American College of Cardiology.
“Hemoglobin A1c may be better considered as a continuous measure of risk rather than dichotomized” as either less than 6.5%, or 6.5% or higher, the usual threshold defining people with type 2 diabetes, said Dr. Honigberg, a cardiologist at Massachusetts General Hospital in Boston.
‘Prediabetes is not a benign entity’
“Our findings reinforce the notion that A1c represents a continuum of risk, with elevated risks observed, especially for atherosclerotic cardiovascular disease [ASCVD], at levels where some clinicians wouldn’t think twice about them. Prediabetes is not a benign entity in the middle-aged population we studied,” Dr. Honigberg said in an interview. “Risks are higher in individuals with type 2 diabetes,” he stressed, “however, prediabetes is so much more common that it appears to confer similar cardio, renal, and metabolic risks at a population level.”
Results from prior observational studies also showed elevated incidence rate of cardiovascular disease events in people with prediabetes, including a 2010 report based on data from about 11,000 U.S. residents, and in a more recent meta-analysis of 129 studies involving more than 10 million people. The new report by Dr. Honigberg “is the first to comprehensively evaluate diverse cardio-renal-metabolic outcomes across a range of A1c levels using a very large, contemporary database,” he noted. In addition, most prior reports did not include chronic kidney disease as an examined outcome.
The primary endpoint examined in the new analysis was the combined incidence during a median follow-up of just over 11 years of ASCVD events (coronary artery disease, ischemic stroke, or peripheral artery disease), CKD, or heart failure among 336,709 adults in the UK Biobank who at baseline had none of these conditions nor type 1 diabetes.
The vast majority, 82%, were normoglycemic at baseline, based on having an A1c of less than 5.7%; 14% had prediabetes, with an A1c of 5.7%-6.4%; and 4% had type 2 diabetes based on an A1c of at least 6.5% or on insulin treatment. Patients averaged about 57 years of age, slightly more than half were women, and average body mass index was in the overweight category except for those with type 2 diabetes.
The primary endpoint, the combined incidence of ASCVD, CKD, and heart failure, was 24% among those with type 2 diabetes, 14% in those with prediabetes, and 8% in those who were normoglycemic at entry. Concurrently with the report, the results appeared online. Most of these events involved ASCVD, which occurred in 11% of those in the prediabetes subgroup (roughly four-fifths of the events in this subgroup), and in 17% of those with type 2 diabetes (nearly three-quarters of the events in this subgroup).
In an analysis that adjusted for more than a dozen demographic and clinical factors, the presence of prediabetes linked with significant increases in the incidence rate of all three outcomes compared with people who were normoglycemic at baseline. The analysis also identified an A1c level of 5.0% as linked with the lowest incidence of each of the three adverse outcomes. And a very granular analysis suggested that a significantly elevated risk for ASCVD first appeared when A1c levels were in the range of 5.4%-5.7%; a significantly increased incidence of CKD became apparent once A1c was in the range of 6.2%-6.5%; and a significantly increased incidence of heart failure began to manifest once A1c levels reached at least 7.0%.
Need for comprehensive cardiometabolic risk management
The findings “highlight the importance of identifying and comprehensively managing cardiometabolic risk in people with prediabetes, including dietary modification, exercise, weight loss and obesity management, smoking cessation, and attention to hypertension and hypercholesterolemia,” Dr. Honigberg said. While these data cannot address the appropriateness of using novel drug interventions in people with prediabetes, they suggest that people with prediabetes should be the focus of future prevention trials testing agents such as sodium-glucose cotransporter 2 inhibitors.
“These data help us discuss risk with patients [with prediabetes], and reemphasize the importance of guideline-directed preventive care,” said Vijay Nambi, MD, PhD, a preventive cardiologist and lipid specialist at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston, who was not involved with the study.
An additional analysis reported by Dr. Honigberg examined the risk among people with prediabetes who also were current or former smokers and in the top tertile of the prediabetes study population for systolic blood pressure, high non-HDL cholesterol, and C-reactive protein (a marker of inflammation). This very high-risk subgroup of people with prediabetes had incidence rates for ASCVD events and for heart failure that tracked identically to those with type 2 diabetes. However. the incidence rate for CKD in these high-risk people with prediabetes remained below that of patients with type 2 diabetes.
Dr. Honigberg had no disclosures. Dr. Nambi has received research funding from Amgen, Merck, and Roche.
in a study of nearly 337,000 people included in the UK Biobank database.
The findings suggest that people with prediabetes have “heightened risk even without progression to type 2 diabetes,” Michael C. Honigberg, MD, said at the annual scientific sessions of the American College of Cardiology.
“Hemoglobin A1c may be better considered as a continuous measure of risk rather than dichotomized” as either less than 6.5%, or 6.5% or higher, the usual threshold defining people with type 2 diabetes, said Dr. Honigberg, a cardiologist at Massachusetts General Hospital in Boston.
‘Prediabetes is not a benign entity’
“Our findings reinforce the notion that A1c represents a continuum of risk, with elevated risks observed, especially for atherosclerotic cardiovascular disease [ASCVD], at levels where some clinicians wouldn’t think twice about them. Prediabetes is not a benign entity in the middle-aged population we studied,” Dr. Honigberg said in an interview. “Risks are higher in individuals with type 2 diabetes,” he stressed, “however, prediabetes is so much more common that it appears to confer similar cardio, renal, and metabolic risks at a population level.”
Results from prior observational studies also showed elevated incidence rate of cardiovascular disease events in people with prediabetes, including a 2010 report based on data from about 11,000 U.S. residents, and in a more recent meta-analysis of 129 studies involving more than 10 million people. The new report by Dr. Honigberg “is the first to comprehensively evaluate diverse cardio-renal-metabolic outcomes across a range of A1c levels using a very large, contemporary database,” he noted. In addition, most prior reports did not include chronic kidney disease as an examined outcome.
The primary endpoint examined in the new analysis was the combined incidence during a median follow-up of just over 11 years of ASCVD events (coronary artery disease, ischemic stroke, or peripheral artery disease), CKD, or heart failure among 336,709 adults in the UK Biobank who at baseline had none of these conditions nor type 1 diabetes.
The vast majority, 82%, were normoglycemic at baseline, based on having an A1c of less than 5.7%; 14% had prediabetes, with an A1c of 5.7%-6.4%; and 4% had type 2 diabetes based on an A1c of at least 6.5% or on insulin treatment. Patients averaged about 57 years of age, slightly more than half were women, and average body mass index was in the overweight category except for those with type 2 diabetes.
The primary endpoint, the combined incidence of ASCVD, CKD, and heart failure, was 24% among those with type 2 diabetes, 14% in those with prediabetes, and 8% in those who were normoglycemic at entry. Concurrently with the report, the results appeared online. Most of these events involved ASCVD, which occurred in 11% of those in the prediabetes subgroup (roughly four-fifths of the events in this subgroup), and in 17% of those with type 2 diabetes (nearly three-quarters of the events in this subgroup).
In an analysis that adjusted for more than a dozen demographic and clinical factors, the presence of prediabetes linked with significant increases in the incidence rate of all three outcomes compared with people who were normoglycemic at baseline. The analysis also identified an A1c level of 5.0% as linked with the lowest incidence of each of the three adverse outcomes. And a very granular analysis suggested that a significantly elevated risk for ASCVD first appeared when A1c levels were in the range of 5.4%-5.7%; a significantly increased incidence of CKD became apparent once A1c was in the range of 6.2%-6.5%; and a significantly increased incidence of heart failure began to manifest once A1c levels reached at least 7.0%.
Need for comprehensive cardiometabolic risk management
The findings “highlight the importance of identifying and comprehensively managing cardiometabolic risk in people with prediabetes, including dietary modification, exercise, weight loss and obesity management, smoking cessation, and attention to hypertension and hypercholesterolemia,” Dr. Honigberg said. While these data cannot address the appropriateness of using novel drug interventions in people with prediabetes, they suggest that people with prediabetes should be the focus of future prevention trials testing agents such as sodium-glucose cotransporter 2 inhibitors.
“These data help us discuss risk with patients [with prediabetes], and reemphasize the importance of guideline-directed preventive care,” said Vijay Nambi, MD, PhD, a preventive cardiologist and lipid specialist at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston, who was not involved with the study.
An additional analysis reported by Dr. Honigberg examined the risk among people with prediabetes who also were current or former smokers and in the top tertile of the prediabetes study population for systolic blood pressure, high non-HDL cholesterol, and C-reactive protein (a marker of inflammation). This very high-risk subgroup of people with prediabetes had incidence rates for ASCVD events and for heart failure that tracked identically to those with type 2 diabetes. However. the incidence rate for CKD in these high-risk people with prediabetes remained below that of patients with type 2 diabetes.
Dr. Honigberg had no disclosures. Dr. Nambi has received research funding from Amgen, Merck, and Roche.
FROM ACC 2021
Mohs surgery favorable as monotherapy for early Merkel cell carcinomas
Pittsburgh.
The results compare favorably with the standard treatment approach, wide local excision with or without radiation, which has a local recurrence rate of 4.2%-31.7% because of incomplete excision or false negative margins, said Vitaly Terushkin, MD, a Mohs surgeon who presented the findings of the study, a retrospective chart review, at the annual meeting of the American College of Mohs Surgery.
Mohs surgery as monotherapy offered “survival at least as good as historical controls treated with wide local excision plus radiation therapy, and because of the superior local control, Mohs surgery may obviate the need for adjuvant radiation and decrease the chance for additional surgery for the treatment of local recurrence,” said Dr. Terushkin, now in practice in the New York City area.
“We hope this data fuel additional studies with larger cohorts to continue to explore the value of Mohs for Merkel cell carcinoma,” he said.
The findings add to a growing body of literature supporting Mohs for many types of rare tumors. “Micrographic surgery or complete circumferential peripheral and deep margin analysis has been shown to be superior to wide local excision in a variety of tumors and clinical scenarios,” said Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington.
“When the entire margin is able to be evaluated over random bread-loafed sections, there is growing evidence that this leads to superior outcomes and disease specific mortality,” he said when asked for comment on the study results.
In all, 56 primary Merkel cell carcinomas were treated in the 53 patients from 2001 to 2019; about two-thirds of the patients had stage 1 tumors and the rest stage 2a.
They were treated with Mohs alone, without radiation. Average follow up was 4.6 years, with about a third of patients followed for 5 or more years.
The average age of the patients was 78 years, and just over half were men. In more than half the cases, tumors were located on the head and neck (62.5%), and the mean tumor size was 1.7 cm. Patients were negative for lymphadenopathy and declined lymph node biopsy.
Although there was no local recurrence, defined as tumor reemerging within or adjacent to the surgery site, 7 patients (12.7%) developed in-transit metastases, 13 (23.6%) developed nodal metastases, and 3 developed distant metastases.
The 5-year disease-specific survival rate was 91.2% for stage 1 and 68.6% for stage 2a patients, which compared favorably with historical controls treated with wide local excision with or without radiation, with reported 5-year disease-specific survival rates of 81%-87% for stage 1 disease and 63%-67% for stage 2. Although radiation wasn’t used in the study, Dr. Patel noted that more investigation is needed about the role of adjuvant radiation therapy after Mohs surgery “given recent publications showing improved outcomes in patients with narrow margin excision and postoperative radiation therapy.”
No external funding of the study was reported. Dr. Terushkin had no disclosures. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
Pittsburgh.
The results compare favorably with the standard treatment approach, wide local excision with or without radiation, which has a local recurrence rate of 4.2%-31.7% because of incomplete excision or false negative margins, said Vitaly Terushkin, MD, a Mohs surgeon who presented the findings of the study, a retrospective chart review, at the annual meeting of the American College of Mohs Surgery.
Mohs surgery as monotherapy offered “survival at least as good as historical controls treated with wide local excision plus radiation therapy, and because of the superior local control, Mohs surgery may obviate the need for adjuvant radiation and decrease the chance for additional surgery for the treatment of local recurrence,” said Dr. Terushkin, now in practice in the New York City area.
“We hope this data fuel additional studies with larger cohorts to continue to explore the value of Mohs for Merkel cell carcinoma,” he said.
The findings add to a growing body of literature supporting Mohs for many types of rare tumors. “Micrographic surgery or complete circumferential peripheral and deep margin analysis has been shown to be superior to wide local excision in a variety of tumors and clinical scenarios,” said Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington.
“When the entire margin is able to be evaluated over random bread-loafed sections, there is growing evidence that this leads to superior outcomes and disease specific mortality,” he said when asked for comment on the study results.
In all, 56 primary Merkel cell carcinomas were treated in the 53 patients from 2001 to 2019; about two-thirds of the patients had stage 1 tumors and the rest stage 2a.
They were treated with Mohs alone, without radiation. Average follow up was 4.6 years, with about a third of patients followed for 5 or more years.
The average age of the patients was 78 years, and just over half were men. In more than half the cases, tumors were located on the head and neck (62.5%), and the mean tumor size was 1.7 cm. Patients were negative for lymphadenopathy and declined lymph node biopsy.
Although there was no local recurrence, defined as tumor reemerging within or adjacent to the surgery site, 7 patients (12.7%) developed in-transit metastases, 13 (23.6%) developed nodal metastases, and 3 developed distant metastases.
The 5-year disease-specific survival rate was 91.2% for stage 1 and 68.6% for stage 2a patients, which compared favorably with historical controls treated with wide local excision with or without radiation, with reported 5-year disease-specific survival rates of 81%-87% for stage 1 disease and 63%-67% for stage 2. Although radiation wasn’t used in the study, Dr. Patel noted that more investigation is needed about the role of adjuvant radiation therapy after Mohs surgery “given recent publications showing improved outcomes in patients with narrow margin excision and postoperative radiation therapy.”
No external funding of the study was reported. Dr. Terushkin had no disclosures. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
Pittsburgh.
The results compare favorably with the standard treatment approach, wide local excision with or without radiation, which has a local recurrence rate of 4.2%-31.7% because of incomplete excision or false negative margins, said Vitaly Terushkin, MD, a Mohs surgeon who presented the findings of the study, a retrospective chart review, at the annual meeting of the American College of Mohs Surgery.
Mohs surgery as monotherapy offered “survival at least as good as historical controls treated with wide local excision plus radiation therapy, and because of the superior local control, Mohs surgery may obviate the need for adjuvant radiation and decrease the chance for additional surgery for the treatment of local recurrence,” said Dr. Terushkin, now in practice in the New York City area.
“We hope this data fuel additional studies with larger cohorts to continue to explore the value of Mohs for Merkel cell carcinoma,” he said.
The findings add to a growing body of literature supporting Mohs for many types of rare tumors. “Micrographic surgery or complete circumferential peripheral and deep margin analysis has been shown to be superior to wide local excision in a variety of tumors and clinical scenarios,” said Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington.
“When the entire margin is able to be evaluated over random bread-loafed sections, there is growing evidence that this leads to superior outcomes and disease specific mortality,” he said when asked for comment on the study results.
In all, 56 primary Merkel cell carcinomas were treated in the 53 patients from 2001 to 2019; about two-thirds of the patients had stage 1 tumors and the rest stage 2a.
They were treated with Mohs alone, without radiation. Average follow up was 4.6 years, with about a third of patients followed for 5 or more years.
The average age of the patients was 78 years, and just over half were men. In more than half the cases, tumors were located on the head and neck (62.5%), and the mean tumor size was 1.7 cm. Patients were negative for lymphadenopathy and declined lymph node biopsy.
Although there was no local recurrence, defined as tumor reemerging within or adjacent to the surgery site, 7 patients (12.7%) developed in-transit metastases, 13 (23.6%) developed nodal metastases, and 3 developed distant metastases.
The 5-year disease-specific survival rate was 91.2% for stage 1 and 68.6% for stage 2a patients, which compared favorably with historical controls treated with wide local excision with or without radiation, with reported 5-year disease-specific survival rates of 81%-87% for stage 1 disease and 63%-67% for stage 2. Although radiation wasn’t used in the study, Dr. Patel noted that more investigation is needed about the role of adjuvant radiation therapy after Mohs surgery “given recent publications showing improved outcomes in patients with narrow margin excision and postoperative radiation therapy.”
No external funding of the study was reported. Dr. Terushkin had no disclosures. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
FROM ACMS 2021
Noses can be electronic, and toilets can be smart
Cancer loses … by a nose
Since the human nose is unpredictable at best, we’ve learned to rely on animals for our detailed nozzle needs. But researchers have found the next best thing to man’s best friend to accurately identify cancers.
A team at the University of Pennsylvania has developed an electronic olfaction, or “e-nose,” that has a 95% accuracy rate in distinguishing benign and malignant pancreatic and ovarian cancer cells from a single blood sample. How?
The e-nose system is equipped with nanosensors that are able to detect the volatile organic compounds (VOCs) emitted by cells in a blood sample. Not only does this create an opportunity for an easier, noninvasive screening practice, but it’s fast. The e-nose can distinguish VOCs from healthy to cancerous blood cells in 20 minutes or less and is just as effective in picking up on early- and late-stage cancers.
The investigators hope that this innovative technology can pave the way for similar devices with other uses. Thanks to the e-nose, a handheld device is in development that may be able to sniff out the signature odor of people with COVID-19.
That’s one smart schnoz.
Do you think this is a (food) game?
Dieting and eating healthy is tough, even during the best of times, and it has not been the best of times. With all respect to Charles Dickens, it’s been the worst of times, full stop. Millions of people have spent the past year sitting around their homes doing nothing, and it’s only natural that many would let their discipline slide.
Naturally, the solution to unhealthy eating habits is to sit down and play with your phone. No, that’s not the joke, the Food Trainer app, available on all cellular devices near you, is designed to encourage healthy eating by turning it into a game of sorts. When users open the app, they’re presented with images of food, and they’re trained to tap on images of healthy food and pass on images of unhealthy ones. The process takes less than 5 minutes.
It sounds really simple, but in a study of more than 1,000 people, consumption of junk food fell by 1 point on an 8-point scale (ranging from four times per day to zero to one time per month), participants lost about half a kilogram (a little over one pound), and more healthy food was eaten. Those who used the app more regularly, along the lines of 10 times per month or more, saw greater benefits.
The authors did acknowledge that those who used the app more may have been more motivated to lose weight anyway, which perhaps limits the overall benefit, but reviews on Google Play were overall quite positive, and if there’s one great truth in this world, it’s that Internet reviewers are almost impossible to please. So perhaps this app is worth looking into if you’re like the LOTME staff and you’re up at the top end of that 8-point scale. What, pizza is delicious, who wouldn’t eat it four times a day? And you can also get it from your phone!
It’s time for a little mass kickin’
The universe, scientists tell us, is a big place. Really big. Chromosomes, scientists tell us, are small. Really small. But despite this very fundamental difference, the universe and chromosomes share a deep, dark secret: unexplained mass.
This being a medical publication, we’ll start with chromosomes. A group of researchers measured their mass with x-rays for the first time and found that “the 46 chromosomes in each of our cells weigh 242 picograms (trillionths of a gram). This is heavier than we would expect, and, if replicated, points to unexplained excess mass in chromosomes,” Ian K. Robinson, PhD, said in a written statement.
We’re not just talking about a bit of a beer belly here. “The chromosomes were about 20 times heavier than the DNA they contained,” according to the investigators.
Now to the universe. Here’s what CERN, the European Council for Nuclear Research, has to say about the mass of the universe: “Galaxies in our universe … are rotating with such speed that the gravity generated by their observable matter could not possibly hold them together. … which leads scientists to believe that something we cannot see is at work. They think something we have yet to detect directly is giving these galaxies extra mass.”
But wait, there’s more! “The matter we know and that makes up all stars and galaxies only accounts for 5% of the content of the universe!”
So chromosomes are about 20 times heavier than the DNA they contain, and the universe is about 20 times heavier than the matter that can be seen. Interesting.
We are, of course, happy to share this news with our readers, but there is one catch: Don’t tell Neil deGrasse Tyson. He’ll want to reclassify our genetic solar system into 45 chromosomes and one dwarf chromosome.
A photo finish for the Smart Toilet
We know that poop can tell us a lot about our health, but new research by scientists at Duke University is really on a roll. Their Smart Toilet has been created to help people keep an eye on their bowel health. The device takes pictures of poop after it is flushed and can tell whether the consistency is loose, bloody, or normal.
The Smart Toilet can really help people with issues such as irritable bowel syndrome and inflammatory bowel disease by helping them, and their doctors, keep tabs on their poop. “Typically, gastroenterologists have to rely on patient self-reported information about their stool to help determine the cause of their gastrointestinal health issues, which can be very unreliable,” study lead author Deborah Fisher said.
Not many people look too closely at their poop before it’s flushed, so the fecal photos can make a big difference. The Smart Toilet is installed into the pipes of a toilet and does its thing when the toilet is flushed, so there doesn’t seem to be much work on the patient’s end. Other than the, um, you know, usual work from the patient’s end.
Cancer loses … by a nose
Since the human nose is unpredictable at best, we’ve learned to rely on animals for our detailed nozzle needs. But researchers have found the next best thing to man’s best friend to accurately identify cancers.
A team at the University of Pennsylvania has developed an electronic olfaction, or “e-nose,” that has a 95% accuracy rate in distinguishing benign and malignant pancreatic and ovarian cancer cells from a single blood sample. How?
The e-nose system is equipped with nanosensors that are able to detect the volatile organic compounds (VOCs) emitted by cells in a blood sample. Not only does this create an opportunity for an easier, noninvasive screening practice, but it’s fast. The e-nose can distinguish VOCs from healthy to cancerous blood cells in 20 minutes or less and is just as effective in picking up on early- and late-stage cancers.
The investigators hope that this innovative technology can pave the way for similar devices with other uses. Thanks to the e-nose, a handheld device is in development that may be able to sniff out the signature odor of people with COVID-19.
That’s one smart schnoz.
Do you think this is a (food) game?
Dieting and eating healthy is tough, even during the best of times, and it has not been the best of times. With all respect to Charles Dickens, it’s been the worst of times, full stop. Millions of people have spent the past year sitting around their homes doing nothing, and it’s only natural that many would let their discipline slide.
Naturally, the solution to unhealthy eating habits is to sit down and play with your phone. No, that’s not the joke, the Food Trainer app, available on all cellular devices near you, is designed to encourage healthy eating by turning it into a game of sorts. When users open the app, they’re presented with images of food, and they’re trained to tap on images of healthy food and pass on images of unhealthy ones. The process takes less than 5 minutes.
It sounds really simple, but in a study of more than 1,000 people, consumption of junk food fell by 1 point on an 8-point scale (ranging from four times per day to zero to one time per month), participants lost about half a kilogram (a little over one pound), and more healthy food was eaten. Those who used the app more regularly, along the lines of 10 times per month or more, saw greater benefits.
The authors did acknowledge that those who used the app more may have been more motivated to lose weight anyway, which perhaps limits the overall benefit, but reviews on Google Play were overall quite positive, and if there’s one great truth in this world, it’s that Internet reviewers are almost impossible to please. So perhaps this app is worth looking into if you’re like the LOTME staff and you’re up at the top end of that 8-point scale. What, pizza is delicious, who wouldn’t eat it four times a day? And you can also get it from your phone!
It’s time for a little mass kickin’
The universe, scientists tell us, is a big place. Really big. Chromosomes, scientists tell us, are small. Really small. But despite this very fundamental difference, the universe and chromosomes share a deep, dark secret: unexplained mass.
This being a medical publication, we’ll start with chromosomes. A group of researchers measured their mass with x-rays for the first time and found that “the 46 chromosomes in each of our cells weigh 242 picograms (trillionths of a gram). This is heavier than we would expect, and, if replicated, points to unexplained excess mass in chromosomes,” Ian K. Robinson, PhD, said in a written statement.
We’re not just talking about a bit of a beer belly here. “The chromosomes were about 20 times heavier than the DNA they contained,” according to the investigators.
Now to the universe. Here’s what CERN, the European Council for Nuclear Research, has to say about the mass of the universe: “Galaxies in our universe … are rotating with such speed that the gravity generated by their observable matter could not possibly hold them together. … which leads scientists to believe that something we cannot see is at work. They think something we have yet to detect directly is giving these galaxies extra mass.”
But wait, there’s more! “The matter we know and that makes up all stars and galaxies only accounts for 5% of the content of the universe!”
So chromosomes are about 20 times heavier than the DNA they contain, and the universe is about 20 times heavier than the matter that can be seen. Interesting.
We are, of course, happy to share this news with our readers, but there is one catch: Don’t tell Neil deGrasse Tyson. He’ll want to reclassify our genetic solar system into 45 chromosomes and one dwarf chromosome.
A photo finish for the Smart Toilet
We know that poop can tell us a lot about our health, but new research by scientists at Duke University is really on a roll. Their Smart Toilet has been created to help people keep an eye on their bowel health. The device takes pictures of poop after it is flushed and can tell whether the consistency is loose, bloody, or normal.
The Smart Toilet can really help people with issues such as irritable bowel syndrome and inflammatory bowel disease by helping them, and their doctors, keep tabs on their poop. “Typically, gastroenterologists have to rely on patient self-reported information about their stool to help determine the cause of their gastrointestinal health issues, which can be very unreliable,” study lead author Deborah Fisher said.
Not many people look too closely at their poop before it’s flushed, so the fecal photos can make a big difference. The Smart Toilet is installed into the pipes of a toilet and does its thing when the toilet is flushed, so there doesn’t seem to be much work on the patient’s end. Other than the, um, you know, usual work from the patient’s end.
Cancer loses … by a nose
Since the human nose is unpredictable at best, we’ve learned to rely on animals for our detailed nozzle needs. But researchers have found the next best thing to man’s best friend to accurately identify cancers.
A team at the University of Pennsylvania has developed an electronic olfaction, or “e-nose,” that has a 95% accuracy rate in distinguishing benign and malignant pancreatic and ovarian cancer cells from a single blood sample. How?
The e-nose system is equipped with nanosensors that are able to detect the volatile organic compounds (VOCs) emitted by cells in a blood sample. Not only does this create an opportunity for an easier, noninvasive screening practice, but it’s fast. The e-nose can distinguish VOCs from healthy to cancerous blood cells in 20 minutes or less and is just as effective in picking up on early- and late-stage cancers.
The investigators hope that this innovative technology can pave the way for similar devices with other uses. Thanks to the e-nose, a handheld device is in development that may be able to sniff out the signature odor of people with COVID-19.
That’s one smart schnoz.
Do you think this is a (food) game?
Dieting and eating healthy is tough, even during the best of times, and it has not been the best of times. With all respect to Charles Dickens, it’s been the worst of times, full stop. Millions of people have spent the past year sitting around their homes doing nothing, and it’s only natural that many would let their discipline slide.
Naturally, the solution to unhealthy eating habits is to sit down and play with your phone. No, that’s not the joke, the Food Trainer app, available on all cellular devices near you, is designed to encourage healthy eating by turning it into a game of sorts. When users open the app, they’re presented with images of food, and they’re trained to tap on images of healthy food and pass on images of unhealthy ones. The process takes less than 5 minutes.
It sounds really simple, but in a study of more than 1,000 people, consumption of junk food fell by 1 point on an 8-point scale (ranging from four times per day to zero to one time per month), participants lost about half a kilogram (a little over one pound), and more healthy food was eaten. Those who used the app more regularly, along the lines of 10 times per month or more, saw greater benefits.
The authors did acknowledge that those who used the app more may have been more motivated to lose weight anyway, which perhaps limits the overall benefit, but reviews on Google Play were overall quite positive, and if there’s one great truth in this world, it’s that Internet reviewers are almost impossible to please. So perhaps this app is worth looking into if you’re like the LOTME staff and you’re up at the top end of that 8-point scale. What, pizza is delicious, who wouldn’t eat it four times a day? And you can also get it from your phone!
It’s time for a little mass kickin’
The universe, scientists tell us, is a big place. Really big. Chromosomes, scientists tell us, are small. Really small. But despite this very fundamental difference, the universe and chromosomes share a deep, dark secret: unexplained mass.
This being a medical publication, we’ll start with chromosomes. A group of researchers measured their mass with x-rays for the first time and found that “the 46 chromosomes in each of our cells weigh 242 picograms (trillionths of a gram). This is heavier than we would expect, and, if replicated, points to unexplained excess mass in chromosomes,” Ian K. Robinson, PhD, said in a written statement.
We’re not just talking about a bit of a beer belly here. “The chromosomes were about 20 times heavier than the DNA they contained,” according to the investigators.
Now to the universe. Here’s what CERN, the European Council for Nuclear Research, has to say about the mass of the universe: “Galaxies in our universe … are rotating with such speed that the gravity generated by their observable matter could not possibly hold them together. … which leads scientists to believe that something we cannot see is at work. They think something we have yet to detect directly is giving these galaxies extra mass.”
But wait, there’s more! “The matter we know and that makes up all stars and galaxies only accounts for 5% of the content of the universe!”
So chromosomes are about 20 times heavier than the DNA they contain, and the universe is about 20 times heavier than the matter that can be seen. Interesting.
We are, of course, happy to share this news with our readers, but there is one catch: Don’t tell Neil deGrasse Tyson. He’ll want to reclassify our genetic solar system into 45 chromosomes and one dwarf chromosome.
A photo finish for the Smart Toilet
We know that poop can tell us a lot about our health, but new research by scientists at Duke University is really on a roll. Their Smart Toilet has been created to help people keep an eye on their bowel health. The device takes pictures of poop after it is flushed and can tell whether the consistency is loose, bloody, or normal.
The Smart Toilet can really help people with issues such as irritable bowel syndrome and inflammatory bowel disease by helping them, and their doctors, keep tabs on their poop. “Typically, gastroenterologists have to rely on patient self-reported information about their stool to help determine the cause of their gastrointestinal health issues, which can be very unreliable,” study lead author Deborah Fisher said.
Not many people look too closely at their poop before it’s flushed, so the fecal photos can make a big difference. The Smart Toilet is installed into the pipes of a toilet and does its thing when the toilet is flushed, so there doesn’t seem to be much work on the patient’s end. Other than the, um, you know, usual work from the patient’s end.